Abstract
Hemorrhagic shock causes oxidative stress that leads to tissue injuries in various organs including the lung, liver, kidney and intestine. Excess amounts of free heme released from destabilized hemoproteins under oxidative conditions might constitute a major threat because it can catalyze the formation of reactive oxygen species. Cells counteract this by rapidly inducing the rate-limiting enzyme in heme breakdown, heme oxygenase-1 (HO-1), which is a low-molecular-weight stress protein. The enzymatic HO-1 reaction removes heme. As such, endogenous HO-1 induction by hemorrhagic shock protects tissues from further degeneration by oxidant stimuli. In addition, prior pharmacological induction of HO-1 ameliorates oxidative tissue injuries induced by hemorrhagic shock. In contrast, the deletion of HO-1 expression, or the chemical inhibition of increased HO activity ablated the beneficial effect of HO-1 induction, and exacerbates tissue damage. Thus, HO-1 constitutes an essential cytoprotective component in hemorrhagic shock-induced oxidative tissue injures. This article reviews recent advances in understanding of the essential role of HO-1 in experimental models of hemorrhagic shock-induced oxidative tissue injuries with emphasis on the role of its induction in tissue defense.
Keywords: heme, heme oxygenase, shock, oxidative stress
Table of Contents
Introduction ---- 29
Induction of HO-1 and Its Protective Role in Hemorrhagic Shock-Induced Oxidative Tissue Injury ---- 30
-
Hemorrhagic shock-induced hepatic injury and HO-1
-
1-1.
Induction of hepatic HO-1 by hemorrhagic shock-induced ROS generation
-
1-2.
Protective role of HO-1 in hemorrhagic shock-induced hepatic injury
-
1-2-1.
Dobutamine ameliorates hemorrhagic shock-induced hepatic dysfunction through induction of HO-1
-
1-2-1.
-
1-1.
-
Hemorrhagic shock-induced renal injury and HO-1
-
2-1.
Heme-mediated HO-1 induction in the kidney after hemorrhagic shock
-
2-2.
HO-1 induction is necessary in restoring ischemic renal injury
-
2-2-1.
HO-1 deficiency exacerbates ischemic renal injury and augments systemic inflammation induced by renal ischemia
-
2-2-1.
-
2-3.
Amelioration of ischemic renal injury by HO-1 induction specific to renal epithelial cells
-
2-4.
Attenuation of ischemic renal injury by HO-1 induced in infiltrating macrophages by statins
-
2-1.
-
Hemorrhagic shock-induced intestinal tissue injury and HO-1
-
3-1.
Site-specific induction of HO-1 in hemorrhagic shock-induced intestinal tissue injury
-
3-1-1.
Concordant expression of HO-1 and hypoxia inducible factor-1α in the intestine after hemorrhagic shock
-
3-1-1.
-
3-2.
Protection of the hemorrhagic shock-induced intestinal tissue injury by glutamine by its HO-1 induction
-
3-1.
-
Hemorrhagic shock-induced lung injury and HO-1
-
4-1.
Amelioration of hemorrhagic shock-induced acute lung injury by HO-1 induction
-
4-1.
Critical Role of Free Heme in Hemorrhagic Shock-Induced Oxidative Tissue Injury ---- 34
Free heme as an activator of inflammatory process
-
Role of Bach1 in ho-1 gene regulation and oxidative tissue injury
-
2-1.
Bach1 inactivation leads to myocardial cytoprotection involving HO-1 against ischemia/reperfusion injury
-
2-1.
Metabolites of HO Reaction Protect Against Hemorrhagic Shock-Induced Oxidative Tiuuse Injury ---- 36
-
Role of bile pigments in the protective response against oxidative injury
-
1-1.
Protective effects of biliverdin against endotoxin-induced shock
-
1-1.
-
Protective role of carbon monoxide against oxidative tissue injury
-
2-1.
Inhalation of carbon monoxide attenuates HS-induced systemic inflammation and ameliorates end organ injuries
-
2-1.
Conclusion ---- 37
Acknowledgements ---- 37
Introduction
Hemorrhagic shock (HS) triggers a systemic inflammatory response that culminates in multiple organ failure with significantly high mortality and morbidity [1]. Exaggerated systemic inflammatory responses comprise an important component of HS-induced tissue injury [2]. Both hypoxia-reoxygenation and neutrophil activation enhance this systemic inflammatory response [2] by generating reactive oxygen species (ROS), which ultimately leads to tissue injury [3].
Heme is a prosthetic group in hemoproteins, such as hemoglobin, myoglobin, cytochrome c, cytochrome P450, catalase and peroxidase, all of which are critically important for cellular vitality [4, 5]. In contrast, “free heme”, which is not bound to a protein and can be released from destabilized hemoprotein by oxidative stimuli such as HS, is quite toxic, since it catalyzes ROS production [5]. The body is equipped with various protective mechanisms to defend against excessive free heme concentrations. Heme oxygenase (HO) is a key factor in the defense mechanism, and it plays a fundamental role against oxidative processes mediated by free-heme, including HS [6].
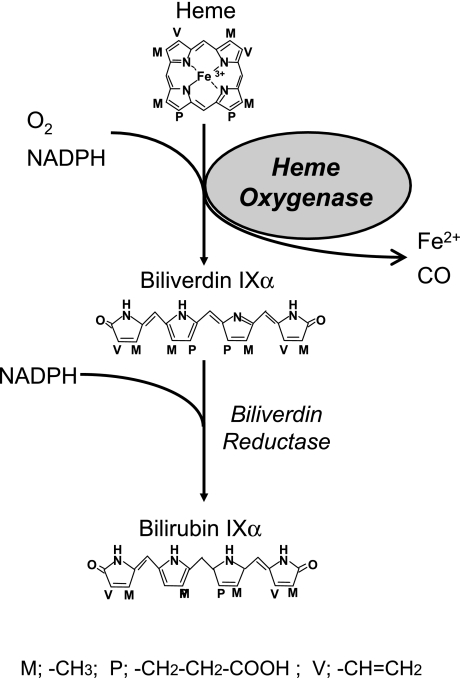
Heme Oxygenase-1 is the rate-limiting enzyme in heme catabolism. It oxidatively cleaves heme to yield one molecule each of iron, carbon monoxide (CO), and biliverdin IXα (biliverdin), the last of which is subsequently reduced by biliverdin reductase to bilirubin IXα (bilirubin) (Fig. 1) [7]. At least two isozymes for HO have been identified; HO-1 is induced in response to various stimuli [7], whereas HO-2 is constitutively expressed and not inducible [8]. The expression of HO-1 is upregulated in various cell types not only by its substrate heme but also by a vast array of stressful stimuli including oxidative stress [6].
Fig. 1.
Oxidative catabolism of heme. HO catalyzes the oxidative conversion of heme, to biliverdin IXα, releasing carbon monoxide (CO) and ferrous iron as reaction products. Biliverdin IXα is subsequently reduced to bilirubin IXα by biliverdin reductase.
The two enzymatic products of the HO reaction, i.e., biliverdin and CO can be toxic at very high concentrations. However, recent evidence indicates that they are not toxic at physiological concentrations in normal cells, and that they may also have important anti-oxidant, anti-inflammatory, or anti-apoptotic properties [9, 10]. Thus, in addition to the removal of free heme, i.e., a potent pro-oxidant, HO-1 induction results in the production of anti-oxidant molecules that function the protective response and contribute to the suppression of oxidative tissue injuries [6, 11–13]. The importance of HO-1 in protection against oxidative stresses is further substantiated by previous studies that show a reduction in the protective responses against oxidant stress of ho-1 knockout mutant mice and in a patient with an inherited HO-1 deficiency [14, 15]. In the case of the patient, the absence of HO-1 was associated with abnormally elevated serum heme concentrations (~500 µM), and various intensive oxidative as well as massive inflammatory complications [15]. The powerful adaptive response to oxidative stress suggests an entirely new role for HO-1 in protection against inflammatory processes induced by HS and suggests that HO-1 is an essential component in the biological defensive response against HS-induced oxidative tissue injuries.
