Summary
Sympathetic nervous system development depends upon many factors that mediate neuron migration, differentiation and survival. Target tissue-derived nerve growth factor (NGF) signaling-induced gene expression is required for survival, differentiation and target tissue innervation of post-migratory sympathetic neurons. However, the transcriptional regulatory mechanisms mediated by NGF signaling are very poorly defined. Here, we identify Egr3, a member of the early growth response (Egr) family of transcriptional regulators, to have an important role in sympathetic nervous system development. Egr3 is regulated by NGF signaling and it is expressed in sympathetic neurons during development when they depend upon NGF for survival and target tissue innervation. Egr3-deficient mice have severe sympathetic target tissue innervation abnormalities and profound physiologic dysautonomia. Unlike NGF which is essential for sympathetic neuron survival and axon branching within target tissues, Egr3 is required for normal terminal axon extension and branching, but not neuron survival. The results indicate that Egr3 is a novel NGF signaling effector which regulates sympathetic neuron gene expression required for normal target tissue innervation and function. Egr3-deficient mice have a phenotype that is remarkably similar to humans with sympathetic nervous system disease, raising the possibility that it may have a role in some forms of human dysautonomia, most of which have no known cause.
Keywords: Egr3, development, sympathetic, ptosis, neurotrophin, physiology
Introduction
The sympathetic nervous system (SNS) is a division of the autonomic nervous system with an important role in maintaining organ and tissue homeostasis. A detailed understanding of the factors involved in establishing and maintaining the stability of sympathetic target tissue innervation is of considerable importance because the SNS is the target of a wide variety of debilitating developmental and degenerative diseases in many vertebrate species, including humans. Many molecules are known to have important roles in sympathoadrenal precursor migration, phenotype specification and target tissue innervation during development. For example, migration of sympathoadrenal precursors from the neural crest is dependent upon signaling through ErbB2, ErbB3 (Britsch et al., 1998) and Ret (Enomoto et al., 2001) tyrosine kinase receptors as well as guidance molecules such as Semaphorin3a (Kawasaki et al., 2002). In addition, specification of the noradrenergic phenotype of post-migratory sympathoadrenal precursors depends upon diffusible factors such as bone morphogenetic proteins (BMPs) and transcriptional regulators such as Mash1, Phox2a and b, dHand and GATA3 (for review see, (Goridis and Rohrer, 2002; Howard, 2005)). To establish their connections in the periphery, sympathetic neuroblasts also require several diffusible factors such as hepatocyte growth factor (HGF) (Maina et al., 1998), Artemin (Honma et al., 2002), neurotrophin-3 (NT-3) (Francis et al., 1999) and nerve growth factor (NGF) (Glebova and Ginty, 2004) that promote their survival, axon extension along blood vessels and target tissue innervation during development.
Of the diffusible factors known to be involved in establishing sympathetic neuron connections with peripheral target tissues, NGF has emerged as the most important. Its role in sympathetic neuron survival and differentiation has been known for many decades (Levi-Montalcini and Cohen, 1960), but more recently, a particularly important role in terminal axon extension and branching during target tissue innervation has been identified (Glebova and Ginty, 2004). NGF may act locally to facilitate axon extension and target tissue innervation by directly regulating neurofilament protein stabilization (Veeranna et al., 1998), but it also alters gene expression in sympathetic neurons which facilitates their survival and axon outgrowth (Milbrandt, 1987; Riccio et al., 1997). However, specific transcriptional regulators that control NGF-dependent gene expression during SNS development have not been well defined.
Early growth response-1 (Egr1) protein is among a relatively small number of transcriptional regulators induced by NGF signaling in sympathetic neurons (Milbrandt, 1987). Disruption of Egr mediated gene transcription using either a dominant negative molecule (Levkovitz et al., 2001) or the Egr co-repressor molecule Nab2 (Qu et al., 1998) inhibits NGF-mediated neurite outgrowth and differentiation in sympathetic neuron-like PC12 cells. Similarly, antisense oligonucleotide knockdown of Egr1 protein translation inhibits neurite outgrowth, whereas over expression of Egr1 enhances neurite outgrowth in N2A neuroblastoma cells (Pignatelli et al., 1999). Thus, it is surprising that Egr1-deficient mice develop normally and have no apparent SNS abnormalities ((Lee et al., 1995) and unpublished observations). However, considering that Egr3 and Egr4, two closely related transcriptional regulators, have been shown to functionally cooperate with Egr1 to regulate some target genes such as luteinizing hormone β-peptide (LHβ), the neuroplasticity associated protein Arc, and the low-affinity neurotrophin receptor p75NTR (Gao et al., 2007; Li et al., 2005; Tourtellotte et al., 2000), it seemed plausible that other Egr proteins may have important roles in sympathetic neuron differentiation in vivo.
Here, we identify Egr3 to have an unexpected but important role in SNS development. Egr3-deficient mice exhibit sympathetic neuron loss, target tissue innervation defects and profound dysautonomia. Egr3 expression is induced by NGF signaling and it is up-regulated in sympathetic neurons during a developmental period when NGF signaling is required for normal sympathetic neuron survival and target tissue innervation in vivo. Thus, Egr3 appears to be a physiologically important effector of NGF signaling with an essential role in sympathetic neuron target tissue innervation and terminal axon branching.
MATERIALS AND METHODS
Animals
Egr3-deficient mice were genotyped as previously described (Tourtellotte et al., 2001). Perinatal and adult (age 12–35 weeks) mice were used as indicated. Transgenic DβH-τlacZ (DτlZ) reporter mice were generated by cloning the τlacZ cDNA (Callahan and Thomas, 1994) into the BamHI site of the previously described 2949A12 DβH promoter plasmid (Hoyle et al., 1993). F1 progeny from transgenic founder mice were screened to establish a single reporter line with τlacz expression in >99% of sympathetic neurons and their axons (Fig. S3 and data not shown). DτlZ transgenic reporter mice were genotyped by PCR using primers: 5′-GATTCCCCGCTAGACAAATGTGA-3′ and 5′-CATGTCACCCTCCTGGTCTT-3′. All experimental procedures complied with protocols approved by The Northwestern University Institutional Animal Care and Use Committee.
Tissue preparation
Embryos from timed pregnant females were isolated by cesarean section. Postnatal mice were perfused through the heart with 0.1M phosphate buffered 4% paraformaldehyde (PFA, pH=7.2) and embryos were immersion fixed. Tissues were either processed for paraffin embedding or cryoprotected overnight in graded (15–30%) phosphate-buffered sucrose and embedded in OCT. Serial paraffin tissue sections (16 μm) or frozen sections (12–16 μm) were analyzed.
Ganglion neuron counts and volume estimation
SCG neuron numbers were determined using unbiased stereology and optical dissector methods (StereoInvestigator, Microbrightfield) on every fifth serial section from various developmental ages as previously described (Albert et al., 2005). The total number of neurons per SCG was estimated using an optical fractionator probe and ganglionic volume measurements were estimated using the planimetry function of the StereoInvestigator software.
NGF neutralization
Newborn Swiss Webster mice were injected with PBS (N=5) or antibody (N=5; 50 mg/kg, i.p., mouse anti-NGF, clone AS-18, Exalpha Biologicals) which has been previously well-characterized for its specificity and NGF neutralizing effects (Wild et al., 2007). SCG were isolated 14 hours after injection and subjected to qPCR.
Primary SCG neuron cultures
For SCG neuron cultures, E19 or P0 Swiss Webster (Charles River Laboratories) mice or E18.5 Egr3+/+ and littermate Egr3−/− mice were used. SCG neurons were dissociated in 1mg/mL Type IV Collagenase (Worthington Biochemical Corporation), followed by 0.25% Trypsin-EDTA and plated on collagen-coated 35mm dishes in Minimal Essential Media (MEM) containing 10% Fetal Bovine Serum (FBS), 1% penicillin/streptomycin, and 100 ng/mL of NGF. For signaling studies, neurons were differentiated in the presence of 100 ng/mL of NGF for 7 days, deprived of NGF for 3 hours and then re-stimulated with either vehicle (control), NGF (100 ng/mL), NT-4 (30 ng/mL) or BDNF (30 ng/mL) for 45 minutes. In some wells, DMSO (control) or the MAP Kinase Kinase (MEK) inhibitor U0126 (20 μM, Promega) were added to the cultures.
