Abstract
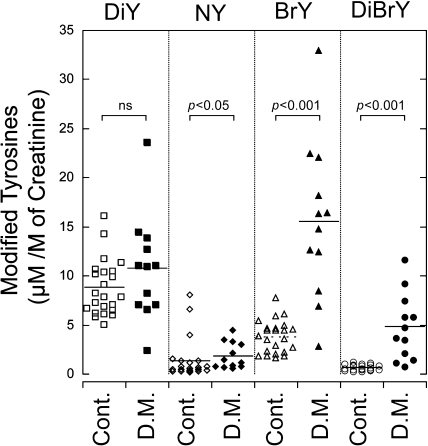
The quantification of urinary oxidized tyrosines, dityrosine (DiY), nitrotyrosine (NY), bromotyrosine (BrY), and dibromotyrosine (DiBrY), was accomplished by quadruple liquid chromatography-tandem mass spectrometry (LC/MS/MS). The sample was partially purified by solid phase extraction, and was then applied to the LC/MS/MS using multiple-reaction monitoring (MRM) methods. The analysis for the DiY quantification was done first. The residual samples were further butylated with n-butanol/HCl, and the other modified tyrosines were then quantified with isotopic dilution methods. MRM peaks of the modified tyrosines (DiY, NY, BrY, and DiBrY) from human urine were measured and the elution times coincided with the authentic and isotopic standards. The amounts of modified tyrosines in healthy human urine (n = 23) were 8.8 ± 0.6 (DiY), 1.4 ± 0.4 (NY), 3.8 ± 0.3 (BrY), and 0.7 ± 0.1 (DiBrY) µmol/mol of creatinine, respectively. A comparison of the modified tyrosines with urinary 8-oxo-deoxyguanosine, pentosidine, and Nε-(hexanoyl)lysine was also performed. Almost all products, except for NY, showed good correlations with each other. The amounts of the modified tyrosines (NY, BrY, and DiBrY) in the diabetic urine were higher than those in the urine from healthy people.
Keywords: dityrosine, nitrotyrosine, halotyrosines, urine, diabetes
Introduction
Oxidative stress may be involved in the initiation and/or progression of various diseases. For example, the pathogenesis of diabetic complications is known to involve oxidative stress [1]. Sources of reactive oxygen species (ROS) include the Maillard reaction products [2, 3] as well as the activated inflammatory system [4]. ROS cause modification of biomolecules such as proteins, lipids, and nucleic acids. The monitoring of oxidatively degraded biomolecules, which can become oxidative stress biomarkers, may act as an alarm/warning for the presence of diseases like diabetes. To monitor these markers, human urine is one of the most suitable sources for non-invasive sampling without inducing pain.
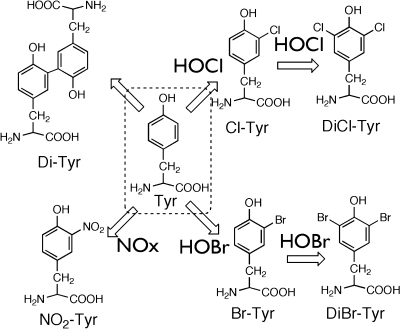
The oxidative modification of proteins often induces structural damage to amino acid residues [5, 6]. Of the twenty amino acids, tyrosine is a unique target for oxidizing species because the profiles of the tyrosine oxidation products are different from ROS. For example, 3-nitrotyrosine (NY) is a marker for reactive nitrating species such as peroxynitrite and NO2− [7, 8], which originate from excess nitric oxide (·NO); NO should be generated from nitric oxide synthase. Therefore, NY may be a marker of activated macrophages. The halotyrosines, 3-chlorotyrosine (ClY) and 3-bromotyrosine (BrY), appear to be markers of inflammatory tissue damage because eosinophil peroxidase (EPO) and neutrophil myeloperoxidase (MPO) generate halogenating species such as HOCl/HOBr [9–13]. Dityrosine (DiY) is one of the cross-linkers of proteins. DiY can thus become one of the universal protein oxidation markers because DiY can be generated by various ROS, such as peroxynitrite (ONOO−), metal-catalyzed oxidation, and UV-irradiation [14]. These products that originate from tyrosine may serve as oxidative stress biomarkers (Scheme I).
Scheme I.
Generation of tyrosine-derived modified products
Immunochemical approaches have been used to detect the modified tyrosines NY, DiY, and ClY. Antibodies to NY have been prepared and are widely used for immunohistochemical staining, enzyme-linked immunosorbent assay (ELISA), and Western blot analysis [15]. DiY has been immunochemically identified in the lipofuscin of pyramidal neurons in the aged human brain [16] and in atherosclerotic lesions in Apo-E deficient mice [17]. Positive staining of DiY has been reported in models of Alzheimer and Parkinson diseases [18, 19]. The antibody to ClY was recently prepared and used in immunohistochemical staining [20, 21]. The preparation of an antibody recognizing di-halotyrosines (both dichloro- and dibromotyrosines) has also been done and the positive immunostaining of the liver of lipopolysaccharide (LPS)-treated mice has already been described [22]. Although these immunochemical approaches can visually show the localization of modified tyrosines, this method is not suitable for the “rigid” quantification and chemical identification of the modified tyrosines.
To estimate the exact amount of these tyrosine-derived products, gas-chromatography mass spectrometry (GC/MS) and high performance lipid chromatography (HPLC)-electrochemical detection (ECD) have been used [23–25]. Urinary DiY has also been quantified by GC/MS [26] and by tandem liquid chromatography-mass spectrometry (LC/MS/MS) [27]. A more recent study has reported the simultaneous quantification of DiY, including other modified tyrosines in urine using LC/MS/MS [28]. NY has also been quantified by GC-MS, HPLC-ECD, and LC/MS/MS [24, 25, 29, 30]. To avoid the artificial generation of NY, GC/MS in the negative ion chemical ionization mode has been applied [31]. Halotyrosines, such as ClY, BrY, 3,5-dichlorotyrosine (DiClY), and 3,5-dibromotyrosine (DiBrY), have also been detected in tissues using GC/MS [9, 13, 32–34]. ClY and BrY in human urine have been detected by GC/MS [35]. However, there are few reports on the simultaneous quantification of these several modified tyrosines [28].
In this study, we serially and simultaneously quantified the modified tyrosines and other oxidative stress markers (including advanced glycation end-products [AGEs] and an oxidized nucleoside) in the urine of healthy controls. Moreover, as a preliminary study, we compared the levels of modified tyrosines between healthy controls and people with diabetes.
Materials and Methods
Materials
3-Nitrotyrosine (NY) and 3-chlorotyrosine (ClY) were purchased from Sigma Chemical Company. 3,5-Dibromotyrosine (DiBrY) was prepared as described below and also obtained from Aldrich. N-Bromosuccinimide (NBS), tyrosine, sodium hypochloride (NaOCl), and other reagents were purchased from Wako Chemicals Co., Ltd. Stable isotopic tyrosines, [13C6]tyrosine and [13C9]tyrosine, were obtained from Cambridge Isotope Laboratories, Inc.
