Abstract
Medical gases are pharmaceutical gaseous molecules which offer solutions to medical needs and include traditional gases, such as oxygen and nitrous oxide, as well as gases with recently discovered roles as biological messenger molecules, such as carbon monoxide, nitric oxide and hydrogen sulphide. Medical gas therapy is a relatively unexplored field of medicine; however, a recent increasing in the number of publications on medical gas therapies clearly indicate that there are significant opportunities for use of gases as therapeutic tools for a variety of disease conditions. In this article, we review the recent advances in research on medical gases with antioxidant properties and discuss their clinical applications and therapeutic properties.
Keywords: medical gas, antioxidant, carbon monoxide, hydrogen, nitric oxide
Introduction
The use of medical gases to treat oxidative stress is an exciting and evolving therapeutic possibility. Oxidative stress is the process of cellular injury caused by excessive levels of reactive oxygen species (ROS). ROS are highly reactive, unstable molecules generated during a variety of energy-generating biochemical reactions and cellular functions. ROS function as necessary signaling molecules, critically modulate the activation of the immune system and, thus, participate in antibacterial defense [1]. However, when ROS formation is unbalanced in proportion to protective antioxidants, the excess ROS can cause toxic effects that damage all components of the cell including lipids, proteins, and DNA and ultimately lead to cell death. Antioxidants prevent oxidant formation or scavenge the ROS produced under conditions of oxidative stress by binding and inactivating them. Most antioxidants are electron donors and react with ROS, which are free radicals, to form innocuous end products, such as water.
The primary sources of oxidative injury include superoxide anion (·O2−) and hydrogen peroxide (H2O2), which undoubtedly play important roles; however, these species also produce highly reactive hydroxyl radicals (·OH) and peroxynitrite (ONOO−) through the Haber-Weiss or Fenton reactions [2, 3]. Oxidative stress resulting from an imbalance between pro-oxidant and antioxidant systems, has been implicated in many diseases including cardiovascular disease, cancer [4], chronic inflammatory disease [5], hypertension [6], ischemia/reperfusion injury [7], acute respiratory distress syndrome (ARDS) [8], and neurodegerative diseases such as Parkinson’s disease and Alzheimer’s disease [9, 10], as well as in aging [11]. Exogenous administration of antioxidants has been utilized as a therapeutic approach and there is evidence that antioxidants protect against oxidative stress and prevent the pathological processes of a wide range of diseases.
Medical gases are pharmaceutical gaseous molecules which offer solutions to medical needs. They include traditional gases, like oxygen and nitrous oxide, as well as gases with recently discovered roles as biological messenger molecules including nitric oxide, carbon monoxide and hydrogen sulphide. In this article, we will review recent advances and current knowledge pertaining to antioxidant medical gases including the three gaseous signaling molecules (nitric oxide, carbon monoxide and hydrogen sulphide), as well as hydrogen, xenon and ozone. Additionally, we will discuss their clinical applications and therapeutic properties (Table 1). These gases may be toxic, hazardous or poisonous at a higher concentration, however; they are safe and potentially therapeutic at lower concentrations. Medical gases can be administered in a straightforward way simply by providing the gas for the patients to inhale using a ventilator circuit, facemask, or nasal cannula. While there are a variety of delivery systems presently in use and even more under development, the basic design and goal of each system is to provide safe gas delivery and precision gas analysis or monitoring. In addition to the development of safe devices for inhaled medical gas, potential clinical application may include a parenteral injectable or a drug containing a gas-releasing moiety. Medical gas therapy is a relatively unexplored field of medicine; however, a recent increase in publications in the medical gas field clearly indicates that there are significant opportunities for the use of medical gases as therapeutic tools [12, 13].
Table 1.
Antioxidant medical gas
| nitric oxide | carbon monoxode | hydrogen | hydrogen sulphide | xenon | ozone | |
|---|---|---|---|---|---|---|
| formula | NO | CO | H2 | H2S | Xe | O3 |
| color, odor | colorless, a mild, sweet odor | colorless, odorless | colorless, odorless | colorless, smell like rotten egg | colorless, odorless | pale blue a sharp, cold, irritating odor |
| receptors | heme proteins K/Ca channel | heme proteins K/Ca channel | unknown | KATP channels | N-methyl-D- aspartate (NMDA) | not determined |
| flammable | no | no | yes | yes | yes | no |
| toxicity | yes | yes | no | yes | yes | yes |
| produced in mammalian cells? | Yes. From L- arginine by nitric oxide synthase (NOS), or reduction of nitrite | Yes. Through heme degradation by heme oxygenase | No | Yes. From L- cysteine by CBS and CBE | No | Yes. In the white blood cells and other biological systems |
| application except medical use | rare except medical use | industrial | fuels | analytical chemistry | light emitting devise | bleaching substances |
| effects for vessels | vasodilation | vasodilation | unknown | vasodilation | no change | vasodilation |
| anti-apoptotic effects | yes | yes | yes | yes | yes | yes (preconditioning) |
| anti-inflammatory effects | yes | yes | yes | yes | yes | yes (preconditioning) |
| therapeutic human use | pulmonary hypertension, lung transplantation ARDS | not available | decompression sickness in divers, breath test for mal-absorption | not available | general anesthesia, medical imaging (133Xe) | cancers, chronic fatigue syndrome, infectious disease |
Nitric Oxide (NO)
Nitric oxide (NO) is a colorless and poisonous gas which is generated by automobile and thermal power plants and causes a serious air pollutant. NO concentration in unpolluted air is approximately 0.01 parts per million (ppm). NO, together with NO2, participates in damage of ozone layer by absorbing high frequency ultraviolet light from the sun. However, NO is an important signaling molecule in the body of mammals and was named “Molecule of the Year” in 1992 [14]. Inhaled NO is a relatively new United States Food and Drug Administration (FDA) investigational drug and numerous facilities are involved in clinical trials utilizing NO.
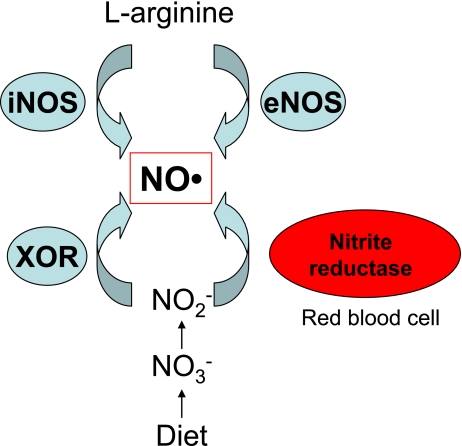
In normoxic conditions, NO is synthesized endogenously from the amino acid, L-arginine, by nitric oxide synthase (NOS). NO is also generated by the reaction of near-physiological levels of nitrite with deoxyhemoglobin, a nitrite reductase, along the physiological oxygen gradient [15]. In hypoxic or acidotic conditions, NO is generated by reduction of nitrite. Under these conditions of very low tissue pH and oxygen tension, nitrite may be reduced to NO by disproportionation (acid reduction) or by the enzymatic action of xanthine oxidoreductase (XOR) (Fig. 1) [16, 17]. The half life of NO is measured in seconds and NO is eliminated rapidly as nitrite and nitrate in urine. Nitrite is also considered to have a role as an endocrine pool of NO [18].
Fig. 1.
Biological NO production in human body.
NO is generated from L-arginine by inducible nitric oxide synthase (iNOS) and endothelial NOS (eNOS). Alternatively, NO is produced from diet-derived nitrates, which are reduced to nitrite by bacteria in the alimentary tract. At very low tissue pH and oxygen tension, nitrite may be reduced to NO by the enzymatic action of xanthine oxidoreductase (XOR). Also, physiological levels of nitrite are reduced to NO by reaction with deoxyhemoglobin, a nitrite reductase, in the along the physiological oxygen gradient.
Blood vessel dilation is one of the most well-known effects of NO. NO stimulates soluble guanylate cyclase (sGC) and increases cGMP content in vascular smooth muscle cells, resulting in relaxation of vascular tone and vasodilation. Sildenafil (Viagra®), a selective inhibitor of phosphodiesterase type 5 (PDE5) in vascular smooth muscle, blocks the degradation of cGMP, leading to relaxation of blood vessels by increasing levels of cGMP. This action of sildenafil has been used for stimulation of erections primarily by enhancing signaling through the NO pathway [19]. People taking nitrate medications that are converted to NO in the body, such as nitroglycerin, are contraindicated for PDE5 inhibitor treatment due to the potential of overstimulating the NO-cGMP pathway. In addition to its vasorelaxative effect, NO has a complex spectrum of actions including the regulation of platelet activity and the preservation of the normal structure of the vessel wall. NO, produced by endothelial nitric oxide synthase (eNOS) in vascular endothelial cells, activates sGC and plays a key anti-inflammatory role by inhibiting P-selectin expression and leukocyte recruitment [20]. Thus, the actions of NO on blood vessels may increase tissue blood supply and abate the inflammatory response, leading to protection of the tissues from oxidative insults.