Immunohistochemistry and Western Blotting
Immunohistochemistry for tyrosine hydroxylase (TH; Chemicon) or β-galactosidase (βgal; ICN Pharmaceuticals) was performed on frozen tissue sections to identify sympathetic axons. For proliferation and apoptosis assays, tissue sections were incubated with cleaved caspase-3 antibody (Cell Signaling) to identify apoptotic neurons or anti-5-bromo-2-deoxyuridine (BrdU) antibody (Sigma) to identify proliferating cells. Species appropriate Alexa488-conjugated (Invitrogen) or Cy3-conjugated (Jackson Immunoresearch) secondary antibodies were used to visualize the proteins. Western blotting was performed as described in detail (Li et al., 2005) using the following antibodies: anti-Egr3 (sc-191), anti-ERK1/2 (sc-94), anti-phosphorylated ERK1/2 (sc-16982) and anti-actin (sc-1616) (all from Santa Cruz Biotechnology). The antibodies were detected using species appropriate horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) and SuperSignal West Pico chemiluminescent substrate (Pierce).
LacZ enzyme histochemistry
Tissues were dissected and postfixed in 2% PFA, 0.2% glutaraldehyde, 5mM EGTA, 0.01% NP-40 in PBS-Mg at 4°C and reacted for 6–12 hours at 37°C in reaction buffer (1mg/mL X-gal, 5mM potassium ferrocyanide, 5mM potassium ferricyanide). After reaction, the tissues were postfixed/dehydrated in Methanol, and cleared in 2:1 benzyl benzoate:benzyl alcohol.
In situ hybridization
In situ hybridization was performed on frozen sections using digoxygenin-labeled anti-sense and sense riboprobes for Egr3 (GenBank NM018781, nt 345-746) as described previously in detail (Albert et al., 2005).
Apoptosis and proliferation analysis
Newborn (P0) pups received injections with 50mg/kg, i.p. BrdU (Sigma) and euthanized after 2 hours. BrdU and caspase-3 were detected by immunohistochemistry and the number of immunopositive cells in every fifth section from E15, E16, E17, E18, or P0 mice were quantified using unbiased stereology and optical fractionator methods (StereoInvestigator, Microbrightfield, Williston, VT). The data were reported as caspase-3+ or BrdU+ neurons per unit volume of sampled ganglion.
qPCR
Total RNA was isolated from cultured sympathetic neurons or whole SCG using Trizol extraction (Invitrogen). Reverse transcription and qPCR was performed as previously described in detail (Albert et al., 2005). The primer sequences used for expression analysis are available upon request.
AANAT expression analysis and light cycling
Adult wild type and Egr3−/− mice were housed in a controlled lighting environment (10 hours of dark, 14 hours of light [10D:14L]) for at least three weeks prior to analysis. ZT0 was defined as the time of dark-light transition. Some animals were sacrificed in the dark during the dark phase of the light-dark cycle and pineal glands were rapidly dissected. Gene expression was determined by qPCR and compared at three time points (ZT9, ZT13 and ZT21) between wild type and Egr3−/− mice.
Cardiac physiology
Male and female adult wild type and Egr3−/− mice, weighing 18–23g, were used. Pressure measurements from a 1.4 French micromanometer-tipped Millar pressure transducer (SPR839, Millar Instruments) were calibrated against a mercury manometer. The right jugular vein of anesthetized mice was cannulated for fluid administration and the pressure catheter was inserted into the right carotid artery and advanced into the left ventricle of the heart. Heart rate and myocardial contractility (change in pressure over time; dP/dt) measurements were compared before and after injection of the α2-adrenoreceptor antagonist, Yohimbine (YOH; 2 mg/kg, i.v.). Heart rate and pressure data were analyzed using Millar data acquisition and analysis software.
Statistical analysis
For SCG neuron survival assays, the data were analyzed by two-way ANOVA using Genotype (WT and Egr3−/−) and NGF concentration as grouping factors. All values were expressed as mean ± s.e.m. with p < 0.05 considered statistically significant.
RESULTS
Egr3 is regulated during development and by NGF signaling in sympathetic neurons
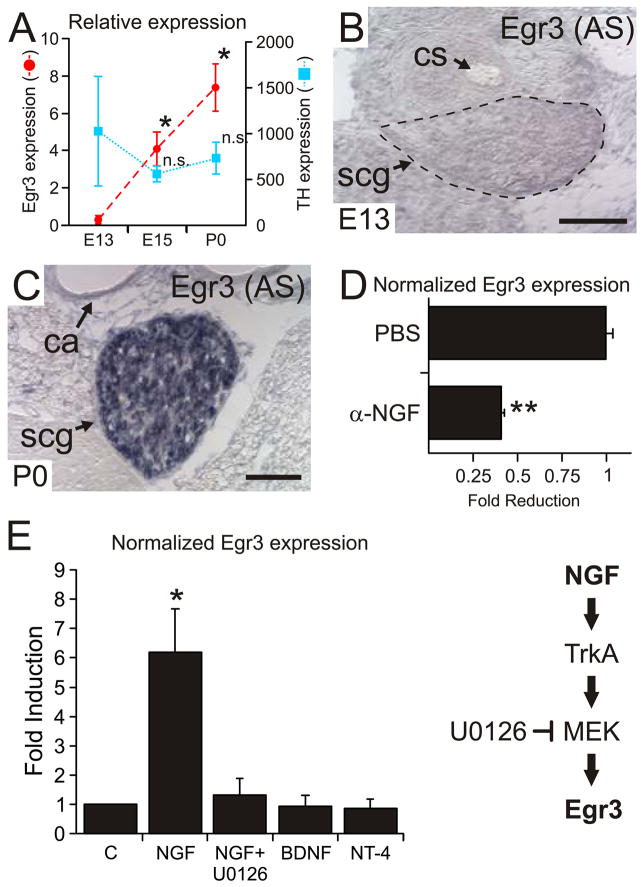
Previous reports indicate that Egr1 may have a role in NGF-mediated sympathetic neuron differentiation, but the results were not confirmed in Egr1-deficient mice. We became intrigued by the possibility that Egr3 may have a role in SNS development after identifying blepharoptosis (drooping of the upper eyelid) in Egr3-deficient mice (Tourtellotte and Milbrandt, 1998), since it is often associated with abnormal sympathetic innervation to the eyelid musculature in both humans and rodents. Since nothing is known about Egr3 expression in sympathetic neurons, we first examined whether it is expressed in the superior cervical ganglion (SCG), which contains sympathetic neurons that innervate cephalic tissues including the superior and inferior tarsal muscles of the eyelids. At embryonic day 13 (E13), when sympathetic neurons are not dependent upon NGF signaling and have not yet innervated target tissues (Francis and Landis, 1999; Wyatt and Davies, 1995; Wyatt et al., 1997), Egr3 expression was low in the SCG. Egr3 expression was induced greater than 7-fold at E15 and greater than 11-fold by birth (P0), two developmental time points after which sympathetic neurons are dependent upon NGF signaling for survival and target tissue innervation (Fig. 1A). Consistent with the qPCR results, Egr3 expression was undetectable by in situ hybridization at E13 (Fig. 1B and S1B′) and markedly upregulated in most SCG neurons by birth (P0; Fig. 1C and S1C′). Thus, Egr3 expression is developmentally regulated in the SCG and the timing of expression coincides with the onset of NGF dependence and target tissue innervation. Moreover, Egr3 is regulated by NGF signaling in vivo since neutralization of NGF function significantly decreased Egr3 expression in SCG neurons (Fig. 1D).
Figure 1.
Egr3 expression is developmentally regulated and coupled to NGF signaling in SCG neurons. (A) At E13, Egr3 expression low in SCG neurons and by E15, Egr3 expression was markedly upregulated when sympathetic neurons begin to express TrkA and respond to NGF. Tyrosine hydroxylase (TH) expression confirmed the representation of sympathetic neurons in all of the RNA samples. (results from 5–8 pooled ganglia for each developmental time point and qPCR performed in triplicate, * = p < 0.001 and n.s = no significant difference, Student’s T test relative to E13 time point). (B) Egr3 expression was not detectable at E13 by in situ hybridization whereas at (C) birth (P0) it was expressed by most, if not all SCG neurons. (cs = carotid sinus; ca = carotid artery; scg = superior cervical ganglion; scale bar = 100 μm). (D) Treatment with NGF function neutralizing antibody resulted in a 60% reduction in Egr3 expression relative treatment with PBS indicating that Egr3 expression was coupled to NGF signaling in SCG neurons in vivo (results from N=5 PBS and NGF treated P0 mice and qPCR performed in triplicate; ** = p < 0.01, Mann-Whitney U test). (E) Egr3 expression was induced approximately 6-fold by NGF treatment, but not after treatment with the neurotrophins NT-4 or BDNF. NGF-dependent Egr3 induction was abrogated using the MEK inhibitor U0126. (results from 3 experimental replicates and qPCR performed in triplicate; * = p < 0.001, Student’s t-test relative to control).