Injury after HS is unique in that a global insult is delivered to all organ systems, such as the liver, kidney, intestine and lung, indicating that HS is a systemic injury. The role of HO-1 in single organ ischemia/reperfusion (I/R) injury including transplant-related injury has already been extensively reviewed [16–19]. Thus, the present review emphasizes the protective role of HO-1 in a model of HS-induced oxidative tissue injury in vivo.
Induction of HO-1 and Its Protective Role in Hemorrhagic Shock-Induced Oxidative Tissue Injury
Ischemia-induced oxidative tissue injury can frequently cause destabilization of hemoproteins which results in an increase in intracellular free heme concentration. Both hemin, an oxidized form of heme that is available as a chemical, and tissue inflammation obviously induce HO-1 [6, 11–13], suggesting a possible role of HO-1 in the protection of HS-induced oxidative stress. Recent evidence indicates that HO-1 induction is essential to maintain the physiological milieu against oxidant stimuli in animal models of HS.
1. Hemorrhagic shock-induced hepatic injury and HO-1
HS elicits the formation of significant amount of ROS in the liver not only by hepatic hypoxia-reoxygenation itself, but also by immune activation due to gut bacterial translocation [2], which leads to oxidative hepatic injury. Thus, HS induces HO-1 in the liver, and this process plays a significant protective role against HS-induced hepatic injury.
1-1. Induction of hepatic HO-1 by hemorrhagic shock-induced ROS generation.
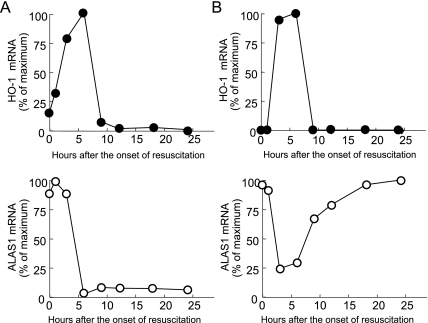
HS leads to a substantial increase in hepatic HO-1 mRNA and protein primarily in parenchymal cells at the midzonal and pericentral region in rats [20]. The potent antioxidant “Trolox” significantly attenuates the HS-induced increase of HO-1 in hepatocytes [21]. Moreover, Kupffer cell blockade by the treatment of gadolium chloride and antioxidants abolished HO-1 mRNA and protein induction after HS [22]. These results suggest that oxygen-free radicals released by Kupffer cells serve as paracrine regulator of HO-1 gene expression. We also found that HO-1 mRNA and protein levels obviously increased in hepatocyte after HS (Fig. 2A) [23], whereas mRNA levels of non-specific 5-aminolevulinate synthase (ALAS1), which is down-regulated by heme [24], significantly decreased after HS (Fig. 2B). Cytochrome P450, a major hemoprotein in the liver, undergoes a rapid degradation upon ischemia [25], which results in the release of pro-oxidant free heme. Collectively, these findings suggest that increased levels of free heme in hepatocytes also contribute to the induction of HO-1 after HS mediated through the enhanced production of oxygen free radicals.
Fig. 2.
Effect of hemorrhagic shock on gene expression of heme oxygenase-1 (HO-1) and non-specific 5-aminolevulinate synthase (ALAS1) in the liver and kidney. Rats were subjected to HS for 60 min followed by resuscitation. After the onset of resuscitation, time course changes in HO-1 (closed circle) and ALAS1 (open circle) mRNA expression were examined by Northern blot analysis. HO-1 mRNA was rapidly and markedly increased in the liver (A) and kidney (B) after HS, while ALAS1 mRNA levels were drastically decreased immediately after HS, suggesting that there may be an abnormal increase in “free heme” concentrations in the liver and kidney after HS.
1-2. Protective role of HO-1 in hemorrhagic shock-induced hepatic injury.
The role of hepatic HO-1 induction after HS has been evaluated in animals with HS administered with tin-protoporphyrin (SnPP), a specific competitive inhibitor of HO activity [26] at 5 h after the onset of resuscitation [20]. Increases in the incidence of pericentral necrosis and in the level of serum α-glutathione-S-transferase, a sensitive marker of hepatocyte injury indicates aggravated hepatic injury [20]. These findings suggest that HO-1 induced by HS protects hepatocytes from HS-induced, tissue injury.
1-2-1. Dobutamine ameliorates hemorrhagic shock-induced hepatic dysfunction through induction of HO-1.
Recent evidence suggests that increased intracellular cyclic AMP levels induce HO-1 via protein kinase A-dependent pathway [27]. Intracellular cyclic AMP levels are increased in the liver by several receptor-dependent pathways including adrenoceptor agonists. The β1-receptor agonist dobutamine induces HO-1 mRNA and protein via protein kinase A pathway in hepatocytes in vitro and in vivo [28]. Whereas HS significantly impairs liver function judged as a decrease in the disappearance rate of indocyanine green in rat plasma, prior dobutamine administration induces significant amounts of HO-1 in hepatocytes around the central vein and improves hepatic dysfunction after HS [29]. This protective effect was abrogated by blocking the HO pathway with tin-mesoporphyrin (SnMP), a specific competitive inhibitor of HO activity [30]. A blockade of β1-adorenoceptors with esmolol also attenuates the induction of HO-1 and the disappearance rate of indocyanine green from plasma [29]. These results suggest that the β1-adrenocepter-dependent up-regulation of HO-1 induced by dobutamine contributes to improved hepatic function after HS.
2. Hemorrhagic shock-induced renal injury and HO-1
The kidney is vulnerable to oxidative damage caused by ischemia, ultimately progressing to acute renal failure [31]. Thus, ROS and HO-1 have both been implicated in HS/ischemia-induced renal injury.
2-1. Heme-mediated HO-1 induction in the kidney after hemorrhagic shock.
HS resulted in increased renal HO-1 mRNA and protein expression in tubular epithelial cells (Fig. 2B) [23] whereas the level of ALAS1 mRNA in the kidney rapidly fell (Fig. 2B). Ischemia of the rat kidney decreases the amount of microsomal cytochrome P450 [32]. Cytochrome P450 represents the major source of heme in the kidney because of its high content and rapid turnover [33]. Collectively, the increase in the concentration of free heme presumably released from microsomal cytochrome P450 may contribute to HO-1 induction after HS. Levels of microsomal heme and HO-1 mRNA concomitantly increase in the rat kidney after 30 min of bilateral renal ischemia [32], suggesting that HO-1 induction is also mediated by heme in this model.
2-2. HO-1 induction is necessary in restoring ischemic renal injury.
The significance of HO-1 induction after HS has been examined using the rat model of renal ischemia. Renal ischemia for 40 min in uni-nephrectomized rats significantly induces HO-1 mRNA and its enzyme activity after kidney reperfusion [34]. The inhibition of HO activity by SnMP results in a rapid, obvious increase in the intracellular heme content, and in the aggravation of renal function [34]. Thus, HO-1 induction in this model also plays a critical role in protecting the kidney against oxidative damage induced by ischemia.
2-2-1. HO-1 deficiency exacerbates ischemic renal injury and augments systemic inflammation induced by renal ischemia.
The protective role of HO-1 in acute renal injury caused by ischemia has been further examined in HO-1 knockout mice. Renal function in HO-1 knockout mice shows obvious deterioration after relatively mild renal ischemia, reflected by increased blood urea nitrogen and serum creatinine levels [35]. Renal ischemia also causes increased mortality in HO-1 knockout mice, unlike the wild type mice. The surviving HO-1 knockout mice show distinct histological renal injury, which is accompanied by increased activation of NF-κB and NF-κB-dependent proinflammatory genes such as monocyte chemoattractant protein-1 [35]. Moreover, IL-6 mRNA is clearly induced not only in the kidney, but also in the lung and heart of HO-1 deficient mice after renal ischemia. Systemic levels of IL-6 after ischemia are also increased in HO-1 knockout mice compared with wild-type mice [36]. The administration of an antibody to IL-6 protects against the renal dysfunction and mortality observed in HO-1 knockout mice after ischemia [36], suggesting that augmented systemic inflammation following renal ischemia in an HO-1-deficient state is responsible for the exaggerated vulnerability observed in this setting.