NO abates oxidative injury via several mechanisms. NO reacts with peroxy and oxy radicals generated during the process of lipid peroxidation. These peroxy and oxy radical adducts continue the chain reactions in lipid peroxidation, resulting in compromised cell membranes [21]. The reactions between NO and these ROS can terminate lipid peroxidation and protect tissues from ROS-induced injuries [22]. Through the Fenton reaction, hydrogen peroxide oxidizes iron (II) and in the process generates an extremely reactive intermediate (the hydroxyl radical) which then carries out oxidations of various substrates [H2O2 + Fe2+ → Fe3+ + OH− + hydroxyl radical (·OH)]. NO prevents hydroxyl radical formation by blocking the predominant iron catalyst in the Fenton reaction [2]. Furthermore, NO reacts with iron and forms an iron-nitrosyl complex, inhibiting iron’s catalytic functions in the Fenton reaction [23]. Treatment of rat hepatocytes with NO imparts resistance to H2O2-induced cell death by induction of the rate-limiting antioxidant enzyme, heme oxygenase (HO)-1 [24]. In bacteria, NO activates the redox-sensitive transcriptional regulator protein (OxyR), resulting in the subsequent expression of proteins protective against ROS [25]. In addition, NO prevents the induction of some ROS-induced genes during tissue injury such as early growth response-1 (Egr-1), which activates a number of adhesion molecules and accelerates oxidative tissue injuries [26]. Thus, multiple mechanisms underlie the antioxidant properties of NO.
Identification of the cytoprotective abilities of NO to mitigate oxidative injuries in many model systems led to numerous formal studies that examined the effect NO on human patients. Multiple single-center studies demonstrated the ability of inhaled NO to improve the outcome of patients with ARDS. NO inhalation was associated with a redistribution of blood flow to well-ventilated areas in the lungs and improved oxygen levels in the blood [27, 28]. On the other hand, several studies showed that NO had no affects on ARDS [27, 29]. Similarly, some studies advocate inhalation of NO as a method to prevent graft injury due to ischemia/reperfusion injury after human lung [30] and liver transplantation [31], while others have demonstrated detrimental or marginal effects [32–34]. Some of the adverse effects of NO may be due to the formation of peroxynitrite (ONOO−), a powerful pro-oxidant capable of causing organ injuries, by reaction of NO with superoxide anion [35]. Thus, studies examining the delivery of NO in experimental and clinical studies have shown discrepant results. NO may be linked both to protective and toxic effects after oxidative insults, depending on NO levels, NO source, timing of NO administration and the environment, suggesting a narrow therapeutic window for NO administration in the treatment of oxidative injuries [36].
Carbon Monoxide (CO)
CO occurs in nature as a product of oxidation or combustion of organic matter. CO is an invisible, chemically inert, colorless and odorless gas and is commonly viewed as a poison. CO avidly binds to hemoglobin and forms carboxyhemoglobin (COHb) with an affinity 240 times higher than that of oxygen, resulting in interference with the oxygen-carrying capacity of the blood and consequent tissue hypoxia. COHb levels of 10–30% can cause headache, shortness of breath and dizziness, and higher levels (30–50%) produce deleterious toxicity, such as severe headache, vomiting, syncope and arrhythmia, possibly death [37]. Thus, CO is widely known to be toxic at high concentrations.
Similar to NO, the gaseous molecule CO is endogenously and physiologically generated in mammalian cells via the catabolism of heme in the rate-limiting step by heme oxygenase (HO) systems [38]. Catabolism of heme by HO-1 is highly induced in a variety of tissues in response to diverse stress-related conditions [39], and provides generalized endogenous cytoprotection [40]. The specific mechanisms by which HO-1 can mediate endogenous cytoprotective functions are not clear, but byproducts generated during the heme catabolism such as CO, iron and biliverdin, have been suggested as potential protective mediators [41, 42]. In fact, CO, generated by HO-1 or exogenously administered, has beneficial biological and physiological functions. Potent therapeutic efficacies of CO have been demonstrated using experimental models for many conditions, including paralytic ileus [43], hemorrhagic shock [44], hyperoxic lung injury [45], and endotoxiemia [46], supporting the new paradigm that, at low concentrations, CO functions as a signaling molecule that exerts significant cytoprotection.
Several possible mechanisms have been postulated to explain the antioxidant effects of CO. CO binds to the heme moiety of mitochondrial cytochrome c oxidase and substantially decreases mitochondria-derived ROS [47, 48]. Additionally, this inhibition of mitochondria-derived ROS can result in low level ROS generation and trigger adaptive responses and cell survival, a novel mechanism to explain redox signaling by CO [49–51]. The strong affinity of CO for the heme moiety of other heme-containing proteins (referred to collectively as heme proteins) may also account for the antioxidant effects afforded by CO. During hemorrhage, hemolysis, or ischemia/reperfusion injury, damaged heme proteins are prone to degrade and release heme, which is highly lipophilic and detrimental. Heme can directly induce tissue injury by rapidly promoting peroxidation of the lipid membranes of the cells [52, 53]. Furthermore, free heme derived from degraded heme proteins during cellular injury is implicated as the source of catalytic iron that would participate in the Fenton reaction, converting H2O2 to more reactive hydroxyl radicals and promoting severe tissue damage by propagating lipid peroxidation. CO may prevent the degradation of heme proteins by binding to the heme moiety.
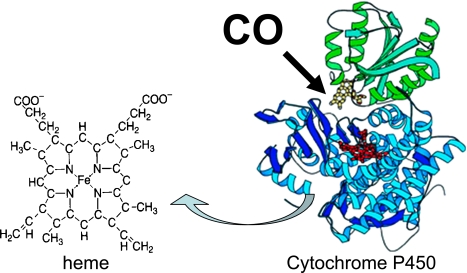
Cytochrome P450s (CYP), a large group of heme proteins which are abundant in many organs, are prone to degradation and the release heme and iron and play a critical roles during organ injury by various insults (Fig. 2) [54–62]. Recently, our group demonstrated that CO in the organ preservation solution used during transplantation can bind to and stabilize renal CYP and prevent CYP degradation and detrimental heme/iron release in renal grafts. This resulted in potent protection from transplant-induced ischemia/reperfusion injury [63]. Syngenic orthotopic kidney transplantation (KTx) with 24 h cold ischemia in UW solution (Viaspan®, Du Pont, Wilmington, DE) was performed using inbred male LEW (RT.1l) rats. The excised graft was flushed with and preserved in UW or CO-supplemented UW (CO content; 40.6 ± 1.6 µmol/L). The graft functions treated in UW with CO showed better renal functions and fewer inflammatory events. The grafts stored in control preservation solution exhibited a markedly decreased total CYP levels in rat kidney 3 h after reperfusion, indicating CYP degradation during ischemia/reperfusion injury [63]. In contrast, the grafts stored in UW with CO maintained CYP enzyme at the levels comparable to those seen in normal kidney [63]. These data indicate that ex vivo organ-targeted CO delivery during cold storage prevents CYP breakdown during the ischemia/reperfusion process.
Fig. 2.
Hypothetic scheme for the binding of CO to the heme moiety of cytochrome P450.
Cytochrome P450 (CYP) proteins are susceptible to oxidative stress and are liable to degrade and release prooxidant heme during cellular damage. CO may bind in the heme pocket of CYP and stabilize CYP, thereby prevent CYP degradation.