Synthesis of modified tyrosines
Synthesis of DiY was done as previously reported [36]. Briefly, l-tyrosine (5 mg) was incubated for 1 h with 75 µg of horseradish peroxidase (HRP) in the presence of 5 µl of 30% H2O2 in 5 ml of 0.1 M borate buffer (pH 9.0). The mixture was acidified by the addition of 500 µl of 6 N HCl and 100 µl of trifluoroacetic acid (TFA) and then applied to a solid phase extraction (SPE) column (Supelco Spelclean ENVI-18, 500 mg solid). The column was then washed with 2 ml of 0.1% TFA, and the sample was eluted with 1 ml of 0.1% TFA/CH3OH (1:3). The eluate was concentrated and then further purified by reversed-phase HPLC [36]. BrY and DiBrY were simultaneously prepared by treating l-tyrosine (2 mM) with 2 mM of NBS in water. The reaction was terminated by the addition of l-methionine (final concentration 3 mM). The crude reaction mixture was acidified by the addition of a bolus of TFA solution. The reaction mixture was then applied to an SPE ENVI-18 column, which was washed with 2 ml of 0.1% TFA, and the sample was then eluted with 2 ml of CH3OH. BrY and DiBrY in the eluate were concentrated and further purified by reversed-phase HPLC using Develosil ODS-HG-5 (8 × 250 mm) equilibrated with 0.1% acetic acid/CH3CN at a flow rate of 2 ml/min. In addition, commercially-obtained DiBrY was also used. DiClY was prepared from N-acetyl-tyrosine (NAY) in several steps [22]. NAY (2 mM) was chlorinated by exposure to 2 mM NaOCl in 0.1 M phosphate buffer (pH 7.4) for 24 h at room temperature. The reaction was terminated by the addition of 5 mM L-methionine. The reaction mixture was concentrated by freeze-drying and N-acetyl-3,5-dichlorotyrosine (NADiClY) in the sample was purified by reversed-phase HPLC. The sample was applied to the ODS-HG-5 (8 × 250 mm) using 0.1% TFA/CH3OH (1:1) at a flow rate of 2.0 ml/min, and the peak was collected and further purified. The purified NADiClY was then hydrolyzed by 6 N HCl at 105°C for 24 h. The generated DiClY was purified by reversed phase HPLC using the ODS-HG-5 (8 × 250 mm) with 0.1% acetic acid/CH3OH (7:3) as the eluent at a flow rate of 2.0 ml/min. The concentration was estimated by quantification of the amino residues using trinitrobenzenesulfonic acid (TNBS) [37].
As internal standards, stable isotopic DiY ([13C18]DiY) was prepared from [13C9]tyrosine by treatment with HRP and H2O2, and then purified as described above. To prepare the isotopic mono- and di-bromotyrosines, [13C9]tyrosine was modified by NBS as described above and the formed bromotyrosines were purified by HPLC. To prepare the isotopic mono- and di-chlorotyrosines, N-acetyl-[13C9]tyrosine was prepared first. Briefly, [13C9]tyrosine (70 µM) was acetylated by acetic anhydrate (0.36 mM) in a saturated sodium acetate solution for 24 h at room temperature. The sample was partly purified using the SPE column (ENVI-18, 500 mg), which was then washed with 1 ml of 0.1% TFA and eluted with 3 ml of CH3OH. The eluate was concentrated and purified by reversed phase HPLC using an ODS-HG-5 (8 × 250 mm) with 0.1% TFA/CH3OH (75:25) at a flow rate of 2.0 ml/min by monitoring at UV 215 nm. The N-acetyl-O-acetyl-[13C9]tyrosine obtained was treated for 24 h with 0.1 M borate buffer (pH 9.0) to remove the O-acetyl moiety and the resulting N-acetyl-[13C9]tyrosine was purified by reversed phase HPLC using the ODS-HG-5 (8 × 250 mm) with a solvent of 0.1% acetic acid/CH3OH (7:3) at a flow rate of 2.0 ml/min. The purified stable NAY was further modified by NaOCl as described above. After purifying the chlorinated N-acetyl-[13C9]tyrosines by HPLC, N-acetyl-[13C9]chlorotyrosine and N-acetyl-[13C9]dichlorotyrosine were de-acetylated by acid hydrolysis, and further purified as described above. [13C9]NY was synthesized by treating [13C9]tyrosine with peroxynitrite. Briefly, peroxynitrite was prepared as previously described [38, 39]. The tyrosine (0.2 mg/ml) was reacted with 1 mM peroxynitrite in 0.1 M phosphate buffer containing 1 mM Fe/ethylenediaminetetraacetic acid (EDTA) (pH 7.4) [15]. The reaction mixture was acidified by the addition of TFA and then applied to a solid-phase extraction (SPE) column (ENVI-18). The SPE column was washed with 2 ml of 0.1% TFA and then eluted with 2 ml of 0.1%TFA/methanol (1:1). The eluate was collected and further purified by reversed-phase HPLC using the ODS-HG-5 (8 × 250 mm) with the solvent 0.1% acetic acid/CH3OH (7:3) at a flow rate of 2.0 ml/min by monitoring at UV 274 nm.
Sample preparation
Human urine was collected as a spot sample after approval by the Nakatsugawa Municipal Hospital committee. The urine was frozen at −70°C until use. The sample was dissolved and centrifuged to remove any insoluble materials. The supernatant was collected and 300 µl of the sample was used for the analysis of the modified tyrosines. An internal standard cocktail (containing 500 nM of [13C6]tyrosine and 50 nM of [13C9]DiY, [13C9]ClY, [13C9]DiClY, [13C9]NY, [13C9]BrY, and [13C9]DiBrY) was added to the sample and the proteins in the urine were precipitated by 0.1% TFA and 10% trichloroacetic acid (TCA). The sample was centrifuged to remove the proteins and the supernatant underwent solid phase extraction (ENVI-18 [500 mg]), which had been preconditioned by 2 ml of methanol, followed by 6 ml of 0.1% TFA. The SPE column was washed by 3 ml of 0.1% TFA and the sample was eluted in three 750 µl aliquots of 0.1% TFA/methanol (2:3). The combined eluate (approx. 2.25 ml) was concentrated by centrifugal evaporation, and the dried sample was dissolved in 250 µl of 2 mM ammonium formate and analyzed by LC/MS/MS as described below. As another method to remove urinary proteins, CH3CN (9:1) was added to the urine and the sample was centrifuged. The upper layer was evaporated using a centrifugal evaporator, dissolved in 0.1% TFA and applied to an ENVI-18 SPE column. While a previous report showed the possibility of an artifact generation of NY by acid plus nitrite [40], NY formation by TCA precipitation could not be confirmed in our urine samples.
First LC/MS/MS analysis for tyrosine and dityrosine (Method I)
The SPE-purified urine (10 µl) was analyzed twice using an electrospray-ionization quadruple tandem mass spectrometer (API-3000, Applied Biosystems Co.) connected to an Agilent 1100 HPLC system. Data were calculated from the averages of two serial analyses of one sample. For the DiY and tyrosine analyses, HPLC was done by gradient systems using solvent A (0.1% acetic acid) and solvent B (CH3CN). The separation was performed using the ODS-HG-3 (2 × 50 mm). The gradient program is as follows: 0 min (A100%), 2 min (A100%), 7 min (A50%), 7.1 min (A100%), 15 min (A100%). The positive mode was used for the electrospray ionization (ESI). To reduce possible contamination of the ion source, the flow was separated by a Valco switching valve using the following program: 0 min (waste), 2 min (mass), 7 min (waste). The four multiple reaction monitoring (MRM) transitions used for the DiY were as follows: 379.2/332.1 (internal standard, [13C18]DiY-1), 379.2/253.1 (internal standard, [13C18]DiY-2), 361.2/315.1 (native DiY-1), and 361.2/237.1 (native DiY-2). The two MRM combinations for one molecule showed a similar sensitivity. The use of two MRMs on one molecule is meaningful for the identification of DiY. To estimate urinary tyrosine, the analyte was diluted 100-fold by a solution of 2 mM ammonium formate with an additional internal standard (500 nM of [13C6]tyrosine) and then analyzed separately. Two MRMs 182.2/136.1 (tyrosine) and 188.1/142.1 ([13C6]tyrosine) were used for the analysis. To check for artifact formation of DiY during sample preparation, the MRM of the [13C6]tyrosine-derived DiY was also determined regularly. A summary of the MRM scan settings is listed in Table 1. To avoid a decrease in the S/N ratio, the MRM scan program for the [13C6]tyrosine-derived DiY was omitted in routine analyses.
Table 1.
