Abstract
Radioimmunotherapy (RIT) for treatment of hematological malignancies frequently fails because of disease recurrence. We therefore conducted pretargeted (P)RIT studies to augment the efficacy in mice of therapy using a pretargeted anti-human (h)CD45 antibody (Ab)-streptavidin (SA) conjugate followed by a biotinylated clearing agent and radiolabeled-DOTA-biotin. Tumor-to-blood ratios at 24 hours were 20:1 using pretargeted anti-hCD45 RIT and <1:1 with conventional RIT. In vivo imaging studies confirmed that the PRIT approach provided high-contrast tumor images with minimal blood-pool activity, whereas directly-labeled anti-hCD45 Ab produced distinct tumor images but the blood pool retained a large amount of labeled Ab for a prolonged time. Therapy experiments demonstrated that 90Y-DOTA-biotin significantly prolonged survival of mice treated with pretargeted anti-hCD45 Ab-SA compared to mice treated with conventional RIT using 90Y-labeled anti-hCD45 Ab at 200 μCi. Since human CD45 antigens are confined to xenograft tumor cells in this model, and all murine tissues are devoid of hCD45 and will not bind anti-hCD45 Ab, we also compared one-step and PRIT using an anti-murine (m)CD45 Ab where the target antigen is present on normal hematopoietic tissues. After 24 hours, 27.3 ± 2.8% of the injected dose of activity was delivered per gram (% ID/g) of lymph node using 131I-A20-Ab compared with 40.0 ± 5.4% ID/g for pretargeted 111In-DOTA-biotin. These data suggest that pretargeted methods for delivering RIT may be superior to conventional RIT when targeting CD45 for the treatment of leukemia and may allow for the intensification of therapy, while minimizing toxicities.
Keywords: Radioimmunotherapy, CD45, leukemia
INTRODUCTION
Antibodies (Ab) conjugated to radionuclides have been used for over a decade to deliver targeted radiation to bone marrow, spleen, and other sites of acute myeloid leukemia (AML) while sparing normal organs.(1–4) At least 90% of myeloid leukemias express CD45 and the antigen is not found on tissues of non-hematopoietic origin, making CD45 an attractive target for treatment of leukemia.(5–10) Our group has performed clinical studies treating acute leukemias using myeloablative doses of an anti-CD45 radiolabeled Ab in combination with hematopoietic cell transplantation to reconstitute hematopoiesis.(11–13) These studies suggested that a greater radiation absorbed dose delivered to marrow may lead to lower post-transplant relapse rates for patients with high-risk AML.(14) Although these higher doses of radioimmunotherapy (RIT) appear to be more efficacious, many patients treated with this approach still relapse. Since acute leukemias have a relatively steep dose-response curve with no apparent radiation threshold for cell injury, this therapy could be even more effective and less toxic if the tumor-to-normal organ dose ratios could be improved.
While the use of RIT may lead to the accumulation of DNA damage in leukemia cells by delivering a prolonged, exponentially decreasing low dose-rate of radiation exposure, the suboptimal therapeutic index (risk-to-benefit ratio) currently achievable with conventional RIT methodologies is a general limitation of RIT. Pretargeted (P)RIT is a method that might improve the delivery of a cytotoxic radionuclide to target cells while reducing non-specific radiation to normal organs and thus minimizing toxicity.(15–23) PRIT first allows for maximal Ab targeting prior to delivery of the therapeutic radionuclide. The delivery of the radionuclide is mediated via attachment to a small molecule that allows for rapid tumor uptake and rapid excretion of non-tumor bound radioactivity. Synthetic clearing agents (CA) have been introduced as an additional refinement to PRIT studies to remove non-targeting immunoconjugates lingering in the bloodstream prior to administration of the radioactive moiety.(17–19)
To assess the merits of CD45 PRIT for leukemia, we here report comparative in vivo imaging, biodistribution, and therapy experiments using human leukemia xenografts implanted in athymic mice. In a series of fluorescent imaging studies we have demonstrated significantly superior localization to HEL leukemia tumor sites using PRIT compared with conventional RIT. In addition, a single treatment of pretargeted anti-human (h)CD45 Ab-streptavidin (SA) BC8 conjugate followed by a single dose of radio-biotin resulted in tumor-to-blood and tumor-to-normal organ radioactivity concentration ratios that improved by as much as 15-fold over those seen with a directly radiolabeled anti-hCD45 Ab, resulting in markedly enhanced therapeutic efficacy with the PRIT method. The murine tumor xenograft model has limitations, however, since only the human tumor cells bear the target antigen and the host immune system is defective. Furthermore, the HEL xenograft model consists of a single subcutaneous nodule, which is analogous to a chloroma, but is dissimilar from the disease pattern in most leukemia patients who have blood and marrow based disease. To counterbalance these limitations, we also report experiments in a syngeneic murine system utilizing an anti-murine (m)CD45 Ab A20 that targets normal hematopoietic CD45+ tissues, as is the case in human patients. Results from these syngeneic experiments demonstrated marked improvement in the hematopoietic organ to non-hematopoietic organ radioactivity concentration and absorbed dose ratios using PRIT due primarily to elimination of the initial non-specific radioactivity from circulating blood- when directly-labeled Abs were employed. Radiation dose calculations for the syngeneic model showed that at least twice as much radiation absorbed dose can be delivered to the marrow, and five times more to the spleen, using PRIT compared to doses delivered by a conventional radiolabeled Ab. These data suggest that anti-CD45 PRIT may be highly effective and may allow for intensification of the targeted radiotherapy, with diminished toxicity, to sites of leukemic involvement in order to decrease the risk of relapse.
MATERIALS AND METHODS
Mice
Female BALB/c athymic mice, 6 to 8 weeks old, were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Male B6 Ly5a mice were bred at the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA) and housed in a pathogen-free environment with acidified water and autoclaved chow. The animals were housed under protocols approved by the FHCRC Institutional Animal Care and Use Committee. Results from all mouse studies are representative of at least 2 experiments.