In addition to its oxidative abilities, described above, free heme activates vascular endothelial cells and upregulates adhesion molecules such as intercellular adhesion molecule (ICAM)-1 and E-selectin. These heme-induced adhesive events cause massive cellular infiltration and increased vascular permeability, contributing to the pathogenesis of local inflammatory processes by induction of monocyte chemoattractant protein (MCP-1) and nuclear factor (NF)-kappaB [64]. To cope with the problems caused by high free heme concentrations, the body is equipped with various defense mechanisms, namely the HO system. CO treatment can induce HO-1 in cells to protect against injury [65–67]. Thus, a detrimental excess of heme can be immediately removed by HO-1 enzymatic activity, induced by CO. The adverse effects of inhaled CO are a major concern for clinical use. CO combines with hemoglobin, interferes with the oxygen-carrying capacity of the blood and leads to tissue hypoxia. Soluble forms of CO, such as CO-releasing molecules, may overcome this problem and allow clinical application [68, 69]. Currently, several human clinical trials are ongoing for various pathophysiologic disease states testing the therapeutic effects of inhaled CO administered at concentrations similar to those used in animal transplantation models [70, 71]. A recent study demonstrated that the application of CO to animals at low concentrations approximating cigarette-smoke exposure caused no apparent lung pathology [72]. However, Myer et al. failed to obtain similar anti-inflammatory effects of CO in a human endotoxemia model as were seen in small and large animal experiments [71]. These discrepancies may be attributed to species-specific differences in the affinity of CO for hemoglobin, or physiological differences such as respiratory rate and sensitivity to lipopolysaccharides (endotoxins) [73, 74].
Hydrogen (H2)
Hydrogen (H2) is the lightest and most abundant of chemical elements, constituting nearly 90% of the universe’s elemental mass. In contrast, earth’s atmosphere contains less that 1 ppm of hydrogen. In concentrations over 5%, hydrogen can form explosive mixtures with air, as typified by the 1937 Hindenberg Zeppelin disaster. Although hydrogen is known to be highly flammable and to violently react with oxidizing elements, it is noteworthy that hydrogen has no risk of explosion at concentrations less than 4.6% (Safety and Standard for hydrogen and hydrogen systems; National Aeronautics and Space Administration “NASA”, 1997). Hydrogen has wide applications in physics and engineering. As hydrogen is a highly potent energy source, the industrial use of hydrogen is expanding, such as the use of hydrogen fuel cells for zero-emission vehicles.
In fact, hydrogen is physiologically and continuously produced in our body during fermentation of non-digestible carbohydrates, primarily in the large intestine, by numerous strains of intestinal bacteria and is excreted as flatus, further metabolized by flora or exhaled as a natural component of abdominal gas [75, 76], the basis for the routinely-used hydrogen breath test for gastrointestinal transit [77, 78]. Increased excretion of hydrogen in the breath after carbohydrate ingestion is considered a consequence of bacterial fermentation in the colon [79, 80]. Hydrogen gas is routinely administered to divers as hydreliox, which contains 49% hydrogen [81].
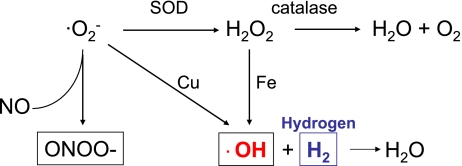
Gharib et al. reported that animals maintained in a hydrogen-supplemented hyperbaric chamber were significantly protected from schistosomiasis-associated chronic liver injury [82]. Hydrogen treatment significantly increased antioxidant enzyme activity, decreased lipid peroxide levels and decreased circulating proinflammatory cytokine levels. Consistent with this first report showing the antioxidant effect of hydrogen, Ohsawa et al. demonstrated that inhaled hydrogen gas (~4%) has antioxidant and anti-apoptotic properties that can protect the brain against ischemia-induced injury and stroke by selectively neutralizing the detrimental ROS [83]. Hydrogen selectively reduces the levels of hydroxyl radicals (·OH) mainly generated through the Fenton reaction and peroxynitrite (ONOO−) in vitro (Fig. 3). This report also suggests that hydrogen easily crosses the brain-blood barrier and can be safely administered to human patients [81]. More recently, our group has shown that hydrogen treatment ameliorates transplant-induced intestinal injuries including mucosal erosion and mucosal barrier breakdown. Perioperative inhalation of 2% hydrogen mitigated intestinal dysmotility following transplantation and reduced upregulation of inflammatory mediators, such as chemokine (C-C motif) ligand 2 (CCL2), interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α. Hydrogen significantly diminished lipid peroxidation compared to air-treated grafts, as indicated by elevated levels of malondialdehyde, a lipid peroxidation product, in air-treated grafts demonstrating an antioxidant effect of hydrogen [84]. Thus, treatment with hydrogen has several potential advantages over current therapies used for ischemia/reperfusion or oxidative injury.
Fig. 3.
Hydrogen scavenges hydroxyl radicals.
Hydrogen selectively neutralize hydroxyl radicals generated by the Fenton reaction.
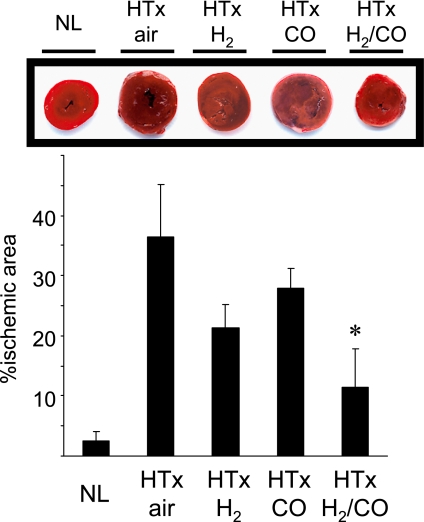
The positive results of our experiments with CO inhalation and hydrogen inhalation alone led us to conduct experiments toward potentially more potent therapeutic gas strategy combined CO and hydrogen. The rationale behind this approach was based on our unpublished data and on previous observations demonstrating several differences in the biological actions of CO and hydrogen, even though both ameliorate cardiac cold IRI [85, 86]. There is no chemical reaction between CO and hydrogen at room temperature, and the gases can be administered together safely. The combined effects of CO and hydrogen were evaluated using our established model of cold ischemia/reperfusion injury. In this model, syngeneic heart transplantation (HTx) is conducted from Lewis (RT1l) rats to Lewis rats with 18 h of cold ischemic time in Celsior® (Pasteur Merieux Serums et Vaccins, Lyon, France). HTx is performed into the abdomen by anastomosing the graft aorta and recipient infrarenal aorta and the graft pulmonary artery and recipient inferior vena cava in an end-to-side manner [86, 87]. Myocardial injury is evaluated by measurement of the ischemic area of the grafts as visualized by 2,3,5-triphenyltetrazolium chloride (TTC) staining 3 h after reperfusion [87, 88]. Cold ischemia/reperfusion injury results in significant damage to the cardiomyocytes. However, when both donor and recipient were treated with mixed gas therapy with 250 ppm of CO, 2% hydrogen in balanced air, the ischemic area was significantly reduced, while either CO or hydrogen alone did not significantly decrease the infarct area compared to untreated controls (Fig. 4). These results suggested that dual gas therapy with CO and hydrogen may have more potent protective effects against cardiac cold ischemia/reperfusion injury compared to single treatment.
Fig. 4.
Hydrogen inhalation reduced ischemic area following heart grafts with prolonged cold ischemia.
The extent of gross structural damage to heart graft was evaluated by TTC staining 3 h after reperfusion. Normal naïve hearts had negligible infarct area. The infarct area was significantly reduced by dual-treatment with CO and hydrogen, while either CO or hydrogen alone did not significantly decrease the infarct area compared to untreated controls. (NL; normal naïve heart, HTx: heart transplant, n = 4–5 for each group, *p<0.05 vs HTx/air).
Hydrogen Sulphide (H2S)
Hydrogen sulphide (H2S) is a colorless, toxic and flammable gas. It is a naturally occuring gas found in volcanic gases and some well waters and is also responsible for the foul odor of rotten eggs and flatulence. Toxic effects of hydrogen sulphide in humans include eye irritation, shortness of breath, and chest tightness at concentrations <100 ppm [89, 90]. Exposure to hydrogen sulphide at >1000 ppm may cause severe adverse effects, ranging from loss of consciousness to fatality [91]. Measurement of the concentration of thiosulfate in blood and urine is useful for determining hydrogen sulphide poisoning [92].