We next examined whether NGF, the principle neurotrophin required for sympathetic neuron survival and target tissue innervation, could induce Egr3 expression in vitro. Egr3 expression was induced 6 to 7-fold by NGF, but not by related neurotrophins NT-4 or BDNF relative to untreated (control) neurons (Fig. 1E). Moreover, Egr3 induction was dependent upon MAPK signaling since pretreatment with the MEK inhibitor U0126 abrogated NGF-dependent Egr3 induction (Fig. 1E). Similarly, we found that SH-SY5Y/TrkA human neuroblastoma cells, which have been previously shown to induce Egr1 expression and to differentiate in response to NGF treatment (Edsjo et al., 2001), also induced Egr3 expression after NGF treatment (Fig. S2A). Also similar to primary sympathetic neurons, NGF-mediated Egr3 expression was completely abrogated by the MEK inhibitor U0126 (Fig. S2B). Taken together, these results indicate that Egr3 expression is modulated by NGF signaling in sympathetic neurons, consistent with a potential role in gene regulation related to their survival, differentiation and/or target tissue innervation.
Sympathetic neuron loss in postnatal Egr3-deficient mice
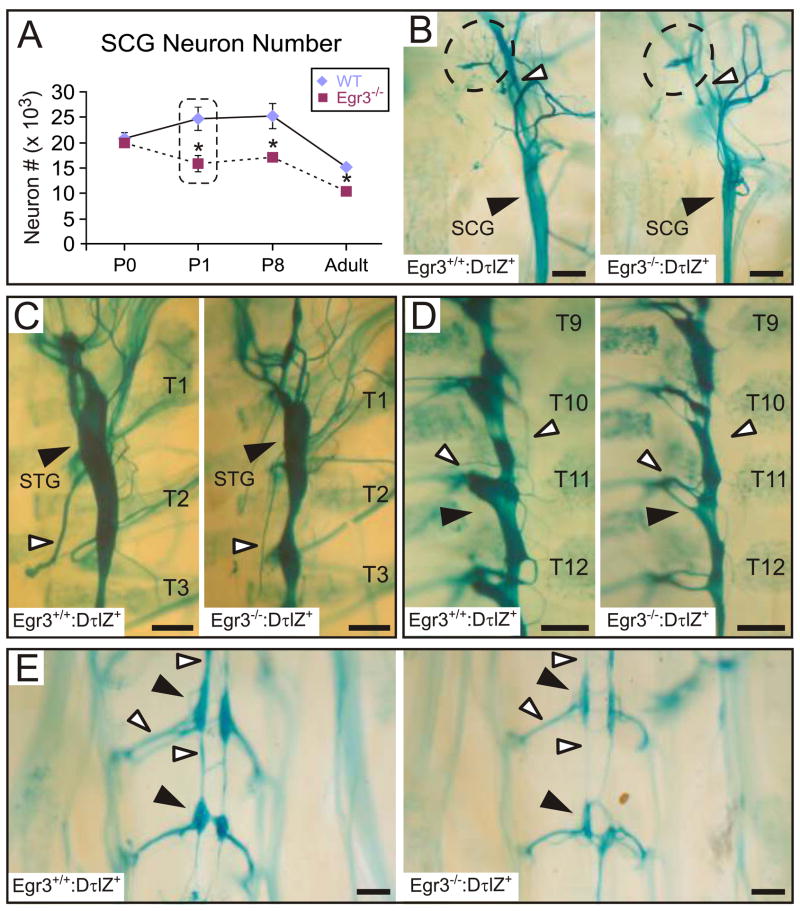
Egr3-deficient mice have blepharoptosis similar to mice with widespread sympathetic neuron loss in the absence of neurotrophins NGF or NT-3, or their cognate tyrosine kinase receptor, TrkA (Crowley et al., 1994; Ernfors et al., 1994; Farinas et al., 1994; Smeyne et al., 1994). Unlike neurotrophin signaling-deficient mice in which most sympathetic neuron loss occurs during late embryonic development, no significant neuron loss was observed in Egr3−/− mice until one day after birth (P1; Fig. 2A). At P1, Egr3−/− mice had approximately 30% less SCG neurons relative to wild type (Fig. 2A). Moreover, consistent with previously published observations, the total number of SCG neurons decreased with age in both wild type and Egr3−/− mice (Gatzinsky et al., 2004; Jansen et al., 2007). Viability of senescent SCG neurons is dependent upon pro-NGF and Sortilin signaling (Jansen et al., 2007) which does not appear to be disrupted in Egr3−/−mice since roughly similar amounts of neuron attrition was observed in both adult wild type and Egr3−/− mice (Fig. 2A).
Figure 2.
Widespread sympathetic neuron loss without migration defects in postnatal Egr3-deficient mice. (A) Sympathetic neuron loss was detected in the SCG from Egr3−/− mice starting 1 day after birth (P1). Approximately 30% of SCG neurons were lost between P0 and P1, and the difference between wild type and Egr3−/− mice persisted in aged adult mice when the overall numbers declined due to physiologic attrition of senescent neurons. (results from 3–4 SCG per genotype and developmental time point; * = p < 0.05, Student’s t-test relative to wild type). (B–E) At P1, the sympathetic ganglia were properly positioned along the rostral-caudal axis. (B) The SCG was smaller (black arrowhead) and many axon bundles emanating from it were either absent (dashed contour) or markedly atrophic (white arrowhead) in Egr3−/− mice. Similarly, (C) the stellate ganglion (STG; black arrowhead), (D) the thoracic paravertebral chain ganglia and (E) caudal sympathetic ganglia were consistently smaller (black arrowheads) and there was atrophy and loss of projections emanating from them (white arrowheads). (representative results from 3 P1 Egr3+/+:DτlZ and Egr3−/−:DτlZ mice; scale bars = 250 μm).
To characterize the impact of sympathetic neuron loss on target tissue innervation in Egr3−/− mice, transgenic reporter mice were generated to visualize sympathetic neurons and their axons using the human dopamine β-hydroxylase (DβH) promoter (Hoyle et al., 1993) to regulate expression of a τ-β-galactosidase fusion protein (tlacZ) (Callahan and Thomas, 1994) in all sympathetic neurons (Fig. S3A). Whole mount lacZ histochemistry (Fig. S3B–D) and double-labeling immunofluorescence for TH and β-gal (Fig. S3E) confirmed that the neuron/axon localized τlacZ protein was a reliable and sensitive marker for all sympathetic neurons and their axons in DβH-τlacZ+ (DτlZ+) transgenic mice.
Whole mount lacZ histochemistry performed on P3 mice showed atrophy of the paravertebral sympathetic chain ganglia throughout its rostral-caudal extent in Egr3−/−:DτlZ+ mice relative to Egr3+/+:DτlZ+ (wild type) littermates. The SCG were markedly smaller in Egr3−/− mice compared to wild type littermates (Fig. 2B, black arrowhead), consistent with a 30% neuron loss (Fig. 2A) and a 36% decrease in ganglion volume (N=3 SCG of each genotype, p < 0.01). Many axons emanating from Egr3−/− SCG were either atrophic or absent (Fig. 2B, white arrowhead and dashed contour, respectively). In addition, neurons that remained in the Egr3−/− SCG were generally smaller compared to wild type (Fig. S4A), most likely due to atrophy since diameter-frequency analysis of SCG neurons showed a loss of large diameter neurons that was accompanied by an increase in small diameter neurons (Fig. S4B). Widespread abnormalities of the sympathetic nervous system were apparent as the stellate (STG; Fig. 2C, black arrowhead), thoracic (Fig. 2D, black arrowhead) and caudal paravertebral ganglia (Fig. 2E, black arrowheads) were all smaller in Egr3−/−: DτlZ+ mice, and many axon bundles emanating from them were also markedly thin or completely absent compared to wild type mice (Fig. 2C–E, white arrowheads).
