Abstract
Ubiquinol-10 (QH2), the reduced form of Coenzyme Q10 (CoQ10) serves as a potent antioxidant of lipid membranes. Because many antioxidants reveal potent anti-inflammatory effects, the influence of QH2 on lipopolysaccharide (LPS)-induced pro-inflammatory cytokines and chemokines were determined in the human monocytic cell line THP-1. Stimulation of cells with LPS resulted in a distinct release of Tumour necrosis factor-alpha (TNF-α), Macrophage inflammatory protein-1 alpha (MIP-1α), Regulated upon activation, normal T cell expressed and secreted (RANTES) and Monocyte chemotattractant protein-1 (MCP-1). The LPS-induced responses were significantly decreased by pre-incubation of cells with QH2 to 60.27 ± 9.3% (p = 0.0009), 48.13 ± 6.93% (p = 0.0007) and 74.36 ± 7.25% (p = 0.008) for TNF-α, MIP-1α and RANTES, respectively. In conclusion, our results indicate anti-inflammatory effects of the reduced form of CoQ10 on various proinflammatory cytokines and chemokines in vitro.
Keywords: coenzyme Q10, ubiquinol-10, inflammation, monocytes
Introduction
Exposure of cells to the pro-inflammatory lipopolysaccharide (LPS) triggers TLR4-dependent phosphorylation cascades which lead to activation of NFκB. This central transcription factor induces the expression and subsequent secretion of various pro-inflammatory cytokines and chemokines [1–3]. Reactive oxygen species (ROS) are important for the activity of the TLR4-signalling pathway [4]. Accordingly, antioxidants are described as anti-inflammatory agents [5, 6]. Because Coenzyme Q10 (CoQ10) is a potent antioxidant, we postulated that this molecule possesses anti-inflammatory properties. More recently was shown that CoQ10 supplementation minimizes oxidative stress during statin drug therapy [7]. Indeed, we found a reduction of LPS-induced cytokine release by CoQ10 in murine and human monocytic cell lines [8]. In the latter study, we treated cells with the oxidized form of CoQ10 (ubiquinone-10) which is converted intracellular to ubiquinol-10 (QH2). As only the reduced form of CoQ10 can act as an antioxidant, here we studied effects on secretion of the cytokine TNF-α and different chemokines in LPS-stimulated THP-1 cells that were directly incubated with QH2.
Material and Methods
Reagents
Lipopolysaccharide (LPS, E.coli O55:B5) was obtained from Sigma-Aldrich (Taufkirchen, Germany). The aqueous solutions of ubiquinol-10 (PEG-60 hydrogenated castor oil, ubiquinol-10, glycerol, water) and the corresponding vehicle (no ubiquinol-10 supplement) were received from KANEKA Corporation (Osaka, Japan).
Cell culture
Cultivation of THP-1 cells occurred routinely in RPMI medium 1640 supplemented with 10% FCS and 1% antibiotics (penicillin/streptomycin) in a humidified incubator containing 5% CO2 at 37°C. For determination of TNF-α and chemokines, cells were plated at a density of 0.5 × 106 cells in a 12-well plate for 24 h before pre-incubation. Subsequently, cells were preincubated with either 10 µM ubiquinol-10 or the reference substances pyrrolidine-dithiocarbamate (PDTC) or N-acetyl-cysteine (NAC), or the respective vehicle control. After 24 h, cell culture medium was removed and fresh LPS-containing medium (1 µg/ ml) was added for 4 h. Finally, for cytokine determination via ELISA, supernatants were kept and stored at −80°C. For protein determination via the BRADFORD method, cells were collected into NET-buffer.
Cytotoxicity
For determination of cell viability, the Cell-Titer Glo® Luminescent Assay was used. Thus, total ATP levels were measured as an index of the viable cell number. The luminescence was detected on a GloMax® (Promega, Mannheim, Germany).
Determination of TNF-α and chemokines
Using TNF-α as an internal control, this cytokine was determined by DuoSet ELISA (R&D Systems, Wiesbaden, Germany) as well as multiplex suspension array technology (BioRad, Munich, Germany) according to the manufacturer’s instructions. The chemokines MCP-1, MIP-1α and RANTES were determined by the multiplex suspension array system.
