Abstract
BACKGROUND:
Deficits in motivated behavior and decision-making figure prominently in the behavioral syndrome that characterizes schizophrenia and are difficult both to treat and to understand. One explanation for these deficits is that schizophrenia decreases sensitivity to rewards in the environment. An alternate explanation is that sensitivity to rewards is intact but that poor integration of affective with cognitive information impairs the ability to use this information to guide behavior.
METHODS:
We tested reward sensitivity using a modified version of an existing signal detection task with asymmetric reinforcement [Pizzagalli, D.A., Jahn, A.L., & O'Shea, J.P. (2005) Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological Psychiatry, 57(4), 319-27], and decision-making using a probabilistic decision-making task in 40 participants with schizophrenia and 26 healthy participants.
RESULTS:
Results showed normal sensitivity to reward in participants with schizophrenia, but differences in choice patterns on the decision-making task. A logistic regression model of the decision-making data showed that participants with schizophrenia differed from healthy participants in the ability to weigh potential outcomes, specifically potential losses, when choosing between competing response options. Deficits in working memory ability accounted for group differences in ability to use potential outcomes during decision-making.
CONCLUSIONS:
These results suggest that the implicit mechanisms that drive reward-based learning are surprisingly intact in schizophrenia, but that poor ability to integrate cognitive and affective information when calculating the value of possible choices may hamper the ability to use such information during explicit decision-making.
Keywords: schizophrenia, reward sensitivity, decision-making, learning
Introduction
Many patients with schizophrenia demonstrate significant functional disability with prominent impairments in motivation and the ability to pursue long-term goals [1-6]. In seeking to understand the origins of this disability, impairments in reward processing and decision-making are logical candidate processes. For example, if schizophrenia muted the experience of rewards resulting from goal attainment, the failure to initiate and sustain goal directed behavior would be understandable. Similarly, adaptive behavior deficits could be a consequence of deficient ability to learn from rewards (and/or punishments). The same sort of inertia and behavioral limitations could result from decision-making abnormalities. If patients have difficulty weighing the risks and benefits associated with different choices, impairments in adaptive behavior would inevitably result.
A review of the literature suggests that such simple formulations are probably inadequate. In the area of reward, there is replicated evidence that patients have surprisingly normative experiences of emotionally evocative stimuli [7-13] and that they successfully use rewards to guide learning in procedural learning tasks [14-17], although contrary findings exist [18,19]. Despite evidence for spared reward-learning, patients show dramatic impairments in the ability to use feedback to guide behavior on tasks including the Wisconsin Card Sorting Test [20-23]. Thus, it appears that the illness compromises some, but not all, aspects of reward processing.
The literature on decision-making in schizophrenia is similarly complex. Although several reports show that patients make relatively normal choices [24-26], research often shows that they make “impulsive” decisions [27-30] and are more myopic with respect to future outcomes than healthy participants [31]. These findings suggest that poor decision-making in schizophrenia likely occurs in the context of spared implicit reinforcement learning.
The explanation for this discrepancy may lie along the intersection of affect and cognition. Both the ability to adapt to rapid trial-to-trial alterations in reward contingencies and the ability to maintain the longer-term average reward value of a choice are critical to decision-making [16]. In schizophrenia, it may be the case that cognitive impairments (e.g., working memory deficits) reduce the ability to use immediate reinforcements to shift behavior from trial-to-trial [32], despite adequate ability to acquire a stable response pattern based on a longer-term reinforcement history [16]. This incongruity might manifest itself in poor performance early in a task, with later performance approximating normal levels [14,16]. Cognitive/affective integration deficits might also explain low coherence between affective experience and motivated behavior when rewards must be maintained in working memory [13], and would predict difficulty in making decisions based on subjective values of possible outcomes.
The subjective value of an outcome is important when choosing among competing response options [33-35]. For example, it is likely that people choose options with higher subjective value, determined by a combination of the magnitude and valence (gain or loss) of an outcome, its likelihood of occurrence, and some affective preference weighting [36,37]. Consistent with this idea, many studies document the involvement of reward circuitry in decision-making [38,39], especially when participants choose among uncertain or temporally distant outcomes [40-42]. These studies suggest that prefrontal systems carrying both cognitive and reward information (dorsolateral prefrontal cortex and orbitofrontal cortex respectively) are required to optimize task performance [43,44]. In schizophrenia, poor integration of affective with cognitive information [45] might impair the ability to assign preference values to competing actions, particularly when actions are not immediately associated with reinforcement.
In this paper, we propose that decision-making difficulties in schizophrenia relate to the ability to assign subjective value to potential outcomes and not to general reward sensitivity deficits. We therefore predict that patients with schizophrenia will show normal responses to experienced rewards but worse ability to assign subjective value to response options, thereby affecting explicit decision-making.
We measured reward sensitivity using a signal detection task with asymmetric reinforcement. In signal detection tasks, participants report which of two stimuli was present on each trial by making one of two responses [46]. By rewarding one stimulus more frequently than the other, it is possible to induce a response bias such that on trials where participants are uncertain about which stimulus they saw, they tend to respond as though the more frequently rewarded stimulus was present [47]. Pizzagalli et al [48] used this paradigm to demonstrate that depressed individuals show reduced reward sensitivity compared with non-depressed individuals. Specifically, they found that non-depressed participants developed biased responding toward the frequently rewarded stimulus but depressed participants did not [48]. If patients have intact reward sensitivity, they should develop a similar response bias as comparison participants.
A probabilistic decision-making task assessed how the value of a gamble related to participants' willingness to choose it [49]. On each trial, participants chose one of two gambles that differed in reward magnitude, outcome (potential loss or no loss possible) and probability of winning. To understand how participants determined the subjective value of an outcome, we estimated the degree to which potential gains, losses, and uncertainty about outcomes contributed to decision-making.
