Abstract
By labeling surface carbohydrates we found that a pool of lipoglycans, cell wall associated, is exposed at the cell surface of mycobacteria and thus, most probably, inserted in the outer leaflet of the outer membrane. In contrast, plasma membrane anchored lipoglycans are not accessible to surface labeling. This result supports the role of lipoglycans as key immunomodulatory molecules but raises the question of their transport from the plasma membrane, where they are synthesized, to the outermost layers of the envelope, where they can act as modulins. The data are discussed in term of consequences for cell envelope organization.
Keywords: Mycobacterium, lipoglycan, localization, cell envelope, transporter
The lipoglycans, lipoarabinomannan (LAM) and lipomannan (LM), are major immunomodulatory molecules of the mycobacterial cell envelope 1–4. Mannose-capped LAM (ManLAM) is a potential ligand for the entry of Mycobacterium tuberculosis into phagocytic cells via the host C-lectins 5. In addition, purified ManLAM reproduces several properties of M. tuberculosis that may contribute to the inhibition of the host defense response and define ManLAM as a major virulence factor of the pathogen 5. In contrast, phosphoinositol-capped LAM (PILAM) and LM stimulate innate immunity via signaling through Toll-like receptor 2 (TLR2) 5. Lipoglycans are delivered from infected macrophages, via exosomes or apoptotic vesicles, to non-infected bystander dendritic cells 6–8 and thus can modulate the functions of the latter, via binding C-lectins 9 or TLR-2, even though they are not necessarily receptor ligands on the whole bacterium 10. Nevertheless, their role as mycobacterial cell surface adhesins or as soluble molecules released by phagocytic cells implies that they are exposed to the cell surface or, at least, located in the outermost part of the cell envelope. However, their precise localization remains unclear. They are not covalently attached to the cell envelope, but they have never been found in culture supernatants or in the surface-exposed material obtained by gentle mechanical treatment of cells with glass beads and/or detergent treatment 11,12, suggesting that they are instead imbedded in the cell wall.
In the present study, the exposition of lipoglycans to the cell surface of mycobacteria was investigated by cell labeling with biotin. The validity of this approach relies on the assumption that labeling is indeed restricted to surface components. BCG cells were grown as surface pellicles in Sauton’s medium for 20–25 days. We assumed that these cells had an intact envelope since the culture supernatant was found to be devoid of the cytosolic heat shock protein 65 and of traces of the cell wall polysaccharide, arabinogalactan (AG). Cells were thus submitted to periodate oxidation to generate aldehydic functions in surface exposed-carbohydrates, by incubation for 20 min, at 4°C in the dark with 0.1 M sodium acetate buffer pH 5.5 (buffer A) containing 15 mM sodium metaperiodate (Merck). Oxidized cells were then labeled for 2 h at room temperature in buffer A containing 5 mM of biotin-hydrazide (Sigma) 6,13. Bacteria retained around 98% viability and electron microscopy examinations showed that the whole bacilli morphology (Figures 1B and C) as well as cell wall organization (not shown) of labeled cells were undistinguishable of those of untreated control cells. Moreover, AG, known to be imbedded in the cell wall, was not labeled by biotin as determined by dot-blot and alkaline phosphatase-conjugated streptavidin (AP-streptavidin) detection (Figure 1A, lane f) whereas the crude ethanol/water extract (lane c) of treated cells gave an intense response. Altogether these data led us to conclude that biotin labeling did not affect the integrity of the bacilli and that it was indeed restricted to cell surface-exposed molecules.
Figure 1. Biotin labeling of various BCG sub-fractions (A) and scanning electron microscopy of control (B) and biotin-hydrazide labeled (C) BCG cells.
A) 1 μg of each fraction (10 μg for arabinogalactan) were dot-blotted and probed with AP-streptavidin. 1, control cells; 2, biotinylated cells. HIC, hydrophobic interaction chromatography.