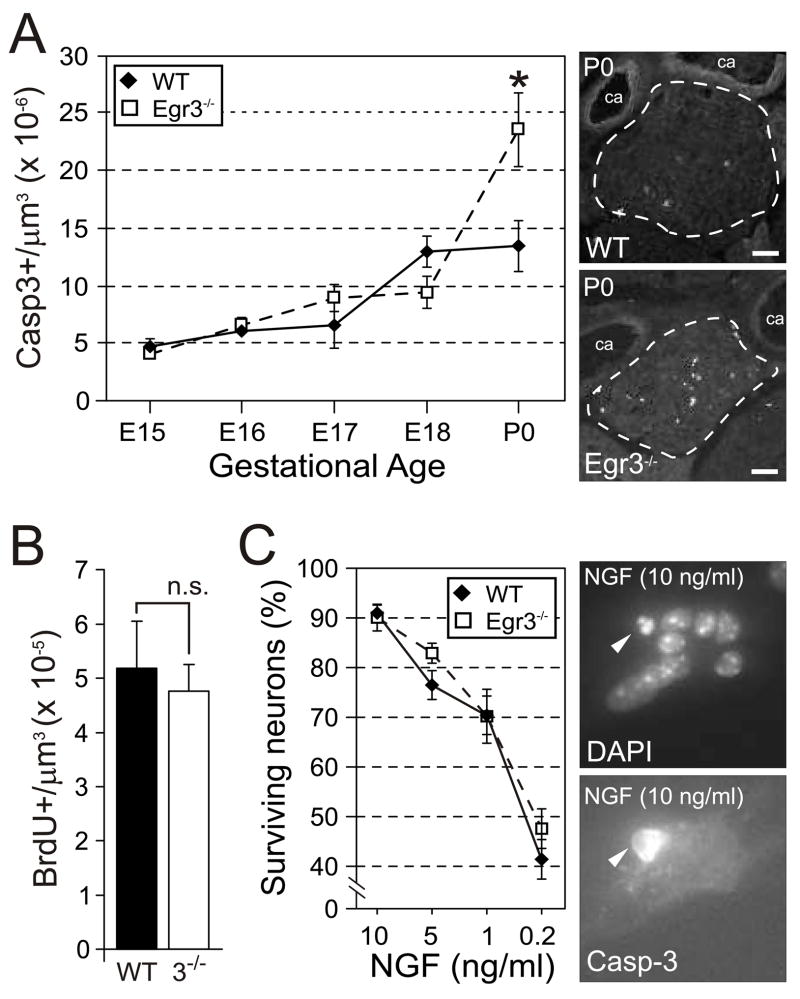
Perinatal sympathetic neuron apoptosis in Egr3−/− mice
In NGF, NT-3 and TrkA deficient mice, sympathetic neurons are generated in normal numbers but undergo increased apoptosis during prenatal and postnatal development (Crowley et al., 1994; Ernfors et al., 1994; Farinas et al., 1994; Smeyne et al., 1994). Sympathetic neurons were also generated in normal numbers in Egr3−/− mice since no differences in SCG neuron number were observed between wild type and Egr3−/− newborn mice (Fig. 2A). However, significantly increased apoptosis was found in newborn Egr3−/− SCG (Fig. 3A) which correlated with differences in SCG neuron number that were detected slightly later at P1. In addition, BrdU incorporation studies showed no difference in cell proliferation between newborn wild type and Egr3−/− SCG that could have otherwise contributed to differences in the number of sympathetic neurons in postnatal mice (Fig. 3B). Thus, similar to neurotrophin- and neurotrophin signaling-deficient mice, sympathetic neuron death in Egr3−/− mice occurs by apoptosis at a developmental time point that coincides with active target tissue innervation and acquisition of NGF dependence.
Figure 3.
Increased apoptosis in sympathetic neurons Egr3−/− mice. (A) No significant differences in apoptosis were observed until P0, at which point apoptosis in Egr3−/− SCG was nearly double that of wild type. (results of Casp3+ cells from 2–6 ganglia of each genotype and developmental time point, * = p < 0.01, Student’s t test). (B) Cellular proliferation (BrdU+ cells) was not altered in the SCG between wild type and Egr3−/− mice at P0. (C) There was no autonomous defect in NGF-dependent survival of Egr3−/− SCG neurons in vitro. There was a highly significant correlation between neuron survival and NGF concentration, but no significant difference in the amount of survival between wild type and Egr3−/− neurons at any NGF concentration tested. Representative images of sympathetic neurons stained with Hoechst (right, top) and Casp3 (right, bottom). (results from cultures performed in triplicate)
Embryonic and early postnatal sympathetic neurons depend upon NGF for their survival in vitro and in vivo, and >90% of them can be rescued from apoptosis in vitro when 10 ng/mL of NGF is present in the culture medium (Belliveau et al., 1997). To examine whether Egr3−/−sympathetic neurons have an autonomous defect in survival, wild type and Egr3−/− SCG were isolated at E18.5, prior to the onset of increased apoptosis that occurs in Egr3−/− mice in vivo, and sympathetic neurons were dissociated into culture media containing differing amounts of NGF. As expected, there was a highly significant effect of NGF concentration on sympathetic neuron survival for both wild type and Egr3−/− neurons 24 hours after plating (F3,23 = 123.9, p < 0.0001). However, there was no significant effect of genotype on neuron survival (F1,23 = 1.27, p = 0.28), indicating that Egr3−/− sympathetic neurons do not have a cell autonomous defect in survival (Fig. 3C).
Abnormal sympathetic target tissue innervation and terminal axon branching in Egr3−/−mice
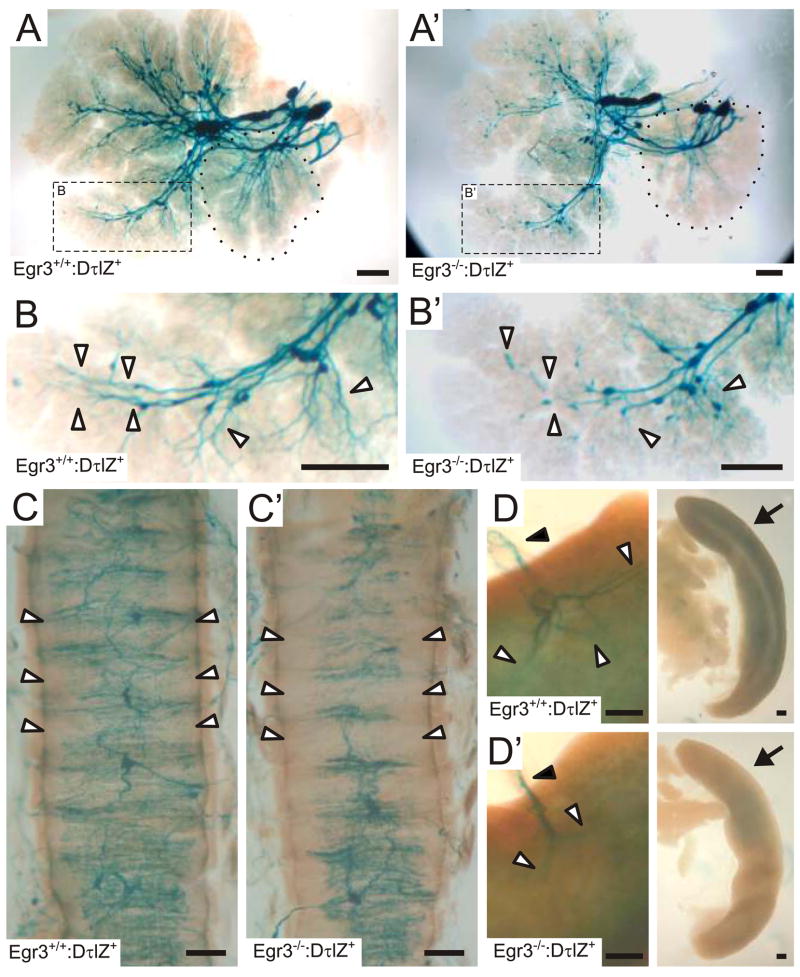
Egr3-deficient neurons do not have an autonomous survival defect and yet some of them die in vivo during a period of active target tissue innervation. These observations raise the possibility that neuron death in vivo results from a failure to normally innervate target tissues and acquire adequate trophic factor support. To address this hypothesis, sympathetic target tissue innervation was analyzed in adult Egr3+/+:DτlZ+ and Egr3−/−:DτlZ+ mice. In all Egr3−/−:DτlZ+ target tissues examined, there was a decrease in the overall sympathetic innervation compared to Egr3+/+:DτlZ+ target tissues, consistent with sympathetic neuron loss. However, the remaining sympathetic axons also showed abnormal axon extension and branching patterns within tissues. For example, in Egr3+/+:DτlZ+ submandibular and sublingual salivary glands (Fig. 4A), which express high levels of NGF required for normal sympathetic innervation and terminal axon branching (Glebova and Ginty, 2004), whole mount LacZ histochemistry highlighted robust sympathetic axon innervation and axon branching deep into the glandular parenchyma (Fig. 4B, arrowheads). By contrast, in Egr3−/−:DτlZ+ glands there was decreased innervation (Fig. 4A′) that was accompanied by attenuated terminal axon extension and branching into the glandular parenchyma (Fig.. 4B′, arrowheads). Similarly, in the trachea where there is robust sympathetic innervation to smooth muscle and submucosal glands, the axons entered the dorsal midline and branched to form a dense circumferential plexus in Egr3+/+:DτlZ+ mice (Fig. 4C, white arrowheads). By contrast, innervation to the trachea of Egr3−/−:DτlZ+ mice was generally decreased but remaining axons also failed to branch efficiently to form a comparatively elaborate sympathetic plexus. In some regions of the trachea the axons appeared to barely branch at all, leaving the corresponding tracheal segments nearly devoid of sympathetic innervation (Fig. 4C′, arrowheads). Sympathetic innervation to several major organs including, kidneys, bowel and spleen was also abnormal. For example, sympathetic axons in wild type spleen entered the organ along the splenic artery (Fig. 4D, black arrowhead) and then branched considerably upon entering the splenic parenchyma (Fig. 4D, white arrowheads). By contrast, although some axons also reached the spleen along the splenic artery in Egr3−/−:DτlZ+ mice (Fig. 4D′, black arrowhead), they failed to normally branch and invade the splenic parenchyma (Fig. 4D′, white arrowheads). This correlated with an overall decrease in sympathetic innervation to the spleen as indicated by an overall decrease in the lacZ reaction product in Egr3−/−:DτlZ+ spleens (Fig. 4D′, arrow) compared to wild type (Fig. 4D, arrow). Thus, in the absence of Egr3, there is decreased innervation to many target tissues due to sympathetic neuron loss and innervation defects from residual axons that fail to normally branch and invade target tissues.