Methods
Participants
Participants included 40 clinically stable outpatients with chronic schizophrenia (SC) and 26 healthy comparison participants (HC), matched on age and paternal education. Patient diagnoses were confirmed with the Structured Clinical Interview for DSM-IV [SCID, 50]. All patients received antipsychotic medications and none had had prescription changes during the month before participation. Patients were capable of providing informed consent, as documented by a set of standard probes. Symptom assessments included the Brief Psychiatric Rating Scale [BPRS, 51] and Scale for the Assessment of Negative Symptoms [SANS, 52]. Comparison participants were free of psychiatric diagnoses, as indicated by the SCID, received no psychiatric medications, and had no family history of psychosis (see Table 1 for sample characteristics). Participants were free of substance abuse/dependence, except nicotine, for at least 6 months. After a complete description of study procedures, participants gave written informed consent. The University of Maryland's institutional review board approved the study.
Table 1.
Sample Characteristics and Neuropsychological Performance
| Healthy Participants (N = 26) |
Participants with Schizophrenia (N = 40) |
p-value | ||
|---|---|---|---|---|
| Age | 48.26 (9.93) | 45.8 (10.21) | .40 | |
| Age at illness onset | ---- | 22.75 (7.26) | ---- | |
| Participant Education | 14.26 (1.97) | 12.25 (1.96) | < .001 | |
| Paternal Education | 12.64 (4.38) | 12.47 (4.34) | .90 | |
| Gender (M:F) * | 18:8 | 31:9 | .45 | |
| Race * | .67 | |||
| African American | 9 | 11 | ||
| Caucasian | 17 | 27 | ||
| Other | 0 | 2 | ||
| Antipsychotic Medication | ||||
| Atypical | ---- | 29 | ---- | |
| Typical | ---- | 6 | ---- | |
| Typical + Atypical | ---- | 5 | ---- | |
| Clinical Ratings | ||||
| BPRS | ---- | 36.83 (8.46) | ---- | |
| SANS | ---- | 25.94 (14.11) | ---- | |
| Neurocognitive Test Results | ||||
| Spatial Span | 10.81 (3.06) | 7.95 (3.23) | .001 | |
| Letter-Number Sequencing | 16.22 (3.83) | 11.70 (3.22) | < .001 | |
| Hopkins Verbal Learning Test | 28.36 (4.08) | 20.84 (5.19) | < .001 | |
| Wechsler Test of Adult Reading | 106.78 (15.52) | 95.27 (16.35) | .005 | |
Note: Table includes means and standard deviations. Except where noted, group differences tested with T-tests. BPRS, Brief Psychiatric Rating Scale; SANS, Scales for the Assessment of Negative Symptoms.
Comparison tested with chi-square
Procedures
Reward Sensitivity Task
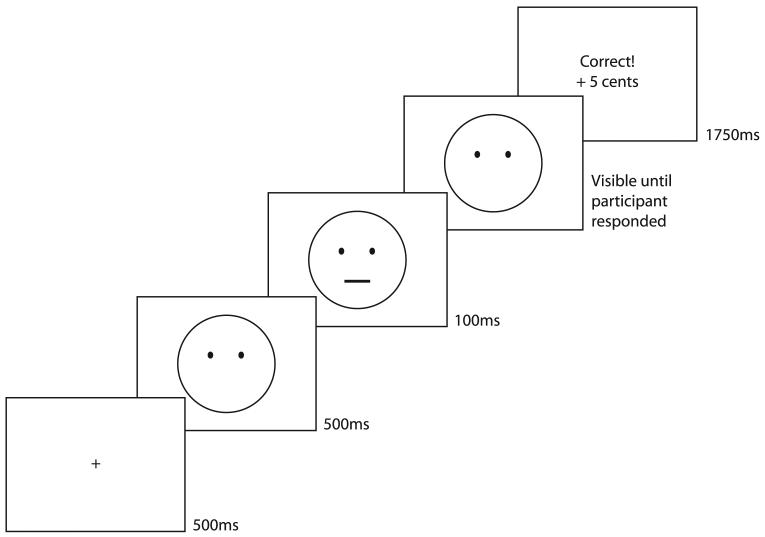
We used a line discrimination task, closely modeled after that reported in Pizzagalli et al [48]. At the start of each trial, participants viewed a fixation cross for 500ms. A cartoon face with no mouth then appeared (see Figure 1). After 500ms, either a short (22mm) or a long mouth (24mm) appeared on the face and remained for 100ms. The face, without the mouth, remained on the screen until participants responded. On feedback trials, participants saw feedback for 1750ms. On trials without feedback, the screen was blank for 1750ms before the start of the next trial. Participants responded with a left or right button press on a game-controller to indicate which mouth they had seen.
Figure 1.
Trial timeline for a feedback trial on the reward sensitivity task.
Participants completed a practice block of 20 trials (10 short-mouth, in random order) to ensure that they understood task instructions. In the practice, participants saw the words “correct” or “incorrect” after each trial but received no bonus money. Neither group showed a preference for any stimulus following practice (ps>.43). During the task, participants completed three blocks of 100 trials. Each mouth was presented 50 times per block in pseudorandom order such that there were no more than four consecutive trials of the same mouth. Feedback (“Correct, +5 cents”) appeared on 40 correct responses per block. Of the 40 rewards, 30 were provided to one of the mouths and 10 to the other. The more frequently rewarded mouth (short or long) and the response mappings (left and right buttons) were randomly determined for each participant before the task and remained consistent across all blocks. Reinforcements were pseudo-randomly scheduled such that no more than three trials in a row were reinforced. If participants responded incorrectly on a trial with scheduled reinforcement, the reinforcer was dispensed on the next correct identification of the same stimulus [see 48]. The maximum bonus was $6.
Trials in which participants' reaction times were shorter than 300ms and longer than 3000ms were excluded from analyses. One HC participant was excluded for treating the task as a reaction-time task (>25% of trials faster than 300ms). Across the task, we excluded 0.58% (SD=1.14) trials per HC participant and 4.10% (SD=5.24) trials per SC participant. We also excluded one SC participant who confused the response buttons. Debriefing confirmed that no SC participants and two HC participants were aware of the reinforcement asymmetry.