Conditions for urinary dityrosine and tyrosine analyses by LC/MS/MS
| Sample | Q1 | Q3 | Scan time (msec) |
|---|---|---|---|
| Tyr | 182.2 | 136.1 | 150 |
| [13C6]Tyr | 188.1 | 142.1 | 150 |
| DiY-1 | 361.2 | 237.1 | 150 |
| [13C18]DiY-1 | 379.2 | 253.1 | 150 |
| [13C12]DiY-1* | 373.2 | 327.1 | 150 |
| DiY-2 | 361.2 | 315.1 | 150 |
| [13C18]DiY-2 | 379.2 | 332.1 | 150 |
| [13C12]DiY-2* | 373.2 | 249.1 | 150 |
* Asterisks show omission of setting for actual routine analysis.
Second LC/MS/MS analysis for butylated modified tyrosines (Method II)
The rest of the DiY analysis was used for analysis of butylated modified tyrosine. The sample, which had already been enriched with the stable isotopes, was recovered in a glass tube and concentrated by centrifugal evaporation. The dried sample was butylated with 500 µl of n-butanol/HCl (2:1) at 65°C for 30 min. The sample was again evaporated by centrifugal evaporation and then dissolved with 200 µl of 0.05% formic acid/CH3CN (95:5). The HPLC was done by gradient systems using 0.05% formic acid (solvent A) and CH3CN (solvent B). The separation was performed using the ODS-SR-5 (2 × 150 mm), with a gradient program as follows: 0 min (A80%), 2 min (A 80%), 22 min (A70%), 23 min (A50%), 25 min (A50%), 25.1 min (A80%), and 35 min (A80%). The positive mode was used for the ionization. Twenty MRM transitions, which were derived from the liberation of the butyl moiety, were used for the analysis. The switching valve was set as follows: 0 min (waste), 3 min (mass), 20 min (waste). To check the artifact formation of the modified tyrosines during sample preparation, the MRM of the modified [13C6]tyrosines was also determined. A summary of the MRM scan settings for butylated samples is listed in Table 2.
Table 2.
Conditions for butylated urinary NY, ClY, BrY, DiClY, and DiBrY analyses by LC/MS/MS
| Butylated Sample | Q1 | Q3 | Scan time (msec) |
|---|---|---|---|
| NY | 283.2 | 181.0 | 30 |
| [13C9]NY | 292.5 | 189.0 | 30 |
| [13C6]NY* | 289.2 | 187.0 | 30 |
| 35ClY | 272.2 | 170.0 | 30 |
| [13C9]35ClY | 281.0 | 178.0 | 30 |
| [13C6] 35ClY* | 278.2 | 176.0 | 30 |
| 37ClY | 274.2 | 172.0 | 30 |
| [13C9]37ClY | 283.0 | 180.0 | 30 |
| 79BrY | 316.0 | 214.0 | 30 |
| [13C9]79BrY | 325.0 | 222.0 | 30 |
| [13C6] 79BrY* | 322.1 | 220.0 | 30 |
| 81BrY | 318.0 | 216.0 | 30 |
| [13C9]81BrY | 327.0 | 224.0 | 30 |
| [13C6] 81BrY* | 324.1 | 222.0 | 30 |
| 35Cl • 35ClY | 306.1 | 204.0 | 30 |
| [13C9]35Cl • 35ClY | 315.0 | 212.0 | 30 |
| [13C6] 35Cl • 35ClY* | 312.1 | 210.0 | 30 |
| 79Br • 79BrY | 394.0 | 292.0 | 30 |
| [13C9]79Br • 79BrY | 403.0 | 300.0 | 30 |
| [13C6] 79Br • 79BrY* | 400.0 | 298.0 | 30 |
| 79Br • 81BrY | 396.0 | 294.0 | 30 |
| [13C9]79Br • 81BrY | 405.0 | 302.0 | 30 |
| [13C6] 79Br • 81BrY* | 402.0 | 300.0 | 30 |
| 81Br • 81BrY | 398.0 | 296.0 | 30 |
| [13C9]81Br81BrY | 407.0 | 304.0 | 30 |
| [13C6] 81Br81BrY* | 404.0 | 302.0 | 30 |
* Asterisks (*) show omission of setting for actual routine analysis.
Addition and recovery experiments
The coefficient of variance (CV) was estimated using 12 urine sample that were enriched with 50 nM of authentic modified tyrosines. The samples were processed and analyzed by LC/MS/MS as described above. Estimation of the recovery of the added modified tyrosines was done as follows. The authentic modified tyrosines (final concentration 0.325, 0.651, 1.30, 2.60, 5.21, 10.4, 20.8, 41.7, 83.3, 167, 333 nM) were added into the urine samples and then processed for the LC/MS/MS analysis as described above.
Estimation of other biomarkers
Urinary 8-oxo-deoxyguanosine (8-Oxo-dG) was determined using an ELISA kit (JAICA, Nikken Zeil Co.). It has been reported that the heating procedure of frozen urine improves the recovery of urinary 8-Oxo-dG [41]. Because we could not obtain constant results by the heating, the supernatant of centrifuged urine without heat was used for the estimation of urinary 8-Oxo-dG. MRX (Maillard Reaction Product X) and pentosidine in the urine were determined by an HPLC-fluorescence method [42]. Urinary creatinines were determined by the Creatinine Test Wako (WAKO Pure Chemicals). Urinary Nε-(hexanoyl)lysine (HEL) was estimated by LC/MS/MS as described elsewhere [43, 44].
Statistical analysis
Values (urinary biomarkers/creatinine) are expressed as means ± SEM. Spearman’s rank correlation was used to evaluate the relationship between the two biomarkers. Comparisons between two groups were made using the Mann-Whitney test.
Results
To develop the detection method of trace amounts of urinary modified tyrosines by LC/MS/MS with stable isotopic methods, the pattern of the collision-induced dissociation was investigated by infusion analysis of authentic modified tyrosines. To prevent the artificial conversion of the isotopic internal standard of tyrosine into the isotopic modified tyrosine, we used [13C6]tyrosine as the internal standard for the tyrosine analysis itself, and [13C9]tyrosine was used as the parent molecule for the synthesis of the six stable modified tyrosine isotopes. Several fragments, which occurred owing to the collision-induced fragmentation of DiY, were observed [45]. Of these, the combination of Q1/Q3 ions, 361/273 (for stable DiY) and 361/315 (for native DiY) are similar and have a good sensitivity. The detection limit of DiY was 1 nM and the calibration curve of DiY was linear (R = 0.999) over a concentration range of 1–300 nM. Using the selected MRM transitions, the DiY in human urine was estimated by the LC/MS/MS. DiY in some urine samples cannot be determined owing to contaminants in the urine as described elsewhere [28]. Therefore, before the LC/MS/MS analyses, the urine samples were uniformly purified by solid-phase extraction (SPE). The DiY in all the SPE-treated urines can be detected by the mass spectrometer (a typical chromatogram is shown in Fig. 1). The CV for supplemented 50 nM DiY into the urine was 2.4% and the recovery of authentic DiY in the urine was significantly high (R = 0.998). We called the determination methods as “Method I” and the MRM combination is shown in Table 1. Because we did not prepare the standards of the [13C6]tyrosine-derived modified tyrosines including [13C12]DiY, the accurate quantification of these modified products, which have a backbone of [13C6]tyrosine, could not be done. The artifact DiY ([13C12]DiY) was observed at very low levels on the MRM chart. For tyrosine and the other modified tyrosines, including stable isotopes, the estimated MRM combinations are derived from the liberation of HCOOH (−46) from the precursor ions. For example, the MRMs 182.1/136.1 for the native and 188.1/142.1 for the stable isotope tyrosine, [13C6]tyrosine, were used to quantify tyrosine levels. By checking the formation of the [13C6]tyrosine-derived modified tyrosine, artifact generation of the modified tyrosine can be examined during sample preparation. We attempted the quantification of NY and halogenated tyrosines (such as ClY and BrY) in urine by Method I. However, the free halotyrosines could not be determined in the SPE-treated human urine. NY cannot be clearly detected because of a co-eluting contaminating large unknown product, which has the same signal of MRM 227.2/181.2 for NY (data not shown).