Cell Lines, Abs, and production of DOTA-Ab and Ab-SA conjugates
All cells were maintained as described previously.(19) The human erythroleukoblastic leukemia (HEL) cell line was obtained from American Type Culture Collection (Bethesda, MD). The BC8 hybridoma cell line expressing the anti-human IgG1 CD45 Ab was a gift from Claudio Anasetti (FHCRC). The hybridoma cell line secreting murine IgG2a A20 Ab, which recognizes the Ly5.1 epitope encoded by the Ly5a allotype of murine CD45, was a gift from Dr. Shoji Kimura of Memorial Sloan Kettering Cancer Center (New York, NY). The A20 Ab was produced from mouse ascitic fluid as previously described.(19) Isotype matched (IgG1) human anti-bovine herpesvirus-1 (BHV-1) Ab was produced from a hybridoma obtained from ATCC and employed as non-specific negative control Ab for human HEL xenograft experiments. Hybridoma culture supernatants from the BC8 and BHV-1 Abs were produced using hollow fiber bioreactor systems in the FHCRC. Rat polyclonal IgG (MS163) was obtained from Biomeda (Foster City, CA) and employed as non-specific negative control for murine syngeneic studies. DOTA-Ab and Ab-SA conjugates were produced as described previously.(19)
Radiolabeling
Antibodies were iodinated with Na131I or Na125I (PerkinElmer, Inc., Waltham, MA) by the chloramine-T method as previously published.(19) 111Indium (PerkinElmer, Inc.) radiolabeling of intact Ab-SA conjugates for PRIT was performed as described.(19) Radiochemical purity was typically greater than 99% as determined by HPLC for each construct, and labeling efficiencies were >90%. DOTA-biotin was synthesized as described.(19)
Biotinylated clearing agent
A synthetic biotinylated CA containing 16 N-acetyl-galactosamine-residues per dendrimeric molecule was a gift from NeoRx Corporation (Seattle, WA) and was used for PRIT experiments to eliminate excess Ab-SA molecules from the circulation prior to the administration of radiolabeled biotin. The N-acetyl galactosamine residues have a high affinity for hepatic asialoglycoprotein receptors and thus facilitate the rapid hepatic clearance of residual Ab-SA conjugates from the bloodstream and their endocytosis into liver cells.(17)
In vivo imaging studies
Anti-hCD45 BC8 whole Ab was used for conventional studies and was labeled with Xenofluor 680 (Caliper Life Sciences; Hopkinton, MA) according to the manufacturer’s instructions. The fluoresceinated BC8 Ab (1.4 nmol) was delivered i.v. via tail vein to athymic mice (n = 3) bearing HEL tumor xenografts at time = 0 hours. The group of pretargeted mice (n = 3) received 1.4 nmol of unlabeled anti-hCD45 BC8 Ab-SA i.v. at time = −27 hours followed by 5.8 nmol of CA at time = −3 hours. At time = 0 hours the pretargeted mice were injected i.v. with 100 μg of R-phycoerythrin biotin conjugate (Invitrogen, Carlsbad, CA). All mice were anesthetized with 2.5% isofluorane in O2. Each animal was imaged on a Xenogen IVIS 100 System (Caliper Life Sciences) at 12, 24, and 48 hours. Images were analyzed with Living Image v 2.50.2 imaging software (Caliper Life Sciences). Background fluorescence of each image was removed after calculation of a region of interest (ROI) located on negative control (untreated) mice, using the formula A-B*k; with A indicating fluorescent excitation measured, B indicating fluorescent emission measured, and k being the constant calculated by determining the ratio of the ROI from the excitation to the emission image. Fluorescence intensity and tumor-to-total body ratios were determined for each mouse using background-corrected fluorescent whole body images and a standard background ROI for comparison to measurements obtained from a ROI on each tumor.
Biodistribution studies
Mice were injected with 1 × 107 HEL cells subcutaneously (s.c.) in each flank to generate human leukemia xenograft tumors. Mice were selected for experimentation at a time when similar HEL tumor sizes (~100 mm3) were detectable (approximately 7–10 days after the s.c. administration of the HEL cells). Mice were placed on a biotin-free diet (Harlan Teklad, Madison, WI) for 5 days prior to receiving an Ab construct. Groups of 5 mice were injected i.v. via the tail vein with 1.4 nmol of 111In-labeled (50 μCi) anti-hCD45 (BC8) Ab or non-binding control BHV-1 Ab (conventional RIT) or unlabeled Ab-SA or BHV-1 Ab-SA conjugate (PRIT). Mice in PRIT groups were subsequently administered 5.8 nmol (50 μg) of CA i.v. 22 hours later followed by i.v. delivery of 1.2 nmol (1 μg) of 111In-DOTA biotin (50 μCi) 2 hours after CA. At 24 or 48 hours after injection, mice were bled from the retro-orbital venous plexus, euthanized, and tumors and normal organs were excised, weighed, and gamma counted for 111In activity using a Packard 5000 gamma counter (Packard Instrument Co, Downers Grove, IL). The percent administered activities per gram of tumor or organ (% ID/g) were determined (after correcting for radioactive decay using an aliquot of the injectate at each time point), as were the tumor-to-normal organ absorbed dose ratios.
To determine the biodistribution of anti-mCD45 (A20) DOTA-Ab and Ab-SA conjugates in syngeneic B6 Ly5a mice, animals were injected i.v. with either 0.67 nmol of A20 DOTA-Ab trace-labeled with 131I (50 μCi) or an equal molar amount of unlabeled A20 Ab-SA conjugate. Twenty hours after the injection of the Ab-SA conjugate these mice received 5.8 nmol of CA i.v. followed 4 hours later by i.v. delivery of 1.2 nmol of 111In-DOTA biotin. Control groups were injected with 0.67 nmol of the non-binding rat polyclonal IgG 131I-DOTA-Ab or Ab-SA conjugate followed by CA and 111In-DOTA-biotin. Groups of 5 mice were sacrificed at multiple time points from 2 to 96 hours after injection, and tissues were sampled, weighed, and counted as described above.
Radiation dosimetry
Time-activity curves were constructed for each organ from the syngeneic murine biodistribution data and the infinite-time integrals were calculated for the areas under curve to estimate the total number of radioactive transformations for dosimetry assuming that both A20 DOTA-Ab and A20 Ab-SA conjugates were labeled with 131I. Absorbed fractions of β-particle energy for 131I were calculated by applying electron transport theory to a dosimetric model for the laboratory mouse.(24) This model accounts for the size, shape, position, and density of organs, as well as for the overlap and contact from surrounding organs and tissues. The absorbed fractions included contributions from same-organ irradiation as well as from cross-organ irradiation. Contributions to the absorbed dose from 131I penetrating gamma radiation in mouse organs were negligible (<1% of the total dose) and were neglected. The femoral marrow was assumed to be a cylinder of 0.5 mm in radius; therefore, only a proportion (46%) of the radioiodine present in marrow was assumed to deposit its energy inside the marrow. The β-particle absorbed fraction for lymph nodes was estimated to be 73%. Results were expressed as organ absorbed dose (Gy).