B) Bacteria were fixed with 2% glutaraldehyde (EMS, Washington PA) in 0.1 M cacodylate buffer pH 7.4 during 1 hour at 4°C. Fixed bacteria were washed in 0.2 M cacodylate buffer (pH 7.4), postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h and dehydrated in graded ethanol series. After dehydration samples were critical point dried with an emscope CPD 750 apparatus, mounted on stubs, coated with gold-palladium alloy with a JEOL JFC 1100 ion sputtering apparatus and examined with a Hitachi S-450 scanning electron microscope at an accelerating voltage of 15 kV. Bars, 0.5 μm.
Analysis of the lipoglycan fractions
We previously set up a purification protocol where a first lipoglycan fraction, tentatively called “parietal” is obtained directly by ethanol/water extraction of the delipidated cells (Figure 1A), whereas a second one, termed “cellular”, can be obtained only after sonication of the resulting cells 14. Both fractions, prepared from labeled cells, gave a positive response when probed with AP-streptavidin (Figure 1A, lanes a and b) suggesting that, in contrast to our first hypothesis, molecules contained in these fractions do not differ by a particular localization in the cell envelope but rather by the strength of their association to cell wall material after delipidation, the molecules recovered in the “cellular” fraction being more firmly attached, possibly as a result of a higher acylation degree 15,16. It is well-known that cell wall purification requires SDS extraction steps to remove molecules, including lipoglycans, that remain strongly associated to mAGP complex after cell lysis.
Further analyses were performed on the total lipoglycan pool obtained by water/ethanol extraction of the cells disrupted immediately after delipidation 13 (Figure 1A, lane c). Contaminating proteins, glucan and nucleic acids were enzymatically removed yielding a fraction enriched in glycans and lipoglycans, which were further resolved by hydrophobic interaction chromatography (HIC) 13. As expected, the glycan fraction, which contains the surface-exposed arabinomannan (AM) and mannan 11,12, was labeled (Figure 1A, lane e). More interestingly, the lipoglycan fraction, found by SDS-PAGE and silver staining to contain only ManLAM and LM, was also biotin positive (Figure 1A, lane d). As previously reported 13, its analysis by western blot with AP-streptavidin detection revealed two bands that unambiguously corresponded to biotin-labeled ManLAM and LM. Similar results were obtained with Mycobacterium xenopi 13 and Mycobacterium smegmatis (see further), indicating that ManLAM and LM are exposed to the cell surface of these mycobacteria, where they are accessible to small reagent molecules such as biotin, and thus possibly to host immune receptors.
Lipoglycan localization and transport within the mycobacterial cell envelope
The mycobacterial cell envelope is composed of a plasma membrane, a wall and a capsule 12. The wall contains an outer membrane (OM) where the inner leaflet is made of arabinogalactan-bound mycolic acids and the outer leaflet contains the extractable lipids 17–22. In this context, three hypotheses have been proposed regarding the localization of LAM. LAM molecules could be secreted and have no permanent position 1 or could be inserted through their lipidic anchor, i) in the outer leaflet of the OM among other lipids 23, and/or ii) in the plasma membrane 24,25. The later scenario has been favored in the past years 1 owing to