Protein concentration
Cells were collected into NET-buffer (50 mM TRIS [pH 7.5], 150 mM NaCl, 1 mM EDTA [pH 8.0], 0.5% NP-40) and the cell suspension was treated with ultrasonics and then centrifugated by 14000 rpm at 4°C for 20 min. Determination of protein concentration occurred in the resulting supernatant by the Bradford method according to the manufacturer’s instructions.
Statistics
All data are results of two (PDTC, NAC) or three (QH2) independent biological experiments performed in duplicate and expressed as means ± standard error of the mean (SEM). Results were analyzed by an unpaired two-sided Student’s t-test using SPSS 11.5 for Windows and GraphPad Prism 4.0 software. p-values less than or equal to 0.05 were considered statistically significant.
Results and Discussion
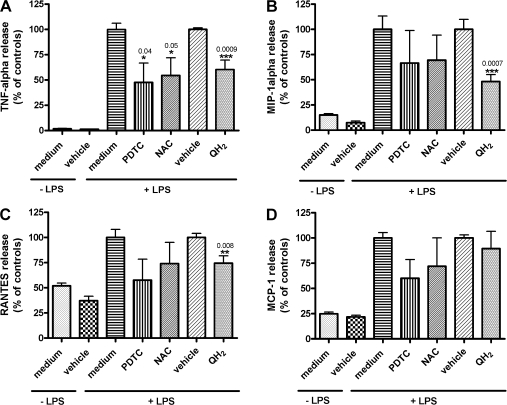
NFκB is a multisubunit transcription factor that is ubiquitously expressed in different cell types and can be activated by several agents such as LPS, TNF-α or the oxidant hydrogen peroxide (H2O2) [9]. This activation process includes phosphorylation of the IκB subunit and its dissociation from the inactive cytoplasmic complex. Thus, the active dimer of p50 and p65 translocates into the nucleus where specific target genes of pro-inflammatory mediators and cytokines become immediately up-regulated [9, 10]. However, this NFκB-activating cascade was shown to be inhibited by antioxidants such as PDTC and NAC. This has led to the hypothesis that oxygen radicals are key players in the activation of NFκB through an redox-dependent mechanism [9, 11, 12]. Because many antioxidants reveal potent anti-inflammatory effects, the influence of QH2 on LPS-induced pro-inflammatory cytokines and chemokines was determined in the human monocytic cell line THP-1. All experiments were performed with the well known radical scavengers PDTC and NAC to validate the putative anti-inflammatory effects of QH2. To implement culture conditions that do not lead to unspecific side effects, cell vitality was measured at different medium concentrations of QH2. As shown in Table 1, no cytotoxic effects were found for PDTC (100 µM), NAC (100 µM) and QH2 (1, 10, 100 µM). For further experiments we used 10 µM QH2, because this concentration leads to a significantly higher intracellular QH2 content in THP-1 cells and is also achievable in human serum through QH2 supplementation (unpublished results). As shown in Figure 1A-D, unstimulated THP-1 monocytes secrete low amounts of the pro-inflammatory cytokine TNF-α and chemokines MIP-1α, RANTES and MCP-1 into the medium. However, stimulation with LPS induces approximately 58-(TNF-α), 7-(MIP-1α), 2-(RANTES) and 4-(MCP-1) fold higher levels of these pro-inflammatory agents in the cell culture medium within 4 h, respectively. Next, we tested the effect of pre-incubation of cells with 10 µM QH2 for 24 h. Thus, as shown in Figure 1A–C, the LPS-induced responses were significantly decreased to 60.27 ± 9.3%, 48.13 ± 6.93% and 74.36 ± 7.25% for TNF-α, MIP-1α and RANTES, respectively (Fig. 1A–C). No significant effect was found for MCP-1 (Fig. 1D). Pre-incubation of cells with 10 µM PDTC or 10 µM NAC decreased TNF-α levels significantly to 47.69 ± 19.07% and 54.43 ± 17.64%, respectively (Fig. 1A). No significant effects of PDTC and NAC were found on LPS-induced secretion levels of other pro-inflammatory mediators.
Table 1.