RIT of leukemia xenografts
To compare the therapeutic efficacy of pretargeted and conventional radiolabeled Abs, groups of 10 HEL leukemia tumor-bearing mice were placed on biotin-free chow for 7 days and injected with 1.4 nmol (215 μg; 200–400 μCi) of directly labeled 90Y-anti-hCD45 BC8 Ab, control 90Y-BHV-1 Ab, or equimolar amounts (300 μg) of anti-hCD45 BC8 Ab-SA or BHV-1 Ab-SA conjugates followed 22 hours later by 5.8 nmol CA and 2 hours later by 1.2 nmol (1 μg) 90Y-DOTA-biotin labeled with 400, 800, or 1600 μCi of 90Y. Mice were monitored every other day for general appearance, tumor volume measurements, and body weight. Mice were euthanized if xenografts exceeded 10% of total body weight, caused obvious discomfort, or impaired ambulation. Tumor volumes (mm3) were calculated as previously described using the following equation: 4/3 A × B × C, where A, B, and C are the three dimensional radii in mm.(19) Time-to-tumor growth (taken as time to development of tumor volume >400 mm3) and time-to-death were treated as time-to-event endpoints, and Cox regression was used to test for a dose-response effect. For mice that received PRIT, levels of 1–5 were assigned to the groups that received no treatment, the control Ab, PRIT at the dose of 400 μCi, PRIT at the dose of 800 μCi, and PRIT at the dose of 1600 μCi 90Y-DOTA-biotin, respectively. For mice that received conventional RIT, levels of 1–4 were assigned to the groups that received no treatment, the control Ab, RIT at 200 μCi, and RIT at 400 μCi 90Y-anti-hCD45 Ab, respectively. A factor treating these levels as a continuous linear variable was then included in a Cox regression model to test for a dose-response effect (i.e., to ask if the risk of failure was associated with an decrease in dose).
RESULTS
In vivo fluorescent imaging
In vivo fluorescent imaging was performed in athymic mice bearing HEL leukemia xenografts at multiple time points after injection of either 1.4 nmol fluorophore-labeled anti-hCD45 DOTA-Ab (conventional) or 100 μg R-Phycoerythrin-biotin following delivery of 1.4 nmol unlabeled anti-hCD45 Ab-SA and CA (pretargeted), as described in the methods section (Fig. 1). In the conventional RIT group, most of the fluorescence after 12 hours was found in the blood pool and minimal tumor localization was observed (Fig. 1a). Although tumors were visible in the conventional RIT group at 24 and 48 hours, a large amount of fluorophore remained in the blood pool. The ratio of fluorescence in tumor-to-total body was 1:8.5 (Fig. 1c). Rapid uptake of fluorophore was observed in tumors after 12 hours in the pretargeted group (Fig. 1b) as well as rapid urinary excretion of unbound PE-biotin, manifested by a large amount of fluorescence in the bladder. By 24 hours, the majority of fluorescent activity in the bladder had cleared in the pretargeted group, resulting in high-contrast tumor images with a relative ratio of fluorescence in tumor-to-total body of 1:3 (Fig. 1c).
Fig. 1. Fluorescent images of HEL xenograft-bearing athymic mice treated with either conventional or PRIT.
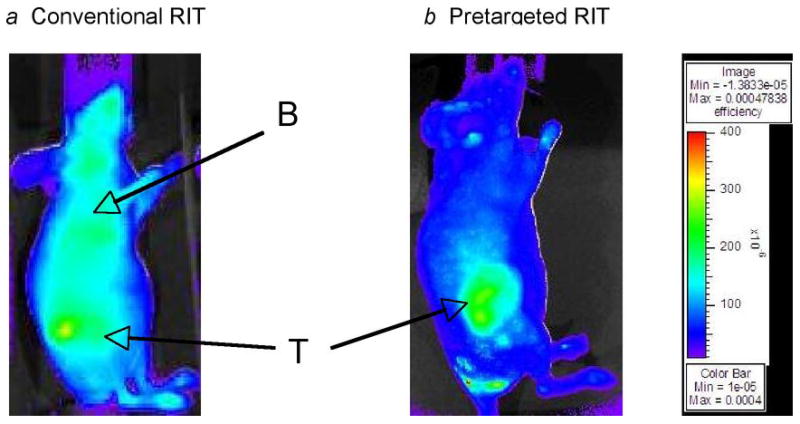
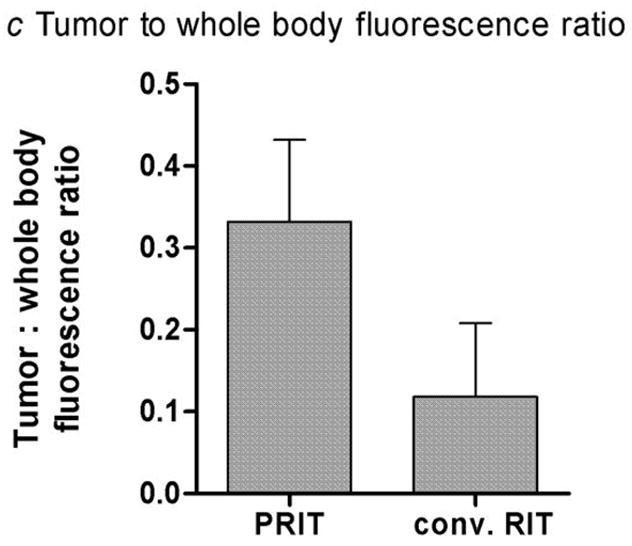
Mice were injected with either (a) 1.4 nmol anti-hCD45 Ab directly labeled with fluorophore or (b) pretargeted anti-hCD45 Ab-SA conjugate (1.4 nmol) followed 22 hours later by CA and then by 100 μg R-Phycoerythrin-biotin. Images are shown at same camera intensity settings. Images of mice are shown at time = 12 hours after injection of fluorophore. Arrows indicate fluorophore in tumor (T) and blood pool (B). (c) Tumor-to-total body fluorescence ratios 8 hours after-injection in conventional and PRIT mice.
Biodistributions of 111In in HEL leukemia tumor-bearing mice treated with directly-labeled 111In-anti-hCD45 Ab
Groups of 5 mice bearing human HEL xenografts were injected i.v. with 1.4 nmol of 111In-anti-hCD45 Ab or negative control 111In-anti-BHV-1 Ab. Mice were sacrificed 24 and 48 hrs following 111In-Ab administration (Fig. 2a). The peak concentration levels of 111In obtained in HEL tumors following conventional RIT employing 111In-anti-hCD45 Ab after 24 and 48 hours were 11.7 ± 1.0% ID/g and 17.4 ± 3.3% ID/g, respectively. The concentrations of radioactivity in normal organs at 24 and 48 hours using directly-labeled 111In-anti-hCD45 Ab ranged from 2.5% ID/g in stomach to 14.1% ID/g in kidney. In addition, circulating blood radioactivities after 24 and 48 hours were relatively high in mice receiving 111In-anti-hCD45 Ab. For example, concentrations in blood of 111In- anti-hCD45 Ab were 19.1 ± 0.29% and 20.5 ± 1.5% ID/g at 24 and 48 hours, respectively. At each time point, control animals injected with the non-binding 111In-BHV-1 Ab had low tumor uptake of radioactivity in HEL xenografts at 24 and 48 hours (5.6 ± 1.1% and 5.9 ± 1.3% ID/g, respectively), demonstrating the specificity of targeting.