Probabilistic Decision-Making Task
We based our probabilistic choice task on one developed by Rogers et al [49]. In the task, participants chose between two simultaneously presented gambles involving hypothetical monetary rewards/penalties [for a comparison of actual and hypothetical rewards, see 53]. Each gamble showed the possible reward; the possible loss; and the likelihood of winning the gamble (see Figure 2). The two gambles varied in magnitude (gamble1 randomly varied between $3 and $7; gamble2 between $13 and $17). There were two outcome conditions. In the no-loss condition, losing the chosen gamble was worth $0. In the loss-possible condition, losing a gamble incurred a variable penalty of the same magnitude as the win (gamble1: $3 to $7; gamble2: $13 to $17). A second condition allowed us to determine how uncertainty about an outcome's likelihood affected behavior [54]. In this condition, a mask over gamble2 concealed the probability of winning. Participants completed 12 of each trial type, in random order.
Figure 2. Example of each type of trial on the probabilistic choice task.
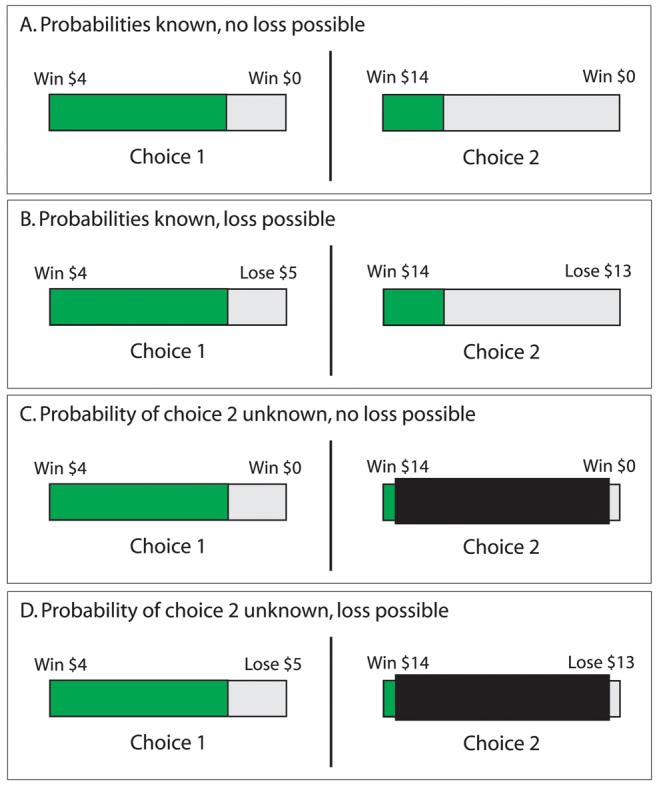
Gamble 1 in each example depicts an 80% chance of winning $4 and a 20% change of losing either $0 or $5. In half the trials, the odds of winning gamble 2 were visible. The examples show a 30% chance of winning $14, versus a 70% chance of losing either $0 (A) or $16 (B). In the remaining trials, the odds of winning gamble 2 were hidden from participants with a mask (C and D). Participants chose gamble 1 with the left button of a game controller and gamble 2 with the right.
At the start of the task, gamble1 had high- and gamble2 low probabilities of winning. To induce participants to choose the riskier gamble2, we altered the probabilities of winning the gambles after each choice using an adaptive procedure. This was implemented such that if a participant chose gamble1, the probability of winning gamble1 on the next trial decreased by 10% and the probability of winning gamble2 increased by 10%. With each choice reversal (e.g., the participant chose gamble1 on trialn-1 and gamble2 on trialn), the magnitude of the change in probability reduced by 20% and the direction of the change reversed (e.g., if the probability of winning gamble2 had been increasing by 10%, it now decreased by 8%). Each trial type adapted independently. We chose an adaptive procedure because these generate reliable estimates of performance in fewer trials than do fixed stimulus sets [55]. Groups did not differ in the number of reversals per condition (MeanHC=4.68 (SD=.74), MeanSC=4.78 (SD=1.26); F(1,62)=.43, p=.88), the mean expected value difference required for a shift from gamble1 to gamble2 (MeanHC=6.65 (SD=.51), MeanSC=6.68 (SD=.63); F(1,61)=.01, p=.94), or the overall proportion of choices to gamble2 (MeanHC=.40 (SD=.08), MeanSC=.42 (SD=.10); F(1,62)=1.13, p=.29), suggesting that they treated the task similarly.
Additional Measures
Participants additionally completed working memory measures [spatial span and letter-number sequencing 56], the Hopkins Verbal Learning Test [HVLT, 57] and the Wechsler Test of Adult Reading [WTAR, 58]. Table 1 displays these scores. Because the working memory measures were highly correlated (r=.53, p<.001), we made a working memory composite score, by normalizing and averaging participants' scores.
Statistical Analysis
To assess task performance and reward sensitivity in the reward sensitivity task, we used a signal-detection theory approach. We indexed performance according to participants' ability to discriminate between the long and short mouths by calculating discrimination accuracy ( d'1) [46]. Participants' tendency to over-report seeing the frequently rewarded stimulus (bias2), served to measure reward sensitivity [47].
In the probabilistic decision-making task, we examined behavior in terms of both objective performance and subjective value. In objective terms, optimal decision-making involves choosing the gamble with the highest expected value. Expected value is the probability of winning multiplied by the amount of a win, minus the probability of losing multiplied by the amount of a loss [59]. We calculated participants' proportion of optimal choices for each gamble.
To examine relative contributions of wins, losses and uncertainty to subjective value, we used a logistic regression model to estimate the degree to which each contributed to choice behavior. This model estimated each participant's conditional probability of choosing gamble2, based on potential outcomes (wins and losses) and the presence of uncertainty (mask over gamble2), using maximum likelihood estimation. This technique allowed us to estimate how strongly each decision component contributed to decision-making. We used the logistic response function:
where PGamble2 is a participant's probability of choosing gamble2 given the linear term θ, which is based on potential outcomes. We estimated θ with the following equation:
where V+1 is the probability of winning gamble1 multiplied by the value of a win; V+2 is the probability of winning gamble2 multiplied by the value of a win; V−1 and V−2 are the probabilities of losing gamble1 and gamble2 respectively, multiplied by the value of that gamble's loss; and U is presence/absence of the mask over gamble2. The estimated βs are participants' subjective weightings of each variable (β1: subjective-value of potential gains; β2: subjective-value of potential losses; β3: subjective-value of uncertainty). All post-hoc comparisons are Bonferroni corrected.
