Abstract
In dietary iron overload, excess hepatic iron promotes liver damage. The aim was to attenuate free radical-induced liver damage using vitamins. Four groups of 60 Wistar rats were studied: group 1 (control) was fed normal diet, group 2 (Fe) 2.5% pentacarbonyl iron (CI) followed by 0.5% Ferrocene, group 3 (Fe + V gp) CI, Ferrocene, plus vitamins A and E (42× and 10× RDA, respectively), group 4 (Fe – V gp) CI, Ferrocene diet, minus vitamins A and E. At 20 months, glutathione peroxidase (GPx), superoxide dismutase (SOD), Oxygen Radical Absorbance Capacity (ORAC), Ames mutagenicity test, AST, ALT and 4-hydroxynonenal (4-HNE) immunohistochemistry were measured. 8OHdG levels of the Fe + V and Fe – V groups were 346 ± 117 and 455 ± 151, ng/g w.wt, respectively. Fe + V and Fe – V differences were significant (p<0.005). A positive correlation between DNA damage and mutagenesis existed (p<0.005) within the iron-fed gps. AST levels for Fe + V and Fe – V groups were 134.6 ± 48.6 IU and 202.2 ± 50.5 IU, respectively. Similarly, ALT levels were 234.6 ± 48.3 IU and 329.0 ± 48.6 IU, respectively. However, Fe – V and Fe + V groups transaminases were statistically insignificant. 4-HNE was detected in Fe + V and Fe – V gp livers. Vitamins A and E could not prevent hepatic damage.
Keywords: iron overload, antioxidants, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) occurs commonly in sub-Saharan Africa, with age-adjusted incidences as high as 113/100 000 of the population and annum [1]. Chronic hepatitis B virus (HBV) infection is the major cause of the tumor in sub-Saharan Africa [2], although a significant percentage of the patients cannot be accounted for by this causal association [3, 4]. Dietary exposure to aflatoxin is another important etiological association of HCC in some parts of sub-Saharan Africa [5], particularly in tandem with HBV infection [6]. A more recently identified risk factor to HCC is dietary iron overload. This condition occurs in some rural areas of sub-Saharan Africa [7], with prevalence as high as 10% [8–12].
Iron is a ubiquitous metal in cells, and a component of many enzymes and proteins. As a transition element, its ionic form participates in one-electron transfer reactions, and this is an important attribute in its role as a prosthetic group in enzymes that catalyze redox reactions. However, this capacity also enables iron to generate free radicals. For example, iron participating in the Fenton reaction will react with less potent reactive oxygen species (ROS) and free radicals to produce more potent secondary ROS. Under pathological conditions, when more ROS are generated, iron catalyzes the reaction of superoxide (O2•−) and hydrogen peroxide (H2O2), resulting in hydroxyl radical (•OH) formation [13], which is more damaging to biological molecules, including lipids, proteins, and nucleic acids [14]. The oxidation products arising from these macromolecules can initiate tissue injury either directly or indirectly.
Free radicals and ROS are normally removed or inactivated in vivo by an antioxidant defence system. Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) protect cells from the toxic effects of ROS and free radicals [13]. SOD converts O2•− into H2O2, while CAT and GPx convert H2O2 to oxygen and water. Other antioxidants of importance are vitamins C, E and A. Vitamin E acts as a potent scavenger of signet oxygen and a chain breaker during lipid peroxidation (LPO). When vitamin E interacts with a lipid peroxyl radical it becomes a radical itself. Vitamin C reacts with vitamin E radical to regenerate vitamin E.
Early epidemiological studies indicated that a diet rich in yellow vegetables is associated with decreased risk of cancer [15–17]. In 1981, it was hypothesized that the beta-carotene present in these vegetables might be responsible for the observed decrease in cancer rates [18]. Since then, more than 100 case-control and cohort studies have examined the association between the intake of vitamins and the risk of developing various forms of cancers [19]. Five large scale clinical trials of the effects of different vitamins reached different conclusions on the effects of antioxidants and cancer. The Cancer Prevention Study showed that a combination of beta-carotene, vitamin E and selenium significantly reduced the occurrence of cancers in healthy individuals [20]. However, in the Alpha-Tocopherol (vitamin E) and Beta-Carotene Cancer Prevention Study (ATBC) the incidence of the cancers studied was not affected by vitamin E [21]. On the contrary, beta-carotene increased lung cancer risk by 18% [21]. Similarly, the Physician’’ Health Study 1 (PHS) found no change in cancer rates associated with beta-carotene [22] and neither did the Women’s Health Study (WHS) find any benefit or harm (with respect to cancers) from beta-carotene and vitamin E supplementation [23, 24]. On the contrary, beta-carotene and retinol increased the risk of cancer in the Beta-Carotene and Retinol (vitamin A) Efficacy Trial (CARET) [25].
These long-term randomised trials raise concerns that vitamins might actually stimulate the development of cancer in high-risk individuals [26, 27]. For example, β-carotene itself may act as an anticarcinogen, but its oxidized products at high concentrations may facilitate carcinogenesis [28]. Furthermore, vitamins A and E, have been found to be damaging to mice with brain tumours, probably by preventing cancer cells from self-destruction through apoptosis [29]. The effectiveness of vitamin antioxidants in carcinogenesis is therefore still debatable [26, 30].
The aim of this study was to determine whether high doses of a combination of vitamins A and E could attenuate the hepatocarcinogenic process initiated by ROS as a result of dietary iron overload in our animal model [31] of hepatic iron overload complicated by HCC.