Fig. 2. Biodistributions of radioactivity in HEL-xenograft bearing athymic mice injected with 111In-anti-hCD45 (BC8) DOTA-Ab conjugate for (a) conventional RIT or with 111In-DOTA-biotin after anti-hCD45 Ab-SA conjugate for (b) PRIT.
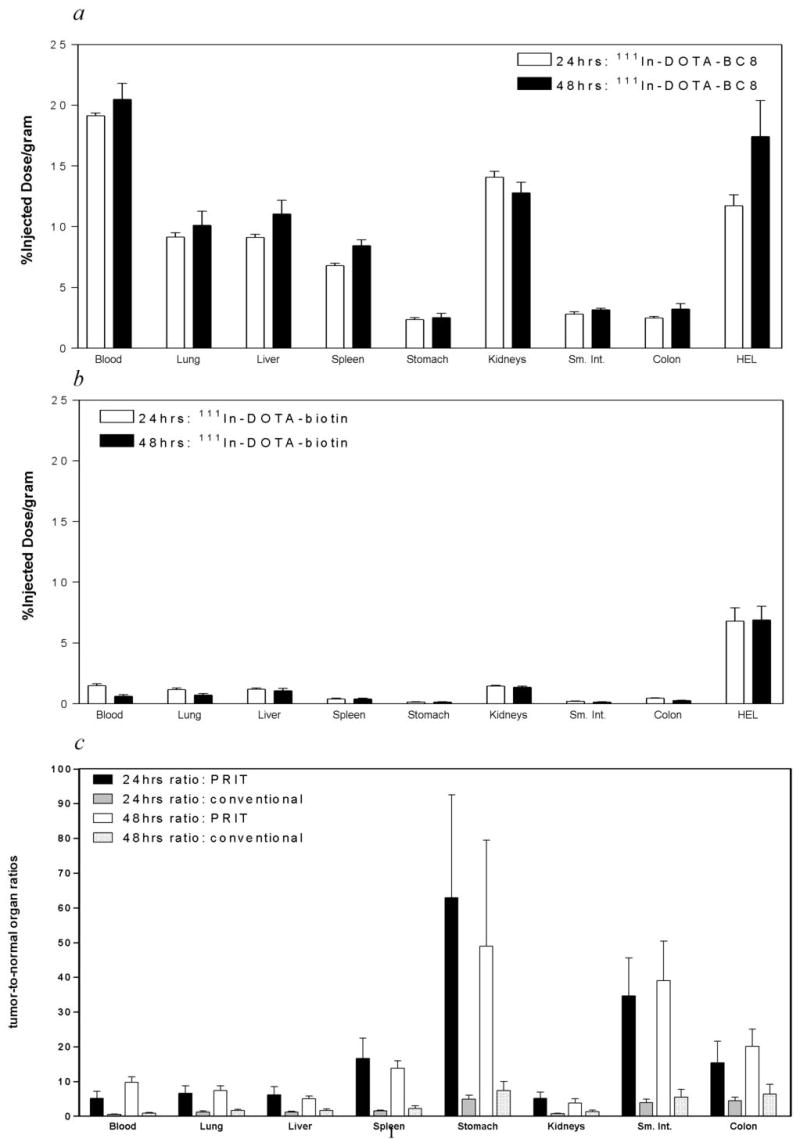
HEL xenograft bearing mice were injected i.v. via the tail vein with 1.4 nmol of either: (a) conventional trace-labeled 111In-anti-hCD45 DOTA-Ab or (b) anti-hCD45 Ab-SA conjugate followed 22 hours later with 5.8 nmol of CA and 2 hours subsequent with 1.2 nmol of 111In-DOTA-biotin. Groups of 5 mice were euthanized 24 (open bars) and 48 (solid bars) hours after injection of 111In. The radioactivity in blood, tumor, and normal organs was quantified by gamma counting, corrected for decay and expressed as % ID/g of tissue. (c) Tumor-to-normal organ ratios of radioactivity using either directly-labeled 111In-anti-hCD45 DOTA-Ab or pretargeted 111In-DOTA-biotin delivered after CA are shown 24 and 48 hrs after injection of radioactivity. In panel c, the first two bars represent results at 24 hours after administration of pretargeted 111In-DOTA-biotin (■) and directly-radiolabeled anti-hCD45 DOTA-Ab conjugate (▨), whereas the third and fourth bars represent tumor-to-normal organ ratios at 48 hours after pretargeted 111In-DOTA-biotin injection (□) and directly-radiolabeled anti-hCD45 DOTA-Ab conjugate (
 ).
).
Biodistributions of radioactivity after PRIT using anti-hCD45 Ab-SA conjugate
Pretargeted biodistribution studies were performed using HEL leukemia-bearing mice injected with 1.4 nmol of anti-hCD45 (BC8) Ab-SA, followed 22 hours later by 5.8 nmol of CA and 2 hours after that by 1.2 nmol 111In-DOTA-biotin. Groups of 5 mice each were sacrificed 24 and 48 hours after administration of the Ab-SA conjugate (Fig. 2b). The uptake of radioactivity in tumors of mice treated with pretargeted anti-hCD45 Ab-SA conjugate reached a maximum of 6.7 ± 2.4% ID/g 24 hours after administration of 111In-DOTA-biotin, and was still 6.9 ± 2.5% ID/g after 48 hours. The specificity of tumor targeting by anti-hCD45 Ab-SA conjugate in these experiments was demonstrated by comparison to control groups treated with BHV-1-Ab-SA followed by CA and 111In-DOTA-biotin. Control mice did not exhibit any appreciable radiobiotin uptake in tumors at any time point (e.g., 1.0 ± 0.25% ID/g at 24 hours).
At all time points assessed, the tumor-to-normal organ ratios were superior with the pretargeted anti-hCD45 Ab-SA conjugate compared to the ratios seen using the DOTA-Ab conjugate (Fig. 2c). At the 48-hour time point, the tumor-to-blood ratio was 10:1 using pretargeted anti-hCD45 Ab-SA but only 0.8:1 with directly-labeled DOTA-Ab conjugate. Additional tumor-to-normal organ ratios using the pretargeted approach with the BC8 Ab-SA conjugate at 48 hours varied from 50:1 in stomach to greater than 3.5:1 in kidney. At the same time point using conventional radiolabeled BC8 DOTA-Ab, the tumor-to-stomach ratio was 7.3:1 and the tumor-to-kidney ratio was approximately 1:1. These data suggest that PRIT produces markedly superior tumor-to-normal organ radioactivity concentration ratios compared to conventional radiolabeling, despite lower uptake of radioactivity into tumor xenografts.