the relatively strong conditions needed to extract LAM and recently reinforced by the finding that glycosyltransferases involved in the last steps of LAM biosynthesis are membrane-associated 26–28. Accordingly, upon subcellular fractionation, LAM has been detected in BCG membranes by ELISA 29. Similarly, by SDS-PAGE and silver nitrate staining, we detected apparently mature LAM and LM in BCG and M. smegmatis (Figure 2B) membrane fractions after protein digestion (Figure 2A). They represented around 5–10% of the total lipoglycans of the cells and showed the same migration pattern as cell wall associated lipoglycans; LAM reacted with CS35 antibody (Figure 2D) and BCG LAM had mannose caps as revealed by mild acid hydrolysis and capillary electrophoresis analysis (not shown). The plasma membrane might be the primary location for lipoglycans since they are also found in actinomycetes species that do not produce mycolic acids, and thus do not harbor an OM 21,22, such as Amycolaptosis sulphurea 30, Turicella otitidis 31 or Saccharothrix aerocolonigenes 32. However, in contrast to cell wall associated lipoglycans, membrane anchored lipoglycans from labeled cells were not positive for biotin tagging (Figure 2C), indicating that they do not reach the cell surface. This is in agreement with the estimated size of LAM. Indeed, BCG ManLAM in solution forms micelles about 30 nm in diameter 33. Recent electron microscopy analyses on unperturbed cells revealed the thickness of the cell wall plus the plasma membrane to be around 40 nm 21,22. Depending on the mycobacterial species, the thickness of the capsule is estimated to be 20 to 100 nm 12,19,34,35. Thus, LAM, with an estimated “length” of 15 nm, is not large enough to cross the cell envelope completely (Figure 3). This implies that lipoglycans accessible from the outside, and associated to cell wall, constitute a second pool of these molecules that might be anchored in the OM, as proposed by Rastogi 23 (Figure 3). This later population of lipoglycans is likely to be the one involved in host-pathogen interactions, acting as adhesins in C-type lectin-mediated phagocytosis or trafficking inside phagocytic cells after their release from the mycobacterial cell surface 6,7. The present results are not necessarily contradictory with the fact that lipoglycans, contrary to their lipid-free counterparts AM and mannan, have never been found in the surface-exposed material recovered after gentle treatment of mycobacterial cells with glass beads and/or detergent 11,12. They probably only reflect the fact that, although not covalently-bound to the cell envelope, lipoglycans are sufficiently strongly attached to the cells not to be extracted by these gentle treatments.
Figure 2. LAM from cell wall but not plasma membrane fractions is labeled with biotin.
The cell wall (cw) and plasma membrane (pm) fractions were prepared from M. smegmatis cells as described in A) and submitted to α-amylase and protease digestions. Lipoglycans were recovered after HIC and LAM (5μg) was analyzed by: B) SDS-PAGE revealed by periodic acid-silver nitrate staining; C) western blot probed with AP-streptavidin; D) western blot probed with anti-LAM CS35 antibody. 1, LAM from non-labeled control cells; 2, LAM from biotin-hydrazide labeled cells; Sc, in vitro biotinylated M. smegmatis total LAM standard; Sd, M. smegmatis total LAM standard.
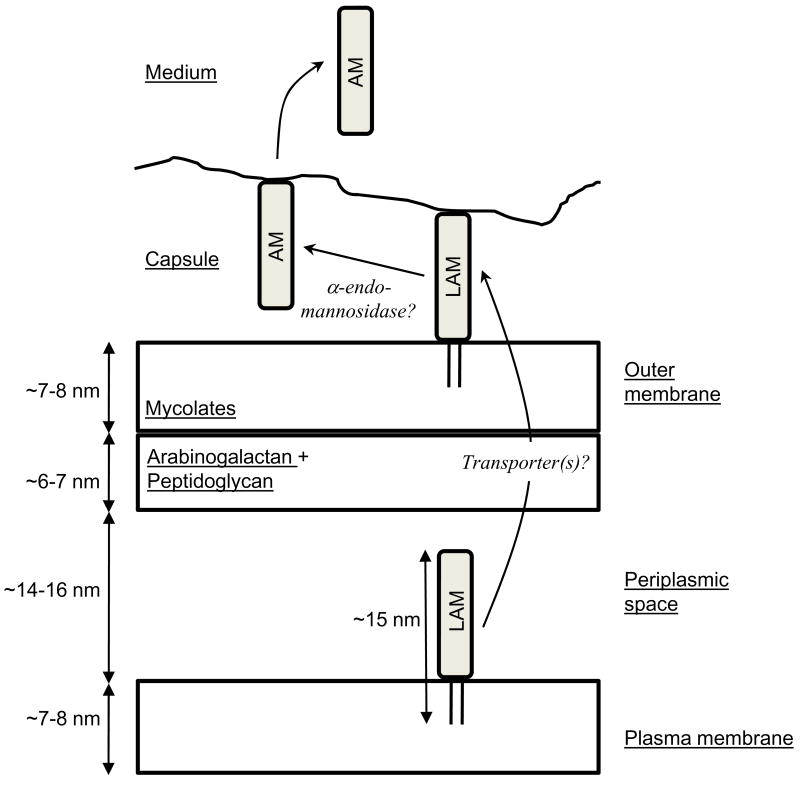
Figure 3. LAM localization in the cell envelope.