Materials and Methods
Experimental animals
Eighty pregnant Wistar albino (rattus Norvegicus) rats (of initial weight 200 g ± 5 g before pregnancy) were fed (ad libitum) a standard chow diet (AIN-93G Formulation). Four groups made up of 20 pregnant rats each were set up prior to littering. Immediately after littering, rats were fed the following dietary regimen: The first group, the control group, was designated C gp. Groups 2 to 4 were fed a high iron diet. Group 2 was designated the iron group (Fe gp). Group 3 (Fe + V gp) received iron plus vitamins A and E supplementation; 50mg vitamin A per kg diet ≡ 166 500 IU all trans-retinol (42× RDA) [32–35] and 500 mg vitamin E per kg diet ≡ 750 IU α-tocopherol (10× RDA) [36–38]. (Vitamins were purchased from Sigma, Munich, Germany). Group 4 (Fe – V gp) received a diet enriched with iron but totally devoid of vitamins A and E. Sixty weaned male rats (weighing 80 ± 5 g) were selected from each group for the study and continued on the same diet regimen assigned to the group. The control group (C) received the standard diet. In the groups receiving a high iron diet, the diet was supplemented with 2.5% pentacarbonyl iron (CI—98.0% purity) (Sigma, Munich, Germany) according to the protocol of Plummer et al. [39]. At 12mo, the 2.5% CI was replaced by 0.5% dicyclopentadienyl iron [Fe(C5H5)2] (CAS-102-54-5) (because of poor iron loading). The rats received humane care in accordance with the guidelines of the Animal Ethics Committee of the University. The rats were studied for 32 months. Five (5) rats from each group were sacrificed at 2 months, 4 months and then, every 4 months up until 24 months for blood and liver. One portion of the liver was snap-frozen in liquid nitrogen (and later stored at −70°C) and the other kept in 10% buffered formalin. After 24 months, rats were kept solely for histological purposes. All other experiments were attenuated at 24 months.
Determination of serum iron, blood GPx and SOD
Iron kit (Sigma) was used to determine serum iron concentrations. Randox commercial kits (Randox, Laboratories, Crumlin, UK) were used for blood GPx and SOD. Procedures were followed according to the manufacturer’s instructions.
Determination of liver total antioxidants
Oxygen Radical Absorbance Capacity (ORAC) was performed on fresh serum to discriminate between water-soluble and lipid-soluble total antioxidants. ORAC was measured by a system made up of β-phycoerythrin as a fluorescent indicator protein, 2-2'-azo-bis (2-amidinpropane) dihydrochloride as a peroxyl radical generator, and the water-soluble vitamin E analogue Trolox (Aldrich-Sigma, Munich, Germany) as a reference standard. The total antioxidant capacity was expressed as ORAC units, where one unit equals the net protection by 1 µmol Trolox/l [40].
Determination of 8-hydroxy-2'-deoxy-guanosine (8OHdG) concentration
Hepatic levels of 8OHdG were determined using a commercial kit from the Japan Institute for the Control of Aging (Fukuroi, Japan). The 8OHdG test is a competitive in vitro enzyme-linked immunosorbent assay for the quantitative measurement of 8OHdG in tissue, serum and plasma. The test was performed according to the manufacturer’s instructions for liver tissue and the absorbance read at 450 nm on a microplate reader.
Determination of Aspartate Transaminase (AST) and Alanine Transaminase (ALT)
Serum AST and ALT were measured using an auto-analyser (Cobas Integra 400, Holliston, MA) and kits from Roche Diagnostics (Indianapolis, IN).
Ames mutagenicity test
Ames test was performed according to the method of Maron and Ames [41]. The Salmonella typhimurium TA 102 tester strain was used for the tests. Assays were carried out on samples of fractionated liver homogenate, namely, whole homogenate, S9 fraction, nucleosomes, microsomes and the cytosolic fraction. Samples were assayed according to the standard plate incorporation test without S9 mix. Dimethyl sulfoxide (5%) and daunorubicin hydrochloride (5 µg/plate) were used as negative and positive controls respectively.
Immunohistochemistry
The protocol of Ma et al. [42] was used for 4-hydroxy-2'-nonenol (4-HNE) detection from paraffin embedded sections (3 µm-thick). Briefly, slides were incubated at 56–60°C for 15 min, for antigen retrieval. Sections were deparaffinized in two changes of xylene (5 min each) followed by two changes of absolute ethanol (5 min each), and briefly in water. Sections were incubated with 0.3% H2O2 in methanol for 30 min to block endogenous peroxidases. This was followed by a similar wash in phosphate buffered saline (PBS). After incubation in PBS for 15 min, sections were blocked, for monospecific binding of the antibody, by incubation in 5% bovine serum albumin (BSA) for 15 min at room temperature. Sections were then covered in a humidified chamber with 300–500 µl of 2 µg/ml anti-4HNE rabbit primary antibody in PBS, overnight at room temperature. PBS without primary antibody was used as a negative control. Slides were rinsed with PBS and left in the buffer for 5 min. 300 µl of biotinylated secondary antibody [biotinylated rabbit IgG (Sigma) diluted in PBS (1/300)] was applied onto the sections for at least 30 min at room temperature. Slides were washed with PBS and covered with 300 µl of ExtrAvidin peroxidase diluted in PBS (1:100) for at least 30 min of incubation time at room temperature. After rinsing briefly with PBS, freshly prepared diaminobenzidine (DAB) substrate mixture was ‘pipetted’ unto the slides to cover the sections, and incubated for 5–10 min. Slides were gently rinsed and counter-stained with Meyer’s Haematoxylin solution for 10 min, followed by brief washing under running water. [Primary antibodies were obtained from the Japan Institute for the Control of Aging].
Histology
Formalin-fixed liver specimens for histology were embedded in paraffin wax. 1 µm sections were stained with haematoxylin-eosin (H & E) and Perls’ Prussian blue stain. The degree of iron loading was assessed histologically in coded sections and graded on a scale of 0–4. Iron-free foci (IFF) were confirmed by placental-glutathione-sulfhydryl-transferase (GST-P) immunohistochemical staining.
Statistical analyses
The SAS statistical package was used. All data was expressed as the mean ± SEM. Statistical analysis was performed by independent Student’s t test at the level of 0.05 for paired and unpaired data. A probability value of p<0.05 was considered statistically significant. For multiple groups, analysis of variance (ANOVA) was performed by Bonferroni (Dunn) Multiple comparison t test. Pearson’s correlation coefficient was determined within groups. Multiple regression analysis was performed to determine predictive indicators of oxidative stress.
