Abstract
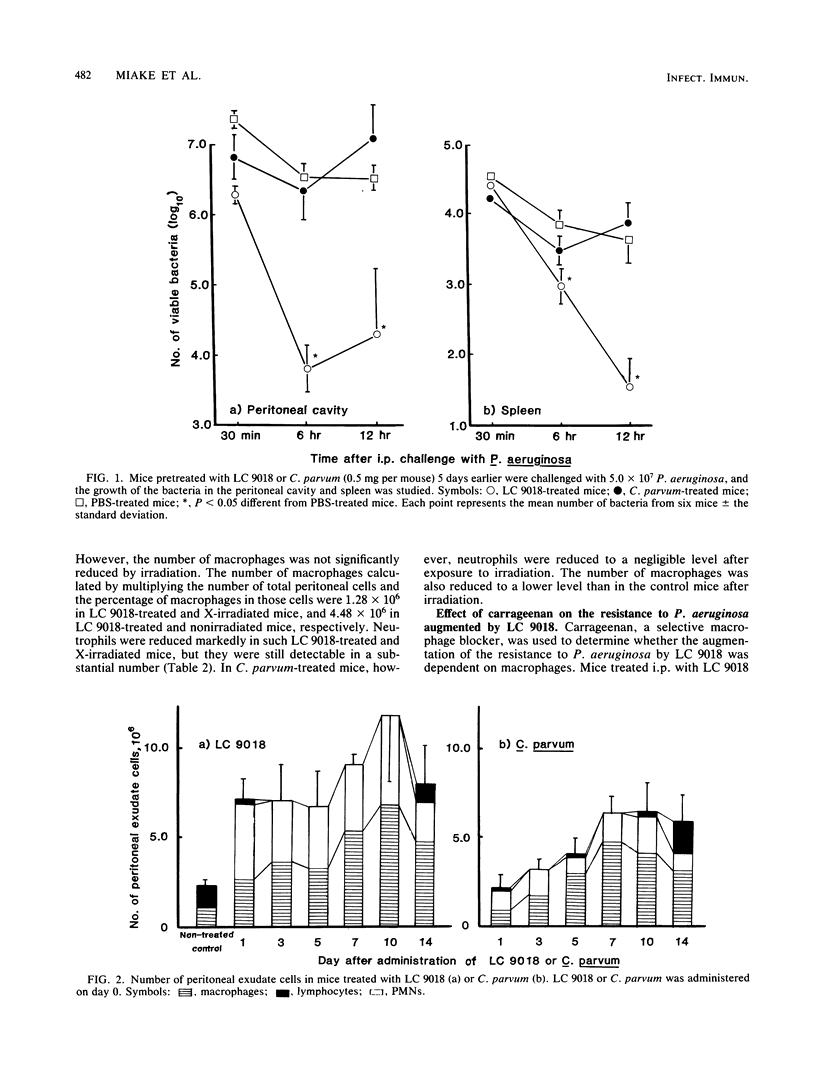
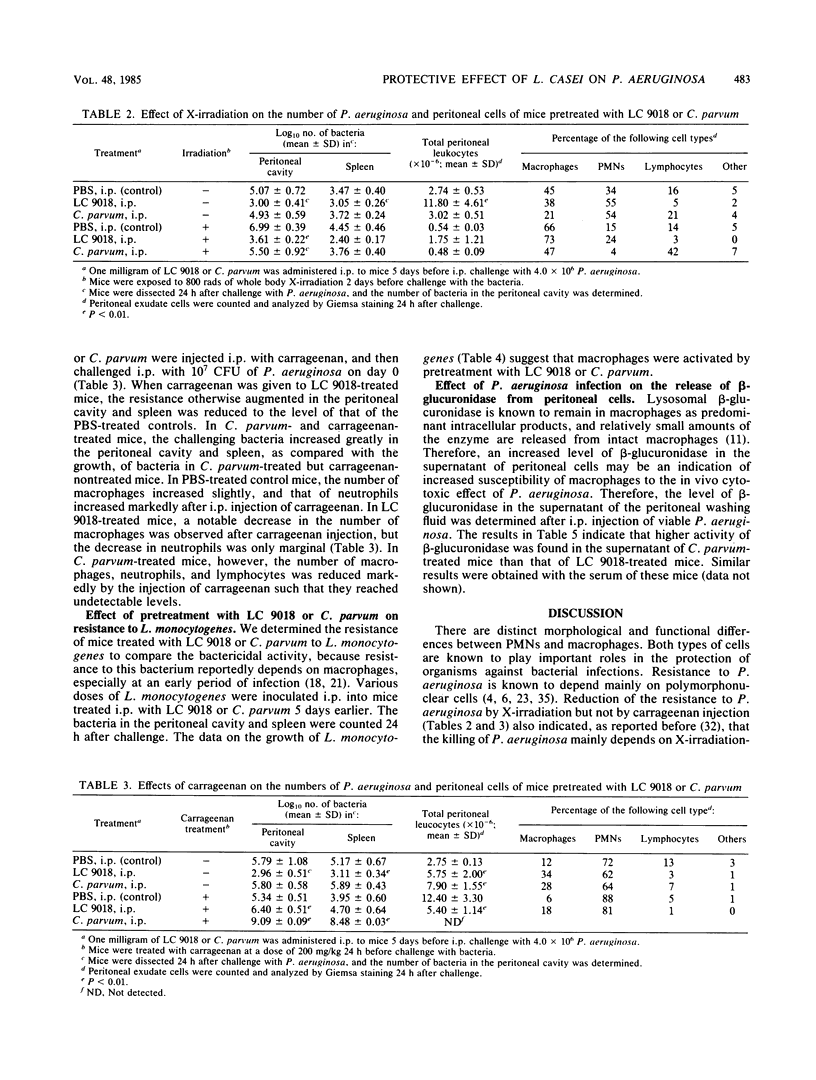
The protective effect of heat-killed Lactobacillus casei YIT9018 (LC 9018) against Pseudomonas aeruginosa infection in mice was compared with that of Corynebacterium parvum. Survival of mice after intraperitoneal (i.p.) infection with P. aeruginosa was augmented in mice that had been pretreated i.p. with LC 9018 5 days previously. Similar treatment of mice with C. parvum, however, was not effective at all. Moreover, mice became more susceptible to infection with P. aeruginosa after such treatment. Growth of P. aeruginosa in the peritoneal cavity and spleen was markedly inhibited in LC 9018-pretreated mice, whereas such inhibition of bacterial growth was not observed in C. parvum-treated mice. The protective effect of LC 9018 was observed in mice subjected to 800 rads of whole body irradiation but was abrogated when mice were treated with carrageenan. These results suggest that augmentation of the resistance of mice to P. aeruginosa was caused by the induction of activated macrophages. The number of macrophages detectable in the peritoneal cavity was almost the same in LC 9018- and C. parvum-treated mice. Growth of Listeria monocytogenes was inhibited by pretreatment with LC 9018. Inhibition of L. monocytogenes was also observed after the same pretreatment with C. parvum. It was suggested that macrophages activated with LC 9018 were involved in the protective immunity to P. aeruginosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., Page R. C. Effects of endotoxin on macrophages and other lymphoreticular cells. J Infect Dis. 1973 Jul;128(Suppl):212–219. doi: 10.1093/infdis/128.supplement_1.s212. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Monocyte function in children with neutropenia and chronic infections. Blood. 1972 Jul;40(1):31–41. [PubMed] [Google Scholar]
- Baltch A. L., Griffin P. E. Pseudomonas aeruginosa bacteremia: a clinical study of 75 patients. Am J Med Sci. 1977 Sep-Oct;274(2):119–129. [PubMed] [Google Scholar]