Figure 4.
Decreased sympathetic innervation to target organs is accompanied by abnormalities in axon extension and terminal axon branching in Egr3−/− mice. (A) In the submandibular gland and sublingual gland (dotted contour) from Egr3+/+:DτlZ+ mice, lacZ histochemistry revealed robust sympathetic innervation. (B) Inset shown in A: Sympathetic axons branched into the distal lobules of the glands (arrowheads). (A′) In Egr3−/−:DτlZ+ glands, there was a relative decrease in sympathetic innervation, consistent with sympathetic neuron loss. (B′) Inset shown in A′: however, there was less complex axon branching and numerous axons that failed to extend to the distal lobules of the glands (arrowheads). (C) In trachea from Egr3+/+:DτlZ+ mice, sympathetic innervation entered along the dorsal midline and branched circumferentially to innervate smooth muscle and submucosal glands (arrowheads). (C′) In trachea from Egr3−/−:DτlZ+ mice however, sympathetic axon branching was consistently decreased and the branching of remaining axons was markedly diminished. (D, D′) Sympathetic axons entered the splenic parenchyma along the splenic arteries (black arrowhead) and in (D) Egr3+/+:DτlZ+ spleens the axons branched extensively after entering the organ parenchyma (white arrowheads). By contrast, in (D′) Egr3−/−:DτlZ+ mice, sympathetic axons branched poorly as they entered the parenchyma (white arrowheads). Global sympathetic innervation to the spleen could be observed in (D, right) Egr3+/+:DτlZ+ spleens which showed a diffuse blue reaction product (arrow) that was consistently diminished in (D′, right) Egr3−/−:DτlZ+ spleens (arrow). (representative results from 3 adult mice for each genotype and organ analyzed; magnification bar= 250μm)
Sympathetic target tissue innervation defects lead to physiologic dysautonomia in Egr3−/−mice
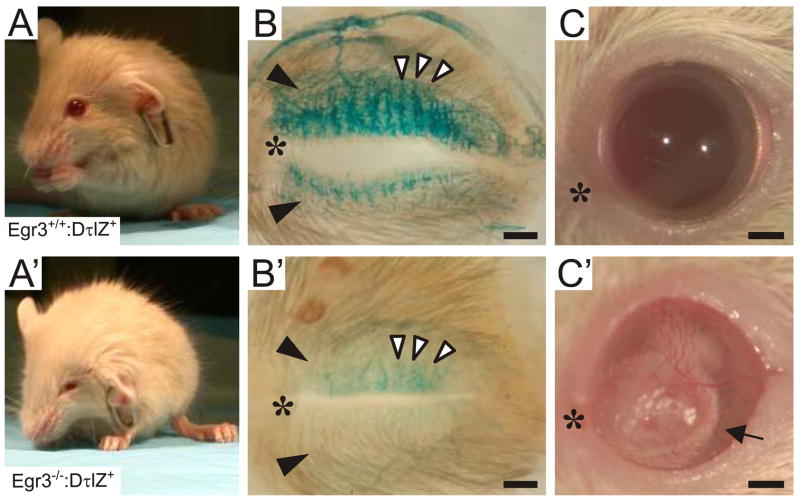
We next examined the extent to which moderate sympathetic neuron loss accompanied by prominent target tissue innervation abnormalities lead to dysautonomia in several well-characterized sympathetic target tissues in Egr3−/− mice. For example, in humans with Horner’s syndrome and NGF-, NT-3- and TrkA-deficient mice with SNS defects, blepharoptosis results from loss of sympathetic innervation to the eye (Crowley et al., 1994; Ernfors et al., 1994; Gurwood, 1999; Smeyne et al., 1994). Compared to wild type (Fig. 5A), Egr3−/− mice have prominent blepharoptosis (Fig. 5A′), suggesting that sympathetic innervation to tarsal musculature may be impaired. Indeed, compared to Egr3+/+:DτlZ+ mice, which showed dense sympathetic innervation to the superior and inferior tarsal muscles in the eyelids (Fig. 5B, black arrowheads) and the secretory Meibomian glands (Fig. 5B, columnar innervation, white arrowheads), innervation to the same structures in Egr3−/−:DτlZ+ mice was nearly absent (Fig.. 5B′). Also similar to humans with familial dysautonomia, the blepharoptosis in adult Egr3−/− mice was accompanied by corneal ulcerations, which were most likely the result of a chronic lack of corneal lubrication from Meibomian glands which require sympathetic innervation for normal secretomotor function (Fig. C′, arrow).
Figure 5.
Physiologic blepharoptosis in Egr3−/− mice. (A) Compared to wild type mice, (A′) Egr3−/− mice have prominent blephoroptosis (drooping eyelids). (B) Whole mount lacZ histochemistry of the inner eyelids from adult Egr3+/+:DτlZ+ mice showed dense sympathetic innervation to the superior and inferior tarsal muscles (black arrowheads) and Meibomian glands in the inner eyelids (columnar innervation, white arrowheads). (B′) Sympathetic innervation was markedly decreased to the upper and lower eyelids (black arrowheads) and the Meibomian glands (white arrowheads) in Egr3−/−:DτlZ+mice. (C) Compared to wild type, (C′) adult Egr3−/−mice developed corneal ulceration (arrow), most likely due to impaired secretomotor function of the Meibomian glands. (scale bars = 0.5 mm; * = nasal canthus)
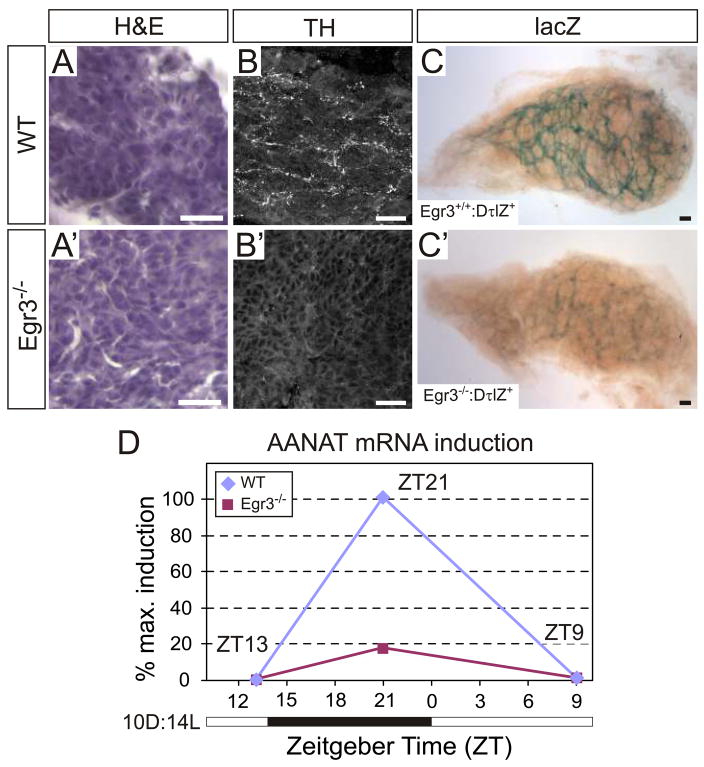
The pineal gland, an SCG target tissue that produces the hormone melatonin, is involved in synchronizing circadian rhythms with environmental light cues. During the dark phase of the light-dark cycle, arylalkylamine N-acetyl transferase (AANAT), the rate-limiting enzyme for melatonin synthesis, is transcriptionally upregulated in pinealocytes by increased sympathetic activity (Borjigin et al., 1995; Foulkes et al., 1997). Wild type (Fig. 6A) and Egr3−/− (Fig. 6A′) pineal glands were morphologically indistinguishable. Sympathetic innervation detected by TH immunohistochemistry showed that wild type pineal glands were innervated by a dense network of TH+ axon terminals (Fig. 6B), whereas pineal glands from Egr3−/− mice had almost none (Fig. 6B′). Similarly, a dense plexus of lacZ+ (sympathetic) axons was observed in whole mount wild type pineal glands (Fig. 6C) which was markedly diminished in Egr3−/− pineal glands from DτlZ+ mice (Fig. 6C′). To examine whether sympathetic denervation was also associated with deregulation of AANAT expression during the light-dark cycle, wild type and Egr3−/− mice were entrained to a 10D:14L light-dark cycle (see methods). Pineal glands were dissected for qPCR analysis at three time points during the 24 hour photo period: ZT13, ZT21 and ZT9, with ZT0 defined as the temporal onset of the dark-light transition. Wild type pineal glands showed a characteristic pattern of AANAT expression associated with the entrained light-dark cycle. Very low basal AANAT expression was observed near the end of the light cycle (ZT13), it was highly induced during the dark phase when sympathetic activity to the pineal gland is maximal (ZT21, defined as 100% maximal induction in this paradigm) and it rapidly declined to basal levels after the onset of the entrained light phase (ZT9; Fig. 6D). By contrast, in Egr3−/− pineal glands AANAT induction reached less than 20% of the maximal wild type induction (ZT21) after the onset of the dark phase before returning to baseline during the light phase of the cycle (Fig. 6D). These results are consistent with decreased sympathetic innervation to the pineal gland which leads to impaired AANAT induction during the dark phase of the light cycle in Egr3−/− mice. Moreover, the results raise the possibility that Egr3−/− mice have altered circadian rhythms as a consequence of sympathetic dysautonomia and impaired AANAT cycling and melatonin synthesis.