Results
It should be noted that time/course analysis was done for antioxidants as seen in the graphs because of its importance with regards to the study. However, 20 months’ statistical analysis was chosen in order to compare results of oxidative damage to the immunohistochemical changes and commencement of neoplasm observed at 20 months.
Serum and liver iron
Serum iron levels at 20 months were as follows: Control group (C) = 62.5 ± 6.2 µmol/l; Fe ± V group = 119.1 ± 9.2 µmol/l; Fe – V group = 117.4 ± 8.5 µmol/l and Fe group = 122.0 ± 10.5 µmol/l. Differences between the iron-loaded groups compared to the control were significant (p<0.05, respectively). These results are consistent with similar published results [31]. Examination of the livers demonstrated slow progressive iron loading with a gradient commencing from zone 1. By 20 months all livers showed grade 4 iron overload, with hepatocytes in all zones showing uniform positivity. Furthermore, iron-free foci (IFF) were observed and detailed histology has been published elsewhere [43].
GPx
GPx levels of iron-overloaded and control rats are shown in Fig. 1. The curves of all the groups except the Fe – V group had a similar undulating nature. The control group’s curve remained the highest up to 4 months. Later the Fe + V group continued to be the highest from 16 months to the end. Peak values occurred at 4 and 16 months and declined between 8 and 12 months. GPx level of the Fe – V group declined from 2 months, reaching its lowest at 12 months and increased gradually to a maximum. Furthermore, it was the lowest at several time-points. Group differences between 4–8 and 12–16 months were statistically significant (p<0.05 each), but not at 20 months. Peak GPx values in the various groups were as follows: C group = 214 ± 11.9 U/g Hb (4 months), Fe group = 179.1 ± 16.1 U/g Hb (20 mo), Fe + V gp = 228.8 ± 17.6 U/g Hb (16 mo). Maximum GPx value for Fe – V group was 176.9 ± 24.9 U/g Hb (24 mo).
Fig. 1.
Interaction Plot of time and group: Blood GPx levels of the Fe group; Fe + V group; Fe – V group; and the control group (C). Peak values for Fe + V group; C group; and Fe group occurred at 16 mo, 4 mo, ≈18 mo, respectively. *indicates that values are different (p<0.05) for Fe, Fe + V, Fe – V, groups at 8 and 16 mo compared to the control group. Furthermore, p<0.05 between the Fe + V and Fe – V groups at 16 mo. ‡indicates p>0.05; there were no statistical differences between the various groups at 20 mo.
SOD
Fig. 2 shows SOD levels of iron-overloaded and control rats. Curves for all the groups including the control group showed an undulating pattern. SOD for the C group remained the highest throughout and the Fe – V group, the lowest. SOD levels for the Fe + V group were directly below the C group (4–16 mo) but later declined and was below the Fe group. Peak values observed between 12 and 16 months were as follows: C gp = 2165 ± 444 U/g Hb (12 mo), Fe gp = 1584 ± 360 U/g Hb (16 mo), Fe + V = 1626 ± 199 U/g Hb (12 mo), Fe – V = 1242 ± 201 U/g Hb (16 mo). At 20 months, group differences were statistically insignificant (p>0.05) comparing Fe + V and Fe – V groups.
Fig. 2.
Interaction Plot of time and group: Blood SOD levels of the Fe group; Fe + V group; Fe – V group; and the control group (C). Peak values for C group, Fe + V group, Fe – V group occurred around 12 mo. However, peak value for Fe group occurred at 16 mo. * indicates that values of groups are different (p<0.05) for Fe group, Fe + V group, Fe – V group at 12 mo compared to the control group; ‡ indicates p>0.05 when Fe + V and Fe – V groups were compared at 20 mo.
ORAC
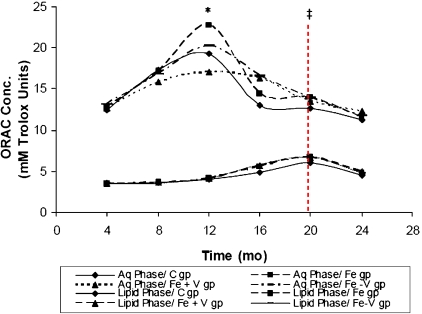
Fig. 3 shows ORAC of water- and lipid-soluble fractions. ORAC-Aqueous phase (ORAC-A) and ORAC-Lipid phase (ORAC-L) was in the ratio of about 4–5:1. Curves for ORAC-A showed peaks at 12 months, whilst, ORAC-L showed peaks at 20 months. In both ORAC-A and ORAC-L, the curve for the Fe + V group was the lowest up until 12 months. The curve for the Fe group of the ORAC-A fraction was the highest between 8 and 12 months. In addition, both curves (ORAC-A and ORAC-L) appeared to operate in an inverse relationship. When ORAC-A curves were at peak values, ORAC-L curves were low. Conversely, when ORAC-L curves were at peak values, ORAC-A curves were low. The correlation analysis showed a negative correlation between ORAC-L and ORAC-A in the C group (r = −0.390, p = 0.0412).
Fig. 3.
Interaction Plot of time and group: Serum ORAC-Aqueous phase (ORAC-A) and ORAC-Lipid phase (ORAC-L) of the Fe group; Fe + V group; Fe – V group; and the control group (C). ORAC-A and ORAC-L curves peaked at 12 and 20 mo, respectively. ORAC-A and ORAC-L appears to operate inversely with a negative correlation (r = −0.390, p = 0.0412). * indicates that values are different (p<0.05) at 12 mo when ORAC-A is compared to ORAC-L (group by group). ‡indicates p>0.05 at 20 mo when ORAC-A is compared to ORAC-L (group by group). Furthermore, differences between ORAC-A and ORAC-L were 4-5-fold and 2-fold, respectively, at 12 and 20 mo.