RIT with either conventional or pretargeted anti-hCD45 Ab conjugates
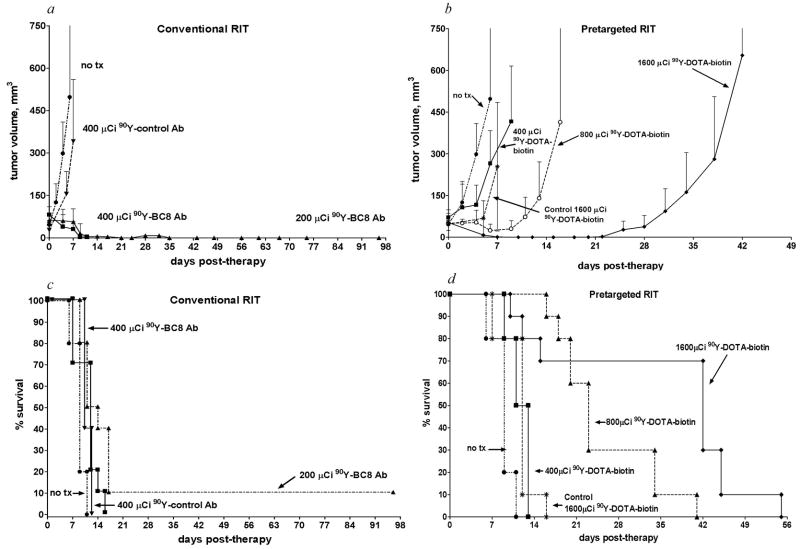
In view of the promising findings of the comparative biodistribution experiments described above, we performed therapy experiments to assess whether the superior biodistributions obtained with the PRIT method would translate to enhanced efficacy compared with conventional one-step RIT. Experimental groups of 10 human leukemia-bearing mice were injected with 1.4 nmol anti-hCD45 (BC8) Ab-SA, followed 22 hours later by 5.8 nmol CA, and then 2 hours after CA by 1.2 nmol 90Y-DOTA-biotin labeled with 400, 800, or 1600 μCi/mouse. Comparison groups were injected with 1.4 nmol directly radiolabeled 90Y-anti-hCD45 Ab (200 or 400 μCi/mouse). Control groups were either untreated or injected with 1.4 nmol of the non-binding BHV-1 Ab labeled with 400 μCi of 90Y or BHV-1 Ab-SA control conjugate followed by 5.8 nmol CA and then 1600 μCi 90Y-DOTA-biotin. All mice in the control groups experienced exponential growth of their leukemia xenografts requiring euthanasia before day 16 (Fig. 3). Mice treated with conventional one-step RIT using 200 μCi 90Y-anti-hCD45 Ab experienced transient remissions with complete regression of tumor seen in 30% of mice by day 11 after treatment (Fig. 3a). However, 90% of mice in this group exhibited huddling behavior, developed extensive petechiae, and died by day 17 (Fig. 3c). Mice treated with 400 μCi conventional one-step 90Y-anti-hCD45 Ab experienced more striking tumor regressions, with complete disappearance of xenografts in 40% of the animals by day 9 after therapy (Fig. 3a). However, all mice in this group experienced lethal toxicity, losing 26.7 ±15.5% of their initial weight and dying, likely from marrow suppression and infection, by day 11 (Fig. 3c). Mice were not treated with doses of direct conjugates higher than 400 μCi as this was a uniformly lethal dose.
Fig. 3. Regression of HEL leukemia xenografts and survival of mice after conventional and PRIT.
Athymic BALB/c mice bearing HEL leukemia xenografts were injected i.v. with either 1.4 nmol (a, c) BC8 Ab directly labeled with 200–400 μCi of 90Y, or (b, d) unlabeled BC8 Ab-SA conjugate followed 20 hours later by 5.8 nmol of CA, and 2 hours after that with 400–1600 μCi 90Y-DOTA-biotin. Control mice bearing xenograft tumors were either left untreated or treated in a similar manner with directly labeled BHV-1 Ab or pretargeted BHV-1-SA, respectively. Tumor volume curves (panel a represents conventional RIT; panel b represents PRIT) are truncated at the time of euthanasia of the first mouse in each group. These mice bearing HEL leukemia xenografts were also analyzed for survival as a function of time (panel c represents conventional RIT; panel d represents PRIT).
Experimental mice pretargeted with BC8 Ab-SA fared much better than other groups in terms of toxicity, tumor responses, and survival (Fig. 3b, d). Transient tumor responses were seen in mice receiving pretargeted anti- hCD45 Ab-SA plus 800 μCi 90Y-DOTA-biotin with a maximal response seen ~6 days after treatment; however, tumors rapidly recurred in all mice, leading to death by day 41 (Fig. 3b and d). In contrast, all mice receiving pretargeted BC8 Ab-SA plus 1600 μCi 90Y-DOTA-biotin achieved complete remissions (CR) before day 15. Three mice in the 1600 μCi 90Y-DOTA-biotin group died by day 16 from toxicity without tumor, while the remaining 7 mice died from tumor relapse between days 42 and 55 (Fig. 3d). There was minimal toxicity in mice pretargeted with anti-hCD45 Ab-SA followed by 400 or 800 μCi 90Y-DOTA-biotin, with only 4.7 ± 2.0 % weight loss in the 800 μCi 90Y-DOTA-biotin by day 4; all mice regained pre-treatment weight by day 11 (data not shown).
Overall, the hazard of tumor growth to >400 mm3 decreased as radionuclide dose increased in each group as dose increased but was statistically significant only for the mice treated with PRIT (p <0.0001 PRIT; p =0.31 for conventional RIT). However, the hazard of death decreased as radionuclide dose increased both for mice who received conventional RIT and for mice who received PRIT (p <0.0001 for each group).
Biodistributions of radioactivity after conventional and PRIT employing a syngeneic murine model using anti-murine DOTA-Ab and Ab-SA conjugates
We first assessed the efficacy of the CA using the PRIT strategy on blood clearance in a relevant syngeneic murine system using an anti-mCD45 Ab (A20) that targets normal hematopoietic CD45+ tissues, as is the case in human therapies directed toward the CD45 antigen. Groups of 5 syngeneic B6 Ly5a mice received 1.4 nmol of either 125I-A20 DOTA-Ab or 125I-A20 Ab-SA administered i.v., followed 22 hours later by 5.8 nmol of CA. The concentration of 125I-A20 Ab-SA in the blood decreased from 31.3 ± 0.29% ID/g of blood to 3.3 ± 2.1% ID/g within 1 hour after CA administration. By 48 hours after CA injection, 2.6 ± 0.4% ID/g of 125I-A20 Ab-SA conjugate was still circulating in blood.