All the constituents are tentatively presented to scale and the drawing is based on the recent electron microscopy analyses on unperturbed cells 21,22. Lipoglycans are synthesized at the level of the plasma membrane where they have not access to cell surface. The hydrophilic part of the molecule is most probably located in the periplasmic space, which has now been clearly evidenced 21,22, or may protrude through the cell wall skeleton via the pores made by cross-linked peptidoglycan strands 20. After synthesis, a part of or all the lipoglycans are transferred, possibly via a transporter(s) that still remains to be discovered, to the outer layers of the cell envelope. At this stage, they are exposed at the cell surface and most probably inserted among other lipids in the outer layer of the outer membrane and can play their roles of modulins and adhesins. Action of an endogenous α-endomannosidase might convert a portion of lipoarabinomannan (LAM) and lipomannan molecules into their lipid free glycan counterparts, arabinomannan (AM) and mannan respectively that can be subsequently released into the culture medium.
The presence of a second pool of lipoglycans exposed at the cell surface and cell wall associated raises the question of their transport from the plasma membrane, where they are synthesized, to the outermost layers of the envelope. Recent studies have shown that different families of proteins are involved in the transport of lipids/glycolipids to the cell surface 36,37. In Escherichia coli, LPS transport from the inner membrane (IM) to the OM requires 38–40: i) an ABC transporter at the IM, presumably involved in providing the energy to extract LPS from the IM, ii) a periplasmic chaperone that escorts LPS through the periplasm, and iii) an OM assembly protein complex, whose supposed function is to receive LPS at inner leaflet of the OM and to flip it across the membrane. It is tempting to envision that translocation of mycobacterial lipoglycans could be realized by analogous machinery. We thus investigated the possible involvement of a putative polysaccharide ABC transporter encoded by the genes Rv3781 and Rv3783 41 by disrupting by homologous recombination 42 the ortholog of Rv3783 in M. smegmatis. The lipoglycans of the mutant were, however, as efficiently tagged by biotin as in its wild-type parent. Moreover, the mutant secreted similar amounts of glucan as well as the LAM- and LM-derived glycans, AM and mannan. Thus, the existence of lipoglycan translocation machinery still remains an open but crucial question, since the virulence of bacilli may depend on the export of immunomodulatoty molecules to the cell surface, as recently shown for phthiocerol dimycocerosates 43.