8OHdG
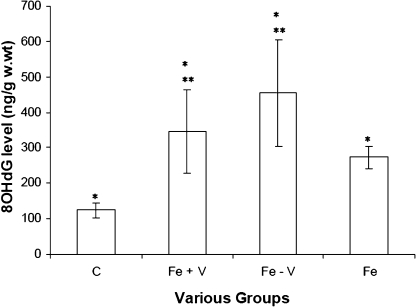
In Fig. 4, 8OHdG levels of the Fe + V and Fe – V groups were 346 ± 117 ng/g & 455 ± 151 ng/g at 20 months. 8OHdG level in the non-vitamin group was 1.3 times higher compared to the vitamin-supplemented group. Control values were (123 ± 21 ng/g w.wt). Differences between the Fe – V and Fe + V groups were also statistically significant (p<0.005). Furthermore, 8OHdG levels of the iron-loaded groups were 2–3 times higher compared to the control group.
Fig. 4.
Bar chart of liver 8OHdG levels of Wistar albino rats fed different diets. Chart indicates 8OHdG levels of the Fe group; Fe + V group; Fe – V group; and the control group (C). * indicates that values are different (p<0.05) when Fe, Fe + V and Fe – V groups, are compared to the control group (C). ** indicates that values are different (p<0.005) when Fe + V group is compared to Fe – V group.
Ames mutagenicity test
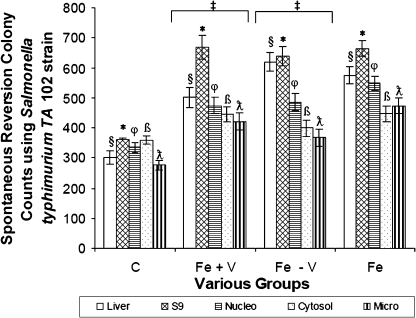
In Fig. 5, differences between fractionated liver samples of the Fe – V and Fe + V groups were not statistically significant. However, all other groups apart from the control group had mutagenicity levels over 600 revertant colonies at 20 months compared to 360 in the control group. The positive control was 570 colonies. Colony differences of any liver fraction (§, *, φ, β, λ) were statistically significant for all the iron-loaded groups compared to the same fraction (§, *, φ, β, λ) in the control group (p<0.05).
Fig. 5.
Bar chart of the level of mutagens produced using Salmonella typhimurium TA 102 strain in the Plate Incorporation method of the Ames Mutagenicity Test on ultracentrifuged liver fractions of C, Fe + V, Fe – V and Fe groups, respectively at 20 months. Fractions from the same group whose values are symbolized by §, *, φ, β, λ, are statistically different (p<0.05) compared to fraction from the control group. Furthermore, ‡ indicates that values are not different (p>0.05) for fractions §, *, φ, β, λ, of the Fe + V group compared to the Fe – V group.
AST/ALT
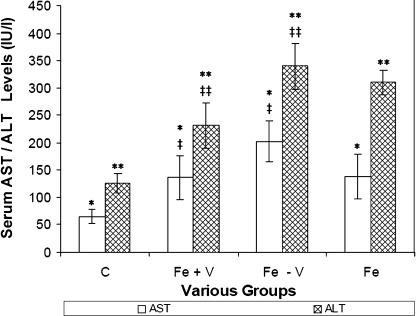
In Fig. 6, ALT levels of the Fe + V and Fe – V groups at 20 months were 234.6 ± 48.3 IU & 329.0 ± 48.6 IU, respectively. AST levels for the Fe + V and Fe – V groups at 20 months were 134.6 ± 48.6 IU & 202.2 ± 50.5 IU, respectively. AST/ ALT levels were 2.0–2.5× higher than the control group and statistically significant (p<0.05, p<0.05). Differences between the Fe – V and Fe + V groups were not statistically significant.
Fig. 6.
Bar chart of serum AST/ALT levels of the control (C) group, Fe + V group, Fe – V group and Fe group at 20 mo using the Cobas Integra 400 auto analyzer. */** indicate that AST/ALT values are different (p<0.05) when compared to the control group. ‡/‡‡indicate that AST/ALT values are not statistically different (p>0.05) when Fe + V group is compared to Fe – V group.
Histology and immunohistochemistry
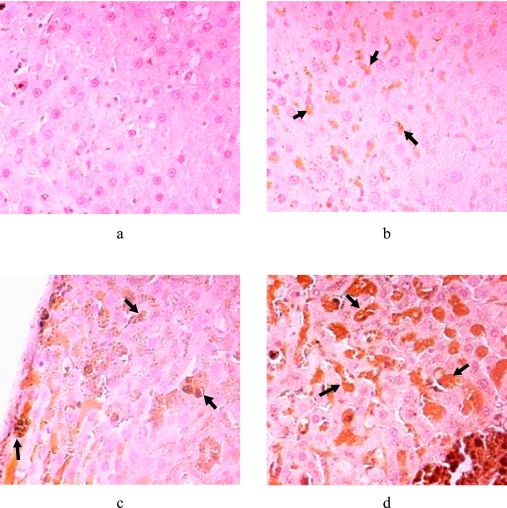
Detailed histology is presented elsewhere [43]. In summary, liver tissues in all the iron-fed groups were heavily loaded (grade 4). First signs of IFF which are neoplastic nodules were observed at 20 months. IFF have significantly less or no iron in the foci compared with the surrounding parenchyma, and these were seen in this study at 20 months and beyond. In 28 IFF samples out of the 95 iron-overloaded samples examined between 20 and 32 months, 43% belonged to the Fe + V group and 21% belonged to the Fe – V group. The rest belonged to the Fe group. In the control group liver sections’ immunohistochemistry (Fig. 7a), there was hardly any brown labelling observed after exposure to the anti-HNE antibodies. However, intense brown cytoplasmic granular immuno-staining was observed in the hepatocytes of the iron-fed groups (Fig. 7b–d).
Fig. 7.
Immunohistochemical detection of 4-HNE adducts (arrowed) in iron-overloaded liver sections using 2 µg/ml 4-HNE rabbit primary antibodies in PBS and biotinylated rabbit IgG secondary antibodies. Freshly prepared DAB stain and Mayer’s Haematoxylin counter-stain were used. Photomicrograph (a) is negative for 4-HNE (control group). (b) (Fe group); (c) (Fe + V group), (d) (Fe – V group) are all positive for 4-HNE adducts (arrowed) deposited in the cytoplasmic area.