Results
Reward Sensitivity
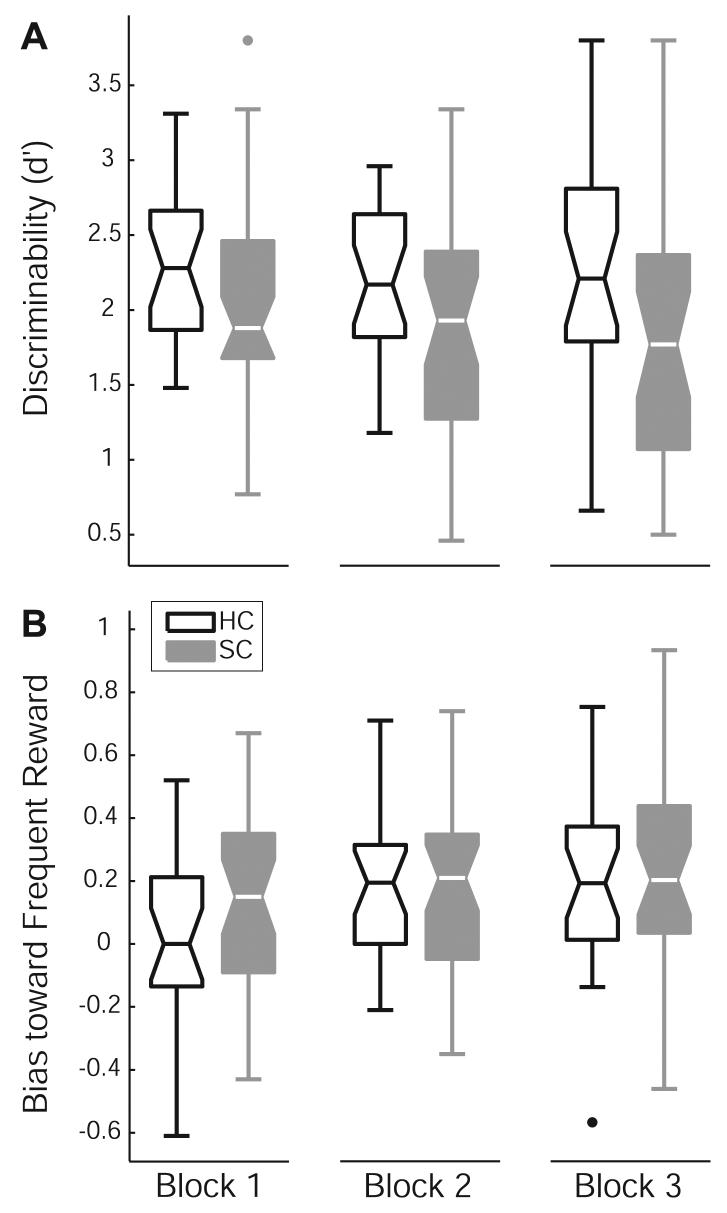
Figure three shows results for the two mixed-model ANOVAs that examined group (HC, SC) differences in measures of discriminability (d') and bias across task blocks (1, 2, 3). SC participants tended to find discriminating between the short and long mouths more difficult than did HC participants (Table 2 shows ANOVA results). Neither the main-effect of block nor the group × block interaction approached significance for d'. However, despite slightly worse performance in the SC group, groups did not differ in the amount of bonus money earned (MeanHC=$5.90, SD=.20; MeanSC=$5.95, SD=.08; t(64)=−1.14, p=.26).
Table 2.
Task Results
| df | F | p-value | Effect Size (η2p) | |||
|---|---|---|---|---|---|---|
| Reward Sensitivity | ||||||
| Bias† | ||||||
| Block (1, 2 or 3) | 2, 55 | 4.08 | .04 | .08 | ||
| Group (HC or SC) | 1, 55 | .15 | .70 | .003 | ||
| Group × Block | 2, 55 | .55 | .46 | .01 | ||
| Discriminability | ||||||
| Block (1, 2 or 3) | 2, 58 | 1.46 | .24 | 03 | ||
| Group (HC or SC) | 1, 58 | 3.68 | .06 | .07 | ||
| Group × Block |
2, 58 |
.71 |
.50 |
.01 |
||
| Probabilistic Choice | ||||||
| Subjective Weightings | ||||||
| Potential Gains (β1) | 1,62 | .37 | .54 | .006 | ||
| Potential Losses (β2) | 1,62 | 7.02 | .01 | .11 | ||
| Uncertainty (β3) | 1,62 | .56 | .46 | .009 | ||
| Proportion of Optimal Choices | ||||||
| Gamble (1 or 2) | 1, 62 | 84.72 | < .001 | .59 | ||
| Group (HC or SC) | 1, 62 | 7.23 | .009 | .13 | ||
| Gamble × Group | 1, 62 | 9.27 | .003 | .11 | ||
Note: Table shows ANOVA results with effect sizes. Bold text indicates significant differences.
Three SC participants did not make errors in one or more task blocks. Bias cannot be calculated in errorless blocks so the missing data excluded these participants from this analysis.
Consistent with hypotheses, we did not find group differences in the development of bias (see Table 2). As Figure 3B illustrates, both groups showed similar development of a reward-seeking response bias, with more pronounced bias in later blocks compared with the first (p-values<.04). Bias did not differ between blocks 2 and 3 (p=.91). Neither the effect of group nor the block × group interaction was significant. These results suggest intact sensitivity to reward in schizophrenia.
Figure 3. Reward sensitivity task results.
Data are shown in Tukey box-plots. The middle line in each box shows the median, the box encloses 50% of the scores and the whiskers show the full range of the data, excluding outliers. Outliers, depicted by dots, are data points that fall more than two standard deviations from the mean [76]. (A) Healthy participants show slightly better ability to discriminate between the two stimuli (85% correct on average) than do participants with schizophrenia (80% correct on average). (B) Both groups show similar development of response bias across the blocks. Note that positive values indicate bias toward the more frequently rewarded stimulus (scores of zero indicate no preference for either stimulus).