2-3. Amelioration of ischemic renal injury by HO-1 induction specific to renal epithelial cells.
Although tin is an essential trace element for some animals, whether it is essential for humans remains unknown. Growth is retarded in rats with tin deficiency syndrome [37], whereas excessive concentrations of tin cause various side effects in humans such as gastrointestinal complaints [38]. Uniquely, treating rats with tin potently and exclusively induces HO-1 in the kidney [39]. Moreover, tin chloride (SnCl2) administration before renal ischemia significantly improves renal dysfunction, since increased serum creatinine levels are suppressed [40]. Proximal tubular cells are significantly damaged in control animals pretreated with vehicle, while minimally damaged with SnCl2 [40]. Elevated levels of renal HO-1 mRNA after SnCl2 administration are followed by increases in HO-1 protein expression and HO activity, and HO-1 protein specifically accumulates in renal tubular epithelial cells [40]. Tin chloride significantly attenuates the sustained increase in the microsomal heme concentration caused by ischemia [40]. In contrast, the inhibition of HO activity induced by SnMP results in microsomal heme accumulation, and abolishes the beneficial effect of SnCl2 on ischemic renal injury, indicating that HO-1 plays a fundamental role in protecting renal epithelial cells from oxidative damage due to ischemic insult by removing the pro-oxidant “free heme” [40].
2-4. Attenuation of ischemic renal injury by HO-1 induced in infiltrating macrophages by statins.
Statins are a class of lipid lowering drugs that inhibit the enzyme, 3-hydroxy-3 methylglutaryl-coenzyme A reductase, and they have recently emerged as potentially powerful inhibitors of the inflammatory process [41]. The mechanism through which statins modulate the immune response is complex, but it is often regarded as being independent of cholesterol lowering activity [41]. Recent findings indicate that the anti-inflammatory effects of statins are linked to HO-1 induction [42, 43]. Administering rats with statins reduces subsequent renal damage and attenuates renal dysfunction after renal ischemia [44]. The protective effect of statins is abolished by the co-administration of SnPP [44]. Renal ischemia increases HO-1 expression both at the transcript and protein level in the kidney. This effect is significantly more evident in animals pretreated with statin. Moreover, infiltrating macrophages comprise the major source of tissue HO-1 production [44]. Collectively, in addition to HO-1 induction in renal epithelial cells, local HO-1 delivery from infiltrating macrophages induced by statins may exert anti-inflammatory effects, thereby reducing oxidative tissue injuries caused by renal ischemia.
3. Hemorrhagic shock-induced intestinal tissue injury and HO-1
The intestine plays a critical role in the pathogenesis of HS-induced multiple organ damage as a site of end-organ injury and as an activator of the immune response via bacterial translocation [45]. ROS greatly contribute to HS-induced gut injury [3]. Therefore, HO-1 induced in the mucosal epithelial cells of the intestine after HS confers protection against oxidative intestinal tissue injury caused by HS [46].
3-1. Site-specific induction of HO-1 in hemorrhagic shock-induced intestinal tissue injury.
Following HS, significant amounts of HO-1 are induced in the mucosal epithelial cells of the rat duodenum, jejunum and colon, but not at all in the ileum [46]. In contrast, intestinal tissue injury and inflammation are more pronounced in the ileum than in other regions of the intestine [46]. These findings suggest that the intestinal sites where HO-1 can be induced after HS are better protected from oxidative tissue injury than sites where HO-1 cannot be induced. In addition, the prior administration of SnMP to animals results in mucosal epithelial cell injury and inflammation in the duodenum, jejunum and colon but not of the ileum according to the augmented gene expression of proinflammatory mediators, such as tumor necrosis factor (TNF)-α and inducible nitric oxide synthase (iNOS) [46]. Injury and inflammation are further indicated by increased apoptotic DNA fragmentation as evidenced by increased number of positive cells in the oligo ligation assay in situ; increased protein expression of activated caspase-3 to carry out the apoptotic process; and decreased gene expression of Bcl-2 for an anti-apoptosis [46]. Thus, HO-1 induction and the maintenance of its activity are critical in the protection of the intestinal epithelial cells from oxidative injury induced by HS.
3-1-1. Concordant expression of HO-1 and hypoxia inducible factor-1α in the intestine after hemorrhagic shock.
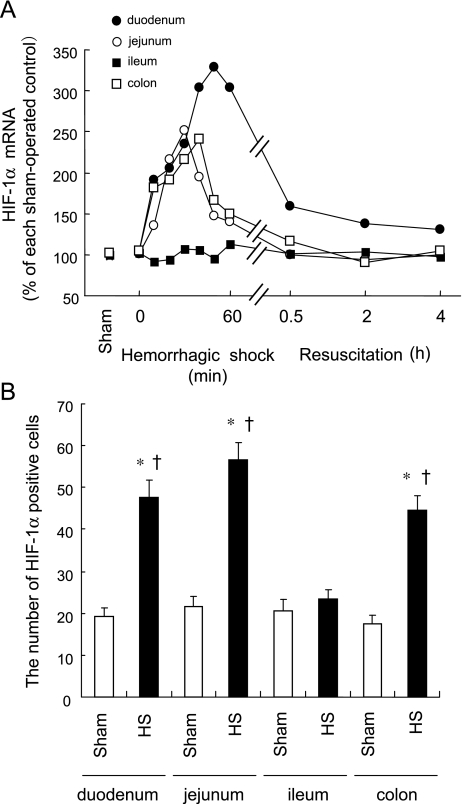
Hypoxia inducible factor (HIF)-1α is a hypoxia responsive helix-turn-helix transcription factor with a binding sequence in the ho-1 promoter [47]. Since HIF-1α coordinates hypoxia-induced HO-1 expression [47], it is probably involved in the site-specific HO-1 expression in the intestine induced by HS. Thus, we examined the effect of HS on expression of HIF-1α in various regions of the intestine. Levels of HIF-1α mRNA rapidly increased in the duodenum, jejunum and colon at the ischemic phase of HS. In contrast, levels in the ileum were not influenced by HS (Fig. 3A). Consistent with enhanced HIF-1α gene expression, HIF-1α protein staining was positive and increased in the duodenum, jejunum and colon after HS, but not in the ileum (Fig. 3B). Positive HIF-1α protein principally stained the mucosal cells of the intestine where HO-1 protein was also expressed. This site-specific induction and the cellular localization of HIF-1α protein are consistent with those of HO-1 induction after HS, suggesting that HIF-1α contributes to the site-specific induction of intestinal HO-1 after HS.
Fig. 3.
Site-Specific induction of hypoxia inducible factor (HIF)-1α in the intestine by hemorrhagic shock (HS). Rats were subjected to hemorrhagic shock for 60 min followed by resuscitation. Intestines were excised after the onset of HS and dissected into the duodenum, jejunum, ileum and colon. (A) Time course changes in HIF-1α mRNA expression were examined in each region of the intestine by Northern blot analysis. (B) HIF-1α protein expression was examined by immunohistochemical analysis 30 min after the onset of HS and the number of HIF-1α positive cells was counted. Sham, sham-operated control animals; HS; HS animals. Data are presented as mean ± SEM (n = 6). *p<0.01 vs. HS-treated ileum. †p<0.01 vs. regional matched Sham. Both HIF-1α mRNA levels and the number of HIF-1α positive cells were markedly increased in the ileum but not in other regions of the intestine at the early phase of HS.
3-2. Protection of the hemorrhagic shock-induced intestinal tissue injury by glutamine by its HO-1 induction.
Glutamine is now recognized as an essential nutrient during serious injury and illness [48]. Glutamine helps to prevent infectious morbidity and mortality in seriously ill patients [48] by maintaining the integrity of the intestinal mucosal epithelium [49]. Levels of HO-1 mRNA and protein are up-regulated in the mucosal epithelial cells of the ileum of rats treated with glutamine [50, 51]. Furthermore, HS-induced intestinal tissue injury in the ileum is also significantly suppressed by glutamine compared with untreated controls. Thus, glutamine significantly ameliorates HS-induced mucosal injury, inflammation and apoptotic cell death by inducing HO-1 [51]. In contrast, SnMP abolishes the beneficial effect of glutamine, indicating that the protective effect of glutamine in HS-treated animals is principally mediated by its ability to induce HO-1 [51].