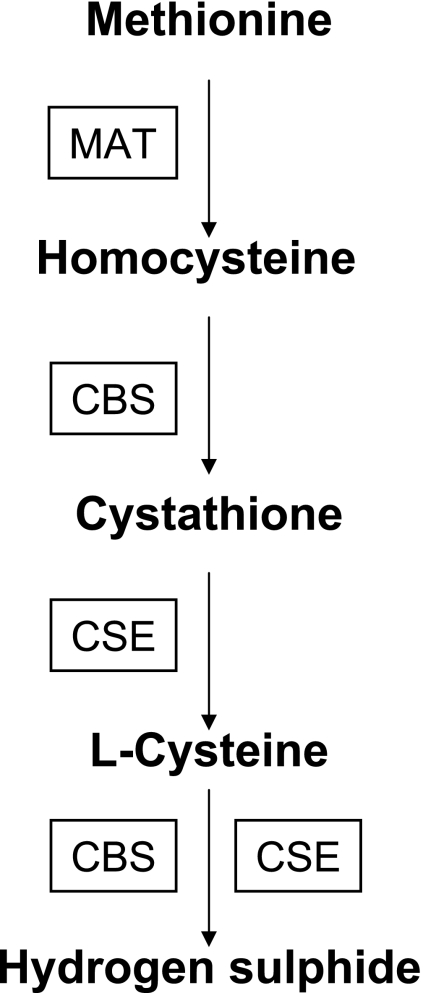
Hydrogen sulphide is endogenously synthesized from L-cysteine, a product of food-derived methionine, by cystathiononeβ-synthase (CBS) and cyctathione-γ-lyase (CSE) (Fig. 5). Methionine adenosyltransferase (MAT) converts methionine to homocystine. CBS catalyses the homocystine to cystathionine conversion. CSE, subsequently, converts cystathionine to L-cysteine. L-cysteine is further metabolized to hydrogen sulphide by CBS and CSE [93, 94]. Hydrogen sulphide is produced normally in vertebrates, and is believed to help regulate body temperature and metabolic activity at physiological concentrations [95, 96]. Also, hydrogen sulphide exerts physiological effects in the cardiovascular system of vertebrates, possibly through modulation of K+-ATP channel opening or as a cellular messenger molecule involved vascular flow regulation [97, 98]. Recent studies have shown that hydrogen sulphide is a physiologic gaseous signaling molecule, as are NO and CO [12].
Fig. 5.
Enzymatic pathways for hydrogen sulphide production.
MAT; methionine adenosyltransferase, CBS; cystathiononeβ-synthase, CSE; cyctathione-γ-lyase
Administration of hydrogen sulphide produced a “suspended animation-like” metabolic status with hypothermia and reduced oxygen demand in pigs (who received it intravenously) [99] and mice (who received hydrogen sulphide via inhalation) [100, 101], thus protecting from lethal hypoxia. This hypometabolic state, which resembles hibernation, induced by hydrogen sulphide may contribute to tolerance against oxidative stress.
Some of the antioxidant effects of hydrogen sulphide can be explained by its effects on cytochome c oxidase and mitochondrial functions, while its effects on gene expression may be related to actions on the the NFkB and extracellular signal-regulated kinase (ERK) pathways [102]. Cardiac protection from oxidative injury is, at least in part, due to the ability of hydrogen sulphide to activate myocardial KATP channels, although this is still being elucidated [103]. Whiteman et al. showed that hydrogen sulphide has the potential to act as an antioxidant inhibitor of peroxynitrite-mediated processes via activation of N-methyl-D-aspartate (NMDA) receptors [104]. Kimura et al. revealed that hydrogen sulphide can shield cultured neurons from oxidative damage by increasing levels of glutathione, an antioxidant enzyme [105]. Similarly, hydrogen sulphide can induce upregulation of HO-1, anti-inflammatory and cytoprotective genes [102, 106]. Hydrogen sulphide also inhibits myeloperoxidase and destroys H2O2 [107] and can reduce ischemia/reperfusion-induced apoptosis via reduction of cleaved caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) [108]. Thus, multiple mechanisms may be involved in the antioxidant properties of hydrogen sulphide.
Xenon
Xenon is a noble gas considered chemically inert and unable to form componds with other molecules. Xenon is a trace gas in Earth’s atmosphere, occurring at <0.001 ppm and is also found in gases emitted from some mineral springs. Xenon is not a “greenhouse gas” and,in fact, is viewed as enviromentally friendly gas [109].
Xenon possesses anesthetic properties in animals and humans [109–111]. Since Cullen first used xenon on human patients in 1951, xenon has been successfully used in a number of surgical operations [110]. Although the cost of xenon has been too high to be used routinely in surgical practice, xenon anesthesia systems are still being proposed and designed for certain cases of patients, as xenon has several advantages including greater circulatory stability, lower analgesic consumption, lower adrenaline levels and better regional perfusion of individual organs [109, 112]. Xenon readily crosses the blood-brain barrier and has low blood/gas solubility, which is advantageous for rapid inflow and washout [113]. Human patients anaesthetized with xenon show good cardiovascular stability and satisfactory sedation [111]. Also, xenon inhalation had no measurable effects on mesenteric vascular resistance in the propofol-sedated pig [114].
In addition to its anesthetic properties, xenon has protective effects against cerebral ischemia [115]. Decreased blood flow to the brain leads to neuronal death through necrotic and apoptotic mechanisms, which are largely dependent on the activation of the NMDA receptor [116]. Since xenon effectively inhibits the NMDA receptor, the neuroprotective effects of xenon may be at least partially due to this inhibition [115, 117–119]. Similarly, Abraini et al. showed that xenon reduced ischemia-induced neuronal death induced by occlusion of the middle cerebral artery in rodents, and decreased NMDA-induced Ca2+ influx, a critical event involved in excitotoxicity, in neuronal cell cultures [120]. Also, xenon has inhibitory effects on the Ca2+-ATPase pump and interferes with the Ca2+ intracellular signaling pathway, which might also be involved in the prevention of ischemic injury [121].
There is evidence suggesting that brief exposure to xenon prevents myocardial ischemia/reperfusion injury [122]. Although exact mechanism involved in prevention of ischemia-induced myocardial injury are still not fully elucidated, xenon significantly activates protein kinase C (PKC)-epsilon and leads to phosphorylation of p38 mitogen-activated protein kinase (MAPK). This signaling may represent a central molecular mechanism for xenon’s protective effects [122, 123]. Interestingly, other noble gases, such as helium, neon and argon, have also been shown to convey protection against cardiac ischemia/reperfusion injury [124].
Ozone
Ozone is a triatomic molecule, consisting of three oxygen atoms. Ozone is a pale blue gas with a sharp, cold, irritating odor and is produced naturally by electrical discharges following thunderstorms or ultraviolet (UV) rays emitted from the sun. Ozone is present in low concentrations throughout the Earth’s atmosphere; however, an ozone layer exists between 10 km and 50 km above from the surface of the earth and plays a very important role filtering UV rays which is critical for the maintenance of biological balance in the biosphere [125, 126]. Ozone gas has a high oxidation potential and is used in as an antimicrobial agent against bacteria, viruses, fungi, and protozoa. In particular, supplementation of ozone into spas and hot tubs can reduce the amount of chlorine or bromine required to maintain cleanliness by reactivating chlorine and bromine to their free states. Also, ozone is also widely used in treatment of water in aquariums and fish ponds to minimize bacterial growth, control parasites, and eliminate transmission of some diseases. Ozone inhalation (0.1 to 1 ppm) can be toxic to the pulmonary system and cause upper respiratory irritation, rhinitis, headache, and occasionally nausea and vomiting. Ozone, administered by rectal insufflation, prior to ischemia/reperfusion injury, prevents the damage induced by ROS and attenuate renal and hepaticischemia/reperfusion injury [127–129]. It is postulated that ozone could prepare the host to face physiopathological events mediated by ROS, through NO-related mechanisms by modulating increases in eNOS and iNOS expression [127, 130, 131]. In addition, ozone has vasodilative effects without affecting any other cardiopulmonary parameters [132]. Al-Dalain et al. demonstrated that the ozone treatment improved glycemic control and prevented oxidative stress in diabetic rats. This study suggested that repeated administration of ozone in non-toxic doses might play a role in the control of diabetes and its complications [133]. Medical applications of blood ozonation via extracorporeal blood oxygenation and ozonation (EBOO) was found to be safe and effective in treating peripheral artery disease in clinical trials [126, 134–136].
Conclusion
As highlighted above, the ability of medical gases to ameliorate oxidative stress plays important roles at the chemical, cellular and physiological levels. Although some medical gases may cause serious adverse effects, there are still many possible applications of these gases as therapeutic tools for various diseases if the concentrations are tightly controlled. The future of medical gas therapy must focus on the establishment of safe and well-defined administration parameters and on randomized controlled trials to determine the precise indications and guidelines for the use of medical gases in the treatment of various pathologies.
Acknowledgement
We thank Shannon L. Wyszomierski PhD, Heart, Lung and Esophageal Surgery Institute, University of Pittsburgh Medical Center for excellent scientific editorial services. We also wish to thank Dr Noriko Murase, Dr Junichi Kohmoto and Dr David R Kaczorowski for valuable supports.