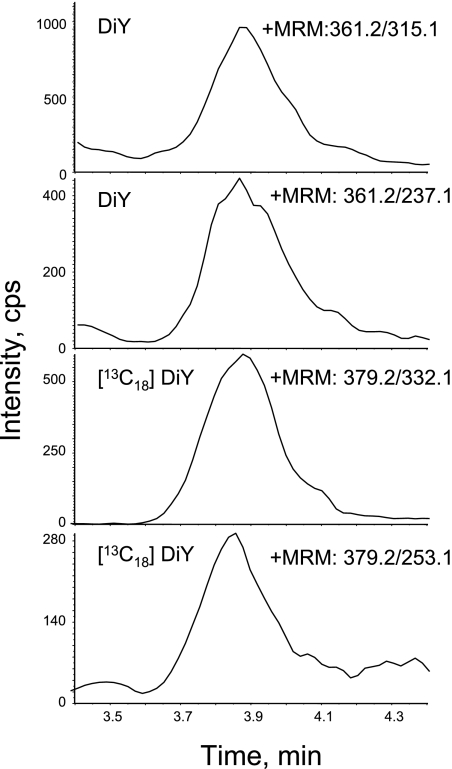
Fig. 1.
DiY in SPE-treated urines was separated by HPLC and then detected using a mass spectrometer. The sample preparation was done as described in the Materials and Methods section. The analysis of the mass spectra was done by “Method I”. The final concentration of the internal standards cocktail including [13C18]DiY is 50 nM. The MRMs of 361.2/315.1 and 361.2/237.1 show the native DiY and those of 379.2/332.1 and 379.2/253.1 derived from the stable internal standard of DiY.
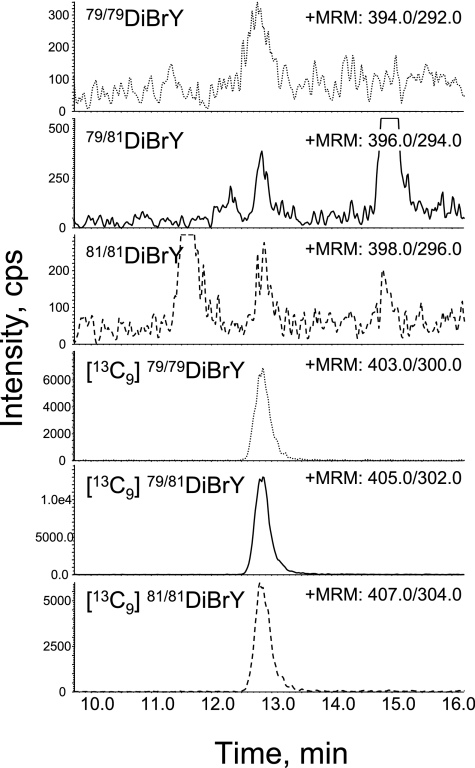
To detect the NY and halotyrosines, the rest of DiY analyte, which included the stable internal standards mixture, was further derivatized by butanol/HCl, and the successive butylated tyrosines were analyzed by LC/MS/MS (Method II) as described in the Materials and Methods section. To evaluate the artifact formation of the modified tyrosines, butylated modified tyrosines with a [13C6] ring were analyzed. The characteristic collision-induced fragmentations caused by the liberation of the butyl moiety (−102 for native, −103 for stable internal standard) were used for the MRM transmission of all the analyses for the butylated modified tyrosines [27]. The detection limits were approximately 0.1 nM (ClY and DiBrY), 0.3 nM (BrY, DiClY), and 1 nM (NY). Calibration curves of Method II were linear (R>0.99) over a concentration range of 1–300 nM for NY, 0.1–300 nM for ClY, 0.3–300 nM for BrY, and 0.1–300 nM for DiBrY. Using Method II, NY, BrY, and DiBrY in the urine were successfully determined simultaneously (Figs. 2, 3, 4 and Table 2). Urinary NY was detected without interference from any unknown contaminants. Each MRM peak was co-eluted with the “partner” internal standard which have a [13C9] backbone. Moreover, the difference in the natural existence ratio of isotopes for 79Br and 81Br (1:1) was also used for identification. Because BrY has one Br atom with an equal ratio of 79Br:81Br, 79BrY and 81BrY show a similar intensity (Fig. 3). DiBrY shows a unique mass profile of three major isotopic peaks (triplet peak, 1:2:1) because DiBrY consists of 79Br•79BrY, 79Br•81BrY, and 81Br•81BrY. Whereas a similar analogy of the isotope ratio is applicable to the identification of chlorotyrosine (ClY), ClY could not be clearly detected from all samples, as successful detection of ClY was dependent on the behavior of the samples. DiClY could not be detected in most samples. It seems likely that the urinary modified tyrosines are excreted as conjugates such as glucuronide conjugates. However, the treatment of a urine sample with β-glucuronidase/sulfatase had no effect on the level of the modified tyrosines (unpublished observation). The recovery of the additional authentic NY, ClY, DiClY, BrY, and DiBrY was also estimated (NY, R = 0.999; ClY 0.991; DiClY, 0.999; BrY, 0.995; DiBrY, 0.998). The CV (%) of the 50 nM standards into the urine were 4.9 (NY), 2.9 (ClY), 4.5 (DiClY), 4.7 (BrY), and 5.1 (DiBrY). The addition of phenol to the urine prior to sample preparation had no effects on the amount of the modified tyrosines (data not shown), suggesting that artifact generation during sample preparation is negligible.
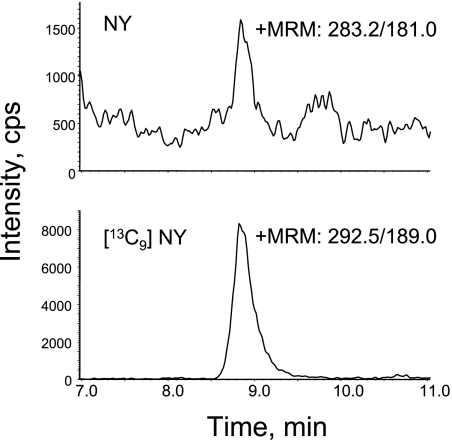
Fig. 2.
MRM analysis of butylated NY from urine. The sample preparation was done as described in the Materials and Methods section. The analysis of the mass spectra was done by “Method II”. Briefly, the rest of the DiY analysis was further derivatized as butylated amino acids and then analyzed by LC/MS/MS. The final concentration of the internal standards cocktail including [13C9]NY is 50 nM. The MRMs of 283.2/181.0 show the native NY and the combination of 292.5/189.0 indicate the MRM of the stable internal standard of NY.
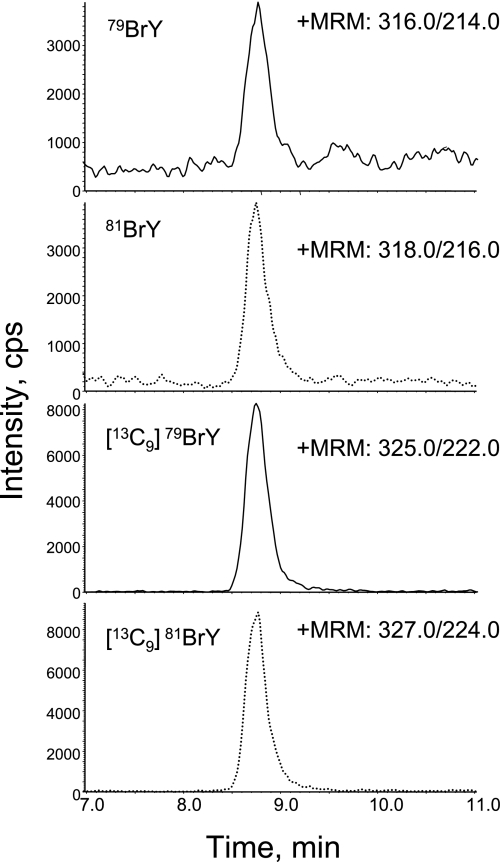
Fig. 3.