Effect of ubiquinol-10 (QH2) and the reference substances PDTC and NAC on viability of THP-1 cells.
| 10% DMSO | Vehicle control | 100 µM PDTC | 100 µM NAC | 1 µM QH2 | 10 µM QH2 | 100 µM QH2 | |
|---|---|---|---|---|---|---|---|
| % | 1.71 | 148.87 | 109.44 | 92.51 | 120.74 | 109.68 | 98.43 |
| ±SEM | ±0.36 | ±15.09 | ±22.33 | ±21.38 | ±13.20 | ±15.70 | ±16.09 |
THP-1 cells were either treated with 1–100 µM QH2 or 100 µM PDTC or NAC for 24 h. Medium was used as negative control (data not shown) and 10% dimethyl sulfoxide (DMSO) as positive control (poco, positive control). The applied amount of the vehicle (veco) was in accordance to 100 µM QH2. The cell viability of the negative control was set to 100% and the other values (means ± SEM) were referenced to it. Three independent experiments were performed in triplicate.
Fig. 1.
Effects of pre-treatment of ubiquinol on LPS-induced release of TNF-α (A), MIP-1α (B), RANTES (C) and MCP-1 (D) in THP-1 cells.
Cells were either pre-treated with 10 µM QH2 or the respective reference substances PDTC or NAC, or medium and vehicle for 24 h. Afterwards, media were removed and cells were treated with LPS (1 µg/ml medium) for 4 h. The resulting concentrations (pg/µg cellular protein) of TNF-α, MIP-1α, RANTES and MCP-1 of the vehicle controls (+LPS) were set to 100% for QH2-pretreated cells and the other values were referenced to it. Values from PDTC- and NAC-pretreated cells were related to medium controls (+LPS) taken as 100%. Statistically significant data (*, p≤0.05; **, p≤0.01; ***, p≤0.001) are means ± SEM of four (ubiquinol, vehicle) or two (PDTC, NAC, medium) independent experiments performed in duplicate.
Inflammation has been related to the pathogenesis of various diseases, such as atherosclerosis [13]. Monocytes play an important role in the response to inflammatory agents, particularly to those derived from gut bacteria and are able to enter the circulation, such as bacterial endotoxins. Thus, endotoxins circulate at low concentrations in the blood of all healthy individuals, but are also increased after a high-fat meal [14]. However, elevated levels are associated with an increased risk of atherosclerosis or sepsis [14–16]. For our experiments we used LPS, a compound of gram-negative bacteria that is also relevant in vivo to trigger a serious medical inflammatory process in vitro. Finally, stimulation of monocytes with LPS induces production of ROS, which in turn activate the transcription factor NFκB [4, 9] that triggers a large amount of genes encoding for inflammatory mediators and cytokines [17]. Numerous studies in monocytes revealed natural occurring antioxidants as compounds with anti-inflammatory effects [18, 19]. Here we used CoQ10 in order to study its putative anti-inflammatory effect in the human monocytic cell line THP-1. For this purpose, we used the reduced form of CoQ10, QH2, which functions as an antioxidant. To our knowledge, effects of QH2 on inflammatory markers have not been investigated so far, because this form of CoQ10 is not commonly available. Thus, only a few studies were published using QH2 in vivo. These studies indicate effects of QH2 on safety and bioavailability [20], sperm kinetic features [21], oxidative imbalance in children with Trisomy 21 [22] and neuroprotection in an animal Parkinson model [23]. In THP-1 cells, we found that QH2 reduces significantly the secretion of the pro-inflammatory agents TNF-α, MIP-1α, and RANTES in response to LPS. This putative anti-inflammatory effect of QH2 could be due to its antioxidant property in cell membranes, because LPS-induced ROS production occurs very closely to the membrane [4]. One important membrane-associated complex that is relevant for generation of ROS in monocytic cells [24] is the NADPH oxidase. This complex is described to consist of four proteins, whereas Rac is the most critical component for a functional NADPH oxidase. Rac is regulated by small GTP-binding proteins [25]. In this context it was shown that LPS induced Rac activity and moreover, the NADPH oxidase-dependent ROS formation [26]. Thus it seems that LPS directly initiates the NADPH oxidase activity by downstream signalling pathways. Results from a further study indicate an up-regulation of the NADPH oxidase complex through a NFκB-dependent TNF-α activation process which finally leads to enhanced ROS production and further NFκB-activation [24]. This in turn might contribute to sustained releases of pro-inflammatory cytokines and mediators. In this context it was also shown that the well known antioxidant and radical scavenger NAC inhibited NFκB-activation via reduction of H2O2 [9], an important reactive oxygen intermediate (ROI) of the NADPH oxidase pathway. These strong radical scavenging effects are also described for PDTC [27], which we used as an additional internal control to describe the putative anti-inflammatory effects of the reduced form of CoQ10 (QH2), a compound with strong antioxidant properties. In general it seems that QH2 mediates stronger anti-inflammatory effects on the tested pro-inflammatory compounds than PDTC and NAC, two well known radical scavengers mediating its anti-inflammatory properties through a diminished NFκB activation. Thus it seems that the reduced form of CoQ10 (QH2) mediates its anti-inflammatory effects at least in part through its strong antioxidant properties. However, these effects may be additionally mediated by gene expression. It has been shown in skeletal muscle of humans [28], heart of mice [29], CaCo-2 [30], and HeLa cells [31] that CoQ10 influences the expression of different genes. These hypotheses should be tested in future studies. In conclusion, our results indicate anti-inflammatory effects of the reduced form of CoQ10 on various proinflammatory cytokines and chemokines in vitro.