Abbreviations
- ARDS
acute respiratory distress syndrome
- CBS
cystathiononeβ-synthase
- cGMP
cyclic guanosine monophosphate
- CO
carbon monoxide
- COHb
carboxyhemoglobin
- CSE
cyctathione-γ-lyase
- CYP
Cytochrome P450s
- ERK
extracellular signal-regulated kinase
- H2O2
hydrogen peroxide
- HO
heme oxygenase
- HTx
heart transplantation
- ICAM
intercellular adhesion molecule
- IL
interleukin
- KTx
kidney transplantation
- MAPK
mitogen-activated protein kinase
- NFκB
nuclear factor-κB
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- NOS
nitric oxide synthase
- ·OH
hydroxyl radicals
- ONOO−
peroxynitrite
- ·O2−
superoxide anion
- PDE
phosphodiesterase
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
- UV
ultraviolet
- XOR
xanthine oxidoreductase
References
- 1.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 2.Menasche P., Grousset C., Gauduel Y., Mouas C., Piwnica A. Prevention of hydroxyl radical formation: a critical concept for improving cardioplegia. Protective effects of deferoxamine. Circulation. 1987;76:180–185. [PubMed] [Google Scholar]
- 3.Bernard M., Menasche P., Pietri S., Grousset C., Piwnica A., Cozzone P.J. Cardioplegic arrest superimposed on evolving myocardial ischemia. Improved recovery after inhibition of hydroxyl radical generation by peroxidase or deferoxamine. A 31P nuclear resonance study. Circulation. 1988;78:164–172. [PubMed] [Google Scholar]
- 4.Cerutti P.A., Trump B.F. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991;3:1–7. [PubMed] [Google Scholar]
- 5.Ha H., Park J., Kim Y.S., Endou H. Oxidative stress and chronic allograft nephropathy. Yonsei. Med. J. 2004;45:1049–1052. doi: 10.3349/ymj.2004.45.6.1049. [DOI] [PubMed] [Google Scholar]
- 6.Watson T., Goon P.K., Lip G.Y. Endothelial Progenitor Cells, Endothelial Dysfunction, Inflammation, and Oxidative Stress in Hypertension. Antioxid. Redox Signal. 2008;10:1079–1788. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 7.Nakao A., Kaczorowski D.J., Sugimoto R., Billiar T.R., McCurry K.R. Application of heme oxygenase-1, carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 2008;42:78–88. doi: 10.3164/jcbn.2008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tasaka S., Amaya F., Hashimoto S., Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid. Redox Signal. 2008;10:739–753. doi: 10.1089/ars.2007.1940. [DOI] [PubMed] [Google Scholar]
- 9.Nunomura A., Moreira P.I., Takeda A., Smith M.A., Perry G. Oxidative RNA damage and neurodegeneration. Curr. Med. Chem. 2007;14:2968–2975. doi: 10.2174/092986707782794078. [DOI] [PubMed] [Google Scholar]
- 10.Loh K.P., Huang S.H., De Silva R., Tan B.K., Zhu Y.Z. Oxidative stress: apoptosis in neuronal injury. Curr. Alzheimer. Res. 2006;3:327–337. doi: 10.2174/156720506778249515. [DOI] [PubMed] [Google Scholar]
- 11.Wei Y.H., Lu C.Y., Wei C.Y., Ma Y.S., Lee H.C. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin. J. Physiol. 2001;44:1–11. [PubMed] [Google Scholar]
- 12.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug. Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 13.Dingley J., Tooley J., Porter H., Thoresen M. Xenon provides short-term neuroprotection in neonatal rats when administered after hypoxia-ischemia. Stroke. 2006;37:501–506. doi: 10.1161/01.STR.0000198867.31134.ac. [DOI] [PubMed] [Google Scholar]
- 14.Culotta E., Koshland D.E. Jr. NO news is good news. Science. 1992;258:1862–1865. doi: 10.1126/science.1361684. [DOI] [PubMed] [Google Scholar]
- 15.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X., Huang K.T., Shields H., Kim-Shapiro D.B., Schechter A.N., Cannon R.O. 3rd, Gladwin M.T. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 16.Godber B.L., Doel J.J., Sapkota G.P., Blake D.R., Stevens C.R., Eisenthal R., Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 2000;275(11):7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 17.Webb A., Bond R., McLean P., Uppal R., Benjamin N., Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 19.Cartledge J., Minhas S., Eardley I. The role of nitric oxide in penile erection. Expert Opin Pharmacother. 2001;2:95–107. doi: 10.1517/14656566.2.1.95. [DOI] [PubMed] [Google Scholar]
- 20.Ahluwalia A., Foster P., Scotland R.S., McLean P.G., Mathur A., Perretti M., Moncada S., Hobbs A.J. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of P-selectin expression and leukocyte recruitment. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1386–13891. doi: 10.1073/pnas.0304264101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am. J. Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 22.Padmaja S., Huie R.E. The reaction of nitric oxide with organic peroxyl radicals. Biochem. Biophys. Res. Commun. 1993;195:539–544. doi: 10.1006/bbrc.1993.2079. [DOI] [PubMed] [Google Scholar]
- 23.Wink D.A., Miranda K.M., Espey M.G., Pluta R.M., Hewett S.J., Colton C., Vitek M., Feelisch M., Grisham M.B. Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox Signal. 2001;3:203–213. doi: 10.1089/152308601300185179. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.M., Bergonia H., Lancaster J.R., Jr. Nitrogen oxide-induced autoprotection in isolated rat hepatocytes. FEBS. Lett. 1995;374:228–232. doi: 10.1016/0014-5793(95)01115-u. [DOI] [PubMed] [Google Scholar]
- 25.Nunoshiba T., deRojas-Walker T., Wishnok J.S., Tannenbaum S.R., Demple B. Activation by nitric oxide of an oxidative-stress response that defends Escherichia coli against activated macrophages. Proc. Natl. Acad. Sci., U. S. A. 1993;90:9993–9997. doi: 10.1073/pnas.90.21.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu J.J., Wung B.S., Hsieh H.J., Lo L.W., Wang D.L. Nitric oxide regulates shear stress-induced early growth response-1. Expression via the extracellular signal-regulated kinase pathway in endothelial cells. Circ. Res. 1999;85:238–246. doi: 10.1161/01.res.85.3.238. [DOI] [PubMed] [Google Scholar]
- 27.Michael J.R., Barton R.G., Saffle J.R., Mone M., Markewitz B.A., Hillier K., Elstad M.R., Campbell E.J., Troyer B.E., Whatley R.E., Liou T.G., Samuelson W.M., Carveth H.J., Hinson D.M., Morris S.E., Davis B.L., Day R.W. Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am. J. Respir. Crit. Care Med. 1998;157:1372–1380. doi: 10.1164/ajrccm.157.5.96-10089. [DOI] [PubMed] [Google Scholar]
- 28.Troncy E., Collet J.P., Shapiro S., Guimond J.G., Blair L., Ducruet T., Francoeur M., Charbonneau M., Blaise G. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am. J. Respir. Crit. Care Med. 1998;157:1483–1488. doi: 10.1164/ajrccm.157.5.9707090. [DOI] [PubMed] [Google Scholar]
- 29.Adhikari N.K., Burns K.E., Friedrich J.O., Granton J.T., Cook D.J., Meade M.O. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334:779. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Date H., Triantafillou A.N., Trulock E.P., Pohl M.S., Cooper J.D., Patterson G.A. Inhaled nitric oxide reduces human lung allograft dysfunction. J. Thorac. Cardiovasc. Surg. 1996;111:913–919. doi: 10.1016/s0022-5223(96)70364-1. [DOI] [PubMed] [Google Scholar]
- 31.Lang J.D. Jr, Teng X., Chumley P., Crawford J.H., Isbell T.S., Chacko B.K., Liu Y., Jhala N., Crowe D.R., Smith A.B., Cross R.C., Frenette L., Kelley E.E., Wilhite D.W., Hall C.R., Page G.P., Fallon M.B., Bynon J.S., Eckhoff D.E., Patel R.P. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J. Clin. Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George I., Xydas S., Topkara V.K., Ferdinando C., Barnwell E.C., Gableman L., Sladen R.N., Naka Y., Oz M.C. Clinical indication for use and outcomes after inhaled nitric oxide therapy. Ann. Thorac. Surg. 2006;82:2161–2169. doi: 10.1016/j.athoracsur.2006.06.081. [DOI] [PubMed] [Google Scholar]
