Abstract
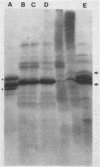
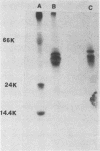
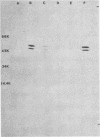
Sodium dodecyl sulfate-urea polyacrylamide gel electrophoresis of outer membranes from a flagellated and an isogenic nonflagellated strain of Vibrio cholerae (classical, Inaba) suggested that two proteins were absent from the nonflagellated strain. Immunoblot examination of such preparations demonstrated that two proteins, present only in outer membrane from the flagellated strain, were associated with flagella. Analysis of purified flagellar cores from strains CA401 and N16961 (El Tor, Inaba) by electron microscopy, sodium dodecyl sulfate-urea polyacrylamide gel electrophoresis, and immunoblotting showed that these two proteins, with apparent molecular weights of 47,000 and 49,000, composed the flagellar core. Antiserum specific for flagellar core proteins did not agglutinate or inhibit the motility of intact V. cholerae. These latter findings suggested that, for intact cells, the flagellar core proteins are not accessible to antibody.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983 May;147(5):864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- Baselski V. S., Medina R. A., Parker C. D. In vivo and in vitro characterization of virulence-deficient mutants of Vibrio cholerae. Infect Immun. 1979 Apr;24(1):111–116. doi: 10.1128/iai.24.1.111-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselski V. S., Upchurch S., Parker C. D. Isolation and phenotypic characterization of virulence-deficient mutants of Vibrio cholerae. Infect Immun. 1978 Oct;22(1):181–188. doi: 10.1128/iai.22.1.181-188.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubanks E. R., Guentzel M. N., Berry L. J. Evaluation of surface components of Vibrio cholerae as protective immunogens. Infect Immun. 1977 Feb;15(2):533–538. doi: 10.1128/iai.15.2.533-538.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLLETT E. A., GORDON J. AN ELECTRON MICROSCOPE STUDY OF VIBRIO FLAGELLA. J Gen Microbiol. 1963 Aug;32:235–239. doi: 10.1099/00221287-32-2-235. [DOI] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER E. W., LYLES S. T., LANKFORD C. E., HAGENS S. J. Vibrio cholerae infection in the embryonated egg. J Infect Dis. 1963 May-Jun;112:264–272. doi: 10.1093/infdis/112.3.264. [DOI] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hranitzky K. W., Mulholland A., Larson A. D., Eubanks E. R., Hart L. T. Characterization of a flagellar sheath protein of Vibrio cholerae. Infect Immun. 1980 Feb;27(2):597–603. doi: 10.1128/iai.27.2.597-603.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Resnick I. G., Ford C. W., Shackleford G. M., Berry L. J. Improved protection against cholera in adult rabbits with a combined flagellar-toxoid vaccine. Infect Immun. 1980 Nov;30(2):375–380. doi: 10.1128/iai.30.2.375-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R., Raska I., Mayer F. Plain and complex flagella of Pseudomonas rhodos: analysis of fine structure and composition. J Bacteriol. 1974 Feb;117(2):844–857. doi: 10.1128/jb.117.2.844-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Willis D. L., Berry L. J. Flagella-induced immunity against experimental cholera in adult rabbits. Infect Immun. 1979 Jul;25(1):220–228. doi: 10.1128/iai.25.1.220-228.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Willis D. L., Berry L. J. Role of motility in experimental cholera in adult rabbits. Infect Immun. 1978 Nov;22(2):387–392. doi: 10.1128/iai.22.2.387-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G. C., Schrank G. D., Freeman B. A. Purification of flagellar cores of Vibrio cholerae. J Bacteriol. 1977 Feb;129(2):1121–1128. doi: 10.1128/jb.129.2.1121-1128.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]