4. Hemorrhagic shock-induced lung injury and HO-1
HS causes neutrophil sequestration in the lung, which leads to acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome, both of which are responsible for the significant morbidity and mortality of critically ill patients [52, 53]. Reactive oxygen species generated from infiltrating neutrophils play pivotal roles in the pathogenesis of ALI [3]. However, a specific therapy for oxidative stress is not available [54]. Several studies have indicated that pharmacologically induced pulmonary HO-1 overexpression protects lung cells from HS-induced oxidative injury.
4-1. Amelioration of hemorrhagic shock-induced acute lung injury by HO-1 induction.
HS significantly increases pulmonary HO-1 mRNA expression in rats. Prior hemoglobin administration further increases HO-1 expression and significantly ameliorates HS-induced pulmonary edema in this model [55]. In contrast, SnPP abolishes the protection induced by hemoglobin [55]. These findings suggest that the pharmacological induction of HO-1 is beneficial in treating HS-induced ALI, whereas the inhibition of HO activity by SnPP abrogates the beneficial effect of prior hemoglobin administration.
Heme arginate (HA) is a water-soluble and stable metabolite of the reaction between hemin and L-arginine [56] that is used to treat acute relapses in patients with acute hepatic porphyria [57]. We found that administering HA to rats induces functional HO-1 protein in pulmonary epithelial cells, and significantly ameliorates subsequent lung injury and inflammation induced by HS [23]. Prior HA administration decreases the expression of proinflammatory genes such as TNF-α and iNOS, the DNA binding activity of NF-κB, pulmonary edema and the neutrophil content in the lungs, whereas subsequent HO-1 inhibition by SnMP mitigates these beneficial effects of HA [23, 58]. Thus, similar to prior administration with hemoglobin, that of HA confers significant tissue protection in this model, suggesting that this type of HO-1 induction significantly inhibits lung damage induced by HS.
Critical Role of Free Heme in Hemorrhagic Shock-Induced Oxidative Tissue Injury
Heme is an essential prosthetic group for hemoproteins, which are vital for life [4, 5]. However, excess free heme is a potentially cytotoxic iron-chelate through its ability to catalyze membrane lipid peroxidation and form ROS [5, 59]. Ischemia of the kidney causes a decrease in the amount of microsomal cytochrome P450, the major hemoprotein in the rat kidney, with a concomitant increase in microsomal heme content, followed by a significant induction of HO-1 mRNA and protein [32]. Moreover, the inhibition of HO activity by SnMP results in an obvious increase in microsomal heme content and in the aggravation of renal dysfunction and injury induced by renal ischemia [34]. Thus, an enhanced and sustained increase in the intracellular free heme concentration, which is presumably derived from hemoprotein degraded by ischemic insult, exacerbates HS-induced oxidative tissue injury; and rapid HO-1 induction plays a key role in protecting cells from further damage due to heme-mediated oxidative injury by removing pro-oxidant free heme.
1. Free heme as an activator of inflammatory process
HS initiates an inflammatory response characterized by up-regulated pro-inflammatory cytokine expression followed by the migration of neutrophils into various tissues via the activation of cell adhesion molecules [60]. Recent studies indicate that free heme is involved in the activation of these inflammatory cascades. Hemin induces neutrophil migration in vivo and in vitro, triggers the oxidative burst, promotes cytoskeleton reorganization and activates interleukin-8 expression in human neutrophils [61], suggesting that hemin functions as a proinflammatory agent that induces neutrophil activation. Moreover, exposing endothelial cells to hemin stimulates the expression of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin, probably through the heme-mediated generation of ROS [62, 63]. Heme also induce TNF-α secretion by mouse peritoneal macrophages dependently on MyD88, toll-like receptor (TLR) 4 and CD14, although heme signaling through TLR4 depends on an interaction distinct from that established between TLR4 and LPS [64]. Collectively, these findings suggest that free heme is a potent activator of the innate immune response.
2. Role of Bach1 in ho-1 gene regulation and oxidative tissue injury
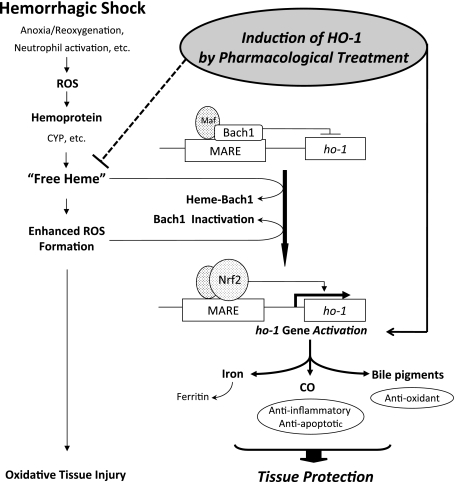
Bach1 is a heme-responsive transcription factor that represses ho-1 gene activation [65, 66]. Bach1 under baseline conditions forms a heterodimer with small maf proteins that represses transcription of the ho-1 gene by binding to maf recognition elements (MARE) in the 5'-untranslated region of the ho-1 promoter. Under conditions of excess heme, increased heme binding to Bach1 causes a conformational change and a decrease in DNA-binding activity followed by nuclear export of Bach1, which in turn leads to transcriptional activation of the ho-1 gene through MARE [65–67]. Heme also induced nuclear translocation of NF-E2-related factor 2 (Nrf2), a partner molecule for the maf family [68], and promotes stabilization of Nrf2 [69]. Thus, an increased intracellular heme concentration displaces Bach1 from the MARE sequences by heme binding, which then permits Nrf2 binding to a member of small maf proteins, ultimately resulting in transcriptional activation of the ho-1 gene (Fig. 4) [66, 70, 71].
Fig. 4.
Protective role of heme oxygenase-1 (HO-1) in hemorrhagic shock-induced oxidative tissue injuries. Hemorrhagic shock (HS) induces oxidative stresses which results in the production of free heme, presumably released from cytochrome P450 (CYP), or from other hemoproteins. Free heme is then involved in the generation of reactive oxygen species (ROS) further to increase oxidative stress. Binding of free heme with Bach1, the transcriptional repressor of ho-1, as well as the inactivation of Bach1 by ROS allows the displacement of Bach1 from maf recognition element (MARE) sequence in the ho-1 promoter. This in turn allows Nrf2 binding with MARE, and induces transcriptional activation of the ho-1 gene. Since HO-1 expression is known to be principally regulated at the transcriptional level, this sequence of events leads to the increase in HO-1 protein and its enzymatic activity. Increased HO-1 activity then metabolizes free heme to iron, CO and biliverdin. Iron is directly sequestered and inactivated by co-induced ferritin. Biliverdin is rapidly converted to bilirubin by biliverdin reductase, and both bile pigments serve as a major anti-oxidant. CO can suppress apoptosis of endothelial cells via the activation of p38 MAPK. In addition, HO-1 is also induced by pharmacological treatment, such as glutamine and tin chloride, in a tissue-specific manner which is targeted to the injured organs, and pre-induction of HO-1 by pharmacological means significantly ameliorates oxidative tissue damages caused by HS. Thus, HO-1 is an essential cytoprotectective component against HS-induced oxidative tissue injuries not only by removing excess amount of cytotoxic free heme but also by producing metabolites from heme which have anti-oxidative and anti-apoptotic properties.
Bach1 may function as a sensor of oxidative stress [72]. Human Bach1 is a thiol-rich protein possessing 34 interspersed cysteine amino acids, of which two are responsible for Bach1 inactivation by oxidants [72]. Furthermore, HO-1 induction elicited by arsenite-mediated oxidative stress follows inactivation of Bach1 and precedes Nrf2 activation [72]. Bach1 repression is dominant over Nrf2-mediated HO-1 transcription and Bach1 inactivation is prerequisite for HO-1 induction [73]. Moreover, Bach1 specifically represses induction of HO-1 elicited by arsenite [74]. These findings collectively suggest that the redox regulation of Bach1 serves as an alternative mechanism to HO-1 induction by oxidative stress (Fig. 4).