Acknowledgments
We thank Dr Mamadou Daffé for helpful discussions and sharing unpublished data. We gratefully acknowledge Dr Patricia Constant for expert advice with cell culture and Ms Lucile Puntil and Anne-Claire Jolivet for helpful technical assistance. Profs G. Marchal (Paris) and A. Basten (Sydney) are thanked for the generous gift of the anti-Apa and HAT5 antibodies respectively. CS-35 monoclonal anti-LAM antibody was obtained from Colorado State University (NIH contract NIAID No-1-AI-40091). This work was supported by grants from CNRS, Agence Nationale de la Recherche (ANR-05-MIIM-038-04), the National Institute of Allergy and Infectious Diseases/National Institutes of Health (grant 5R01AI064798-3) and the IFCPAR (project 2903-3).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chatterjee D, Khoo KH. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–20. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 2.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie. 2003;85:153–66. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 3.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 4.Sutcliffe I. Lipoarabinomannans--structurally diverse and functionally enigmatic macroamphiphiles of mycobacteria and related actinomycetes. Tuberculosis (Edinb) 2005;85:205–6. doi: 10.1016/j.tube.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Gilleron M, Jackson M, Nigou J, Puzo G. Structure, biosynthesis, and activities of the phosphatidyl-myo-inositol-based lipoglycans. In: Daffé M, Reyrat JM, editors. The Mycobacterial Cell Envelope. Washington, DC: ASM Press; 2008. pp. 75–105. [Google Scholar]
- 6.Beatty WL, Rhoades ER, Ullrich HJ, Chatterjee D, Heuser JE, Russell DG. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–47. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 7.Rhoades E, Hsu F, Torrelles JB, Turk J, Chatterjee D, Russell DG. Identification and macrophage-activating activity of glycolipids released from intracellular Mycobacterium bovis BCG. Mol Microbiol. 2003;48:875–88. doi: 10.1046/j.1365-2958.2003.03473.x. [DOI] [PubMed] [Google Scholar]
- 8.Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9:1039–46. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 9.Dulphy N, Herrmann JL, Nigou J, Rea D, Boissel N, Puzo G, Charron D, Lagrange PH, Toubert A. Intermediate maturation of Mycobacterium tuberculosis LAM-activated human dendritic cells. Cell Microbiol. 2008;10:930–44. doi: 10.1111/j.1462-5822.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- 10.Appelmelk BJ, den Dunnen J, Driessen NN, Ummels R, Pak M, Nigou J, Larrouy-Maumus G, Gurcha SS, Movahedzadeh F, Geurtsen J, Brown EJ, Eysink Smeets MM, Besra GS, Willemsen PT, Lowary TL, van Kooyk Y, Maaskant JJ, Stoker NG, van der Ley P, Puzo G, Vandenbroucke-Grauls CM, Wieland CW, van der Poll T, Geijtenbeek TB, van der Sar AM, Bitter W. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell Microbiol. 2008;10:930–44. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 11.Ortalo-Magne A, Dupont MA, Lemassu A, Andersen AB, Gounon P, Daffe M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology. 1995;141:1609–20. doi: 10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- 12.Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 13.Pitarque S, Herrmann JL, Duteyrat JL, Jackson M, Stewart GR, Lecointe F, Payre B, Schwartz O, Young DB, Marchal G, Lagrange PH, Puzo G, Gicquel B, Nigou J, Neyrolles O. Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem J. 2005;392:615–24. doi: 10.1042/BJ20050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delmas C, Gilleron M, Brando T, Vercellone A, Gheorghui M, Rivière M, Puzo G. Comparative structural study of the mannosylated-lipoarabinomannans from Mycobacterium bovis BCG vaccine strains: characterization and localization of succinates. Glycobiology. 1997;7:811–17. doi: 10.1093/glycob/7.6.811. [DOI] [PubMed] [Google Scholar]