The hematolymphoid biodistributions of radioactivity of directly-labeled anti-mCD45 (A20) DOTA-Ab and pretargeted A20 Ab-SA conjugates were then compared in the syngeneic B6 Ly5a mouse model. These biodistribution studies were performed using mice injected with 1.4 nmol of either directly-labeled 131I-A20 Ab alone or pretargeted A20 Ab-SA followed 20 hours later by 5.8 nmol of CA and 4 hours after that by 1.2 nmol 111In-DOTA-biotin. Groups of 5 mice were sacrificed at time points between 2 and 96 hours after injection of radioactivity, and their organs were harvested, washed, weighed and assayed for 131I and 111In activity. Specific localization of conventional 131I-A20 Ab or pretargeted 111In-DOTA-biotin in hematolymphoid organs including blood, bone marrow, lymph node, and spleen is depicted in Fig. 4. At all time points, control animals injected with non-specific 131I-labeled rat polyclonal anti-murine IgG Ab and 111In-Ab-SA exhibited negligible uptake of the radiolabel in hematolymphoid organs demonstrating the specificity of targeting in these experiments (data not shown).
Fig. 4. Biodistributions of radioactivity in blood, bone marrow, spleen, lymph node, and lung of non-tumor bearing B6 Ly5a syngeneic mice injected with directly-radiolabeled anti-mCD45 DOTA-Ab or 111In-DOTA-biotin after pretargeted anti-mCD45 Ab-SA conjugate.
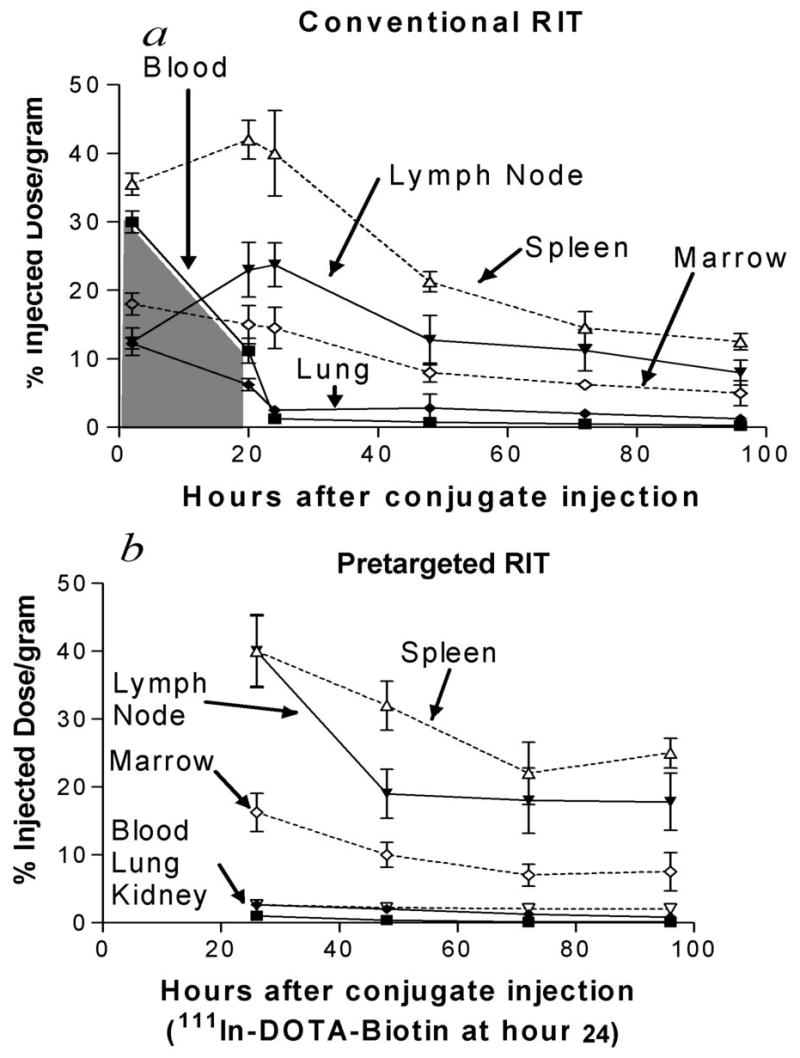
Non-tumor bearing B6 Ly5a mice were injected with 1.4 nmol of (a) conventional trace-labeled 131I-anti-mCD45 (A20) DOTA-Ab or (b) pretargeted anti-mCD45 Ab-SA conjugate followed 20 hours later by 5.8 nmol of CA and 2 hours after that by 1.2 nmol 111In-DOTA-biotin. Groups of 5 mice were euthanized at either 2, 20, 24, 48, 72, and 96 hours after injection of the anti-murine Ab conjugates. The radioactivity concentrations in blood, bone marrow, spleen, lymph node, and lung were quantified by gamma counting and expressed as %ID/g. The shaded area-under-the-curve in panel a represents the initial 20 hours of non-specific radiation dose from blood-borne radiolabeled Ab that can potentially be eliminated with the use of PRIT.
Specific uptake of directly-labeled 131I-anti-mCD45 A20 Ab in target tissues reached a maximum value by 20 hours with 15 ± 2.8% ID/g delivered to marrow and 42 ± 2.7% ID/g to the spleen (Fig. 4a). The targeted concentrations of radioactivity remained at relatively high levels in the bone marrow and spleen using the directly-labeled anti-mCD45 Ab with >8% ID/g present in these target tissues 48 hours after administration of the radiolabeled Ab. The amount of absorbed radioactivity also remained relatively high in the blood and normal organs, however, with 11.2 ± 1.8% ID/g remaining in the blood and 6.2 ± 0.88% ID/g in the lung after 20 hours (Fig. 4a). Despite the relatively rapid clearance of the radiolabeled Ab from the blood, clearance from the circulation may still be too slow for optimal RIT without a pretargeted approach. In particular, PRIT with a CA has the potential to eliminate the initial 20 hours of non-specific radiation exposure from blood-borne directly-labeled A20 Ab (represented by the shaded area-under-the-curve, Fig. 4a). Consequently, a series of biodistribution experiments employing the PRIT strategy were performed in the syngeneic murine CD45 system using non-tumor bearing B6 Ly5a mice to examine the effect of eliminating the non-specific radiation exposure from blood-borne radiolabeled Ab. Pretargeted anti-mCD45 A20 Ab-SA conjugate followed by the CA resulted in favorable biodistributions of radioactivity at each time point assessed (Fig. 4b). After 24 hours, 27.3 ± 2.8% ID/g was delivered to lymph nodes using 131I-A20 Ab compared with 40.0 ± 5.4% ID/g for pretargeted 111In-DOTA-biotin following A20 Ab-SA administration. Superior hematopoietic organ-to-non-hematopoietic organ ratios of radioactivity were seen using the A20 Ab-SA pretargeted conjugate compared to directly-labeled 131I-A20 Ab. The lymph node-to-blood ratios after 24 hours were 40:1 with the pretargeted A20 Ab-SA conjugate compared to 13.5:1 for conventional 131I-A20 Ab. Table I summarizes the mean radiation absorbed doses imparted to each hematolymphoid organ using either conventional targeting of mCD45 or PRIT under conditions delivering 10 Gy to the liver. The calculated radiation absorbed doses to the marrow and lymph node were at least 2-fold greater using the PRIT strategy while 5-times more was delivered to the spleen compared to doses delivered by a directly-labeled anti-mCD45 DOTA-Ab. A liver dose of 10 Gy was selected because this dose produces dose-limiting hepatic toxicity in human clinical trials of 131I-BC8 combined with high-dose chemotherapy and TBI.(11)
Table 1.