Correlation analysis
From Table 1, a strong negative correlation was observed between SOD and AMES (p = 0.0070, r = −0.7320) in the control group. In the Fe – V group, SOD and AMES (p = 0.0349, r = 0.8240); and ORAC-A and ALT (p = 0.0429, r = 0.5390) correlated positively. In the Fe + V group, ORAC-A correlated negatively with AST (p = 0.0400, r = −0.6350). There was a strong positive correlation between oxidative DNA damage and AMES mutagenicity in all iron-fed groups (p<0.005).
Table 1.
Correlation analysis of test parameters was performed to determine predictive indicators of oxidative stress [Bonferroni (Dunn)]: SOD vs AMES; ORAC-A vs AST; ORAC-A vs ALT; 8OHdG vs AMES, within the various experimental groups (Control (C) group; Fe + V group; Fe – V group and Fe group). * indicates negative correlation; ** indicate positive correlation. A strong negative correlation was observed between SOD and AMES (p = 0.0070; r = –0.7320) in the control group. In the Fe – V group, SOD and AMES (p = 0.0349; r = 0.8060); and ORAC-A and ALT correlated positively (p = 0.0429; r = 0.5390). In the Fe + V group, ORAC-A correlated negatively with AST (p = 0.0400; r = –0.6350). There was a strong positive correlation between 8OHdG (oxidative DNA damage) and AMES (mutagenicity) in all iron-fed groups (p<0.005).
| Test | SOD/AMES | ORAC-A/AST | ORAC-A/ALT | 8OHdG/AMES |
|---|---|---|---|---|
| Group | ||||
| C Gp | *p = 0.0070 | p = 0.2108 | p = 0.2335 | p = 0.4560 |
| r = −0.7320 | r = 0.2660 | r = 0.2460 | r = 0.3270 | |
| Fe + V Gp | p = 0.4147 | *p = 0.0400 | p = 0.5917 | **p<0.0001 |
| r = 0.3450 | r = −0.6350 | r = 0.1590 | r = 0.9680 | |
| Fe – V Gp | **p = 0.0349 | p = 0.9457 | **p = 0.0429 | **p = 0.0029 |
| r = 0.8060 | r = 0.0280 | r = 0.5390 | r = 0.8250 | |
| Fe Gp | p = 0.4159 | p = 0.1148 | p = 0.2047 | **p = 0.0039 |
| r = 0.3950 | r = 0.3520 | r = 0.2730 | r = 0.6570 | |
Regression analysis
From the regression analyses results; in the Fe group, serum iron significantly affected 8OHdG formation (p = 0.0121). Furthermore, 8OHdG formation significantly contributed to liver damage (ALT) (p = 0.0000). In the Fe + V group, ORAC-L significantly affected 8OHdG formation (p = 0.0006). Furthermore, ORAC-L significantly affected ALT levels (p = 0.0000). In the Fe – V group, ORAC-L significantly influenced 8OHdG production (p = 0.0050).
Discussion
Iron overload disorders are characterized by the accumulation of Fe in mononuclear phagocytes and parenchymal cells of the liver and other organs. Our biochemistry results show that the iron, iron/vitamin and iron/non-vitamin groups had twice the iron levels of the controls and this was histologically confirmed as iron overload. Fe is a catalyst in the Habar-Weiss reaction and is involved in the initiation and propagation of LPO. Considering the role of iron in iron-reactions, the question is whether antioxidant vitamins all trans-retinol (vitamin A) and α-tocopherol (vitamin E) supplementation, have therapeutic effects in supporting the antioxidant defence system in dietary iron overload against liver damage.
The activity of the antioxidant enzyme, GPx, in the livers of iron-loaded rats fed 1.0%–2.5% carbonyl iron has previously been measured by Fletcher et al. [44]. No significant differences were reported in that study, although there was a trend of increasing GPx activity in the rats receiving 2.5% carbonyl iron. Similarly, no statistically significant differences in hepatic GPx concentration in hereditary hemochromatotosis (HH) patients and controls have been found [45]. In the present study, vitamins A and E increased GPx levels, especially in the second half of the rats’ life. Hamilton et al. [46] also showed that vitamin E supplementation increased GPx activity. However, a homeostatic control of antioxidants has been suggested [47, 48] and this was also observed in the present study by the negative correlation between GPx and ORAC-A. Furthermore, this study group speculates that the higher ORAC-A levels in the iron-overloaded groups suggests that oxidative stress in various tissues lead to an enhanced expression or up-regulation of antioxidant enzymes, as evidenced by others [47, 48].
In this study, the control group obtained the highest SOD levels and the iron/non-vitamin group the lowest, suggesting that oxidative stress may be influenced by vitamin antioxidants in iron loading conditions, and perhaps the importance of SOD in iron-related liver diseases [49]. However, the low antioxidant protection provided by lipid-soluble antioxidants (Fig. 3) and its inability to attenuate LPO is evidenced by the heavy deposition of 4-HNE (Fig. 7b–d), as similarly observed by others [50, 51]. Furthermore, the immunohistochemical findings suggest that 4-HNE formation in the liver could not be attenuated by the combination of vitamins A and E. Of greater consequence is the fact that 4-HNE, which is a by-product of LPO, is able to interact with DNA to form exocyclic guanine adducts [52]. Although the vitamin-supplemented group in this study had significantly reduced levels of the guanidine adduct 8OHdG, compared to the non-vitamin group (Fig. 4), this could not be translated into anti-mutagenic protection as seen in the mutagenesis assay (Fig. 5). Contrary to our studies, a few others have findings to support the role of vitamin E in reducing products of hepatic peroxidation by the subsequent normalization of hepatic LPO products such as malondialdehyde (MDA) in iron-loaded livers [53, 54]. However, it must be pointed out that the reduction in hepatic LPO produced from an initial high level has also been proposed to be a neoplastic marker [55]. The interpretation of biomarkers of oxidative damage must therefore be approached cautiously [56].