- 15.Nigou J, Gilleron M, Cahuzac B, Bounery JD, Herold M, Thurnher M, Puzo G. The phosphatidyl-myo-inositol anchor of the lipoarabinomannans from Mycobacterium bovis bacillus Calmette Guerin. Heterogeneity, structure, and role in the regulation of cytokine secretion. J Biol Chem. 1997;272:23094–103. doi: 10.1074/jbc.272.37.23094. [DOI] [PubMed] [Google Scholar]
- 16.Gilleron M, Himoudi N, Adam O, Constant P, Venisse A, Riviere M, Puzo G. Mycobacterium smegmatis phosphoinositols-glyceroarabinomannans. Structure and localization of alkali-labile and alkali-stable phosphoinositides. J Biol Chem. 1997;272:117–24. doi: 10.1074/jbc.272.1.117. [DOI] [PubMed] [Google Scholar]
- 17.Minnikin DE. Lipids: complex lipids, their chemistry, biosynthesis and role. In: Ratledge C, Standford J, editors. The Biology of the Mycobacteria. London: Academic Press Inc. Ltd; 1982. pp. 95–184. [Google Scholar]
- 18.Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2003;83:91–7. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- 19.Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 20.Dmitriev BA, Ehlers S, Rietschel ET, Brennan PJ. Molecular mechanics of the mycobacterial cell wall: from horizontal layers to vertical scaffolds. Int J Med Microbiol. 2000;290:251–8. doi: 10.1016/S1438-4221(00)80122-8. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci U S A. 2008;105:3963–7. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuber B, Chami M, Houssin C, Dubochet J, Griffiths G, Daffé M. Direct visualization of the outer membrane of native Mycobacteria and Corynebacteria. J Bacteriol. 2008 doi: 10.1128/JB.01919-07. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rastogi N. Recent observations concerning structure and function relationships in the mycobacterial cell envelope: elaboration of a model in terms of mycobacterial pathogenicity, virulence and drug-resistance. Res Microbiol. 1991;142:464–76. doi: 10.1016/0923-2508(91)90121-p. [DOI] [PubMed] [Google Scholar]
- 24.Gaylord H, Brennan PJ. Leprosy and the leprosy bacillus: recent developments in characterization of antigens and immunology of the disease. Annu Rev Microbiol. 1987;41:645–75. doi: 10.1146/annurev.mi.41.100187.003241. [DOI] [PubMed] [Google Scholar]
- 25.McNeil MR, Brennan PJ. Structure, function and biogenesis of the cell envelope of mycobacteria in relation to bacterial physiology, pathogenesis and drug resistance; some thoughts and possibilities arising from recent structural information. Res Microbiol. 1991;142:451–63. doi: 10.1016/0923-2508(91)90120-y. [DOI] [PubMed] [Google Scholar]
- 26.Escuyer VE, Lety M-A, Torrelles JB, Khoo K-H, Tang J-B, Rithner CD, Frehel C, McNeil MR, Brennan PJ, Chatterjee D. The role of the embA and embB gene products in the biosynthesis of the terminal hexaarabinofiuranosyl motif of Mycobacterium smegmatis arabinogalactan. J Biol Chem. 2001;276:48854–62. doi: 10.1074/jbc.M102272200. [DOI] [PubMed] [Google Scholar]
- 27.Berg S, Starbuck J, Torrelles JB, Vissa VD, Crick DC, Chatterjee D, Brennan PJ. Roles of conserved proline and glycosyltransferase motifs of EmbC in biosynthesis of lipoarabinomannan. J Biol Chem. 2005;280:5651–63. doi: 10.1074/jbc.M411418200. [DOI] [PubMed] [Google Scholar]
- 28.Dinadayala P, Kaur D, Berg S, Amin AG, Vissa VD, Chatterjee D, Brennan PJ, Crick DC. Genetic basis for the synthesis of the immunomodulatory mannose caps of lipoarabinomannan in Mycobacterium tuberculosis. J Biol Chem. 2006;281:20027–35. doi: 10.1074/jbc.M603395200. [DOI] [PubMed] [Google Scholar]
- 29.Mehrotra J, Mittal A, Rastogi AK, Jaiswal AK, Bhandari NK, Sinha S. Antigenic definition of plasma membrane proteins of Bacillus Calmette-Guerin: predominant activation of human T cells by low-molecular-mass integral proteins. Scand J Immunol. 1999;50:411–9. doi: 10.1046/j.1365-3083.1999.00616.x. [DOI] [PubMed] [Google Scholar]