- Boggs D. R. Transfusion of neutrophils as prevention or treatment of infection in patients with neutropenia. N Engl J Med. 1974 May 9;290(19):1055–1062. doi: 10.1056/NEJM197405092901906. [DOI] [PubMed] [Google Scholar]
- Catanzaro P. J., Schwartz H. J., Graham R. C., Jr Spectrum and possible mechanism of carrageenan cytotoxicity. Am J Pathol. 1971 Aug;64(2):387–404. [PMC free article] [PubMed] [Google Scholar]
- Fishman W. H., Kato K., Anstiss C. L., Green S. Human serum beta-glucuronidase; its measurement and some of its properties. Clin Chim Acta. 1967 Mar;15(3):435–447. doi: 10.1016/0009-8981(67)90008-3. [DOI] [PubMed] [Google Scholar]
- Flick M. R., Cluff L. E. Pseudomonas bacteremia. Review of 108 cases. Am J Med. 1976 Apr;60(4):501–508. doi: 10.1016/0002-9343(76)90716-6. [DOI] [PubMed] [Google Scholar]
- Gillette R. W., Lance E. M. Kinetic studies of macrophages. IV. Effect of irradiation. J Reticuloendothel Soc. 1973 Jul;14(1):18–25. [PubMed] [Google Scholar]
- Gordon S., Unkeless J. C., Cohn Z. A. Induction of macrophage plasminogen activator by endotoxin stimulation and phagocytosis: evidence for a two-stage process. J Exp Med. 1974 Oct 1;140(4):995–1010. doi: 10.1084/jem.140.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Nomoto K., Matsuzaki T., Yokokura T., Mutai M. Oxygen radical production by peritoneal macrophages and Kupffer cells elicited with Lactobacillus casei. Infect Immun. 1984 Apr;44(1):61–67. doi: 10.1128/iai.44.1.61-67.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Kobayashi S., Yokokura T., Mutai M. Antitumor activity of Lactobacillus casei in mice. Gan. 1981 Aug;72(4):517–523. [PubMed] [Google Scholar]
- Kato I., Yokokura T., Mutai M. Macrophage activation by Lactobacillus casei in mice. Microbiol Immunol. 1983;27(7):611–618. doi: 10.1111/j.1348-0421.1983.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld L., Greenman V. Effects of total-body x-irradiation on peritoneal cells of mice. Radiat Res. 1966 Nov;29(3):433–444. [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal S., Mitsuyama M., Ogata N., Miyata M., Miake S., Nomoto K. BCG-induced susceptibility of mice to challenge with Pseudomonas aeruginosa. J Gen Microbiol. 