Another hepatic preneoplastic marker of greater importance is the development of IFF, which are nodules of hepatocytes found in HH [57]. It has been shown that in iron-overloaded rats with chemically induced liver cancer, early iron-free preneoplastic nodules are thought to be involved in hepatocarcingenesis [58], possibly as a result of mechanisms involving the generation of ROS, LPO and mutagenesis [31]. In this study, 43% IFF livers belonged to the iron/vitamin-supplemented group and 21% belonged to the iron/non-vitamin group. This detrimental effect of high doses of all trans-retinol (vitamin A) and α-tocopherol (vitamin E) on dietary iron overload is our hypothesis, as similarly suggested in other disease conditions by Miller et al. [19] and recently, Bjelakovic [59].
Conclusion
In conclusion, the data presented shows that vitamins A and E reduced the effects of oxidative stress for a limited period of time. However, over a prolonged period, high doses of vitamins A and E could not attenuate the genotoxic effects of the by-products of ROS and its subsequent oxidative damage on the liver. Furthermore, preneoplastic markers of HCC appeared to be more common in the iron/vitamin-supplemented group, than the iron/non-vitamin group, thereby confirming other epidemiological studies (ABTS and CARET) and the recent meta-analysis where high doses of vitamins increased mortality [59].
Abbreviations
- 4-HNE
4-hydroxy-2'-nonenal
- 8OHdG
8-hydroxy-2'-deoxyguanosine
- AIN
American Institute of Nutrition
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- ATBC
Alpha-Tocopherol Beta-Carotene Cancer Prevention Study
- BSA
Bovine serum albumin
- CARET
Beta-Carotene and Retinol Efficacy Trial
- CAT
Catalase
- DAB
Diaminobenzidine
- DNA
Deoxyribonucleic acid
- GPx
Glutathione peroxidase
- GST-P
placental-glutathione-sulfhydryl-transferase
- HBV
Hepatitis B Virus
- HCC
Hepatocellular carcinoma
- HH
hereditary hemochromatotosis
- IFF
Iron free foci
- LPO
Lipid peroxidation
- ORAC
Oxygen radical absorbance capacity
- PBS
Buffered phosphate saline
- ROS
Reactive Oxygen Species
- SAS
Statistical Analysis Software
- SEM
Standard Error of the Mean
- SOD
Superoxide dismutase
References
- 1.Prates M.D., Torresrates F.O. A cancer survey in Lourenco Marques, Portuguese East Africa. J. Natl. Cancer Inst. 1965;35(5):729–757. [PubMed] [Google Scholar]
- 2.Kew M.C., Desmyter J., Bradburne A.F., Macnab G.M. Hepatitis B virus infection in southern African blacks with hepatocellular cancer. J. Natl. Cancer Inst. 1979;62(3):517–520. doi: 10.1093/jnci/62.3.517. [DOI] [PubMed] [Google Scholar]
- 3.Paterson A.C., Kew M.C., Herman A.A.B., Becker P.J., Hodkinson J., Isaacson C. Liver morphology in southern African blacks with hepatocellular carcinoma. A study within the urban environment. Hepatology. 1985;5:72–78. doi: 10.1002/hep.1840050116. [DOI] [PubMed] [Google Scholar]
- 4.Kew M.C., Houghton M., Choo Q.L., Kuo G. Hepatitis C virus antibodies in southern African blacks with hepatocellular carcinoma. Lancet. 1990;14(335(8694)):873–874. doi: 10.1016/0140-6736(90)90474-j. [DOI] [PubMed] [Google Scholar]
- 5.Peers F.G., Linsell C.A. Dietary aflatoxins and human primary liver cancer. Ann. Nutr. Aliment. 1977;31(4–6):1005–1017. [PubMed] [Google Scholar]
- 6.Kew M.C., Kramvis A., Yu M.C., Arakawa K., Hodkinson J. Increased hepato-carcinogenic potential of hepatitis B virus genotype A in Bantu-speaking sub-saharan Africans. J. Med. Virol. 2005;75(4):513–521. doi: 10.1002/jmv.20311. [DOI] [PubMed] [Google Scholar]
- 7.Gordeuk V.R., Boyd R.D., Brittenham G.M. Dietary iron overload persists in rural sub-Saharan Africa. Lancet. 1986;1:1310–1313. doi: 10.1016/s0140-6736(86)91230-4. [DOI] [PubMed] [Google Scholar]
- 8.Gordeuk V.R., Mukiibi J., Hasstedt S.J., Samowitz W., Edwards C.Q., West G., Ndambire S., Emmanuel J., Nkaza N., Chapanduka Z., Randell M., Boone P., Romano P., Martell R.W., Yamashita T., Effler P., Brittenham G. Iron overload in Africa: an interaction between a gene and dietary content. N. Eng. J. Med. 1992;326:95–100. doi: 10.1056/NEJM199201093260204. [DOI] [PubMed] [Google Scholar]
- 9.Gordeuk V.K. Hereditary and nutritional iron overload. Baillière’s Clin. Haematol. 1992;5:169–186. doi: 10.1016/s0950-3536(11)80040-5. [DOI] [PubMed] [Google Scholar]
- 10.Bothwell T.H., Seftel H., Jacobs P., Torrance J.D., Baumslag N. Iron overload in Bantu subjects. Studies on the availability of iron in Bantu beer. Am. J. Clin. Nutr. 1964;14:47–51. doi: 10.1093/ajcn/14.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Mandishona E., McPhail A.P., Gordeuk V.R., Kedda M.A., Paterson C., Rouault T.A., Kew M.C. Dietary iron overload as a risk factor for hepatocellular carcinoma in black Africans. Hepatology. 1998;27:1563–1566. doi: 10.1002/hep.510270614. [DOI] [PubMed] [Google Scholar]
- 12.Moyo V.M., Makunike R., Gangaidzo I.T., Gordeuk V.R., McLaren C.E., Khumalo H., Saungweme T., Rouault T., Kiire C.F. African iron overload and hepatocellular carcinoma (HA-7-0-080) Eur. J. Haematol. 1998;60:28–34. doi: 10.1111/j.1600-0609.1998.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B., Gutteridge J.M.C. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 14.Blakely W.F., Fuciarelli A.F., Wegher B.J., Dizdaroglu M. Hydrogen peroxide-induced base damage in deoxyribonucleic acid. Radiat. Res. 1990;121:338–343. [PubMed] [Google Scholar]