To determine how results related to neurocognitive measures, we examined correlations between cognitive measures, bias and d' (each averaged across task blocks). There was no relationship between bias and any neurocognitive measure in either group (all ps >.16). However, in both groups, d' was significantly related to working memory (HC: r=.49, p=.01; SC: r=.47, p=.005). Among SC participants, d' did not relate to other variables (all ps >.19). Among HC participants, d' related to HVLT (r=.45, p=.03) and WTAR (r=.51, p=.01). These results suggest that cognitive ability, particularly working memory, relates to task performance but is independent of reward sensitivity.
Probabilistic Decision-Making
The probabilistic decision-making task allowed us to estimate participants' subjective weightings of several decision components (potential gains and losses, and uncertainty) based on their decision-making behavior. We subjected our estimates of participants' decision-component weightings (the βs from our logistic regression model) to multivariate ANOVA. As figure 4A shows, groups did not differ in the degree to which they weighted potential gains or uncertainty in their decisions (see Table 2). However, SC participants gave potential losses significantly less weight than did HC participants. That is, the possibility of losing had less influence on SC participants' choices than on HC participants' choices.
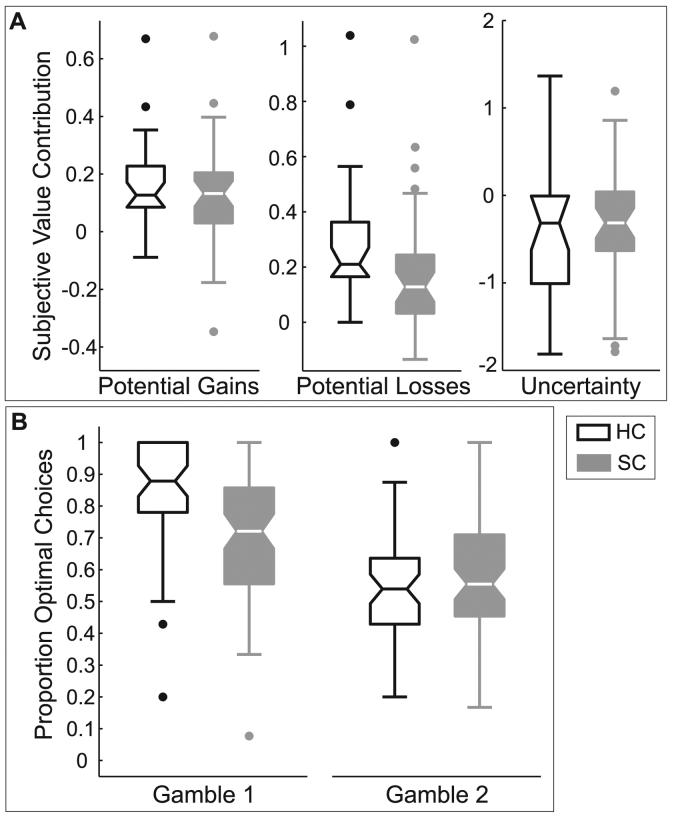
Figure 4. Probabilistic decision-making task results.
Data are shown in Tukey box-plots. The middle line in each box shows the median, the box encloses 50% of the scores and the whiskers show the full range of the data, excluding outliers. Outliers, depicted by dots, are data points that fall more than two standard deviations from the mean [76]. (A) SC Participants weighted potential gains and uncertainty similarly to HC participants when deciding between two uncertain outcomes. However, SC participants gave potential losses significantly less weight when making decisions than do HC participants. (B) HC participants made more optimal choices overall, although the gamble × group interaction shows that HC participants made more optimal choices of gamble1 than did SC participants, but groups did not differ on gamble2 choices.
Recent economic models of decision-making suggest that people's subjective weightings of decision-components influence propensity to choose optimally [33,60]. We therefore examined optimal decision-making with a mixed-model ANOVA (group [HC, SC] × gamble [1, 2]). HC participants made more optimal choices overall (see Table 2 for results). There was a significant main effect of gamble showing that all participants made more optimal gamble1 than gamble2 choices (see Figure 4B). There was also a significant group × gamble interaction such that when gamble1 was optimal, HC participants chose it more frequently but when the higher-stakes gamble2 was optimal, groups chose it with similar frequency. To understand this interaction, we examined participants' cross-gamble difference in optimal choices (optimal gamble2 minus optimal gamble1 choices). Relative to gamble1 behavior, HC participants reduced their optimal gamble2 choices to a greater degree than did SC participants (MeanHC=−.29, SD=.25; MeanSC=−.16, SD=.24; t(64)=−2.08, p=.04), suggesting that gamble2 was subjectively worse for HC participants.
To understand whether subjective weightings of potential outcomes explained group differences in optimal decision-making, we conducted a hierarchical multiple regression with participants' total optimal choices as the criterion variable. We entered participants' subjective weightings of potential gains, losses and uncertainty at step one of the model. Subjective weightings explained significant variance in optimal choice behavior (ΔR2=.35, p<.001). Examination of the regression coefficients showed that subjective weightings of gains (p=.01) and losses (p=.02) related to optimal choice behavior but weighting of uncertainty did not (p=.14). The entry of group (HC, SC) at step two of the model did not significantly account for variance over and above participants' subjective weightings (ΔR2=.03, p=.18). This finding suggests that subjective weightings of decision components relates to how participants choose among competing options.
We have suggested that cognitive impairments might affect the ability to formulate subjective value. We examined this idea using hierarchical multiple regression with average weighting of potential gains and losses (the decision components related to optimal choice) as the criterion variable. At step one of the model, we entered the cognitive measures (working memory, HVLT and WTAR). These accounted for a significant portion of the variance in participants' weightings of potential outcomes (ΔR2=.27, p<.001). The regression coefficients at this step showed that working memory made a significant contribution to the model (p=.01) but neither HVLT (p=.16) nor WTAR (p=.75) did so. We entered group (HC, SC) at step two in the model. Working memory entirely accounted for group differences in participants' subjective valuations of potential outcomes (ΔR2=.02, p=.28).