Figure 6.
Impaired sympathetic innervation and altered AANAT induction in pineal glands from Egr3−/− mice. The pineal glands from (A) wild type and (A′) Egr3−/− mice appeared histologically indistinguishable. Immunohistochemistry for TH showed robust sympathetic innervation to the (B) wild type pineal glands which was (B′) either highly diminished or absent in most pineal glands from Egr3−/− mice. Similar results were obtained with the lacZ sympathetic reporter which showed a robust network of sympathetic axons in (C) wild type pineal glands that was barely detectable in (C′) pineal glands from most Egr3−/− mice. (D) AANAT is induced by increased sympathetic activity to the pineal upon entry into the dark phase of the light-dark cycle. In 10D:14L light cycle entrained wild type mice, AANAT expression was induced as expected during the dark phase of the light-dark cycle and returned to low basal levels when sympathetic activity to the pineal gland decreased with the onset of the light phase of the cycle. However, in Egr3−/− pineal glands AANAT was induced to approximately 18% of the wild type level, consistent with a physiologic impairment of sympathetic innervation to the gland. The white and black bar along the horizontal axis depicts the time during the light-dark cycle when the lights are on and off, respectively. Zeitgeber time (ZT) represents the 24 hour elapsed time in the light-dark cycle relative to the time of dark-light transition (ZT0). (AANAT expression from 3 independent experiments and qPCR performed in triplicate; the peak wild type value at ZT21 was considered 100% maximal AANAT induction in this paradigm; scale = 25 μm)
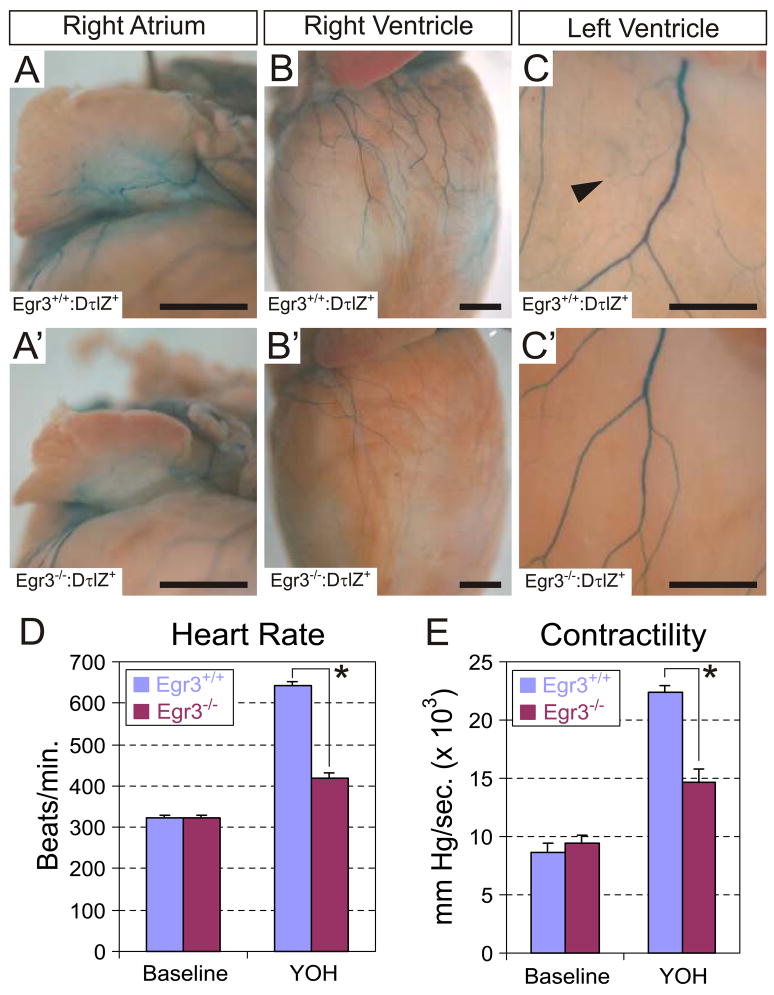
Neurons located in the SCG, STG and upper thoracic sympathetic chain ganglia innervate the heart. Sympathetic axon terminals release norepinephrine to activate myocardiocyte β1 adrenergic receptors and increase heart rate and contractility (Costanzo, 1998). To examine the impact of sympathetic neuron loss on cardiac sympathetic innervation, we compared the pattern of sympathetic innervation in hearts from adult wild type and Egr3−/− mice. In hearts from Egr3+/+:DτlZ+ mice, prominent epicardial innervation to the right atrium (Fig. 7A) and ventricles (Fig. 7B, C) was observed. The ventricular epicardial axons formed highly branched networks of small axons that diffusely innervated the myocardium (Fig. 7C, arrowhead). In Egr3−/−:DτlZ+ hearts, there was a marked decrease in the innervation to the inferior surface of the right atrium and to both ventricles (Fig. 7A′–C′), consistent with sympathetic neuron loss. Similar to other sympathetic target tissues that we examined, there was a conspicuous absence of terminal axon branching on the surface of the ventricles (compare Fig. 7C to 7C′). To determine whether these abnormalities have physiologic significance, heart rate and myocardial contractility were measured in cardiac catheterized adult wild type and Egr3−/− mice. The catheterized mice were administered the central α2-adrenergic receptor antagonist yohimbine (YOH) to increase post-ganglionic sympathetic activity. Whereas the baseline heart rate and myocardial contractility of untreated wild type and Egr3−/− mice were similar, YOH treatment increased heart rate and contractility greater than 2-fold relative to baseline. By contrast, YOH treatment of Egr3−/− mice resulted in a significantly blunted increase in heart rate (Fig. 7D) and contractility (Fig. 7E) compared to wild type. Thus, cardiac hemodynamic abnormalities are present in Egr3−/− mice as a consequence of impaired sympathetic innervation to the heart. Taken together, these results demonstrate that Egr3−/− mice have profound sympathetic dysautonomia associated with the structural SNS abnormalities.
Figure 7.