- 15.MacLennan R., Da Costa J., Day N.E., Law C.H., Ng Y., Shanmugaratnam K. Risk factors for lung cancer in Singapore Chinese, a population with high female incidence rates. Int. J. Cancer. 1977;20(6):854–860. doi: 10.1002/ijc.2910200606. [DOI] [PubMed] [Google Scholar]
- 16.Hirayama T. Diet and Cancer. Nutr. Cancer. 1979;1:67–81. [Google Scholar]
- 17.Block G., Patterson B., Subar A. Fruit, vegetables and cancer prevention: a review of the epidemiological evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 18.Peto R., Doll R., Buckley J.D., Spom M.B. Can dietary beta-carotene materially reduce human cancer rates. Nature. 1981;290(5803):201–208. doi: 10.1038/290201a0. [DOI] [PubMed] [Google Scholar]
- 19.Miller E.R., Pastor-Barrius R., Dalal D., Riemersma R.A., Appel L.J., Guallar E. Mata-analysis: High-dosage vitamin E supplementation may increase all-cases of mortality. Ann. Intern. Med. 2005;142(1):37–47. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 20.Blot W.J., Li J.Y., Taylor P.R., Guo W., Dawsey S., Wang G.Q., Yang C.S., Zheng S.F., Gail M., Li G.Y., Yu Y., Liu B-q., Tangrea J., Sun Y-h., Liu F., Fraumeni J.F., Zhang Y-H., Jr., Li B. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 1993;85(18):1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 21.(ATBC) The A-tocopherol-Tocopherol, Beta Carotene Cancer Prevention Study Group, author. The effect of vitamin E and beta carotene on the incidence of lung cancers in male smokers. N. Engl. J. Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 22.Hennekens C.H., Buring J.E., Manson J.E., Stampfer M., Rosner B., Cook N.R., Belanger C., LaMotte F., Gaziano J.M., Ridker P.M., Willette W., Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 23.Lee I.M., Cook N.R., Manson J.E., Buring J.E., Hennekens C.H. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. J. Natl. Cancer Inst. 1999;91(24):2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 24.Bostick R.M., Potter J.D., McKenzie D.R., Sellers T.A., Kushi L.H., Steinmetz K.A., Folsom A.R. Reduced risk of colon cancer with high intake of vitamin E: the Iowa Women’s Health Study. Cancer Res. 1993;53(18):4230–4237. [PubMed] [Google Scholar]
- 25.Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Meyskens F.L. Jr., Omenn G.S., Valanis B., Williams J.H. Jr. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J. Natl. Cancer Inst. 2004;96(23):1743–1750. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 26.DeLuca L.M., Ross S.A. Beta-carotene increases lung cancer in cigarette smokers. Nutr. Rev. 1996;54:178–180. doi: 10.1111/j.1753-4887.1996.tb03926.x. [DOI] [PubMed] [Google Scholar]
- 27.Albanes D. Beta carotene and lung cancer: a study. Am. J. Clin. Nutr. 1999;69(Suppl):1345S–1530S. doi: 10.1093/ajcn/69.6.1345S. [DOI] [PubMed] [Google Scholar]
- 28.Wang X.D., Russell R.M. Procarcinogenic and anticarcinogenic effects of beta carotene. Nutr. Rev. 1999;57:263–272. doi: 10.1111/j.1753-4887.1999.tb01809.x. [DOI] [PubMed] [Google Scholar]
- 29.Salganik R. Paper presented at: American Society for Cell Biology Annual Meeting; Washington DC.. December 15; 1999. [Google Scholar]
- 30.Diplock A.T. Antioxidant nutrients and disease prevention: an overview. Am. J. Clin. Nutr. 1991;53(1 Suppl):189S–193S. doi: 10.1093/ajcn/53.1.189Sb. [DOI] [PubMed] [Google Scholar]
- 31.Asare G.A., Mossanda K.S.A., Kew M.C., Paterson A.C., Kahler-Venter C.P., Siziba K. Hepatocellular carcinoma caused by iron overload: a possible mechanism of direct hepatocarcinogenicity. Toxicology. 2006;219(1–3):41–52. doi: 10.1016/j.tox.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Dawson H.D., Li N.Q., DeCicco K.L., Nibert J.A., Ross A.C. Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J. Nutr. 1999;129:1510–1517. doi: 10.1093/jn/129.8.1510. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki R., Goda T., Takase S. Consumption of excess vitamin A, but not excess β-carotene, causes accumulation of retinyl that exceeds the binding capacity of cellular retinol-binding protein, type II in rat intestine. J. Nutr. 1995;125:2074–2082. doi: 10.1093/jn/125.8.2074. [DOI] [PubMed] [Google Scholar]
- 34.Rohde C.M., Manatt M., Clagett-Dame M., DeLuca H.F. Vitamin A antagonizes the action of vitamin D in rats. J. Nutr. 1999;129:2246–2250. doi: 10.1093/jn/129.12.2246. [DOI] [PubMed] [Google Scholar]
- 35.Biesalski H.K., Hemmes C., Hanafy M.E., Weiser H., Zschaebitz H., Stofft E. Long term administration of high dose vitamin A to rats does not cause fetal malformations: macroscopic, skeletal and physicochemical findings. J. Nutr. 1996;126:973–983. doi: 10.1093/jn/126.4.973. [DOI] [PubMed] [Google Scholar]
- 36.Cheng W., Valentine B.A., Lei X.G. High levels of dietary vitamin E do not replace cellular glutathione peroxidase (GPX1) in protecting mice from acute oxidative stress. J. Nutr. 1999;129:1951–1957. doi: 10.1093/jn/129.11.1951. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim W., Lee U-S., Yeh C-C., Szabo J., Bruckner G., Chow C.K. Oxidative stress and antioxidant status in mouse liver: effect of dietary lipid, vitamin A and iron. J. Nutr. 1997;127:1401–1406. doi: 10.1093/jn/127.7.1401. [DOI] [PubMed] [Google Scholar]