2-1. Bach1 inactivation leads to myocardial cytoprotection involving HO-1 against ischemia/reperfusion injury.
Cardiac I/R injury induced after hemorrhagic shock is an important example of oxidative tissue injuries [75]. Many reports indicate that HO-1 plays a cytoprotective role against cardiac I/R injury [76–80]. Since Bach1 suppression leads to HO-1 induction [71], Bach1 inactivation is apparently decisive for deployment of the cytoprotective process against oxidative stress, including ho-1 activation. The role of Bach1 in tissue protection against myocardial I/R injury has been investigated in vivo in mice lacking the Bach1 gene [81]. The myocardial expression of HO-1 protein in Bach1-deficient mice is constitutively up-regulated compared with that in wild-type mice [81]. While myocardial I/R induces HO-1 protein in ischemic myocytes from both strains of mice, the extent of induction is significantly greater in the Bach1-deficient, than in wild type mice [81]. Myocardial infarction is clearly reduced in Bach1-deficient mice. Zinc-protoporphyrin, an inhibitor of HO activity [82], abolishes the infarction-reducing effect of Bach1 disruption in Bach1-deficient mice [81], indicating that HO-1 activity mediates the reduction in infarct size to some extent. Thus, Bach1 plays a pivotal role in establishing the levels of both constitutive and inducible myocardial HO-1 expression. Moreover, Bach1 inactivation during I/R appears to be a key mechanism in controlling the activation of cytoprotective processes involving HO-1.
Metabolites of HO Reaction Protect Against Hemorrhagic Shock-Induced Oxidative Tissue Injury
Heme oxygenase breaks down the pro-oxidant free heme into iron, biliverdin and CO, thus resulting in a decrease in oxidative stress. Iron, which is an oxidant, is directly sequestered and inactivated by co-induced ferritin [83]. Heme Oxygenase-1 also prevents cell death by exporting intracellular iron from cells both in vivo [84] and in vitro [85]. Recent evidence suggests that the other two heme metabolites, biliverdin and CO, also have significant anti-oxidant properties. Thus, in addition to the removal of the pro-oxidant free heme via oxidative metabolism, HO-1 in turn produces a series of metabolites from heme, all of which can act as important constituents of the host defense system and contribute to the suppression of oxidative tissue injuries.
1. Role of bile pigments in the protective response against oxidative injury
Biliverdin is rapidly converted by bilirubin reductase to bilirubin. Biliverdin and bilirubin, as well as their glucuronides, are potent anti-oxidants (Fig. 4) [86]. The cellular depletion of bilirubin by RNA interference directed against biliverdin reductase increases tissue levels of ROS and promotes apoptotic cell death, and the potent physiological antioxidant actions of bilirubin reflect an amplification cycle, in which bilirubin acting as an antioxidant is itself oxidized to biliverdin and then recycled by biliverdin reductase back to bilirubin [87]. Several epidemiological studies indicate that mild to moderately elevated serum bilirubin levels are associated with a better outcome in diseases involving oxidative stress [88]. For instances, the incidence of ischemic heart disease among middle-aged individuals with Gilbert’s Syndrome, who have mild unconjugated hyperbilirubinemia because of a genetic disorder of bilirubin conjugation, is reduced <5-fold compared with the general population [89]. High plasma bilirubin levels in the general population are also correlated with a reduced risk of coronary heart disease [90–93].
1-1. Protective effects of biliverdin against endotoxin-induced shock.
Similar to HS, administering endotoxin to animals incites a systemic inflammatory response that mimics septic shock in clinical practice, ultimately leading to multiple organ damage [94]. The effect of biliverdin on endotoxin-induced shock has been investigated in rats administered intravenously with lipopolysaccharide (LPS) [95]. Administering rats with biliverdin before a lethal dose of LPS significantly improves long-term survival, which is associated with reduced serum levels of LPS-induced proinflammatory IL-6 and augmented levels of anti-inflammatory IL-10 [95]. Serum levels of bilirubin generated after this dose of biliverdin are notably increased but remain at high normal levels [95]. Biliverdin also ameliorates LPS-induced lung permeability and lung aloveolitis, at least in part, by blocking NF-κB activation [95]. Moreover, biliverdin administered just after LPS also abrogates lung inflammation [95]. The effects of biliverdin on IL-6 production in mouse macrophage cells in vitro as well as in mouse lung epithelial cells treated with LPS are similar [95]. These findings not only indicate that biliverdin can modulate the LPS-induced inflammatory response and suppress pathophysiological changes in the lung, but also suggest that biliverdin also exerts anti-inflammatory effects on HS-induced tissue inflammation.
2. Protective role of carbon monoxide against oxidative tissue injury
Carbon monoxide exerts anti-inflammatory and anti-apoptotic effects in vitro and in vivo (Fig. 4). These effects are thought to be mediated, at least in part, by activation of the p38 MAPK signaling pathway [96, 97]. Heat shock protein 70 mediates the anti-inflammatory and anti-apoptotic effects of CO with the involvement of P38β MAPK and heat shock factor-1, providing a downstream mechanism for the cytoprotective effect of CO [98]. Moreover, the anti-oxidative effect of CO involves the inhibition of upstream TLR signaling pathways [99]. Very recently, it has been reported that the anti-inflammatory and anti-apoptotic effect of CO can be attributed to its ability to cause a relatively low intensity oxidative burst from the mitochondria by inhibiting the electron transport chain [100, 101]. Mitochondrial ROS induced by CO elicit the activation of HIF-1α [102] and and PPARγ [103] both of which are involved in the cytoprotecitive responses.
2-1. Inhalation of carbon monoxide attenuates HS-induced systemic inflammation and ameliorates end organ injuries.
Carbon monoxide exerts cytoprotective effects in HS-induced systemic inflammation and end-organ damage in a rodent model of HS [104]. The delivery of a low concentration of CO (250 ppm) at the onset of HS decreases levels of HS-induced serum IL-6, but augments HS-induced IL-10 levels in shocked mice, indicating that CO decreases the systemic inflammatory response caused by HS [104]. Carbon monoxide inhalation also ameliorates HS-induced lung, liver, and intestinal tissue injury as judged by the decrease in lung MPO activity, serum ALT levels and the preservation of architecture in the intestinal mucosa, respectively [104]. Moreover, initiation of CO inhalation after resuscitation protected the lung and the liver from HS-induced tissue injury [104]. These results suggest that CO holds promise as a therapeutic adjunct in the treatment of HS-induced oxidative tissue injuries, although the mechanism is not well defined and optimal dose regimens and therapeutic timing need to be determined.
Conclusions
This review summarizes recent evidence in support of HO-1 induction as a major protective response against HS-induced oxidative tissue injury. Pharmacological pre-induction of HO-1 confers significant protection upon cells, tissues and organs against oxidative damage induced by HS. In addition to the protective role of HO-1, both bile pigments and CO, the heme metabolites of the HO reaction, play pivotal protective roles in tissues. These findings suggest that HO-1 constitutes an essential cytoprotective component in HS-induced oxidative tissue injuries, and that appropriate HO-1 induction offers therapeutic promise for treating HS-induced organ damage. However, further studies are warranted to elucidate interspecies, or inter-cell type differences in ho-1 gene expression [105], in order to make better predictions from preclinical to clinical studies.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We would like to dedicate this article to the deceased Dr. Shigeru Sassa.