Abnormal cardiac sympathetic innervation and autonomic dysfunction in Egr3−/− mice. Whole mount lacZ histochemistry was performed on hearts from adult Egr3+/+:DτlZ+ and Egr3−/−:DτlZ+ mice. In Egr3+/+:DτlZ+ mice sympathetic innervation to the inferior surface of the (A) right atrium and the ventral surfaces of the (B) right and (C) left ventricles could be visualized in detail. (C) Diffuse innervation of the myocardium was visualized in Egr3+/+:DτlZ+ hearts (arrowhead). (A′–C′) In hearts from Egr3−/−:DτlZ+ mice however, there was a marked decrease in the overall epicardial sympathetic innervation and the small terminal axon branching was markedly diminished. (results are representative of 3 Egr3+/+:DτlZ+ and Egr3−/−:DτlZ+ hearts analyzed by whole mount lacZ histochemistry). (D, E) Decreased sympathetic innervation to the heart was accompanied by abnormal physiologic response to sympathetic nervous system activation. (D) Heart rate and (E) contractility measurements were similar between untreated (baseline) wild type and Egr3−/− mice. Treatment with the central α2-adrenergic antagonist Yohimbine (YOH) resulted in a greater than 2-fold increase in heart rate and myocardial contractility in wild type mice relative to baseline due to increased sympathetic activity to the heart. In Egr3−/− mice treated with YOH, the increase in heart rate and myocardial contractility was significantly diminished compared to wild type mice. (results from 5 wild type and 7 Egr3−/−catherized mice; * = p < 0.01, student’s t test; scale bar A, B = 1mm. C = 0.5 mm)
DISCUSSION
Egr3 is a member of the early growth response family of zinc finger transcriptional regulators which consists of four structurally similar proteins (Egr1–4). The closely related protein, Egr1 was considered to be a potential mediator of differentiation related gene transcription (Milbrandt, 1987). Egr1 was later shown to have an important role in NGF mediated neurite outgrowth in PC-12 and N2A neuroblastoma cells, but a role in SNS development was not confirmed in Egr1-deficient mice (Lee et al., 1995). Despite the fact that Egr3 is co-expressed with Egr1 in many cell types and they can cooperate to directly regulate some target genes (Carter and Tourtellotte, 2007; Gao et al., 2007; Li et al., 2005), it is surprising to find that Egr3, but not Egr1, has an essential role in SNS development in vivo.
We found that Egr3 expression was upregulated in post-migratory SCG neurons at a time point that coincides with their dependence on NGF for survival and target tissue innervation. This correlation was further substantiated by in vitro and in vivo experiments which showed that Egr3 is regulated in primary and transformed sympathetic neurons by NGF signaling. Moreover, NGF-mediated Egr3 expression was entirely dependent upon MEK (Erk1/2) signaling which is well known to enhance NGF-dependent axon outgrowth in sympathetic neurons (Atwal et al., 2000; Lein et al., 2007; Thompson et al., 2004). Although these results do not rule out non-neurotrophin mediated mechanisms of regulation or a non-neuron autonomous function for Egr3, they clearly demonstrate that Egr3 expression is modulated by NGF signaling and that it is expressed by sympathetic neurons at a time when it could influence gene expression involved in their differentiation and target tissue innervation.
A role for Egr3 in SNS development was confirmed in Egr3-deficient mice. Sympathetic neuron loss was first detected in SCG neurons 1 day after birth which was preceded by a significant increase in apoptosis. Although the quantitative and in vitro studies were focused on SCG neurons, a detailed analysis of Egr3−/− mice demonstrated widespread atrophy of sympathetic chain ganglia and target tissue innervation defects, indicating a global role for Egr3 in SNS development. However, unlike mice lacking NGF or its receptor TrkA, which lose most sympathetic neurons by birth (Crowley et al., 1994; Smeyne et al., 1994), we found that only about 1/3 of SCG neurons were lost in Egr3-deficient mice after birth, despite the fact that it is expressed in most, if not all, SCG neurons. This discrepancy between Egr3 expression and neuron survival may be explained by the fact that Egr3 does not have a neuron-autonomous role in survival since there was no difference in survival between wild type and Egr3−/− neurons when NGF was supplied in culture media in vitro. Yet, sympathetic neuron death is observed in vivo which results in many relatively hypoinnervated target tissues that also have residual innervation with abnormal terminal axon extension and branching. Thus, partial loss of sympathetic neurons in vivo is most likely a consequence of inadequate trophic factor acquisition because of abnormal, but not completely absent, target tissue innervation in Egr3−/− mice (Korsching and Thoenen, 1983). Similarly, sympathetic neuron death which was detected at P0 in Egr3-deficient mice was delayed compared to TrkA-deficient mice where neuron death begins 2 days earlier at E17.5 (Fagan et al., 1996). Sympathetic neuron death may occur later in Egr3-deficient mice because abnormal terminal axon extension and branching within target tissues, which occurs relatively late during SNS development, would be expected to lead to relatively delayed defects in NGF acquisition.
Egr3, a potential transcriptional effector of NGF signaling
The density of sympathetic innervation is correlated with the timing and extent of NGF production by target tissues (Korsching and Thoenen, 1983; Korsching and Thoenen, 1988; Shelton and Reichardt, 1984). Sympathetic neurons depend upon NGF for their survival (Crowley et al., 1994; Levi-Montalcini and Booker, 1960) and for terminal axon extension and branching within target tissues (Glebova and Ginty, 2004; Kuruvilla et al., 2004). Although Egr3 does not appear to have a direct role in sympathetic neuron survival like NGF, it does appear to have a similar role in sympathetic terminal axon extension and branching within target tissues. In the absence of Egr3, we observed a heterogeneous effect on sympathetic target tissue innervation with some tissues showing highly diminished innervation (e.g., eyelids and pineal gland) while in other tissues there was moderate loss of innervation associated with prominent terminal axon extension and branching defects (e.g., salivary glands, trachea, spleen and heart). These results are strikingly similar to NGF/Bax double knockout (dKO) mice, where inhibition of NGF- and Bax-dependent sympathetic neuron death (Deckwerth et al., 1996) made it possible to study target tissue innervation in the absence of NGF (Glebova and Ginty, 2004). Although NGF/Bax dKO mice died shortly after birth before the sympathetic nervous system was completely developed, sympathetic axons extended along vessels and encroached upon target tissues where they either did not innervate them or they innervated them with highly attenuated terminal axon extension and branching. Thus, while Egr3 is just one of many genes regulated by NGF signaling within sympathetic neurons, it is reasonable to assume that it has a particularly important role in NGF-mediated gene expression involved in target tissue innervation. To better understand how Egr3 mediates particular aspects of NGF signaling, it will be important for future studies to identify and characterize the target genes regulated by Egr3 in sympathetic neurons.
Sympathetic dysautonomia in Egr3-deficient mice
Unlike mice lacking NGF or TrkA, most Egr3-deficient mice survive past the perinatal period with many signs and symptoms of sympathetic dysautonomia because of abnormal, but not completely absent, sympathetic target tissue innervation. In Egr3−/− mice, blepharoptosis was a result of impaired sympathetic innervation to the tarsal musculature of the eyelid and corneal ulcerations resulted from abnormal secretomotor function of tarsal Meibomian glands which normally provide lubrication to the surface of the eye. In addition, denervation of the pineal gland was found to disrupt light cycle-dependent and sympathetic activity-induced AANAT expression which is required for normal melatonin synthesis and circadian rhythms. Cardiac function was also impaired by poor sympathetic terminal axon extension and branching to the myocardium which resulted in abnormal regulation of heart rate and contractility.
Egr3 has not been previously implicated in SNS development or human dysautonomia but it is remarkable that Egr3-deficient mice have physiologic abnormalities similar to humans with dysautonomia. The etiology of most human dysautonomias is unknown, but some congenital cases have been associated with abnormal NGF signaling and transcriptional regulation. For example, hereditary sensory and autonomic neuropathy (HSAN) type IV is associated with mutations in the TrkA gene (OMIM #256800), while HSAN type V is associated with mutations in the NGFβ gene (OMIM #608654). Similarly, HSAN type III (Riley-Day syndrome; familial dysautonomia), the most common congenital dysautonomia, is associated with mutations in the IKBKAP gene which encodes a protein component of the halo-transcriptional elongator complex (OMIM #223900). Taken together with this new evidence that Egr3 has an important role in SNS development and NGF signaling, it seems plausible that Egr3-dependent gene regulation could be involved in some forms of congenital and/or acquired human dysautonomia.
Supplementary Material
Acknowledgments
We thank J. Carter, M. Cornwell, and P. Lampe for technical assistance and M. Thomas and R. Palmiter for reagents. This work was supported by NIH grants NS046468 and NS040748 (W.G.T.) and F30-NS047775 and T32-GM008152 (L.C.E).