- 38.Brown E.K., Poulos J.E., Li L. Effect of vitamin E supplementation on hepatic fibrogenesis in chronic dietary iron overload. Am. J. Physiol. 1997;272:G116–G123. doi: 10.1152/ajpgi.1997.272.1.G116. [DOI] [PubMed] [Google Scholar]
- 39.Plummer J.L., Mackinnon M., Cmielewski P.L., Williams P., Ahern M.J., Ilsley A.H., de la M Hall P. Dose related effects of dietary iron supplementation in producing hepatic iron overload in rats. J. Gastroenter. Hepatol. 1997;12:839–884. doi: 10.1111/j.1440-1746.1997.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 40.Cao G., Prior R.L. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol. 1999;299:50–62. doi: 10.1016/s0076-6879(99)99008-0. [DOI] [PubMed] [Google Scholar]
- 41.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y., Zhang D., Kawabata T., Kiriu T., Toyokuni S., Uchida K., Okada S. Copper and iron-induced oxidative damage in non-tumour bearing LEC rats. Pathol. Int. 1997;47(4):203–208. doi: 10.1111/j.1440-1827.1997.tb04481.x. [DOI] [PubMed] [Google Scholar]
- 43.Asare G.A., Paterson A.C., Kew M.C., Khan S., Mossanda K.S.A. Iron-free neoplastic nodules and hepatocellular carcinoma without cirrhosis in Wistar rats fed a diet high in iron. J. Pathol. 2006;208(1):82–90. doi: 10.1002/path.1875. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher L.M., Roberts F.D., Irving M.G., Powell L.W., Halliday J.W. Effects of iron loading on free radical scavenging enzymes and lipid peroxidation in rat liver. Gastroenterology. 1989;97:1011–1018. doi: 10.1016/0016-5085(89)91511-4. [DOI] [PubMed] [Google Scholar]
- 45.Young I.S., Trouton T.G., Torney J.J., McMaster D., Callender M.E., Trimble E. Antioxidant status and lipid peroxidation in hereditary haemochromatosis. Free Rad. Biol. Med. 1994;16(3):393–397. doi: 10.1016/0891-5849(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton I.M.J., Gilmore W.S., Benzie I.F.F., Mulholland C.W., Strain J.J. Interactions between vitamin C and E in human subjects. Br. J. Nutr. 2000;84:261–267. doi: 10.1017/s0007114500001537. [DOI] [PubMed] [Google Scholar]
- 47.Venkatraman J.T., Chandrase B., Kim J.D., Fernandes G. Effects of n-3 and n-6 fatty acids on the activities and expression of hepatic antioxidant enzymes in autoimmune-prone NZB*NZW F1 mice. Lipids. 1994;29:561–568. doi: 10.1007/BF02536628. [DOI] [PubMed] [Google Scholar]
- 48.Brown K.E., Dennery P.A., Ridnour L.A., Fimmel C.J., Kladney E.D., Brunt E.M., Spitz D.R. Effect of iron overload and dietary fat on indices of oxidative stress and hepatic fibrogenesis in rats. Liver Int. 2003;23:232–242. doi: 10.1034/j.1600-0676.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 49.Broide E., Klinowski E., Koukoulis G., Hadzic N., Portmann B., Baker A., Scapa E., Mieli-Vergani G. Superoxide dismutase activity in children with chronic liver diseases. J. Hepatology. 2000;32:188–192. doi: 10.1016/s0168-8278(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 50.Khan M.F., Wu X., Tipnis U.R., Ansari G.A.S., Boor P.J. Protein adducts of malondialdehyde and 4-Hydroxynonenal in livers of iron loaded rats: quantitation and localization. Toxicology. 2002;173:193–201. [PubMed] [Google Scholar]
- 51.Yasa H.M., Kacmaz M., Ozturk S.H., Durak I. Antioxidant status of erythrocytes from patients with cirrhosis. Hepato-Gastro. 1999;46:2460–2463. [PubMed] [Google Scholar]
- 52.Feng Z., Hu W., Tang M.S. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: a possible mechanism for lipid peroxidation-induced carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101(23):8598–8602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bacon B.R., Britton R.S., Neill R. Effects of vitamin E deficiency on hepatic mitochondrial lipid peroxidation and oxidative metabolism in rats with chronic dietary iron overload. Hepatology. 1989;9:398–404. doi: 10.1002/hep.1840090309. [DOI] [PubMed] [Google Scholar]
- 54.Parkkila S., Niemela O., Britton R.S., Brown K.E., Yla-Herttuala S., O’Neill R., Bacon B.R. Vitamin E decreases hepatic levels of aldehyde-derived peroxidation products in rats with iron overload. Gastrointest. Liver Physiol. 1996;33:G376–G384. doi: 10.1152/ajpgi.1996.270.2.G376. [DOI] [PubMed] [Google Scholar]
- 55.Benedetti A., Malvaldi G., Fulceri R., Comporti M. Loss of lipid peroxidation as a histochemical marker of preneoplastic hepatocellular foci of rats. Cancer Res. 1984;44:5712–5717. [PubMed] [Google Scholar]
- 56.Lee C.Y.J., Isaac H.B., Wang H., Huang S.H., Long L.H., Jenner A.M., Kelly R.P., Halliwell B. Cautions in the use of biomarkers of oxidative damage; the vascular and antioxidant effects of dark soy sauce in humans. Biochem. Biophys. Res. Comm. 2006;344:906–911. doi: 10.1016/j.bbrc.2006.03.217. [DOI] [PubMed] [Google Scholar]
- 57.Deugnier Y.M., Charalambous P., Le Quilleuc D., Turlin B., Searle J., Brissot P., Powell L.W., Halliday J.W. Preneoplastic significance of hepatic iron-free foci in genetic hemochromatosis: a study of 185 patients. Hepatology. 1993;18(6):1363–1369. [PubMed] [Google Scholar]
- 58.Terada T., Nakanuma Y. Iron-negative foci in siderotic macroregenerative nodules in human cirrhotic livers. Arch. Pathol. Lab. Med. 1989;113:916–920. [PubMed] [Google Scholar]
- 59.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Motality in randomized trials of antioxidant supplements for primary and secondary prevention. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]