Estimated absorbed radiation doses (Gy) delivered to marrow, spleen, lymph nodes, and thymus using conventional RIT and PRIT when targeting 10 Gy to the liver.
| Conventional RIT | PRIT | |
|---|---|---|
| spleen | 49 Gy | 250 Gy |
| marrow | 27 Gy | 49 Gy |
| lymph node | 31 Gy | 66 Gy |
| thymus | 12 Gy | 12 Gy |
DISCUSSION
The data presented in this study are the first to compare conventional and pretargeted anti-hCD45 RIT in an acute leukemia xenograft model. The results demonstrated clearly that anti-CD45 PRIT provided rapid tumor localization and improved biodistributions of radioactivity with significant improvements in efficacy compared with conventional anti-CD45 RIT. While the concentration of activity in leukemia xenografts decreased with the PRIT strategy compared to conventional RIT (6.7% ID/g vs. 11.7% ID/g, respectively, at 24 hours), the concentration of 111In in the blood at 24 hours decreased by 93% and the level in kidneys by 89%. These findings are likely attributable to the use of the clearing agent and its ability to not only remove excess circulating Ab-SA conjugate from the circulation, but also conjugate localized to tumors. Indium-111 was utilized as a surrogate gamma-emitting radiometal in biodistribution experiments since 90Y does not emit gamma photons.(25, 26) Since the toxic effects on normal tissues must be balanced by the cytotoxic effects on tumor cells, the pretargeted approach was markedly superior to conventional RIT when the therapeutic index (target-to-non-target ratio) of each targeting strategy was considered. Conventional radiolabeled anti-hCD45 Ab achieved low tumor-to-blood ratios of less than 1:1 and tumor-to–normal organ ratios of 7:1 or less. Conversely, the PRIT approach produced tumor-to–normal organ radioactivity concentration ratios that were far superior, with tumor-to-blood ratios of up to 10:1 and tumor-to-normal organ ratios of 50:1 or higher, albeit at the risk of removing a non-negligible amount of pretargeted Ab-SA conjugate from the xenograft. In vivo images displayed excellent tumor imaging with rapid localization of fluorophore-DOTA-biotin into tumors of the pretargeted animals within 5 hours. The efficient clearance of excess circulating immunoconjugate from the vasculature by the CA would suggest that in the setting of radiolabeled-DOTA-biotin, rapid urinary excretion of any excess unbound radionuclide would occur, thus minimizing non-specific irradiation to normal organs and contributing to the superiority of the tumor images with the pretargeted method. Conversely, tumor images generated using conventional RIT with fluoresceinated anti-hCD45 Ab exhibited a high residual level of activity persisting in the blood and other non-target organs. The improved tumor localization of the anti-hCD45 Ab using PRIT was predicted to translate into improved xenograft response rates and survival rates. We thus performed comparative therapeutic RIT studies in mice treated with either directly-labeled anti-hCD45 Ab or anti-hCD45 Ab-SA, followed by CA, and then increasing doses of 90Y-DOTA-biotin. Since the therapeutic index of an anti-neoplastic agent depends on the balance between toxic effects on normal tissues and cytotoxic effects on tumor cells, it is not surprising that the pretargeted approach may be superior to conventional RIT in terms of tumor regressions and improved overall survival.
While these mouse leukemia xenograft experiments with anti-hCD45 PRIT were encouraging and established the ability of a pretargeted anti-hCD45 Ab-SA conjugate to effectively localize to CD45 expressing human tumor cells, this model differs significantly from the state of naturally occurring leukemias in patients. In the xenograft system the human target CD45+ cells are confined to tumors, which are more similar to chloromas than to typical disseminated leukemia. In addition, all other tissues in the xenograft model are devoid of hCD45 and will not bind anti-hCD45 Ab. Leukemia patients, however, display the hCD45 antigen on almost all normal hematopoietic tissues (bone marrow, lymph node cells, Kupfer cells, etc.) as well as on malignant cells. Toxicity profiles, particularly hematopoietic toxicities, in the human may thus not be reliably mimicked in xenograft systems. To overcome these limitations, we tested anti-CD45 PRIT in a complementary murine syngeneic system in which the target antigen was present on normal myeloid, lymphoid, and reticuloendothelial tissues. We have shown in the murine syngeneic model that the CA efficiently reduced circulating anti-mCD45 Ab-SA conjugate from the blood and thus limited the amount of radionuclide delivered to non-target normal organs, resulting in higher target organ to non-target organ radioactivity ratios using PRIT compared to the ratios seen using standard directly-labeled Ab. These data are supported by preliminary studies in a separate pre-clinical syngeneic leukemia model where PRIT appears to improve the therapeutic index over that currently achievable with conventional RIT methodologies.(20)
In addition to the SA-biotin PRIT system, other methods of PRIT have been described including bi-specific Abs that recognize a radiolabeled hapten and the targeted tumor antigen as well as biotinylated Abs capable of binding avidin or SA.(27–29) While these PRIT approaches form a base of noteworthy principles and encouraging results, further advances are required to achieve optimal targeting and elimination of leukemia cells by PRIT. One recent improvement in the anti-CD45 PRIT technology has been the enhanced uniformity of the Ab-SA targeting molecule. Our group has developed a recombinant fusion protein consisting of an anti-CD45 single-chain Ab fused to SA that can be expressed in a soluble tetrameric form in the periplasm of E. coli.(21) This fusion protein has been shown to maintain the full antigen and biotin binding capabilities of its parent molecules, allowing for large-scale cGMP production of the anti-CD45 fusion protein for future PRIT trials.
In conclusion, the data presented in this report suggest that anti-CD45 PRIT has the potential to significantly augment the efficacy of RIT and reduce the radiation exposure to normal organs compared with a conventional, directly labeled anti-CD45 radioimmunoconjugate in patients with AML. The results from this study have prompted large-scale cGMP production of the anti-CD45 fusion protein for PRIT trials to be conducted in patients with advanced myeloid leukemias. It remains to be determined if using an anti-CD45 Ab-SA conjugate in patients with disseminated leukemia will be more effective and less toxic than directly labeled monoclonal Abs as suggested by these murine experiments, however, the current studies pave the way for human clinical trials which we anticipate opening in 2009.