1983 Jan;129(1):93–98. doi: 10.1099/00221287-129-1-93. [DOI] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- Patierno S. R., Costa M., Lewis V. M., Peavy D. L. Inhibition of LPS toxicity for macrophages by metallothionein-inducing agents. J Immunol. 1983 Apr;130(4):1924–1929. [PubMed] [Google Scholar]
- Pennington J. E., Reynolds H. Y., Carbone P. P. Pseudomonas pneumonia. A retrospective study of 36 cases. Am J Med. 1973 Aug;55(2):155–160. doi: 10.1016/0002-9343(73)90163-0. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Markham R. B., Eardley D. Correlation of the biologic responses of C3H/HEJ mice to endotoxin with the chemical and structural properties of the lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981 Jul;127(1):184–191. [PubMed] [Google Scholar]
- Reiss M., Roos D. Differences in oxygen metabolism of phagocytosing monocytes and neutrophils. J Clin Invest. 1978 Feb;61(2):480–488. doi: 10.1172/JCI108959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO K., SUTER E. LYSOSOMAL ACID HYDROLASES AND HYPERREACTIVITY TO ENDOTOXIN IN MICE INFECTED WITH BCG. J Exp Med. 1965 May 1;121:739–749. doi: 10.1084/jem.121.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. HYPERREACTIVITY TO ENDOTOXIN AFTER INFECTION WITH BCG. J Immunol. 1964 Jan;92:49–54. [PubMed] [Google Scholar]
- Sato K. Enhancement of host resistance against Listeria infection by Lactobacillus casei: role of macrophages. Infect Immun. 1984 May;44(2):445–451. doi: 10.1128/iai.44.2.445-451.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa K., Mitsuyama M., Takeya K., Nomoto K. Differing contribution of polymorphonuclear cells and macrophages to protection of mice against Listeria monocytogenes and Pseudomonas aeruginosa. J Gen Microbiol. 1979 Nov;115(1):161–166. doi: 10.1099/00221287-115-1-161. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Mituyama M., Nomoto K. Effects of Corynebacterium parvum on Escherichia coli infection in mice. J Gen Microbiol. 1982 Dec;128(12):2857–2863. doi: 10.1099/00221287-128-12-2857. [DOI] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]