Abbreviations
- ALAS1
non-specific 5-aminolevulinate synthase
- CO
carbon monoxide
- HIF
hypoxia inducible factor
- HO
heme oxygenase
- HS
hemorrhagic shock
- iNOS
inducible nitric oxide synthase
- I/R
ischemia-reperfusion
- LPS
lipopolysaccharide
- MARE
maf recognition element
- Nrf2
NF-E2-related factor 2
- ROS
reactive oxygen species
- SnCl2
tin chloride
- SnMP
tin mesoporphyrin
- SnPP
tin protoporphyrin
- TLR
toll-like receptor
- TNF
tumor necrosis factor
References
- 1.Rushing G.D., Britt L.D. Reperfusion injury after hemorrhage: a collective review. Ann. Surg. 2008;247:929–937. doi: 10.1097/SLA.0b013e31816757f7. [DOI] [PubMed] [Google Scholar]
- 2.Peitzman A.B., Billiar T.R., Harbrecht B.G., Kelly E., Udekwu A.O., Simmons R.L. Hemorrhagic shock. Curr. Probl. Surg. 1995;32:925–1002. doi: 10.1016/s0011-3840(05)80008-5. [DOI] [PubMed] [Google Scholar]
- 3.Fink M.P. Reactive oxygen species as mediators of organ dysfunction caused by sepsis, acute respiratory distress syndrome, or hemorrhagic shock: potential benefits of resuscitation with Ringer’s ethyl pyruvate solution. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:167–174. doi: 10.1097/00075197-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Furuyama K., Kaneko K., Vargas V P.D. Heme as a magnificent molecule with multiple missions: heme determines its own fate and governs cellular homeostasis. Tohoku J. Exp. Med. 2007;213:1–16. doi: 10.1620/tjem.213.1. [DOI] [PubMed] [Google Scholar]
- 5.Sassa S. Biological implications of heme metabolism. J. Clin. Biochem. Nutr. 2006;38:138–155. [Google Scholar]
- 6.Takahashi T., Shimizu H., Morimatsu H., Inoue K., Akagi R., Morita K., Sassa S. Heme oxygenase-1: a fundamental guardian against oxidative tissue injuries in acute inflammation. Mini Rev. Med. Chem. 2007;7:745–753. doi: 10.2174/138955707781024517. [DOI] [PubMed] [Google Scholar]
- 7.Shibahara S. Regulation of heme oxygenase gene expression. Semin. Hematol. 1988;25:370–376. [PubMed] [Google Scholar]
- 8.Maines M.D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB. J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 9.Ryter S.W., Alam J., Choi A.M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 10.Ryter S.W., Kim H.P., Nakahira K., Zuckerbraun B.S., Morse D., Choi A.M. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid. Redox Signal. 2007;9:2157–2173. doi: 10.1089/ars.2007.1811. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T., Morita K., Akagi R., Sassa S. Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr. Med. Chem. 2004;11:1545–1561. doi: 10.2174/0929867043365080. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T., Morita K., Akagi R., Sassa S. Defense against oxidative tissue injury: the essential role played by heme oxygenase-1. Curr. Enzyme Inhib. 2006;2:105–124. [Google Scholar]
- 13.Takahashi T., Shimizu H., Akagi R., Morita K., Sassa S. Heme oxygenase-1: a new drug target in oxidative tissue injuries in critically ill conditions. Drug Dev. Res. 2006;67:130–153. [Google Scholar]
- 14.Poss K.D., Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yachie A., Niida Y., Wada T., Igarashi N., Kaneda H., Toma T., Ohta K., Kasahara Y., Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soares M.P., Bach F.H. Heme oxygenase-1 in organ transplantation. Front. Biosci. 2007;12:4932–4945. doi: 10.2741/2439. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchihashi S., Fondevila C., Kupiec-Weglinski J.W. Heme oxygenase system in ischemia and reperfusion injury. Ann. Transplant. 2004;9:84–87. [PubMed] [Google Scholar]
- 18.Perrella M.A., Yet S.F. Role of heme oxygenase-1 in cardiovascular function. Curr. Pharm. Des. 2003;9:2479–2487. doi: 10.2174/1381612033453776. [DOI] [PubMed] [Google Scholar]
- 19.Nakao A., Kaczorowski D.J., Sugimoto R., Billiar T.R., McCurry K.R. Application of heme oxygenase-1, carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 2008;42:78–88. doi: 10.3164/jcbn.2008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rensing H., Bauer I., Datene V., Patau C., Pannen B.H., Bauer M. Differential expression pattern of heme oxygenase-1/heat shock protein 32 and nitric oxide synthase-II and their impact on liver injury in a rat model of hemorrhage and resuscitation. Crit. Care Med. 1999;27:2766–2775. doi: 10.1097/00003246-199912000-00027. [DOI] [PubMed] [Google Scholar]
- 21.Rensing H., Bauer I., Peters I., Wein T., Silomon M., Jaeschke H., Bauer M. Role of reactive oxygen species for hepatocellular injury and heme oxygenase-1 gene expression after hemorrhage and resuscitation. Shock. 1999;12:300–308. doi: 10.1097/00024382-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Paxian M., Rensing H., Rickauer A., Schonhofen S., Schmeck J., Pannen B.H., Bauer I., Bauer M. Kupffer cells and neutrophils as paracrine regulators of the heme oxygenase-1 gene in hepatocytes after hemorrhagic shock. Shock. 2001;15:438–445. doi: 10.1097/00024382-200115060-00005. [DOI] [PubMed] [Google Scholar]
- 23.Maeshima K., Takahashi T., Uehara K., Shimizu H., Omori E., Yokoyama M., Tani T., Akagi R., Morita K. Prevention of hemorrhagic shock-induced lung injury by heme arginate treatment in rats. Biochem. Pharmacol. 2005;69:1667–1680. doi: 10.1016/j.bcp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M., Kure S., Engel J.D., Hiraga K. Structure, turnover, and heme-mediated suppression of the level of mRNA encoding rat liver delta-aminolevulinate synthase. J. Biol. Chem. 1988;263:15973–15979. [PubMed] [Google Scholar]
- 25.Ferrero M.E., Orsi R., Bernelli-Zazzera A. Effects of ischemia on drug-metabolizing microsomal enzymes in rat liver. Exp. Mol. Pathol. 1978;28:256–266. doi: 10.1016/0014-4800(78)90056-4. [DOI] [PubMed] [Google Scholar]
- 26.Kappas A., Drummond G.S. Control of heme metabolism with synthetic metalloporphyrins. J. Clin. Invest. 1986;77:335–339. doi: 10.1172/JCI112309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Immenschuh S., Kietzmann T., Hinke V., Wiederhold M., Katz N., Muller-Eberhard U. The rat heme oxygenase-1 gene is transcriptionally induced via the protein kinase A signaling pathway in rat hepatocyte cultures. Mol. Pharmacol. 1998;53:483–491. doi: 10.1124/mol.53.3.483. [DOI] [PubMed] [Google Scholar]
- 28.Rensing H., Bauer I., Kubulus D., Wolf B., Winning J., Ziegeler S., Bauer M. Heme oxygenase-1 gene expression in pericentral hepatocytes through beta1-adrenoceptor stimulation. Shock. 2004;21:376–387. doi: 10.1097/00024382-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Raddatz A., Kubulus D., Winning J., Bauer I., Pradarutti S., Wolf B., Kreuer S., Rensing H. Dobutamine improves liver function after hemorrhagic shock through induction of heme oxygenase-1. Am. J. Respir. Crit. Care Med. 2006;174:198–207. doi: 10.1164/rccm.200508-1221OC. [DOI] [PubMed] [Google Scholar]
- 30.Drummond G.S., Galbraith R.A., Sardana M.K., Kappas A. Reduction of the C2 and C4 vinyl groups of Sn-protoporphyrin to form Sn-mesoporphyrin markedly enhances the ability of the metalloporphyrin to inhibit in vivo heme catabolism. Arch. Biochem. Biophys. 1987;255:64–74. doi: 10.1016/0003-9861(87)90294-3. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T., Morita K., Akagi R., Sassa S. Protective role of heme oxygenase-1 in renal ischemia. Antioxid. Redox Signal. 2004;6:867–877. doi: 10.1089/ars.2004.6.867. [DOI] [PubMed] [Google Scholar]
- 32.Maines M.D., Mayer R.D., Ewing J.F., McCoubrey W.K. Jr. Induction of kidney heme oxygenase-1 (HSP32) mRNA and protein by ischemia/reperfusion: possible role of heme as both promotor of tissue damage and regulator of HSP32. J. Pharmacol. Exp. Ther. 1993;264:457–462. [PubMed] [Google Scholar]