Acknowledgment
This work was supported by KANEKA Corporation.
References
- 1.Bubici C., Papa S., Pham C.G., Zazzeroni F., Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol. Histopathol. 2006;21:69–80. doi: 10.14670/HH-21.69. [DOI] [PubMed] [Google Scholar]
- 2.Takashiba S., Van Dyke T.E., Amar S., Murayama Y., Soskolne A.W., Shapira L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappaB. Infect. Immun. 1999;67:5573–5578. doi: 10.1128/iai.67.11.5573-5578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handa O., Naito Y., Takagi T., Shimozawa M., Kokura S., Yoshida N., Matsui H., Cepinskas G., Kvietys P.R., Yoshikawa T. Tumor necrosis factor-alpha-induced cytokine-induced neutrophil chemoattractant-1 (CINC-1) production by rat gastric epithelial cells: role of reactive oxygen species and nuclear factor-kappaB. J. Pharmacol. Exp. Ther. 2004;309:670–676. doi: 10.1124/jpet.103.062216. [DOI] [PubMed] [Google Scholar]
- 4.Sanlioglu S., Williams C.M., Samavati L., Butler N.S., Wang G., McCray P.B. Jr, Ritchie T.C., Hunninghake G.W., Zandi E., Engelhardt J.F. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J. Biol. Chem. 2001;276:30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- 5.Parmentier M., Hirani N., Rahman I., Donaldson K., MacNee W., Antonicelli F. Regulation of lipopolysaccharide-mediated interleukin-1beta release by N-acetylcysteine in THP-1 cells. Eur. Respir. J. 2000;16:933–939. doi: 10.1183/09031936.00.16593300. [DOI] [PubMed] [Google Scholar]
- 6.Geronikaki A.A., Gavalas A.M. Antioxidants and inflammatory disease: synthetic and natural antioxidants with anti-inflammatory activity. Comb. Chem. High Throughput Screen. 2006;9:425–442. doi: 10.2174/138620706777698481. [DOI] [PubMed] [Google Scholar]
- 7.Kettawan A., Takahashi T., Kongkachuichai R., Charoenkiatkul S., Kishi T., Okamoto T. Protective effects of coenzyme q(10) on decreased oxidative stress resistance induced by simvastatin. J. Clin. Biochem. Nutr. 2007;40:194–202. doi: 10.3164/jcbn.40.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsui Y., Schmelzer J.D., Zollman P.J., Mitsui M., Tritschler H.J., Low P.A. Alpha-lipoic acid provides neuroprotection from ischemia-reperfusion injury of peripheral nerve. J. Neurol. Sci. 1999;163:11–16. doi: 10.1016/s0022-510x(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 9.Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. Embo. J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990;344:678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- 11.Schreck R., Bevec D., Dukor P., Baeuerle P.A., Chedid L., Bahr G.M. Selection of a muramyl peptide based on its lack of activation of nuclear factor-kappa B as a potential adjuvant for AIDS vaccines. Clin. Exp. Immunol. 1992;90:188–193. doi: 10.1111/j.1365-2249.1992.tb07926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreck R., Baeuerle P.A. A role for oxygen radicals as second messengers. Trends Cell Biol. 1991;1:39–42. doi: 10.1016/0962-8924(91)90072-h. [DOI] [PubMed] [Google Scholar]
- 13.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 14.Erridge C., Attina T., Spickett C.M., Webb D.J. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 15.Bruunsgaard H., Pedersen A.N., Schroll M., Skinhoj P., Pedersen B.K. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin. Exp. Immunol. 1999;118:235–241. doi: 10.1046/j.1365-2249.1999.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng T., Shen E., Fan J., Zhang Y., Arnold J.M., Feng Q. Disruption of phospholipase Cgamma1 signalling attenuates cardiac tumor necrosis factor-alpha expression and improves myocardial function during endotoxemia. Cardiovasc. Res. 2008;78:90–97. doi: 10.1093/cvr/cvm100. [DOI] [PubMed] [Google Scholar]