MRM analysis of butylated BrY from urine. The sample preparation and analysis were done as described in the Figure 2 legend. The final concentration of internal standards cocktail including [13C9]BrY is 50 nM. The MRMs of 316.0/214.0 and 318.0/216.0 show the native 79BrY and 81BrY, respectively. The MRM combination of 325.0/222.0 and 327.0/224.0 indicates the stable internal standard of 79BrY and 81BrY, respectively.
Fig. 4.
MRM analysis of butylated DiBrY from urine. The sample preparation and analysis was done as described in the Figure 2 legend. The final concentration of the internal standards cocktail including [13C9]DiBrY is 50 nM. The MRMs of 394.0/292.0, 396.0/297.0 and 396.0/294.0 show the native Di79BrY, Di79/81BrY and Di81BrY, respectively. The MRM combination of 403.0/300.0, 405.0/302.0 and 407.0/304.0 indicates the stable internal standard of Di79BrY, Di79/81BrY, and Di81BrY, respectively.
First, we analyzed the urine from healthy adults (n = 23, Table 3) using Methods I and II, and correlations between several tyrosine-derived products were examined. We also determined the amounts of urinary 8-Oxo-dG and Nε-(hexanoyl)lysine (HEL) [43, 44]. Urinary pentosidine and MRX [41], which are advanced glycation end-products, were also determined. The levels of urinary 8-Oxo-dG, HEL, pentosidine, and MRX were 8.8 ± 0.9, 2.3 ± 0.2, 17.0 ± 0.9, and 539 ± 70 µmol/mol of creatinine, respectively. The amounts of the modified tyrosines in healthy human urine (n = 23) were 8.8 ± 0.6 (DiY), 1.4 ± 0.4 (NY), 3.8 ± 0.3 (BrY), and 0.7 ± 0.1 (DiBrY) µmol/mol of creatinine. The relationship between each modified tyrosine and the other biomarkers are summarized in Table 4. Of the modified tyrosines examined, DiY was significantly correlated with BrY, DiBrY, HEL, 8-Oxo-dG, pentosidine, and MRX (p<0.01). NY was not correlated with the other markers, suggesting that the in vivo mechanism of NY production is quite different. Urinary 8-Oxo-dG was significantly correlated with urinary HEL, DiY, BrY, DiBrY, and pentosidine (p<0.01), and urinary HEL was significantly correlated with urinary DiY (p<0.01), DiBrY (p<0.05), 8-Oxo-dG, and pentosidine (p<0.01). Pentosidine and MRX were also significantly correlated (p<0.01). In addition, there were no significant changes in the amounts of modified tyrosines in urines by aging or smoking status.
Table 3.
Baseline characteristics of healthy control subjects
| Healthy Control (n = 23) | |
|---|---|
| Age year (maen ± SD) | 54.1 ± 11.0 |
| Male/female sex | 16/7 |
| Smorkers | 7 |
Table 4.
Correlation efficient between (glyco-)oxidative biomarkers
| DiY | NY | BrY | DiBrY | HEL | 8Oxo | Pentos | MRX | |
|---|---|---|---|---|---|---|---|---|
| DiY | 1 | |||||||
| NY | 0.188 | 1 | ||||||
| BrY | 0.773** | 0.017 | 1 | |||||
| DiBrY | 0.696** | −0.061 | 0.722** | 1 | ||||
| HEL | 0.657** | 0.196 | 0.353 | 0.512* | 1 | |||
| 8Oxo | 0.743** | 0.036 | 0.559** | 0.696** | 0.643** | 1 | ||
| Pentos | 0.923** | 0.015 | 0.677** | 0.587** | 0.673** | 0.621** | 1 | |
| MRX | 0.607** | 0.071 | 0.399 | 0.596** | 0.286 | 0.388 | 0.587** | 1 |
* p<0.05, ** p<0.01
Next, we investigated the effects of diabetes on the generation of these modified tyrosines. People with diabetes (without nephropathy, n = 12) were selected for this pilot study because it is known that the disease is associated with oxidative stress. The concentration of the modified tyrosines was corrected for urinary creatinine. The three markers, NY, BrY, and DiBrY, were significantly increased in diabetic urine compared with the healthy controls (Fig. 5). When the modified tyrosine per tyrosine was calculated, the tendency was similar to the creatinine correction. However, the value from the tyrosine correlation varied widely compared with creatinine correction.
Fig. 5.
Comparison of urinary DiY, NY, BrY, and DiBrY between the control person and diabetics. The evaluation of urines from the control (Cont., n = 23) and diabetics (D. M., n = 12) was performed as described in the Materials and Methods section. Comparisons between the two groups were done using the Mann-Whitney test.
Discussion
The estimation of urinary modified tyrosines would be a useful tool for the evaluation of the oxidation/reduction balance in our body. Indeed, we found differences in the urinary modified tyrosines between healthy people and people with diabetes. Diabetic complications are commonly associated oxidative stress [1]. The simultaneous determination of DiY, halotyrosines, and NY should show a “profile” of oxidative stress. We have also examined the effect of cocoa drink on the contents of urinary modified tyrosines [46].
The simultaneous determination of modified tyrosines, DiY and NY, in human urine by HPLC-atmospheric pressure chemical ionization tandem mass spectrometry (HPLC-APCI-MS/MS) has been reported [28]. The determination was carried out without a pretreatment. However, DiY in some samples (2–3 of eight) could not be determined by this method. Moreover, NY and ClY could not be determined in intact urine. A recent report described an immunoaffinity LC/MS/MS method to measure free 3-NY in various biological fluids at very low levels [47]. However, that method will be difficult to apply for simultaneous determination of several modified tyrosines because the method needs specific antibodies to each modified tyrosine. We have also found that modified tyrosines, except for DiY, could not be detected by LC/MS/MS using urine without the derivatization step in our Method I. Butylation of the samples resolved this problem, except for the detection of the urinary ClYs. Levels of DiY, NY, BrY, and DiBrY were successfully quantified by our serial and simultaneous Methods I–II in all of the urine samples examined. Our HPLC-ESI-LC/MS/MS methods required several processes including protein precipitation, solid phase extraction, and further additional derivatization. We determined DiY separately without any derivatization because it is difficult to control the incorporation of the butyl moiety in a DiY, which has two COOH terminals [27]. It has been reported that DiY, NY, and o-Tyr in cat urine could be quantified by butylation of samples using HPLC-ESI-(quadruple)MS/MS and a quadruple/time of flight (Q-TOF) tandem MS [27]. Using our methods, ClY and DiClY were not completely determined in human urine samples, but the BrYs were. The ClYs (especially DiClY) were below the detection limit in some urine samples, probably because the amount of BrY in human urine is about 10-times higher than that of ClY [35].
In this research study, we detected urinary DiY, NY, BrY, and DiBrY and simultaneously quantified their levels using quadruple mass spectrometry with liquid chromatography. The determination of these modified tyrosines has been performed by GC/MS [24, 25, 31]. Recently, Mita et al. detected ClY and BrY in 2 ml of human urine by GC/MS [35]. They reported that the urinary BrY is 22.6 ± 10.8 ng/mg of creatinine, which can be converted into about 8 µmol/mol of creatinine. On the other hand, we found that the urinary BrY is 3.8 ± 0.3 µmol/mol of creatinine. Our methods require only 300 µl of urine for determination of the four modified tyrosines. The concentration of (non-septic) human control urinary DiY has been shown to be approximately 3.9 ± 1.0 µmol/mol of creatinine by GC/MS [48]. It has also been shown that urinary DiY is 5.8 ± 0.3 µmol/mol of creatinine (morning urine) or 12.5 ± 5 µmol/mol of creatinine (first following urine) by HPLC-APCI-MS/MS [28]. The same group also showed that the mean urinary DiY concentration was 10.1 ± 0.4 µmol/mol of creatinine [49]. We detected DiY at a concentration of 8.8 ± 0.6 µmol/mol of creatinine in the control human urine. The quantification of DiY and NY were also reported as 22.0 to 49.1 DiY/creatinine (µmol/mol) and 0.1 to 0.4 NY/creatinine (µmol/mol) in cat urine by LC/ESI-MS/MS [27].