- 33.Orrenius S., Ellin A., Jakobsson S.V., Thor H., Cinti D.L., Schenkman J.B., Estabrook R.W. The cytochrome P-450-containing mono-oxygenase system of rat kidney cortex microsomes. Drug Metab. Dispos. 1973;1:350–357. [PubMed] [Google Scholar]
- 34.Shimizu H., Takahashi T., Suzuki T., Yamasaki A., Fujiwara T., Odaka Y., Hirakawa M., Fujita H., Akagi R. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit. Care Med. 2000;28:809–817. doi: 10.1097/00003246-200003000-00033. [DOI] [PubMed] [Google Scholar]
- 35.Pittock S.T., Norby S.M., Grande J.P., Croatt A.J., Bren G.D., Badley A.D., Caplice N.M., Griffin M.D., Nath K.A. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: Pathophysiologic correlates. Kidney Int. 2005;68:611–622. doi: 10.1111/j.1523-1755.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 36.Tracz M.J., Juncos J.P., Croatt A.J., Ackerman A.W., Grande J.P., Knutson K.L., Kane G.C., Terzic A., Griffin M.D., Nath K.A. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int. 2007;72:1073–1080. doi: 10.1038/sj.ki.5002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokoi K., Kimura M., Itokawa Y. Effect of dietary tin deficiency on growth and mineral status in rats. Biol. Trace Elem. Res. 1990;24:223–231. doi: 10.1007/BF02917210. [DOI] [PubMed] [Google Scholar]
- 38.Winship K.A. Toxicity of tin and its compounds. Adverse Drug React. Acute Poisoning Rev. 1988;7:19–38. [PubMed] [Google Scholar]
- 39.Kappas A., Maines M.D. Tin: a potent inducer of heme oxygenase in kidney. Science. 1976;192:60–62. doi: 10.1126/science.1257757. [DOI] [PubMed] [Google Scholar]
- 40.Toda N., Takahashi T., Mizobuchi S., Fujii H., Nakahira K., Takahashi S., Yamashita M., Morita K., Hirakawa M., Akagi R. Tin chloride pretreatment prevents renal injury in rats with ischemic acute renal failure. Crit. Care Med. 2002;30:1512–1522. doi: 10.1097/00003246-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Shovman O., Levy Y., Gilburd B., Shoenfeld Y. Antiinflammatory and immunomodulatory properties of statins. Immunol. Res. 2002;25:271–285. doi: 10.1385/IR:25:3:271. [DOI] [PubMed] [Google Scholar]
- 42.Lee T.S., Chang C.C., Zhu Y., Shyy J.Y. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 43.Novack V., Terblanche M., Almog Y. Do statins have a role in preventing or treating sepsis? Crit. Care. 2006;10:113. doi: 10.1186/cc3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gueler F., Park J.K., Rong S., Kirsch T., Lindschau C., Zheng W., Elger M., Fiebeler A., Fliser D., Luft F.C., Haller H. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am. J. Pathol. 2007;170:1192–1199. doi: 10.2353/ajpath.2007.060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassoun H.T., Kone B.C., Mercer D.W., Moody F.G., Weisbrodt N.W., Moore F.A. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 46.Inoue K., Takahashi T., Uehara K., Shimuzu H., Ido K., Morimatsu H., Omori E., Katayama H., Akagi R., Morita K. Protective role of heme oxygenase 1 in the intestinal tissue injury in hemorrhagic shock in rats. Shock. 2008;29:252–261. doi: 10.1097/shk.0b013e3180cab913. [DOI] [PubMed] [Google Scholar]
- 47.Lee P.J., Jiang B.H., Chin B.Y., Iyer N.V., Alam J., Semenza G.L., Choi A.M. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- 48.Wischmeyer P.E. Glutamine: mode of action in critical illness. Crit. Care Med. 2007;35:S541–544. doi: 10.1097/01.CCM.0000278064.32780.D3. [DOI] [PubMed] [Google Scholar]
- 49.De-Souza D.A., Greene L.J. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit. Care Med. 2005;33:1125–1135. doi: 10.1097/01.ccm.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 50.Uehara K., Takahashi T., Fujii H., Shimizu H., Omori E., Matsumi M., Yokoyama M., Morita K., Akagi R., Sassa S. The lower intestinal tract-specific induction of heme oxygenase-1 by glutamine protects against endotoxemic intestinal injury. Crit. Care Med. 2005;33:381–390. doi: 10.1097/01.ccm.0000153407.14237.7f. [DOI] [PubMed] [Google Scholar]
- 51.Umeda K., Takahashi T., Inoue K., Shimizu H., Maeda S., Morimatsu H., Omori E., Akagi R., Katayama H., Morita K. Prevention of Hemorrhagic Shock-Induced Intestinal Tissue Injury by Glutamine Via Heme Oxygenase-1 Induction. Shock. 2008 doi: 10.1097/SHK.0b013e318177823a. In press, [DOI] [PubMed] [Google Scholar]
- 52.Bhatia M., Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 53.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 54.Wiedemann H.P., Arroliga A.C., Komara J.J. Jr. Emerging systemic pharmacologic approaches in acute respiratory distress syndrome. Respir. Care Clin. N. Am. 2003;9:419–435. doi: 10.1016/s1078-5337(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 55.Tamion F., Richard V., Bonmarchand G., Leroy J., Lebreton J.P., Thuillez C. Induction of heme-oxygenase-1 prevents the systemic responses to hemorrhagic shock. Am. J. Respir. Crit. Care Med. 2001;164:1933–1938. doi: 10.1164/ajrccm.164.10.2010074. [DOI] [PubMed] [Google Scholar]
- 56.Tenhunen R., Tokola O., Linden I.B. Haem arginate: a new stable haem compound. J. Pharm. Pharmacol. 1987;39:780–786. doi: 10.1111/j.2042-7158.1987.tb05119.x. [DOI] [PubMed] [Google Scholar]
- 57.Tenhunen R., Mustajoki P. Acute porphyria: treatment with heme. Semin. Liver Dis. 1998;18:53–55. doi: 10.1055/s-2007-1007140. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki T., Takahashi T., Maeshima K., Shimizu H., Toda Y., Morimatsu H., Takeuchi M., Yokoyama M., Akagi R., Morita K. Heme arginate pretreatment attenuates pulmonary NF-kappaB and AP-1 activation induced by hemorrhagic shock via heme oxygenase-1 induction. Med. Chem. 2006;2:271–274. doi: 10.2174/157340606776930781. [DOI] [PubMed] [Google Scholar]
- 59.Ryter S.W., Tyrrell R.M. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic. Biol. Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 60.Hierholzer C., Billiar T.R. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch. Surg. 2001;386:302–308. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graca-Souza A.V., Arruda M.A., de Freitas M.S., Barja-Fidalgo C., Oliveira P.L. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002;99:4160–4165. doi: 10.1182/blood.v99.11.4160. [DOI] [PubMed] [Google Scholar]
- 62.Wagener F.E., de Witte T., Abraham N.G. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc. Soc. Exp. Biol. Med. 1997;216:456–463. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 63.Wagener F.A., da Silva J.L., Farley T., de Witte T., Kappas A., Abraham N.G. Differential effects of heme oxygenase isoforms on heme mediation of endothelial intracellular adhesion molecule 1 expression. J. Pharmacol. Exp. Ther. 1999;291:416–423. [PubMed] [Google Scholar]
- 64.Figueiredo R.T., Fernandez P.L., Mourao-Sa D.S., Porto B.N., Dutra F.F., Alves L.S., Oliveira M.F., Oliveira P.L., Graca-Souza A.V., Bozza M.T. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 65.Ogawa K., Sun J., Taketani S., Nakajima O., Nishitani C., Sassa S., Hayashi N., Yamamoto M., Shibahara S., Fujita H., Igarashi K. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO. J. 2001;20:2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Igarashi K., Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid. Redox Signal. 2006;8:107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki H., Tashiro S., Hira S., Sun J., Yamazaki C., Zenke Y., Ikeda-Saito M., Yoshida M., Igarashi K. Heme regulates gene expression by triggering Crm1-dependent nuclear export of Bach1. EMBO. J. 2004;23:2544–2553. doi: 10.1038/sj.emboj.7600248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim Y.C., Masutani H., Yamaguchi Y., Itoh K., Yamamoto M., Yodoi J. Hemin-induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J. Biol. Chem. 2001;276:18399–18406. doi: 10.1074/jbc.M100103200. [DOI] [PubMed] [Google Scholar]