Symptom correlations
In the SC group, we explored the relationships between symptom ratings and task measures (bias, d', subjective weighting of potential outcomes). D' showed a non-significant, inverse relationship with SANS total (r=−.33, p=.06). No other relationships emerged (all p-values >.19).
Discussion
Consistent with expectations, patients with schizophrenia showed normal sensitivity to rewarding stimuli on a task in which rewards implicitly biased behavior. They also showed altered decision-making relative to healthy participants and relatively worse ability to use subjective valuations of potential outcomes, particularly losses, in their decisions. Deficits in working memory appeared to account for this alteration in subjective valuation. These findings suggest two important ideas. First, when assessed using implicit learning measures, the experience of reward and ability to learn from reinforcement are surprisingly intact in schizophrenia. The fact that patients developed a reward-seeking response bias is evidence of this idea. Second, that degraded working memory in schizophrenia compromises the ability to weigh potential outcomes effectively during decision-making, which in turn limits decision quality. This idea is in keeping with a recent report showing that limbic activity may modulate working memory capacity [61]. Together, these ideas have implications for understanding the functional impairments associated with schizophrenia.
A longstanding explanation for the motivational and behavioral deficits characteristic of schizophrenia is that the illness dampens the experience of reward [62,63]. The present finding, that patients developed normal bias toward a frequently rewarded stimulus, adds to a growing body of literature suggesting spared response to pleasurable stimuli in schizophrenia [10,12,13]. Moreover, the magnitude of the bias our participants showed is similar to that of Pizagalli et al's [48] control participants, suggesting that this result cannot simply be explained by atypical task performance in our sample. These results demonstrate that patients are sensitive to reward contingencies and can modify responses based on reinforcement, despite absence of explicit awareness of reward contingencies. This implies that reward-based implicit learning systems are largely functional in schizophrenia.
In contrast, and consistent with previous reports [30,31,64], patients showed a pattern of altered decision-making that suggests they have difficulty using affective information to guide decision-making. Specifically, patients had more difficulty than did healthy participants choosing a low-risk gamble optimally, although groups did not differ in optimal choices to a high-risk gamble. At first glance, this finding implies a riskier choice strategy among patients [26,27,29], however, our analysis of participants' relative weightings of decision components suggests otherwise. We found no group difference in participants' valuations of potential gains or uncertainty, suggesting that patients are neither overly sensitive to gains nor insensitive to outcome likelihood. Instead, patients gave potential losses less weight in their decisions than did healthy participants, suggesting that “risky” decision-making in schizophrenia relates to the relative undervaluation of potential losses. Working memory significantly related to participants' ability to weigh both types of potential outcomes in their decisions.
Together, results from both tasks suggest an interesting picture of the decision-making deficits so prominent in schizophrenia. To understand this, we examine differences in the tasks themselves, which are important in understanding the relationship of affect and cognition in decision-making. On each trial of the reward sensitivity task, we asked participants to decide which of two stimuli they had seen, implicitly shaping their preference for one stimulus with a series of small reinforcers. On each trial of the decision-making task, participants decided which of two choices they preferred by integrating cognitive (objective value) and affective information (subjective value). Discrimination accuracy in the reward sensitivity task related to working memory, although implicit preference for the frequently rewarded stimulus did not. Conversely, on the probabilistic decision task, working memory was an important determinant of participants' explicit preferences, as evidenced by robust correlations between working memory and task performance. Results suggest that patients are sensitive to immediately present reinforcers but have difficulty formulating preferences based on potential outcomes and/or incorporating these into their choices. In essence, patients have difficulty integrating an affective preference into a cognitive representation that supports optimal decision-making.
In terms of the functional impairments associated with schizophrenia, results imply that deficits in motivated behavior may arise as rewards become more temporally remote [31] or require integration of cognition and affect [28,45,65]. Whereas one might know that working brings a paycheck and exercise improves health, the gap between knowledge and motivation might simply be too large to bridge. Conversely, when rewards are immediately and consistently present in the environment, behavioral deficits may diminish [14,16,66]. Indeed, evidence from token economy studies suggests that making rewards salient aspects of the environment ameliorates some of the motivational deficits associated with schizophrenia [67-69]. Taken together, this implies that consistent and tangible reinforcements may shape motivated behavior in a way that more complex or temporally remote rewards lack the power to do.
This research has several limitations. First, although we found group differences in subjective weightings of potential losses, we did not directly test responses to experienced punishments. Although previous findings show that patients report normal experiences of unpleasant stimuli [7,10,13], punishments may have less salience [23], thereby diminishing their subjective weightings. Second, the external validity of these tasks is unknown. Laboratory tasks are only a proxy for real-world reward sensitivity and decision-making. Moreover, although previous research suggests that participants respond similarly to real and hypothetical rewards [53], our use of hypothetical rewards in the decision-making task might have caused participants to behave differently than if they had played for real prizes. Third, our sample includes more males than females and it might be the case that females treat affective stimuli differently [70-74], although this is not necessarily the case in reward-related decision-making [75]. Finally, all our patients received antipsychotic therapy. Although our results likely generalize to the average treated outpatient with schizophrenia, we do not know whether results would be similar in unmedicated or medication naïve populations.
Conclusions
The present findings demonstrate altered decision-making in the context of intact reward sensitivity in schizophrenia. Patients' decision-making alterations related to their ability to formulate subjective preferences, which itself related to working-memory. These results suggest that implicit learning systems, which rely on long-term reinforcement history, may be intact in schizophrenia but that cognitive deficits impinge on the ability to utilize affective experience in decision-making. Thus, making rewards immediate and salient aspects of the environment may partly mediate the deficits in motivated behavior that characterize schizophrenia.
Acknowledgments
We wish to thank Sharon August, Rebecca Wilbur and Maria Wydra for their invaluable assistance with data collection. A grant (to JMG) from the National Institutes of Mental Health (# 1 R24 MH72647-01A1) made this work possible.
Footnotes
Financial Disclosures
We have no conflicts of interest, biomedical, financial or otherwise, to declare.