- 33.Perrin G., Roch A., Michelet P., Reynaud-Gaubert M., Thomas P., Doddoli C., Auffray J.P. Inhaled nitric oxide does not prevent pulmonary edema after lung transplantation measured by lung water content: a randomized clinical study. Chest. 2006;129:1024–1030. doi: 10.1378/chest.129.4.1024. [DOI] [PubMed] [Google Scholar]
- 34.Meade M.O., Granton J.T., Matte-Martyn A., McRae K., Weaver B., Cripps P., Keshavjee S.H. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am. J. Respir. Crit. Care Med. 2003;167:1483–1489. doi: 10.1164/rccm.2203034. [DOI] [PubMed] [Google Scholar]
- 35.Szabo C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett. 2003;140–141:105–112. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 36.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J. Mol. Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 37.Von Burg R. Carbon monoxide. J. Appl. Toxicol. 1999;19:379–386. doi: 10.1002/(sici)1099-1263(199909/10)19:5<379::aid-jat563>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Tenhunen R., Marver H.S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. U. S. A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dragun D., Hoff U., Park J.K., Qun Y., Schneider W., Luft F.C., Haller H. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001;60:1173–1181. doi: 10.1046/j.1523-1755.2001.0600031173.x. [DOI] [PubMed] [Google Scholar]
- 40.Fujita T., Toda K., Karimova A., Yan S.F., Naka Y., Yet S.F., Pinsky D.J. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat. Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 41.Otterbein L.E., Bach F.H., Alam J., Soares M., Tao L.H., Wysk M., Davis R.J., Flavell R.A., Choi A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 42.Morse D., Sethi J., Choi A.M. Carbon monoxide-dependent signaling. Crit. Care Med. 2002;30:S12–17. [PubMed] [Google Scholar]
- 43.Moore B.A., Otterbein L.E., Turler A., Choi A.M., Bauer A.J. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003;124:377–391. doi: 10.1053/gast.2003.50060. [DOI] [PubMed] [Google Scholar]
- 44.Pannen B.H., Kohler N., Hole B., Bauer M., Clemens M.G., Geiger K.K. Protective role of endogenous carbon monoxide in hepatic microcirculatory dysfunction after hemorrhagic shock in rats. J. Clin. Invest. 1998;102:1220–1228. doi: 10.1172/JCI3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otterbein L.E., Kolls J.K., Mantell L.L., Cook J.L., Alam J., Choi A.M. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J. Clin. Invest. 1999;103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyokane T., Norimizu S., Taniai H., Yamaguchi T., Takeoka S., Tsuchida E., Naito M., Nimura Y., Ishimura Y., Suematsu M. Carbon monoxide from heme catabolism protects against hepatobiliary dysfunction in endotoxin-treated rat liver. Gastroenterology. 2001;120:1227–1240. doi: 10.1053/gast.2001.23249. [DOI] [PubMed] [Google Scholar]
- 47.D’Amico G., Lam F., Hagen T., Moncada S. Inhibition of cellular respiration by endogenously produced carbon monoxide. J. Cell Sci. 2006;119:2291–2298. doi: 10.1242/jcs.02914. [DOI] [PubMed] [Google Scholar]
- 48.Alonso J.R., Cardellach F., Lopez S., Casademont J., Miro O. Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol. Toxicol. 2003;93:142–146. doi: 10.1034/j.1600-0773.2003.930306.x. [DOI] [PubMed] [Google Scholar]
- 49.Bilban M., Bach F.H., Otterbein S.L., Ifedigbo E., de Costa d’Avila J., Esterbauer H., Chin B.Y., Usheva A., Robson S.C., Wagner O., Otterbein L.E. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Taille C., El-Benna J., Lanone S., Boczkowski J., Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J. Biol. Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 51.Zuckerbraun B.S., Chin B.Y., Bilban M., de Costa d’Avila J., Rao J., Billiar T.R., Otterbein L.E. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 52.Nath K.A., Balla J., Croatt A.J., Vercellotti G.M. Heme protein-mediated renal injury: a protective role for 21-aminosteroids in vitro and in vivo. Kidney Int. 1995;47:592–602. doi: 10.1038/ki.1995.75. [DOI] [PubMed] [Google Scholar]
- 53.Kumar S., Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Nakahira K., Takahashi T., Shimizu H., Maeshima K., Uehara K., Fujii H., Nakatsuka H., Yokoyama M., Akagi R., Morita K. Protective role of heme oxygenase-1 induction in carbon tetrachloride-induced hepatotoxicity. Biochem. Pharmacol. 2003;66:1091–1105. doi: 10.1016/s0006-2952(03)00444-1. [DOI] [PubMed] [Google Scholar]
- 55.Toda N., Takahashi T., Mizobuchi S., Fujii H., Nakahira K., Takahashi S., Yamashita M., Morita K., Hirakawa M., Akagi R. Tin chloride pretreatment prevents renal injury in rats with ischemic acute renal failure. Crit. Care Med. 2002;30:1512–1522. doi: 10.1097/00003246-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 56.Paller M.S., Jacob H.S. Cytochrome P-450 mediates tissue-damaging hydroxyl radical formation during reoxygenation of the kidney. Proc. Natl. Acad. Sci. U. S. A. 1994;91:7002–7006. doi: 10.1073/pnas.91.15.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gourishankar S., Halloran P.F. Late deterioration of organ transplants: a problem in injury and homeostasis. Curr. Opin Immunol. 2002;14:576–583. doi: 10.1016/s0952-7915(02)00386-2. [DOI] [PubMed] [Google Scholar]
- 58.Tamura Y., Imaoka S., Gemba M., Funae Y. Effects of ischemia-reperfusion on individual cytochrome P450 isoforms in the rat kidney. Life Sci. 1997;60:143–149. doi: 10.1016/s0024-3205(96)00604-2. [DOI] [PubMed] [Google Scholar]
- 59.Bysani G.K., Kennedy T.P., Ky N., Rao N.V., Blaze C.A., Hoidal J.R. Role of cytochrome P-450 in reperfusion injury of the rabbit lung. J. Clin. Invest. 1990;86:1434–1441. doi: 10.1172/JCI114859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glende E.A., Jr., Hruszkewycz A.M., Recknagel R.O. Critical role of lipid peroxidation in carbon tetrachloride-induced loss of aminopyrine demethylase, cytochrome P-450 and glucose 6-phosphatase. Biochem. Pharmacol. 1976;25:2163–2170. doi: 10.1016/0006-2952(76)90128-3. [DOI] [PubMed] [Google Scholar]
- 61.Maines M.D., Mayer R.D., Ewing J.F., McCoubrey W.K. Jr. Induction of kidney heme oxygenase-1 (HSP32) mRNA and protein by ischemia/reperfusion: possible role of heme as both promotor of tissue damage and regulator of HSP32. J. Pharmacol. Exp. Ther. 1993;264:457–262. [PubMed] [Google Scholar]
- 62.Baliga R., Zhang Z., Baliga M., Shah S.V. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int. 1996;49:362–369. doi: 10.1038/ki.1996.53. [DOI] [PubMed] [Google Scholar]
- 63.Nakao A., Faleo G., Shimizu H., Nakahira K., Choi A.M., McCurry K.R., Takahashi T., Murase N. Ex vivo delivered carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008;74:1009–1016. doi: 10.1038/ki.2008.342. [DOI] [PubMed] [Google Scholar]
- 64.Wagener F.A., Feldman E., de Witte T., Abraham N.G. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc. Soc. Exp. Biol. Med. 1997;216:456–463. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 65.Lee B.S., Heo J., Kim Y.M., Shim S.M., Pae H.O., Chung H.T. Carbon monoxide mediates heme oxygenase 1 induction via Nrf2 activation in hepatoma cells. Biochem. Biophys. Res. Commun. 2006;343:965–972. doi: 10.1016/j.bbrc.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 66.Sawle P., Foresti R., Mann B.E., Johnson T.R., Green C.J., Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br. J. Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hegazi R.A., Rao K.N., Mayle A., Sepulveda A.R., Otterbein L.E., Plevy S.E. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J. Exp. Med. 2005;202:1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Motterlini R., Mann B.E., Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig. Drugs. 2005;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]