- 69.Alam J., Killeen E., Gong P., Naquin R., Hu B., Stewart D., Ingelfinger J.R., Nath K.A. Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. Am. J. Physiol. Renal Physiol. 2003;284:F743–752. doi: 10.1152/ajprenal.00376.2002. [DOI] [PubMed] [Google Scholar]
- 70.Sun J., Brand M., Zenke Y., Tashiro S., Groudine M., Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun J., Hoshino H., Takaku K., Nakajima O., Muto A., Suzuki H., Tashiro S., Takahashi S., Shibahara S., Alam J., Taketo M.M., Yamamoto M., Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO. J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishikawa M., Numazawa S., Yoshida T. Redox regulation of the transcriptional repressor Bach1. Free Radic. Biol. Med. 2005;38:1344–1352. doi: 10.1016/j.freeradbiomed.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 73.Reichard J.F., Motz G.T., Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reichard J.F., Sartor M.A., Puga A. BACH1 is a specific repressor of HMOX1 that is inactivated by arsenite. J. Biol. Chem. 2008 doi: 10.1074/jbc.M801784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goswami S.K., Maulik N., Das D.K. Ischemia-reperfusion and cardioprotection: a delicate balance between reactive oxygen species generation and redox homeostasis. Ann. Med. 2007;39:275–289. doi: 10.1080/07853890701374677. [DOI] [PubMed] [Google Scholar]
- 76.Clark J.E., Foresti R., Sarathchandra P., Kaur H., Green C.J., Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H643–651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 77.Maulik N., Sharma H.S., Das D.K. Induction of the haem oxygenase gene expression during the reperfusion of ischemic rat myocardium. J. Mol. Cell Cardiol. 1996;28:1261–1270. doi: 10.1006/jmcc.1996.0116. [DOI] [PubMed] [Google Scholar]
- 78.Melo L.G., Agrawal R., Zhang L., Rezvani M., Mangi A.A., Ehsan A., Griese D.P., Dell’Acqua G., Mann M.J., Oyama J., Yet S.F., Layne M.D., Perrella M.A., Dzau V.J. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602–607. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 79.Pachori A.S., Melo L.G., Hart M.L., Noiseux N., Zhang L., Morello F., Solomon S.D., Stahl G.L., Pratt R.E., Dzau V.J. Hypoxia-regulated therapeutic gene as a preemptive treatment strategy against ischemia/reperfusion tissue injury. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12282–12287. doi: 10.1073/pnas.0404616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yet S.F., Tian R., Layne M.D., Wang Z.Y., Maemura K., Solovyeva M., Ith B., Melo L.G., Zhang L., Ingwall J.S., Dzau V.J., Lee M.E., Perrella M.A. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ. Res. 2001;89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 81.Yano Y., Ozono R., Oishi Y., Kambe M., Yoshizumi M., Ishida T., Omura S., Oshima T., Igarashi K. Genetic ablation of the transcription repressor Bach1 leads to myocardial protection against ischemia/reperfusion in mice. Genes Cells. 2006;11:791–803. doi: 10.1111/j.1365-2443.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 82.Maines M.D. Zinc. protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim. Biophys. Acta. 1981;673:339–350. doi: 10.1016/0304-4165(81)90465-7. [DOI] [PubMed] [Google Scholar]
- 83.Balla G., Jacob H.S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J.W., Vercellotti G.M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 84.Poss K.D., Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferris C.D., Jaffrey S.R., Sawa A., Takahashi M., Brady S.D., Barrow R.K., Tysoe S.A., Wolosker H., Baranano D.E., Dore S., Poss K.D., Snyder S.H. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat. Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 86.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 87.Baranano D.E., Rao M., Ferris C.D., Snyder S.H. Biliverdin reductase: a major physiologic cytoprotectant. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sedlak T.W., Snyder S.H. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics. 2004;113:1776–1782. doi: 10.1542/peds.113.6.1776. [DOI] [PubMed] [Google Scholar]
- 89.Vitek L., Jirsa M., Brodanova M., Kalab M., Marecek Z., Danzig V., Novotny L., Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis. 2002;160:449–456. doi: 10.1016/s0021-9150(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 90.Schwertner H.A., Jackson W.G., Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin. Chem. 1994;40:18–23. [PubMed] [Google Scholar]
- 91.Hopkins P.N., Wu L.L., Hunt S.C., James B.C., Vincent G.M., Williams R.R. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 1996;16:250–255. doi: 10.1161/01.atv.16.2.250. [DOI] [PubMed] [Google Scholar]
- 92.Djousse L., Levy D., Cupples L.A., Evans J.C., D’Agostino R.B., Ellison R.C. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am. J. Cardiol. 2001;87:1196–1200. doi: 10.1016/s0002-9149(01)01494-1. A1194, 1197, [DOI] [PubMed] [Google Scholar]
- 93.Djousse L., Rothman K.J., Cupples L.A., Levy D., Ellison R.C. Effect of serum albumin and bilirubin on the risk of myocardial infarction (the Framingham Offspring Study) Am. J. Cardiol. 2003;91:485–488. doi: 10.1016/s0002-9149(02)03256-3. [DOI] [PubMed] [Google Scholar]
- 94.Jarrar D., Chaudry I.H., Wang P. Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches (Review) Int. J. Mol. Med. 1999;4:575–583. doi: 10.3892/ijmm.4.6.575. [DOI] [PubMed] [Google Scholar]
- 95.Sarady-Andrews J.K., Liu F., Gallo D., Nakao A., Overhaus M., Ollinger R., Choi A.M., Otterbein L.E. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L1131–1137. doi: 10.1152/ajplung.00458.2004. [DOI] [PubMed] [Google Scholar]
- 96.Otterbein L.E., Bach F.H., Alam J., Soares M., Lu H.T., Wysk M., Davis R.J., Flavell R.A., Choi A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 97.Brouard S., Otterbein L.E., Anrather J., Tobiasch E., Bach F.H., Choi A.M., Soares M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim H.P., Wang X., Zhang J., Suh G.Y., Benjamin I.J., Ryter S.W., Choi A.M. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38 beta MAPK and heat shock factor-1. J. Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 99.Nakahira K., Kim H.P., Geng X.H., Nakao A., Wang X., Murase N., Drain P.F., Sasidhar M., Nabel E.G., Takahashi T., Lukacs N.W., Ryter S.W., Morita K., Choi A.M. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J. Exp. Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bilban M., Haschemi A., Wegiel B., Chin B.Y., Wagner O., Otterbein L.E. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J. Mol. Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 101.Zuckerbraun B.S., Chin B.Y., Bilban M., de Costa d’Avila J., Rao J., Billiar T.R., Otterbein L.E. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB. J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 102.Chin B.Y., Jiang G., Wegiel B., Wang H.J., Macdonald T., Zhang X.C., Gallo D., Cszimadia E., Bach F.H., Lee P.J., Otterbein L.E. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bilban M., Bach F.H., Otterbein S.L., Ifedigbo E., de Costa d’Avila J., Esterbauer H., Chin B.Y., Usheva A., Robson S.C., Wagner O., Otterbein L.E. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 104.Zuckerbraun B.S., McCloskey C.A., Gallo D., Liu F., Ifedigbo E., Otterbein L.E., Billiar T.R. Carbon monoxide prevents multiple organ injury in a model of hemorrhagic shock and resuscitation. Shock. 2005;23:527–532. [PubMed] [Google Scholar]
- 105.Shibahara S. The heme oxygenase dilemma in cellular homeostasis: new insights for the feedback regulation of heme catabolism. Tohoku J. Exp. Med. 2003;200:167–186. doi: 10.1620/tjem.200.167. [DOI] [PubMed] [Google Scholar]