Formula for d': d'=ZCorrectFRS_IDs−ZIncorrectFRS_IDs (where ZCorrectFRS_IDs is the z-transformed probability of correctly identifying the frequently rewarded stimulus [FRS] and ZIncorrectFRS_IDs is the z-transformed probability of incorrectly identifying the FRS, meaning that participants erroneously responded as though the FRS was present; see [47].
References
- 1.Dickinson D, Bellack AS, Gold JM. Social/Communication Skills, Cognition, and Vocational Functioning in Schizophrenia. Schizophr Bull. 2006 doi: 10.1093/schbul/sbl067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velligan DI, Kern RS, Gold JM. Cognitive rehabilitation for schizophrenia and the putative role of motivation and expectancies. Schizophr Bull. 2006;32(3):474–85. doi: 10.1093/schbul/sbj071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaker G, Adami H, Gold J. Functional deterioration in individuals with schizophrenia spectrum personality symptoms. J Personal Disord. 2001;15(3):229–34. doi: 10.1521/pedi.15.3.229.19207. [DOI] [PubMed] [Google Scholar]
- 4.Bleuler E. Monograph Series on Schizophrenia. Vol. 1. International Universities Press; New York, NY: 1950. Dementia Praecox. [Google Scholar]
- 5.McGurk SR, Meltzer HY. The role of cognition in vocational functioning in schizophrenia. Schizophr Res. 2000;45(3):175–84. doi: 10.1016/s0920-9964(99)00198-x. [DOI] [PubMed] [Google Scholar]
- 6.Gold JM, et al. Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry. 2002;159(8):1395–402. doi: 10.1176/appi.ajp.159.8.1395. [DOI] [PubMed] [Google Scholar]
- 7.Earnst KS, Kring AM. Emotional responding in deficit and non-deficit schizophrenia. Psychiatry Res. 1999;88(3):191–207. doi: 10.1016/s0165-1781(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 8.Curtis CE, et al. Acoustic startle reflex in schizophrenia patients and their first-degree relatives: evidence of normal emotional modulation. Psychophysiology. 1999;36(4):469–75. doi: 10.1017/s0048577299980757. [DOI] [PubMed] [Google Scholar]
- 9.Kring AM, Neale JM. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? J Abnorm Psychol. 1996;105(2):249–57. doi: 10.1037//0021-843x.105.2.249. [DOI] [PubMed] [Google Scholar]
- 10.Kring AM, et al. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. J Abnorm Psychol. 1993;102(4):507–17. doi: 10.1037//0021-843x.102.4.507. [DOI] [PubMed] [Google Scholar]
- 11.Myin-Germeys I, Delespaul PA, deVries MW. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr Bull. 2000;26(4):847–54. doi: 10.1093/oxfordjournals.schbul.a033499. [DOI] [PubMed] [Google Scholar]
- 12.Aghevli MA, Blanchard JJ, Horan WP. The expression and experience of emotion in schizophrenia: a study of social interactions. Psychiatry Res. 2003;119(3):261–70. doi: 10.1016/s0165-1781(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 13.Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. 2007;116(2):268–78. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- 14.Weickert TW, et al. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learn Mem. 2002;9(6):430–42. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takano K, et al. Procedural memory in schizophrenia assessed using a mirror reading task. Psychiatry Res. 2002;109(3):303–7. doi: 10.1016/s0165-1781(02)00021-5. [DOI] [PubMed] [Google Scholar]
- 16.Waltz JA, et al. Selective Reinforcement Learning Deficits in Schizophrenia Support Predictions from Computational Models of Striatal-Cortical Dysfunction. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Exner C, et al. State-dependent implicit learning deficit in schizophrenia: evidence from 20-month follow-up. Psychiatry Res. 2006;142(1):39–52. doi: 10.1016/j.psychres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Bigelow NO, et al. Prism adaptation in schizophrenia. Brain Cogn. 2006;61(3):235–42. doi: 10.1016/j.bandc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Polgar P, et al. How to find the way out from four rooms? The learning of “chaining” associations may shed light on the neuropsychology of the deficit syndrome of schizophrenia. Schizophr Res. 2007 doi: 10.1016/j.schres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, et al. The relationship between regional cerebral blood flow and the Wisconsin Card Sorting Test in negative schizophrenia. Psychiatry Clin Neurosci. 2002;56(1):3–7. doi: 10.1046/j.1440-1819.2002.00924.x. [DOI] [PubMed] [Google Scholar]
- 21.Stratta P, et al. Processing of context information in schizophrenia: relation to clinical symptoms and WCST performance. Schizophr Res. 2000;44(1):57–67. doi: 10.1016/s0920-9964(99)00142-5. [DOI] [PubMed] [Google Scholar]
- 22.Hellman SG, et al. Monetary reinforcement and Wisconsin Card Sorting performance in schizophrenia: why show me the money? Schizophr Res. 1998;34(12):67–75. doi: 10.1016/s0920-9964(98)00088-7. [DOI] [PubMed] [Google Scholar]
- 23.Prentice KJ, Gold JM, Buchanan RW. The Wisconsin Card Sorting impairment in schizophrenia is evident in the first four trials. Schizophrenia Research. doi: 10.1016/j.schres.2007.07.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans CE, Bowman CH, Turnbull OH. Subjective awareness on the Iowa Gambling Task: the key role of emotional experience in schizophrenia. J Clin Exp Neuropsychol. 2005;27(6):656–64. doi: 10.1081/13803390490918354. [DOI] [PubMed] [Google Scholar]
- 25.Turnbull OH, et al. A novel set-shifting modification of the iowa gambling task: flexible emotion-based learning in schizophrenia. Neuropsychology. 2006;20(3):290–8. doi: 10.1037/0894-4105.20.3.290. [DOI] [PubMed] [Google Scholar]
- 26.Prentice KJ, Gold JM, Carpenter WT., Jr. Optimistic bias in the perception of personal risk: patterns in schizophrenia. Am J Psychiatry. 2005;162(3):507–12. doi: 10.1176/appi.ajp.162.3.507. [DOI] [PubMed] [Google Scholar]
- 27.Kester HM, et al. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophr Res. 2006;85(13):113–23. doi: 10.1016/j.schres.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, et al. Dissociation of emotional decision-making from cognitive decision-making in chronic schizophrenia. Psychiatry Res. 2007;152(23):113–20. doi: 10.1016/j.psychres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Shurman B, Horan WP, Nuechterlein KH. Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr Res. 2005;72(23):215–24. doi: 10.1016/j.schres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Hutton SB, et al. Decision making deficits in patients with first-episode and chronic schizophrenia. Schizophr Res. 2002;55(3):249–57. doi: 10.1016/s0920-9964(01)00216-x. [DOI] [PubMed] [Google Scholar]