Peroxidases, such as MPO, can modify the tyrosine moiety to DiY [50]. Metal-catalyzed oxidation systems are also considered to be a plausible generator of DiY [51–57]. Peroxynitrite leads to the formation of DiY as well as 3-NY [14] and photo-oxidation is also a plausible source for DiY generation [36]. In this study, urinary DiY was significantly correlated with the various markers examined. These results may indicate that DiY is one of the important universal biomarkers of oxidative stress. The enhancement of DiY in urine samples in people from people with diabetes has been reported [58]. However, in our study, no statistical difference in DiY was observed but the urinary DiY tended to increase in people with diabetes. In addition, we found that the urinary DiY in people with diabetes (n = 12) is significantly higher than in healthy controls (n = 41) (p<0.05, unpublished observation). It has already been shown that the formation of DiY occurs in the kidneys of diabetic Akita mice [59].
On the other hand, a nitrosative stress marker, NY, is specifically formed by the exposure of reactive nitrogen species (RNS) like peroxynitrite, which is derived from the reaction between the superoxide anion radical and NO·. In vivo, peroxidases are plausible and important players for RNS-generation [60]. NY has already been reported to be present in cat urine [27]. It has been reported that urinary NY did not increase in type 1 diabetes [58]. However, we have found enhanced NY excretion in the urine of people with diabetes (Fig. 5). Indeed, the possibility of NY as a useful biomarker for oxidative stress has been described [7].
The halotyrosines, ClY and BrY, are generated from the activation of immune cells such as neutrophils and eosinophils, which contain the peroxidases, MPO and EPO in their granules, respectively. These enzymes generate hypohalous acids, which can halogenate tyrosine. N-Chloramine and Cl2 gas can also chlorinate tyrosine. Therefore, it is difficult to identify the “true” halogenating species in vivo. Estimation of the ratio between the amount of ClY and BrY can not determine the contribution of hypohalous acid (HOCl or HOBr) because of the reaction rate of HOCl and HOBr, and its complicated reaction mechanism [61, 62]. Although the detailed generation mechanism of halotyrosine and the biological significance remains unknown, halotyrosines can be considered as an inflammatory biomarker. The amount of BrY and DiBrY in the urine of people with diabetes was higher than that in the control person (Fig. 5). These results indicate that the immune system is activated in people with diabetes. The increase in di-halotyrosines in LPS-treated mice liver tissues has been characterized immunohistochemically using a specific antibody to the di-halotyrosine residue [22]. Moreover, the increase in the modified tyrosines in tissue was observed chemically and immunochemically during UV-irradiation [63]. However, the metabolic process of excretion of the modified tyrosine into urine is unclear [64, 65].
We have found that the relationship between urinary biomarkers was significantly high (Table 4). This suggests that the mechanism for the generation of the oxidative biomarkers has something in common with each other. We also found that the NY level was not correlated with the levels of other modified tyrosines. This suggests that the mechanism of NY generation is different from that of the other modified tyrosines.
The profile of the quantification of modified tyrosines may become a useful marker for the evaluation of oxidative stress in vivo. Urine is a source suitable for biological sampling because the collection does not induce pain and is a non-invasive process. Detailed studies of the relationship between diabetes and modified tyrosines are now in progress.
Acknowledgements
We thank Fushimi Pharmaceutical Co., Ltd. for determining MRX and pentosidine. We thank Shinsuke Hisaka for his helpful technical assistance. We also thank Dr. Anthony J. Kettle for his critical reading and helpful advice for this manuscript. This work was supported in part by a Grant-in-Aid (Y. K.) for Encouragement of Young Scientists (No. 16780097) from the Japan Society for the Promotion of Science.
Abbreviations
- ROS
reactive oxygen species
- NY
3-nitrotyrosine
- ClY
3-chlorotyrosine
- BrY
3-bromotyrosine
- EPO
eosinophil peroxidase
- MPO
myeloperoxidase
- DiY
3,3'-dityrosine
- ELISA
enzyme-linked immunosorbent assay
- LPS
lipopolysaccharide
- GC/MS
gas chromatography-mass spectrometry
- ECD
electrochemical detection
- LC/MS/MS
liquid chromatography-tandem mass spectrometry
- DiClY
3,5-dichlorotyrosine
- DiBrY
3,5-dibromotyrosine
- AGEs
advanced glycation end-products
- NaOCl
sodium hydrochloride
- NBS
N-bromosuccinimide
- HRP
horseradish peroxidase
- TFA
trifluoroacetic acid
- SPE
solid phase extraction
- NAY
N-acetyl-tyrosine
- NADiClY
N-acetyl-dichlorotyrosine
- TNBS
trinitrobenzenesulfonic acid
- EDTA
ethylenediamine tetraacetic acid
- TCA
trichloroacetic acid
- MRM
multiple-reaction monitoring
- CV
coefficient of variance
- 8-Oxo-dG
8-oxo-7,8-dihydro-2'-deoxyguanosine
- MRX
Maillard reaction product X
- HEL
Nε-hexanoyl-lysine
- APCI
atmospheric pressure chemical ionization
- ESI
electrospray ionization
- Q-TOF
quadruple-time of flight tandem mass spectrometry
References
- 1.Baynes J.W., Thorpe S.R. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Hunt J.V., Smith C.C.T., Wolff S.P. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39:1420–1424. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai T., Sugioka K., Nakano M. O2− generation and lipid peroxidation during the oxidation of a glycated polypeptide, glycated polylysine, in the presence of iron-ADP. Biochim. Biphys. Acta. 1990;1043:27–33. doi: 10.1016/0005-2760(90)90106-8. [DOI] [PubMed] [Google Scholar]
- 4.Rosen G.M., Pou S., Ramos C.L., Cohen M.S., Britigan B.E. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 5.Stadtman E.R. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic. Biol. Med. 1990;9:315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- 6.Berlett B.S., Stadtman E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 7.Gow A.J., Farkouh C.R., Munson D.A., Posencheg M.A., Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am. J. Physiol. Lung Cell Mol. Phyisol. 2004;287:L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 8.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. (Cell Physiol. 40), [DOI] [PubMed] [Google Scholar]
- 9.Vilet V.D., Nguyen M.N., Shigenaga M.K., Eiserich J.P., Marelich G.P., Cross C.E. Myeloperoxidase and protein oxidation in cystic fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L537–546. doi: 10.1152/ajplung.2000.279.3.L537. [DOI] [PubMed] [Google Scholar]
- 10.Hazen S.L., Heinecke J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaut J.P., Yeh G.C., Tran H.D., Byun J., Henderson J.P., Richter G.M., Brennan M.L., Lusis A.J., Belaaouaj A., Hotchkiss R.S., Heinecke J.W. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11961–11966. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W., Samoszuk M.K., Comhair S.A., Thomassen M.J., Farver C.F., Dweik R.A., Kavuru M.S., Erzurum S.C., Hazen S.L. Eosinophils generate brominating oxidants in allergen-induced asthma. J. Clin. Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldridge R., Chan T., van Dalen C., Senthilmohan R., Winn M., Venge P., Town G., Kettle A. Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radic. Biol. Med. 2002;33:847–856. doi: 10.1016/s0891-5849(02)00976-0. [DOI] [PubMed] [Google Scholar]