References
- Albert Y, Whitehead J, Eldredge L, Carter J, Gao X, Tourtellotte WG. Transcriptional regulation of myotube fate specification and intrafusal muscle fiber morphogenesis. J Cell Biol. 2005;169:257–68. doi: 10.1083/jcb.200501156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–77. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Belliveau DJ, Krivko I, Kohn J, Lachance C, Pozniak C, Rusakov D, Kaplan D, Miller FD. NGF and neurotrophin-3 both activate TrkA on sympathetic neurons but differentially regulate survival and neuritogenesis. J Cell Biol. 1997;136:375–88. doi: 10.1083/jcb.136.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjigin J, Wang MM, Snyder SH. Diurnal variation in mRNA encoding serotonin N-acetyltransferase in pineal gland. Nature. 1995;378:783–5. doi: 10.1038/378783a0. [DOI] [PubMed] [Google Scholar]
- Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12:1825–36. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CA, Thomas JB. Tau-beta-galactosidase, an axon-targeted fusion protein. Proc Natl Acad Sci U S A. 1994;91:5972–6. doi: 10.1073/pnas.91.13.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JH, Tourtellotte WG. Early growth response transcriptional regulators are dispensable for macrophage differentiation. J Immunol. 2007;178:3038–47. doi: 10.4049/jimmunol.178.5.3038. [DOI] [PubMed] [Google Scholar]
- Costanzo l. Autonomic Nervous System. In: Company WBS, editor. Physiology. Philadelphia: 1998. pp. 40–47. [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, MacMahon SB, Shelton DL, Levinson AD, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–11. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Jr, Snider WD, Korsmeyer SJ. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–11. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Edsjo A, Hallberg B, Fagerstrom S, Larsson C, Axelson H, Pahlman S. Differences in early and late responses between neurotrophin-stimulated trkA- and trkC-transfected SH-SY5Y neuroblastoma cells. Cell Growth Differ. 2001;12:39–50. [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr, Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–74. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–12. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Zhang H, Landis S, Smeyne RJ, Silos-Santiago I, Barbacid M. TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J Neurosci. 1996;16:6208–18. doi: 10.1523/JNEUROSCI.16-19-06208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–61. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Foulkes NS, Borjigin J, Snyder SH, Sassone-Corsi P. Rhythmic transcription: the molecular basis of circadian melatonin synthesis. Trends Neurosci. 1997;20:487–92. doi: 10.1016/s0166-2236(97)01109-0. [DOI] [PubMed] [Google Scholar]
- Francis N, Farinas I, Brennan C, Rivas-Plata K, Backus C, Reichardt L, Landis S. NT-3, like NGF, is required for survival of sympathetic neurons, but not their precursors. Dev Biol. 1999;210:411–27. doi: 10.1006/dbio.1999.9269. [DOI] [PubMed] [Google Scholar]
- Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–66. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- Gao X, Daugherty RL, Tourtellotte WG. Regulation of low affinity neurotrophin receptor (p75(NTR)) by early growth response (Egr) transcriptional regulators. Mol Cell Neurosci. 2007;36:501–14. doi: 10.1016/j.mcn.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzinsky KP, Thrasivoulou C, Campioni-Noack M, Underwood C, Cowen T. The role of NGF uptake in selective vulnerability to cell death in ageing sympathetic neurons. Eur J Neurosci. 2004;20:2848–56. doi: 10.1111/j.1460-9568.2004.03780.x. [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24:743–51. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;3:531–41. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- Gurwood AS. Horner’s Syndrome. Optometry Today. 1999:36–37. [Google Scholar]
- Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, Milbrandt J. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–82. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 2005;277:271–86. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Mercer EH, Palmiter RD, Brinster RL. Expression of NGF in sympathetic neurons leads to excessive axon outgrowth from ganglia but decreased terminal innervation within tissues. Neuron. 1993;10:1019–34. doi: 10.1016/0896-6273(93)90051-r. [DOI] [PubMed] [Google Scholar]
- Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, Gotthardt M, Lin F, Eilers A, et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10:1449–57. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Bekku Y, Suto F, Kitsukawa T, Taniguchi M, Nagatsu I, Nagatsu T, Itoh K, Yagi T, Fujisawa H. Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development. 2002;129:671–80. doi: 10.1242/dev.129.3.671. [DOI] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: correlation with density of sympathetic innervation. Proc Natl Acad Sci U S A. 1983;80:3513–6. doi: 10.1073/pnas.80.11.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Developmental changes of nerve growth factor levels in sympathetic ganglia and their target organs. Dev Biol. 1988;126:40–6. doi: 10.1016/0012-1606(88)90236-9. [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–55. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Lee SL, Tourtellotte LC, Wesselschmidt RL, Milbrandt J. Growth and differentiation proceeds normally in cells deficient in the immediate early gene NGFI-A. J Biol Chem. 1995;270:9971–7. doi: 10.1074/jbc.270.17.9971. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Guo X, Shi GX, Moholt-Siebert M, Bruun D, Andres DA. The novel GTPase Rit differentially regulates axonal and dendritic growth. J Neurosci. 2007;27:4725–36. doi: 10.1523/JNEUROSCI.5633-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R, Booker B. Destruction of the sympathetic ganglia in mamals by an antiserum to a nerve-growth protein. Proc Natl Acad Sci U S A. 1960;46:384–391. doi: 10.1073/pnas.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R, Cohen S. Effects of the extract of the mouse submaxillary salivary glands on the sympathetic system of mammals. Ann N Y Acad Sci. 1960;85:324–41. doi: 10.1111/j.1749-6632.1960.tb49963.x. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, O’Donovan KJ, Baraban JM. Blockade of NGF-induced neurite outgrowth by a dominant-negative inhibitor of the egr family of transcription regulatory factors. J Neurosci. 2001;21:45–52. doi: 10.1523/JNEUROSCI.21-01-00045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Carter J, Gao X, Whitehead J, Tourtellotte WG. The Neuroplasticity-Associated Arc Gene Is a Direct Transcriptional Target of Early Growth Response (Egr) Transcription Factors. Mol Cell Biol. 2005;25:10286–10300. doi: 10.1128/MCB.25.23.10286-10300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina F, Hilton MC, Andres R, Wyatt S, Klein R, Davies AM. Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron. 1998;20:835–46. doi: 10.1016/s0896-6273(00)80466-3. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–9. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Cortes-Canteli M, Santos A, Perez-Castillo A. Involvement of the NGFI-A gene in the differentiation of neuroblastoma cells. FEBS Lett. 1999;461:37–42. doi: 10.1016/s0014-5793(99)01420-9. [DOI] [PubMed] [Google Scholar]
- Qu Z, Wolfraim LA, Svaren J, Ehrengruber MU, Davidson N, Milbrandt J. The transcriptional corepressor NAB2 inhibits NGF-induced differentiation of PC12 cells. J Cell Biol. 1998;142:1075–82. doi: 10.1083/jcb.142.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Reichardt LF. Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc Natl Acad Sci U S A. 1984;81:7951–5. doi: 10.1073/pnas.81.24.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–9. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Thompson J, Dolcet X, Hilton M, Tolcos M, Davies AM. HGF promotes survival and growth of maturing sympathetic neurons by PI-3 kinase- and MAP kinase-dependent mechanisms. Mol Cell Neurosci. 2004;27:441–52. doi: 10.1016/j.mcn.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, Keller-Peck C, Milbrandt J, Kucera J. The transcription factor Egr3 modulates sensory axon-myotube interactions during muscle spindle morphogenesis. Dev Biol. 2001;232:388–99. doi: 10.1006/dbio.2001.0202. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet. 1998;20:87–91. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, Nagarajan R, Bartke A, Milbrandt J. Functional compensation by Egr4 in Egr1-dependent luteinizing hormone regulation and Leydig cell steroidogenesis. Mol Cell Biol. 2000;20:5261–8. doi: 10.1128/mcb.20.14.5261-5268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC. Mitogen-activated protein kinases (Erk1,2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci. 1998;18:4008–21. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild KD, Bian D, Zhu D, Davis J, Bannon AW, Zhang TJ, Louis JC. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther. 2007;322:282–7. doi: 10.1124/jpet.106.116236. [DOI] [PubMed] [Google Scholar]
- Wyatt S, Davies AM. Regulation of nerve growth factor receptor gene expression in sympathetic neurons during development. J Cell Biol. 1995;130:1435–46. doi: 10.1083/jcb.130.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt S, Pinon LG, Ernfors P, Davies AM. Sympathetic neuron survival and TrkA expression in NT3-deficient mouse embryos. Embo J. 1997;16:3115–23. doi: 10.1093/emboj/16.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.