- 31.Heerey EA, et al. Delay discounting in schizophrenia. Cognit Neuropsychiatry. 2007;12(3):213–21. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: How much and how little we know. Schizophrenia Bulletin. 2005;31(4):875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- 33.Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Brain Res Cogn Brain Res. 2005;23(1):34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304(5678):1782–7. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- 35.Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211(4481):453–8. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- 36.Li J, et al. Policy adjustment in a dynamic economic game. PLoS ONE. 2006;1:e103. doi: 10.1371/journal.pone.0000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorris MC, Glimcher PW. Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron. 2004;44(2):365–78. doi: 10.1016/j.neuron.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–6. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers RD, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. 1999;19(20):9029–38. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalenscher T, et al. Single units in the pigeon brain integrate reward amount and time-to-reward in an impulsive choice task. Curr Biol. 2005;15(7):594–602. doi: 10.1016/j.cub.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 41.Ernst M, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 42.Rogers RD, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55(6):594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 43.McClure SM, et al. Time discounting for primary rewards. J Neurosci. 2007;27(21):5796–804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClure SM, et al. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 45.Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol. 2007;116(1):30–42. doi: 10.1037/0021-843X.116.1.30. [DOI] [PubMed] [Google Scholar]
- 46.Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley; New York, NY: 1966. [Google Scholar]
- 47.Wickens TD. Elementary Signal Detection Theory. Oxford University Press; New York, NY: 2002. [Google Scholar]
- 48.Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–27. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers RD, et al. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28(1):153–62. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- 50.First MB, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen. Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- 51.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:790–812. [Google Scholar]
- 52.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;(7):49–58. [PubMed] [Google Scholar]
- 53.Madden GJ, et al. Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol. 2003;11(2):139–45. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- 54.George S, Rogers RD, Duka T. The acute effect of alcohol on decision making in social drinkers. Psychopharmacology (Berl) 2005;182(1):160–9. doi: 10.1007/s00213-005-0057-9. [DOI] [PubMed] [Google Scholar]
- 55.Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys. 2001;63(8):1279–92. doi: 10.3758/bf03194543. [DOI] [PubMed] [Google Scholar]
- 56.Wechsler D. Wechsler Memory Scale-Third Edition. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 57.Shapiro AM, et al. Construct and concurrent validity of the Hopkins Verbal Learning Test - revised. Clin Neuropsychology. 1999;13(3):348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 58.Wechsler D. Wechsler Test of Adult Reading. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 59.Rolls ET, McCabe C, Redoute J. Expected Value, Reward Outcome, and Temporal Difference Error Representations in a Probabilistic Decision Task. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm097. [DOI] [PubMed] [Google Scholar]
- 60.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11(1):103–7. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt K, et al. Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry. 2001;34(2):66–72. doi: 10.1055/s-2001-15184. [DOI] [PubMed] [Google Scholar]
- 63.Juckel G, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–16. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 64.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93(13):296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herbener ES, et al. What aspects of emotional functioning are impaired in schizophrenia? Schizophr Res. 2007 doi: 10.1016/j.schres.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Juckel G, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;187(2):222–8. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- 67.Dickerson FB, Tenhula WN, Green-Paden LD. The token economy for schizophrenia: review of the literature and recommendations for future research. Schizophr Res. 2005;75(23):405–16. doi: 10.1016/j.schres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 68.Sigmon SC, Higgins ST. Voucher-based contingent reinforcement of marijuana abstinence among individuals with serious mental illness. J Subst Abuse Treat. 2006;30(4):291–5. doi: 10.1016/j.jsat.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Roder V, et al. Improving recreational, residential, and vocational outcomes for patients with schizophrenia. Psychiatr Serv. 2001;52(11):1439–41. doi: 10.1176/appi.ps.52.11.1439. [DOI] [PubMed] [Google Scholar]
- 70.Leon-Carrion J, et al. Differential time course and intensity of PFC activation for men and women in response to emotional stimuli: a functional near-infrared spectroscopy (fNIRS) study. Neurosci Lett. 2006;403(12):90–5. doi: 10.1016/j.neulet.2006.04.050. [DOI] [PubMed] [Google Scholar]
- 71.Orozco S, Ehlers CL. Gender differences in electrophysiological responses to facial stimuli. Biol Psychiatry. 1998;44(4):281–9. doi: 10.1016/s0006-3223(97)00487-3. [DOI] [PubMed] [Google Scholar]
- 72.Sabatinelli D, et al. Affective picture perception: gender differences in visual cortex? Neuroreport. 2004;15(7):1109–12. doi: 10.1097/00001756-200405190-00005. [DOI] [PubMed] [Google Scholar]
- 73.Wild B, Erb M, Bartels M. Are emotions contagious? Evoked emotions while viewing emotionally expressive faces: quality, quantity, time course and gender differences. Psychiatry Res. 2001;102(2):109–24. doi: 10.1016/s0165-1781(01)00225-6. [DOI] [PubMed] [Google Scholar]
- 74.Wrase J, et al. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2003;348(1):41–5. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]
- 75.Epstein LH, et al. Comparison between two measures of delay discounting in smokers. Exp Clin Psychopharmacol. 2003;11(2):131–8. doi: 10.1037/1064-1297.11.2.131. [DOI] [PubMed] [Google Scholar]
- 76.Tukey JW. Exploratory Data Analysis. Addison-Wesley; Reading, MA: 1977. [Google Scholar]