- 14.Malencik D.A., Anderson S.R. Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids. 2003;25:233–247. doi: 10.1007/s00726-003-0014-z. [DOI] [PubMed] [Google Scholar]
- 15.Beckman J.S., Ye Y.Z., Anderson P.G., Chen J., Accavitti M.A., Tarpey M.M., White C.R. Extensive nitration of protein tyrosines in human atherosclerosis detection by immunohistochemistry. Biol. Chem. Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 16.Kato Y., Maruyama W., Naoi M., Hashizume Y., Osawa T. Immunohistochemical detection of dityrosine in lipofuscin pigments in the aged human brain. FEBS Lett. 1998;439:231–234. doi: 10.1016/s0014-5793(98)01372-6. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y., Wu X., Naito M., Nomura H., Kitamoto N., Osawa T. Immunochemical detection of protein dityrosine in atherosclerotic lesion of apo-E-deficient mice using a novel monoclonal antibody. Biochem. Biophys. Res. Commun. 2000;275:11–15. doi: 10.1006/bbrc.2000.3265. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki M., Hasegawa T., Takeda A., Kikuchi A., Furukawa K., Kato Y., Itoyama Y. Histochemical features of stress-induced aggregates in alpha-synuclein overexpressing cells. Brain Res. 2004;1004:83–90. doi: 10.1016/j.brainres.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Atwoodl C.S., Perry G., Zeng H., Kato Y., Jones W.D., Ling K.Q., Huang X., Moir R.D., Wang D., Sayre L.M., Smith M.A., Chen S.G., Bush A.I. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-beta. Biochemistry. 2004;43:560–568. doi: 10.1021/bi0358824. [DOI] [PubMed] [Google Scholar]
- 20.Hataishi R., Kobayashi H., Takahashi Y., Hirano S., Zapol W.M., Jonesm R.C. Myeloperoxidase-associated tyrosine nitration after intratracheal administration of lipopolysaccharide in rats. Anesthesiology. 2002;97:887–895. doi: 10.1097/00000542-200210000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Gujral J.S., Hinson J.A., Jaeschke H. Chlorotyrosine protein adducts are reliable biomarkers of neutrophil-induced cytotoxicity in vivo. Comp. Hepatol. 2004;3 (Suppl. 1):S48. doi: 10.1186/1476-5926-2-S1-S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato Y., Kawai Y., Morinaga H., Kondo H., Dozaki N., Kitamoto N., Osawa T. Immunogenicity of a brominated protein and successive establishment of a monoclonal antibody to dihalogenated tyrosine. Free Radic. Biol. Med. 2005;38:24–31. doi: 10.1016/j.freeradbiomed.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Crow J.P. In: Measurement and Significance of free and protein-bound 3-nitrotyrosine, 3-chlorotyrosine, and free 3-nitro-4-hydroxyphenylacetic acid in biological samples: A high-performance liquid chromatography method using electrochemical detection, in Nitric oxide, part C. Methods in Enzymology, volume 301. Packer L., editor. Academic Press; San Diego: 1999. pp. 151–160. [DOI] [PubMed] [Google Scholar]
- 24.Vilet A.V.D., Jenner A., Eiserich J.P., Cross C.E., Halliwell B. In: Analysis of aromatic nitration, chlorination, and hydroxylation by gas chromatography-mass spectrometry, in Nitric oxide, part C. Methods in Enzymology, volume 301. Packer L., editor. Academic Press; San Diego: 1999. pp. 471–483. [DOI] [PubMed] [Google Scholar]
- 25.Heinecke J.W., Hsu F.F., Crowley J.R., Hazen S.L., Leeuwenburgh C., Mueller D.M., Rasmussen J.E., Turk J. In: Detecting oxidative modification of biomolecules with isotope dilution mass spectrometry: Sensitive and quaititative assays for oxidized amino acids in proteins and tissues, in Oxidants and antioxidants, part B. Methods in Enzymology, volume 300. Packer L., editor. Academic Press; San Diego: 1999. pp. 124–144. [DOI] [PubMed] [Google Scholar]
- 26.Leeuwenburgh C., Wagner P., Holloszy J.O., Sohal R.S., Heinecke J.W. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch. Biochem. Biophys. 1997;346:74–80. doi: 10.1006/abbi.1997.0297. [DOI] [PubMed] [Google Scholar]
- 27.Marvin L.F., Delatour T., Tavazzi I., Fay L.B., Cupp C., Guy P.A. Quantification of o,o'-dityrosine, o-nitrotyrosine, and o-tyrosine in cat urine samples by LC/electrospray ionization-MS/MS using isotope dilution. Anal. Chem. 2003;75:261–267. doi: 10.1021/ac020309w. [DOI] [PubMed] [Google Scholar]
- 28.Orhan H., Vermeulen N.P.E., Tump C., Zappey H., Meerman J.H.N. Simultaneous determination of tyrosine, phenylalaine and deoxyguanosine oxidation products by liquid chromatography-tandem mass spectrometry as non-invasive biomarkers for oxidative damage. J. Chromatography B. 2004;799:245–254. doi: 10.1016/j.jchromb.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 29.Althaus J.S., Schmidt K.R., Fountain S.T., Tseng M.T., Carroll R.T., Galatsis P., Hall E.D. LC-MS/MS detection of peroxynitrite-derived 3-Nitrotyrosine in rat microvessels. Free Radic. Biol. Med. 2000;29:1085–1095. doi: 10.1016/s0891-5849(00)00350-6. [DOI] [PubMed] [Google Scholar]
- 30.Yi D., Ingelse B.A., Duncan M.W., Smythe G.A. Quantification of 3-nitrotyrosine in biological tissues and fluid: generating valid results by eliminating artifactual formation. Am. Soc. Mass Spectrom. 2000;11:578–586. doi: 10.1016/S1044-0305(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 31.Gaut J.P., Byun J., Tran H.D., Heinecke J.W. Artifact-free quantification of free 3-chlorotyrosine, 3-bromotyrosine, and 3-nitrotyrosine in human plasma by electron capture-negative chemical ionization gas chromatography mass spectrometry and liquid chromatography-electrospray ionization tandem mass spectrometry. Anal. Biochem. 2002;300:252–259. doi: 10.1006/abio.2001.5469. [DOI] [PubMed] [Google Scholar]
- 32.Hazen S.L., Heinecke J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaut J.P., Yeh G.C., Tran H.D., Byun J., Henderson J.P., Richter G.M., Brennan M.L., Lusis A.J., Belaaouaj A., Hotchkiss R.S., Heinecke J.W. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11961–11966. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W., Samoszuk M.K., Comhair S.A., Thomassen M.J., Farver C.F., Dweik R.A., Kavuru M.S., Erzurum S.C., Hazen S.L. Eosinophils generate brominating oxidants in allergen-induced asthma. J. Clin. Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mita H., Higashi N., Taniguchi M., Higashi A., Kawagishi Y., Akiyama K. Urinary 3-bromotyrosine and 3-chlorotyrosine concentrations in asthmatic patients: lack of increase in 3-bromotyrosine concentration in urine and plasma proteins in aspirin-induced asthma after intravenous aspirin challenge. Clin. Exp. Allergy. 2004;34:931–938. doi: 10.1111/j.1365-2222.2004.01968.x. [DOI] [PubMed] [Google Scholar]
- 36.Kato Y., Uchida K., Kawakishi S. Aggregation of collagen exposed to UVA in the presence of riboflavin: a plausible role for tyrosine modification. Photochem. Photobiol. 1994;59:343–349. doi: 10.1111/j.1751-1097.1994.tb05045.x. [DOI] [PubMed] [Google Scholar]
- 37.Steinbrecher U.P. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J. Biol. Chem. 1987;262:3603–3608. [PubMed] [Google Scholar]