- 30.Gibson KJ, Gilleron M, Constant P, Puzo G, Nigou J, Besra GS. Identification of a novel mannose-capped lipoarabinomannan from Amycolatopsis sulphurea. Biochem J. 2003;372:821–9. doi: 10.1042/BJ20030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilleron M, Garton NJ, Nigou J, Brando T, Puzo G, Sutcliffe IC. Characterization of a truncated lipoarabinomannan from the Actinomycete Turicella otitidis. J Bacteriol. 2005;187:854–61. doi: 10.1128/JB.187.3.854-861.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson KJ, Gilleron M, Constant P, Sichi B, Puzo G, Besra GS, Nigou J. A lipomannan variant with strong TLR-2-dependent pro-inflammatory activity in Saccharothrix aerocolonigenes. J Biol Chem. 2005;280:28347–56. doi: 10.1074/jbc.M505498200. [DOI] [PubMed] [Google Scholar]
- 33.Riviere M, Moisand A, Lopez A, Puzo G. Highly ordered supra-molecular organization of the mycobacterial lipoarabinomannans in solution. Evidence of a relationship between supra-molecular organization and biological activity. J Mol Biol. 2004;344:907–18. doi: 10.1016/j.jmb.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 34.Paul TR, Beveridge TJ. Preservation of surface lipids and determination of ultrastructure of Mycobacterium kansasii by freeze-substitution. Infect Immun. 1994;62:1542–50. doi: 10.1128/iai.62.5.1542-1550.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul TR, Beveridge TJ. Reevaluation of envelope profiles and cytoplasmic ultrastructure of mycobacteria processed by conventional embedding and freeze- substitution protocols. J Bacteriol. 1992;174:6508–17. doi: 10.1128/jb.174.20.6508-6517.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonden B, Kocincova D, Deshayes C, Euphrasie D, Rhayat L, Laval F, Frehel C, Daffe M, Etienne G, Reyrat JM. Gap, a mycobacterial specific integral membrane protein, is required for glycolipid transport to the cell surface. Mol Microbiol. 2005;58:426–40. doi: 10.1111/j.1365-2958.2005.04847.x. [DOI] [PubMed] [Google Scholar]
- 37.Jackson M, Stadthagen G, Gicquel B. Long-chain multiple methyl-branched fatty acid-containing lipids of Mycobacterium tuberculosis: Biosynthesis, transport, regulation and biological activities. Tuberculosis (Edinb) 2007;87:78–86. doi: 10.1016/j.tube.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Wu T, McCandlish AC, Gronenberg LS, Chng SS, Silhavy TJ, Kahne D. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A. 2006;103:11754–9. doi: 10.1073/pnas.0604744103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperandeo P, Cescutti R, Villa R, Di Benedetto C, Candia D, Deho G, Polissi A. Characterization of lptA and lptB, two essential genes implicated in lipopolysaccharide transport to the outer membrane of Escherichia coli. J Bacteriol. 2007;189:244–53. doi: 10.1128/JB.01126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz N, Gronenberg LS, Kahne D, Silhavy TJ. Identification of two inner-membrane proteins required for the transport of lipopolysaccharide to the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A. 2008;105:5537–42. doi: 10.1073/pnas.0801196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braibant M, Gilot P, Content J. The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS Microbiol Rev. 2000;24:449–67. doi: 10.1111/j.1574-6976.2000.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 42.Pelicic V, Jackson M, Reyrat JM, Jacobs WR, Jr, Gicquel B, Guilhot C. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1997;94:10955–60. doi: 10.1073/pnas.94.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sulzenbacher G, Canaan S, Bordat Y, Neyrolles O, Stadthagen G, Roig-Zamboni V, Rauzier J, Maurin D, Laval F, Daffe M, Cambillau C, Gicquel B, Bourne Y, Jackson M. LppX is a lipoprotein required for the translocation of phthiocerol dimycocerosates to the surface of Mycobacterium tuberculosis. Embo J. 2006;25:1436–44. doi: 10.1038/sj.emboj.7601048. [DOI] [PMC free article] [PubMed] [Google Scholar]