- 69.Nakao A., Toyokawa H., Tsung A., Nalesnik M.A., Stolz D.B., Kohmoto J., Ikeda A., Tomiyama K., Harada T., Takahashi T., Yang R., Fink M.P., Morita K., Choi A.M., Murase N. Ex vivo application of carbon monoxide in university of wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am. J. Transplant. 2006;6:2243–2255. doi: 10.1111/j.1600-6143.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 70.Choi A.M., Dolinay T. “Therapeutic” carbon monoxide may be a reality soon. Am. J. Respir. Crit. Care. Med. 2005;171:1318–1319. doi: 10.1164/ajrccm.171.11.953. [DOI] [PubMed] [Google Scholar]
- 71.Mayr F.B., Spiel A., Leitner J., Marsik C., Germann P., Ullrich R., Wagner O., Jilma B. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am. J. Respir. Crit. Care Med. 2005;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 72.Sorhaug S., Steinshamn S., Nilsen O.G., Waldum H.L. Chronic inhalation of carbon monoxide: effects on the respiratory and cardiovascular system at doses corresponding to tobacco smoking. Toxicology. 2006;228:280–290. doi: 10.1016/j.tox.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Redl H., Bahrami S., Schlag G., Traber DL. Clinical detection of LPS and animal models of endotoxemia. Immunobiology. 1993;187:330–345. doi: 10.1016/S0171-2985(11)80348-7. [DOI] [PubMed] [Google Scholar]
- 74.Klimisch H.J., Chevalier H.J., Harke H.P., Dontenwill W. Uptake of carbon monoxide in blood of miniture pigs and other mammals. Toxicology. 1975;3:301–310. doi: 10.1016/0300-483x(75)90031-1. [DOI] [PubMed] [Google Scholar]
- 75.Christl S.U., Murgatroyd P.R., Gibson G.R., Cummings J.H. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology. 1992;102:1269–1277. [PubMed] [Google Scholar]
- 76.Hammer H.F. Colonic hydrogen absorption: quantification of its effect on hydrogen accumulation caused by bacterial fermentation of carbohydrates. Gut. 1993;34:818–822. doi: 10.1136/gut.34.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schneider A.R., Jepp K., Murczynski L., Biniek U., Stein J. The inulin hydrogen breath test accurately reflects orocaecal transit time. Eur. J. Clin. Invest. 2007;37:802–807. doi: 10.1111/j.1365-2362.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 78.Di Camillo M., Marinaro V., Argnani F., Foglietta T., Vernia P. Hydrogen breath test for diagnosis of lactose malabsorption: the importance of timing and the number of breath samples. Can. J. Gastroenterol. 2006;20:265–268. doi: 10.1155/2006/715459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levitt M.D., Donaldson R.M. Use of respiratory hydrogen (H2) excretion to detect carbohydrate malabsorption. J. Lab. Clin. Med. 1970;75:937–945. [PubMed] [Google Scholar]
- 80.Strocchi A., Corazza G., Ellis C.J., Gasbarrini G., Levitt M.D. Detection of malabsorption of low doses of carbohydrate: accuracy of various breath H2 criteria. Gastroenterology. 1993;105:1404–1410. doi: 10.1016/0016-5085(93)90145-3. [DOI] [PubMed] [Google Scholar]
- 81.Abraini J.H., Gardette-Chauffour M.C., Martinez E., Rostain J.C., Lemaire C. Psychophysiological reactions in humans during an open sea dive to 500 m with a hydrogen-helium-oxygen mixture. J. Appl. Physiol. 1994;76:1113–1118. doi: 10.1152/jappl.1994.76.3.1113. [DOI] [PubMed] [Google Scholar]
- 82.Gharib B., Hanna S., Abdallahi O.M., Lepidi H., Gardette B., De Reggi M. Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. C. R. Acad. Sci. III. 2001;324:719–724. doi: 10.1016/s0764-4469(01)01350-6. [DOI] [PubMed] [Google Scholar]
- 83.Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Katsura K., Katayama Y., Asoh S., Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 84.Buchholz B.M., Kaczorowski D.J., Sugimoto R., Yang R., Wang Y., Billiar T.R., McCurry K.R., Bauer A.J., Nakao A. Hydrogen Inhalation Ameliorates Oxidative Stress in Transplantation Induced Intestinal Graft Injury. Am. J. Transplant. 2008;8:2015–2024. doi: 10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 85.Akamatsu Y., Haga M., Tyagi S., Yamashita K., Graca-Souza A.V., Ollinger R., Czismadia E., May G.A., Ifedigbo E., Otterbein L.E., Bach F.H., Soares M.P. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. Faseb. J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 86.Nakao A., Neto J.S., Kanno S., Stolz D.B., Kimizuka K., Liu F., Bach F.H., Billiar T.R., Choi A.M., Otterbein L.E., Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am. J. Transplant. 2005;5:282–591. doi: 10.1111/j.1600-6143.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- 87.Nakao A., Toyokawa H., Abe M., Kiyomoto T., Nakahira K., Choi A.M., Nalesnik M.A., Thomson A.W., Murase N. Heart allograft protection with low-dose carbon monoxide inhalation: effects on inflammatory mediators and alloreactive T-cell responses. Transplantation. 2006;81:220–230. doi: 10.1097/01.tp.0000188637.80695.7f. [DOI] [PubMed] [Google Scholar]
- 88.Nakao A., Murase N., Ho C., Toyokawa H., Billiar T.R., Kanno S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation. 2005;112:587–591. doi: 10.1161/CIRCULATIONAHA.104.509778. [DOI] [PubMed] [Google Scholar]
- 89.Beauchamp R.O. Jr., Bus J.S., Popp J.A., Boreiko C.J., Andjelkovich D.A. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 90.Reiffenstein R.J., Hulbert W.C., Roth S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 91.Kage S., Kashimura S., Ikeda H., Kudo K., Ikeda N. Fatal and nonfatal poisoning by hydrogen sulfide at an industrial waste site. J. Forensic. Sci. 2002;47:652–655. [PubMed] [Google Scholar]
- 92.Kage S., Nagata T., Kudo K. Determination of polysulphides in blood by gas chromatography and gas chromatography-mass spectrometry. J. Chromatogr. 1991;564:163–169. doi: 10.1016/0378-4347(91)80078-q. [DOI] [PubMed] [Google Scholar]
- 93.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 94.Fiorucci S., Distrutti E., Cirino G., Wallace J.L. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 95.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino. Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 96.Lowicka E., Beltowski J. Hydrogen sulfide (H2S)—the third gas of interest for pharmacologists. Pharmacol. Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 97.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox. Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 98.Leslie M. Medicine. Nothing rotten about hydrogen sulfide’s medical promise. Science. 2008;320:1155–1157. doi: 10.1126/science.320.5880.1155. [DOI] [PubMed] [Google Scholar]
- 99.Simon F., Giudici R., Duy C.N., Schelzig H., Oter S., Groger M., Wachter U., Vogt J., Speit G., Szabo C., Radermacher P., Calzia E. Hemodynamic and Metabolic Effects of Hydrogen Sulfide During Porcine Ischemia/Reperfusion Injury. Shock. 2008 doi: 10.1097/SHK.0b013e3181674185. In press, [DOI] [PubMed] [Google Scholar]
- 100.Blackstone E., Morrison M., Roth M.B. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 101.Blackstone E., Roth M.B. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27:370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 102.Oh G.S., Pae H.O., Lee B.S., Kim B.N., Kim J.M., Kim H.R., Jeon S.B., Jeon W.K., Chae H.J., Chung H.T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 103.Johansen D., Ytrehus K., Baxter G.F. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury—Evidence for a role of K ATP channels. Basic Res. Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 104.Whiteman M., Armstrong J.S., Chu S.H., Jia-Ling S., Wong B.S., Cheung N.S., Halliwell B., Moore P.K. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 105.Kimura Y., Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 106.Qingyou Z., Junbao D., Weijin Z., Hui Y., Chaoshu T., Chunyu Z. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem. Biophys. Res. Commun. 2004;317:30–37. doi: 10.1016/j.bbrc.2004.02.176. [DOI] [PubMed] [Google Scholar]