- 38.Kato Y., Kawakishi S., Aoki T., Itakura K., Osawa T. Oxidative modification of tryptophan residues exposed to peroxynitrite. Biochem. Biophys. Res. Commun. 1997;234:82–84. doi: 10.1006/bbrc.1997.6587. [DOI] [PubMed] [Google Scholar]
- 39.Kato Y., Ogino Y., Aoki T., Uchida K., Kawakishi S., Osawa T. Phenolic antioxidants prevent peroxynitrite-derived collagen modification in vitro. J. Agric. Food Chem. 1997;45:3004–3009. [Google Scholar]
- 40.Oldreive C., Zhao K., Paganga G., Halliwell B., Rice-Evans C. Inhibition of nitrous acid-dependent tyrosine nitration and DNA base deamination by flavonoids and other phenolic compounds. Chem. Res. Toxicol. 1998;11:1574–1579. doi: 10.1021/tx980163p. [DOI] [PubMed] [Google Scholar]
- 41.Weimann A., Belling D., Poulsen H.E. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatographny-electrospray tandem mass spectrometry. Nucleic Acids Res. 2007;30:e7. doi: 10.1093/nar/30.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oya T., Kumon H., Kobayashi H., Hosokawa T., Kakimoto N., Nakamura K., Morimitsu Y., Osawa T. A novel biomarker for hyperglycermia, MRX isolated from hydrolysates of glycated proteins. Biochem. Biophys. Res. Commun. 1998;246:267–271. doi: 10.1006/bbrc.1998.8595. [DOI] [PubMed] [Google Scholar]
- 43.Kato Y., Mori Y., Makino Y., Morimitsu Y., Hiroi S., Ishikawa T., Osawa T. Formation of Nε-(Hexanonyl)lysine in protein exposed to lipid hydroperoxide—a plausible marker for lipid hydroperoxide-derived protein modification. J. Biol. Chem. 1999;274:20406–20414. doi: 10.1074/jbc.274.29.20406. [DOI] [PubMed] [Google Scholar]
- 44.Kato Y., Yoshida A., Naito M., Kawai Y., Tsuji K., Kitamura M., Kitamoto N., Osawa T. Identification and quantification of Nε-(hexanoyl)lysine in human urine by liquid chromatography/tandem mass spectrometry. Free Radic. Biol. Med. 2004;37:1864–1874. doi: 10.1016/j.freeradbiomed.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Takasaki S., Kato Y., Murata M., Homma S., Kawakishi S. Effects of peroxidases and hydrogen peroxide on the dityrosine formation and the mixing characteristics of wheat-flour dough. Biosci. Biotechnol. Biochem. 2005;69:1686–1692. doi: 10.1271/bbb.69.1686. [DOI] [PubMed] [Google Scholar]
- 46.Baba S., Osakabe N., Kato Y., Natsume M., Yasuda A., Kido T., Fukuda K., Muto Y., Kondo K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007;85:709–717. doi: 10.1093/ajcn/85.3.709. [DOI] [PubMed] [Google Scholar]
- 47.Radabaugh M.R., Nemirovskiy O.V., Misko T.P., Aggarwal P., Mathews W.R. Immunoaffinity liquid chromatography-tandem mass spectrometry detection of nitrotyrosine in biological fluids; Development of a clinically translatable biomarker. Anal. Biochem. 2008;380:68–76. doi: 10.1016/j.ab.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharjee S., Pennathur S., Byun J., Crowley J., Mueller D., Gischler J., Hotchkiss R.S., Heinecke J.W. NADPH oxidase of neutrophils elevates o,o'-dityrosine cross-links in proteins and urine during inflammation. Arch. Biochem. Biophys. 2001;395:69–77. doi: 10.1006/abbi.2001.2557. [DOI] [PubMed] [Google Scholar]
- 49.Orhan H., Coolen S., Meerman J.H. Quantification of urinary o,o'-dityroisne, a biomarker for oxidative damage to proteins, by high performance liquid chromatography with triple quadrupole tandem mass spectrometry. A comparison with ion-trap tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;827:104–108. doi: 10.1016/j.jchromb.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 50.Heinecke J.W., Li W., Daehnke H.L. 3rd., Goldstein J.A. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J. Biol. Chem. 1993;268:4069–4077. [PubMed] [Google Scholar]
- 51.Kato Y., Kitamoto N., Kawai Y., Osawa T. The hydrogen peroxide/copper ion system, but not other metal-catalyzed oxidation systems, produces protein-bound dityrosine. Free Radic. Biol. Med. 2001;31:624–632. doi: 10.1016/s0891-5849(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 52.Kato Y., Uchida K., Kawakishi S. Oxidative fragmentation of collagen and prolyl peptide by Cu(II)/H2O2. Conversion of proline residue to 2-pyrrolidone. J. Biol. Chem. 1992;267:23646–23651. [PubMed] [Google Scholar]
- 53.Uchida K., Kato Y., Kawakishi S. A novel mechanism for oxidative cleavage of prolyl peptides induced by the hydroxyl radical. Biochem. Biophys. Res. Commun. 1990;169:265–271. doi: 10.1016/0006-291x(90)91463-3. [DOI] [PubMed] [Google Scholar]
- 54.Huggins T.G., Wells-knecht M.C., Detorie N.A., Baynes J.W., Thrope S.R. Formation of o-tyrosine and dityrosine in proteins during radiolytic and metal-catalyzed oxidation. J. Biol. Chem. 1993;268:12341–12347. [PubMed] [Google Scholar]
- 55.Bayse G.S., Michaels A.W., Morrison M. The peroxidase-catalyzed oxidation of tyrosine. Biochim. Biophys. Acta. 1972;284:34–42. doi: 10.1016/0005-2744(72)90043-5. [DOI] [PubMed] [Google Scholar]
- 56.Ushijima Y., Nakano M., Goto T. Production and identification of bityrosine in horseradish peroxidase-H2O2-tyrosine system. Biochem. Biophys. Res. Commun. 1984;125:916–918. doi: 10.1016/0006-291x(84)91370-6. [DOI] [PubMed] [Google Scholar]
- 57.Giulivi C., Davis K.J.A. Dityrosine and tyrosine oxidation products are endogenous markers for the selective proteolysis of oxidatively modified red blood cell hemoglobin by (the 19 S) proteasome. J. Biol. Chem. 1993;268:8752–8759. [PubMed] [Google Scholar]
- 58.Ahmed N., Babaei-Jadidi R., Howell S.K., Beisswenger P.J., Thornalley P.J. Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia. 2005;48:1590–1603. doi: 10.1007/s00125-005-1810-7. [DOI] [PubMed] [Google Scholar]
- 59.Ueno Y., Horio F., Uchida K., Naito M., Nomura H., Kato Y., Tsuda T., Toyokuni S., Osawa T. Increase in oxidative stress in kidneys of diabetic Akita mice. Biosci. Biotechnol. Biochem. 2002;66:869–872. doi: 10.1271/bbb.66.869. [DOI] [PubMed] [Google Scholar]
- 60.Brennan M.L., Wu W., Fu X., Shen Z., Song W., Frost H., Vadseth C., Narine L., Lenkiewicz E., Borchers M.T., Lusis A.J., Lee J.J., Lee N.A., Abu-Soud H.M., Ischiropoulos H., Hazen S.L. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 61.Pattison D.I., Davies M.J. Kinetic analysis of the reactions of hypobromous acid with protein components: implications for cellular damage and use of 3-bromotyrosine as a marker of oxidative stress. Biochemistry. 2004;43:4799–4809. doi: 10.1021/bi035946a. [DOI] [PubMed] [Google Scholar]
- 62.Senthiimohan R., Kettle A.J. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch. Biochem. Biophys. 2006;445:235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Ishitsuka Y., Maniwa F., Koide C., Douzaki N., Kato Y., Nakamura Y., Osawa T. Detection of modified tyrosines as an inflammation marker in a photo-aged skin model. Photochem. Photobiol. 2007;83:698–705. doi: 10.1562/2006-07-24-RA-978. [DOI] [PubMed] [Google Scholar]
- 64.Ohshima H., Friesen M., Brouet I., Bartsh H. Nitrotyrosine as a new marker for endogenous nitrosation and nitration of proteins. Food Chem. Toxicol. 1990;28:647–652. doi: 10.1016/0278-6915(90)90173-k. [DOI] [PubMed] [Google Scholar]
- 65.Mani A.R., Pannala A.S., Orie N.N., Ollosson R., Harry D., Rice-Evans C.A., Moore K.P. Nitration of endogenous para-hydroxyphenylacetic acid and the metabolism of nitrotyrosine. Biochem. J. 2003;374:521–527. doi: 10.1042/BJ20030670. [DOI] [PMC free article] [PubMed] [Google Scholar]