- 17.Shakhov A.N., Collart M.A., Vassalli P., Nedospasov S.A., Jongeneel C.V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J. Exp. Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonicelli F., Brown D., Parmentier M., Drost E.M., Hirani N., Rahman I., Donaldson K., MacNee W. Regulation of LPS-mediated inflammation in vivo and in vitro by the thiol antioxidant Nacystelyn. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L1319–1327. doi: 10.1152/ajplung.00329.2003. [DOI] [PubMed] [Google Scholar]
- 19.Sacanella E., Vazquez-Agell M., Mena M.P., Antunez E., Fernandez-Sola J., Nicolas J.M., Lamuela-Raventos R.M., Ros E., Estruch R. Down-regulation of adhesion molecules and other inflammatory biomarkers after moderate wine consumption in healthy women: a randomized trial. Am. J. Clin. Nutr. 2007;86:1463–1469. doi: 10.1093/ajcn/86.5.1463. [DOI] [PubMed] [Google Scholar]
- 20.Hosoe K., Kitano M., Kishida H., Kubo H., Fujii K., Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul. Toxicol. Pharmacol. 2007;47:19–28. doi: 10.1016/j.yrtph.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Balercia G., Mosca F., Mantero F., Boscaro M., Mancini A., Ricciardo-Lamonica G., Littarru G. Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil. Steril. 2004;81:93–98. doi: 10.1016/j.fertnstert.2003.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Miles M.V., Patterson B.J., Chalfonte-Evans M.L., Horn P.S., Hickey F.J., Schapiro M.B., Steele P.E., Tang P.H., Hotze S.L. Coenzyme Q10 (ubiquinol-10) supplementation improves oxidative imbalance in children with trisomy 21. Pediatr. Neurol. 2007;37:398–403. doi: 10.1016/j.pediatrneurol.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Cleren C., Yang L., Lorenzo B., Calingasan N.Y., Schomer A., Sireci A., Wille E.J., Beal M.F. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J. Neurochem. 2008;104:1613–1621. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- 24.Gauss K.A., Nelson-Overton L.K., Siemsen D.W., Gao Y., DeLeo F.R., Quinn M.T. Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J. Leukoc. Biol. 2007;82:729–741. doi: 10.1189/jlb.1206735. [DOI] [PubMed] [Google Scholar]
- 25.Bokoch G.M. Regulation of the phagocyte respiratory burst by small GTP-binding proteins. Trends Cell Biol. 1995;5:109–113. doi: 10.1016/s0962-8924(00)88960-6. [DOI] [PubMed] [Google Scholar]
- 26.Hsu H.Y., Wen M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 27.Chandel N.S., Trzyna W.C., McClintock D.S., Schumacker P.T. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J. Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 28.Linnane A.W., Kopsidas G., Zhang C., Yarovaya N., Kovalenko S., Papakostopoulos P., Eastwood H., Graves S., Richardson M. Cellular redox activity of coenzyme Q10: effect of CoQ10 supplementation on human skeletal muscle. Free Radic. Res. 2002;36:445–453. doi: 10.1080/10715760290021306. [DOI] [PubMed] [Google Scholar]
- 29.Lee C.K., Pugh T.D., Klopp R.G., Edwards J., Allison D.B., Weindruch R., Prolla T.A. The impact of alpha-lipoic acid, coenzyme Q10 and caloric restriction on life span and gene expression patterns in mice. Free Radic. Biol. Med. 2004;36:1043–1057. doi: 10.1016/j.freeradbiomed.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Groneberg D.A., Kindermann B., Althammer M., Klapper M., Vormann J., Littarru G.P., Doring F. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int. J. Biochem. Cell Biol. 2005;37:1208–1218. doi: 10.1016/j.biocel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Gorelick C., Lopez-Jones M., Goldberg G.L., Romney S.L., Khabele D. Coenzyme Q10 and lipid-related gene induction in HeLa cells. Am. J. Obstet. Gynecol. 2004;190:1432–1434. doi: 10.1016/j.ajog.2004.01.076. [DOI] [PubMed] [Google Scholar]