- 107.Laggner H., Muellner M.K., Schreier S., Sturm B., Hermann M., Exner M., Gmeiner B.M., Kapiotis S. Hydrogen sulphide: a novel physiological inhibitor of LDL atherogenic modification by HOCl. Free Radic. Res. 2007;41:741–747. doi: 10.1080/10715760701263265. [DOI] [PubMed] [Google Scholar]
- 108.Sodha N.R., Clements R.T., Feng J., Liu Y., Bianchi C., Horvath E.M., Szabo C., Sellke F.W. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur. J. Cardiothorac. Surg. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marx T., Schmidt M., Schirmer U., Reinelt H. Xenon anaesthesia. J. R. Soc. Med. 2000;93:513–517. doi: 10.1177/014107680009301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cullen S.C., Gross E.G. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science. 1951;113(2942):580–582. doi: 10.1126/science.113.2942.580. [DOI] [PubMed] [Google Scholar]
- 111.Bedi A., Murray J.M., Dingley J., Stevenson M.A., Fee J.P. Use of xenon as a sedative for patients receiving critical care. Crit. Care Med. 2003;31:2470–2477. doi: 10.1097/01.CCM.0000089934.66049.76. [DOI] [PubMed] [Google Scholar]
- 112.Boomsma F., Rupreht J., Man in’t Veld A.J., de Jong F.H., Dzoljic M., Lachmann B. Haemodynamic and neurohumoral effects of xenon anaesthesia. A comparison with nitrous oxide. Anaesthesia. 1990;45(4):273–278. doi: 10.1111/j.1365-2044.1990.tb14731.x. [DOI] [PubMed] [Google Scholar]
- 113.Goto T., Suwa K., Uezono S., Ichinose F., Uchiyama M., Morita S. The blood-gas partition coefficient of xenon may be lower than generally accepted. Br. J. Anaesth. 1998;80:255–256. doi: 10.1093/bja/80.2.255. [DOI] [PubMed] [Google Scholar]
- 114.Bogdanski R., Blobner M., Fink H., Kochs E. Effects of xenon on mesenteric blood flow. Eur. J. Anaesthesiol. 2003;20:98–103. doi: 10.1017/s0265021503000188. [DOI] [PubMed] [Google Scholar]
- 115.David H.N., Haelewyn B., Rouillon C., Lecoq M., Chazalviel L., Apiou G., Risso J.J., Lemaire M., Abraini J.H. Neuroprotective effects of xenon: a therapeutic window of opportunity in rats subjected to transient cerebral ischemia. FASEB J. 2008;22:1275–1286. doi: 10.1096/fj.07-9420com. [DOI] [PubMed] [Google Scholar]
- 116.Parsons C.G., Danysz W., Hesselink M., Hartmann S., Lorenz B., Wollenburg C., Quack G. Modulation of NMDA receptors by glycine—introduction to some basic aspects and recent developments. Amino. Acids. 1998;14:207–216. doi: 10.1007/BF01345264. [DOI] [PubMed] [Google Scholar]
- 117.Franks J.J., Horn J.L., Janicki P.K., Singh G. Halothane, isoflurane, xenon, and nitrous oxide inhibit calcium ATPase pump activity in rat brain synaptic plasma membranes. Anesthesiology. 1995;82:108–117. doi: 10.1097/00000542-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 118.Ma D., Wilhelm S., Maze M., Franks N.P. Neuroprotective and neurotoxic properties of the ‘inert’ gas, xenon. Br. J. Anaesth. 2002;89:739–746. [PubMed] [Google Scholar]
- 119.Wilhelm S., Ma D., Maze M., Franks N.P. Effects of xenon on in vitro and in vivo models of neuronal injury. Anesthesiology. 2002;96:1485–1491. doi: 10.1097/00000542-200206000-00031. [DOI] [PubMed] [Google Scholar]
- 120.Abraini J.H., David H.N., Lemaire M. Potentially neuroprotective and therapeutic properties of nitrous oxide and xenon. Ann. N. Y. Acad. Sci. 2005;1053:289–300. doi: 10.1196/annals.1344.025. [DOI] [PubMed] [Google Scholar]
- 121.Petzelt C., Taschenberger G., Schmehl W., Kox W.J. Xenon-induced inhibition of Ca2+-regulated transitions in the cell cycle of human endothelial cells. Pflugers. Arch. 1999;437:737–744. doi: 10.1007/s004240050840. [DOI] [PubMed] [Google Scholar]
- 122.Preckel B., Mullenheim J., Moloschavij A., Thamer V., Schlack W. Xenon administration during early reperfusion reduces infarct size after regional ischemia in the rabbit heart in vivo. Anesth. Analg. 2000;91:1327–1332. doi: 10.1097/00000539-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 123.Weber N.C., Toma O., Wolter J.I., Obal D., Mullenheim J., Preckel B., Schlack W. The noble gas xenon induces pharmacological preconditioning in the rat heart in vivo via induction of PKC-epsilon and p38 MAPK. Br. J. Pharmacol. 2005;144:123–132. doi: 10.1038/sj.bjp.0706063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pagel P.S., Krolikowski J.G., Shim Y.H., Venkatapuram S., Kersten J.R., Weihrauch D., Warltier D.C., Pratt P.F. Jr. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth. Analg. 2007;105:562–569. doi: 10.1213/01.ane.0000278083.31991.36. [DOI] [PubMed] [Google Scholar]
- 125.Nogales C.G., Ferrari P.H., Kantorovich E.O., Lage-Marques J.L. Ozone therapy in medicine and dentistry. J. Contemp. Dent. Pract. 2008;9:75–84. [PubMed] [Google Scholar]
- 126.Bocci V. Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediators. Inflamm. 2004;13:3–11. doi: 10.1080/0962935062000197083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen H., Xing B., Liu X., Zhan B., Zhou J., Zhu H., Chen Z. Ozone Oxidative Preconditioning Protects the Rat Kidney from Reperfusion Injury: The Role of Nitric Oxide. J. Surg. Res. 2008 doi: 10.1016/j.jss.2007.12.756. in press, [DOI] [PubMed] [Google Scholar]
- 128.Ajamieh H., Merino N., Candelario-Jalil E., Menendez S., Martinez-Sanchez G., Re L., Giuliani A., Leon O.S. Similar protective effect of ischaemic and ozone oxidative preconditionings in liver ischaemia/reperfusion injury. Pharmacol. Res. 2002;45:333–339. doi: 10.1006/phrs.2002.0952. [DOI] [PubMed] [Google Scholar]
- 129.Peralta C., Leon OS., Xaus C., Prats N., Jalil EC., Planell ES., Puig-Parellada P., Gelpi E., Rosello-Catafau J. Protective effect of ozone treatment on the injury associated with hepatic ischemia-reperfusion: antioxidant-prooxidant balance. Free Radic. Res. 1999;31:191–196. doi: 10.1080/10715769900300741. [DOI] [PubMed] [Google Scholar]
- 130.Chen H., Xing B., Liu X., Zhan B., Zhou J., Zhu H., Chen Z. Similarities between ozone oxidative preconditioning and ischemic preconditioning in renal ischemia/reperfusion injury. Arch. Med. Res. 2008;39:169–178. doi: 10.1016/j.arcmed.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 131.Chen H., Xing B., Liu X., Zhan B., Zhou J., Zhu H., Chen Z. Ozone oxidative preconditioning inhibits inflammation and apoptosis in a rat model of renal ischemia/reperfusion injury. Eur. J. Pharmacol. 2008;581:306–314. doi: 10.1016/j.ejphar.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 132.Schelegle E.S., Gunther R.A., Parsons G.H., Colbert S.R., Yousef M.A., Cross C.E. Acute ozone exposure increases bronchial blood flow in conscious sheep. Respir. Physiol. 1990;82:325–335. doi: 10.1016/0034-5687(90)90102-5. [DOI] [PubMed] [Google Scholar]
- 133.Al-Dalain SM., Martinez G., Candelario-Jalil E., Menendez S., Re L., Giuliani A., Leon OS. Ozone treatment reduces markers of oxidative and endothelial damage in an experimental diabetes model in rats. Pharmacol. Res. 2001;44:391–396. doi: 10.1006/phrs.2001.0867. [DOI] [PubMed] [Google Scholar]
- 134.Di Paolo N., Bocci V., Salvo D.P., Palasciano G., Biagioli M., Meini S., Galli F., Ciari I., Maccari F., Cappelletti F., Di Paolo M., Gaggiotti E. Extracorporeal blood oxygenation and ozonation (EBOO): a controlled trial in patients with peripheral artery disease. Int. J. Artif. Organs. 2005;28:1039–1050. doi: 10.1177/039139880502801012. [DOI] [PubMed] [Google Scholar]
- 135.Di Paolo N., Bocci V., Gaggiotti E. Ozone therapy. Int. J. Artif. Organs. 2004;27:168–175. doi: 10.1177/039139880402700303. [DOI] [PubMed] [Google Scholar]
- 136.Hernandez F., Menendez S., Wong R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy. Free Radic. Biol. Med. 1995;19:115–119. doi: 10.1016/0891-5849(94)00201-t. [DOI] [PubMed] [Google Scholar]