Acknowledgments
This work was supported by NIH Grants RO1 CA109663, P01 CA44991, and K08 CA095448, and the Frederick Kullman and Penny E. Petersen Memorial Funds. OWP is supported by an endowed Chair from James and Shirley Raisbeck. JMP is supported by Career Development Awards from the Lymphoma Research Foundation and the Damon Runyon Cancer Foundation.
References
- 1.Appelbaum F, Matthews D, Eary JF, et al. The use of radiolabeled anti-CD33 antibody to augment marrow irradiation prior to marrow transplantation for acute myelogenous leukemia. Transplantation. 1992;54:829–33. doi: 10.1097/00007890-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Scheinberg DA, Lovett D, Divgi CR, et al. A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. J Clin Oncol. 1991;9:478–90. doi: 10.1200/JCO.1991.9.3.478. [DOI] [PubMed] [Google Scholar]
- 3.Press OW, Eary JF, Appelbaum FR, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support [see comments] N Engl J Med. 1993;329:1219–24. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 4.Gopal AK, Pagel JM, Rajendran JG, et al. Improving the efficacy of reduced intensity allogeneic transplantation for lymphoma using radioimmunotherapy. Biol Blood Marrow Transplant. 2006;12:697–702. doi: 10.1016/j.bbmt.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Nakano A, Harada T, Morikawa S, Kato Y. Expression of leukocyte common antigen (CD45) on various human leukemia/lymphoma cell lines. Acta Pathol Jpn. 1990;40:107–15. doi: 10.1111/j.1440-1827.1990.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 6.Taetle R, Ostergaard H, Smedsrud M, Trowbridge I. Regulation of CD45 expression in human leukemia cells. Leukemia. 1991;5:309–14. [PubMed] [Google Scholar]
- 7.Friesen C, Glatting G, Koop B, et al. Breaking chemoresistance and radioresistance with [213Bi]anti-CD45 antibodies in leukemia cells. Cancer Res. 2007;67:1950–8. doi: 10.1158/0008-5472.CAN-06-3569. [DOI] [PubMed] [Google Scholar]
- 8.Glatting G, Muller M, Koop B, et al. Anti-CD45 monoclonal antibody YAML568: A promising radioimmunoconjugate for targeted therapy of acute leukemia. J Nucl Med. 2006;47:1335–41. [PubMed] [Google Scholar]
- 9.Jurcic JG. Immunotherapy for acute myeloid leukemia. Curr Oncol Rep. 2005;7:339–46. doi: 10.1007/s11912-005-0060-7. [DOI] [PubMed] [Google Scholar]
- 10.Kotzerke J, Bunjes D, Scheinberg DA. Radioimmunoconjugates in acute leukemia treatment: the future is radiant. Bone Marrow Transplant. 2005;36:1021–6. doi: 10.1038/sj.bmt.1705182. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DC, Appelbaum FR, Eary JF, et al. Phase I study of (131)I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94:1237–47. [PubMed] [Google Scholar]
- 12.Pagel J, Appelbaum F, Sandmaier B, et al. 131I-anti-CD45 antibody plus fludarabine, low-dose total body irradiation and peripheral blood stem cell infusion for elderly patients with advanced acute myeloid leukemia (AML) or high-risk myelodysplastic syndrome (MDS) Blood. 2005:106. doi: 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagel JM, Appelbaum FR, Eary JF, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184–91. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagel J, Gooley T, Rajendran J, et al. Targeted radiotherapy using 131i-anti-CD45 antibody followed by allogeneic hematopoietic cell transplantation (HCT): the relationships among dosimetry, bone marrow uptake, and relapse. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33:S193. [Google Scholar]
- 15.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823–34. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 16.Barbet J, Kraeber-Bodere F, Vuillez JP, Gautherot E, Rouvier E, Chatal JF. Pretargeting with the affinity enhancement system for radioimmunotherapy. Cancer Biother Radiopharm. 1999;14:153–66. doi: 10.1089/cbr.1999.14.153. [DOI] [PubMed] [Google Scholar]
- 17.Axworthy DB, Reno JM, Hylarides MD, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci U S A. 2000;97:1802–7. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Press OW, Corcoran M, Subbiah K, et al. A comparative evaluation of conventional and pretargeted radioimmunotherapy of CD20-expressing lymphoma xenografts. Blood. 2001;98:2535–43. doi: 10.1182/blood.v98.8.2535. [DOI] [PubMed] [Google Scholar]
- 19.Pagel J, Hedin N, Subbiah K, et al. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101:2340–8. doi: 10.1182/blood-2002-03-0874. [DOI] [PubMed] [Google Scholar]
- 20.Pagel JM, Hedin N, Drouet L, et al. Conventional and pretargeted radioimmunotherapy using an anti-murine CD45 monoclonal antibody in a syngeneic, disseminated murine leukemia model. Blood. 2006:108. doi: 10.1182/blood-2007-06-097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y, Pagel JM, Axworthy D, Pantelias A, Hedin N, Press OW. A genetically engineered anti-CD45 single-chain antibody-streptavidin fusion protein for pretargeted radioimmunotherapy of hematologic malignancies. Cancer Res. 2006;66:3884–92. doi: 10.1158/0008-5472.CAN-05-3443. [DOI] [PubMed] [Google Scholar]
- 22.Yao Z, Zhang M, Garmestani K, et al. Pretargeted alpha emitting radioimmunotherapy using (213)Bi 1,4,7,10-tetraazacyclododecane-N, N′,N″,N‴-tetraacetic acid-biotin. Clin Cancer Res. 2004;10:3137–46. doi: 10.1158/1078-0432.ccr-03-0171. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Zhang Z, Garmestani K, et al. Pretarget radiotherapy with an anti-CD25 antibody-streptavidin fusion protein was effective in therapy of leukemia/lymphoma xenografts. Proc Natl Acad Sci U S A. 2003;100:1891–5. doi: 10.1073/pnas.0437788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews DC, Badger CC, Fisher DR, et al. Selective radiation of hematolymphoid tissue delivered by anti-CD45 antibody. Cancer Res. 1992;52:1228–34. [PubMed] [Google Scholar]
- 25.Onthank DC, Liu S, Silva PJ, et al. 90Y and 111In complexes of a DOTA-conjugated integrin alpha v beta 3 receptor antagonist: different but biologically equivalent. Bioconjug Chem. 2004;15:235–41. doi: 10.1021/bc034108q. [DOI] [PubMed] [Google Scholar]
- 26.Leonard JP, Siegel JA, Goldsmith SJ, et al. Comparative physical and pharmacologic characteristics of iodine-131 and yttrium-90: implications for radioimmunotherapy for patients with non-Hodgkin’s lymphoma 90Y and 111In complexes of a DOTA-conjugated integrin alpha v beta 3 receptor antagonist: different but biologically equivalent. Cancer Invest. 2003;21:241–52. doi: 10.1081/cnv-120016421. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg DM, Rossi EA, Sharkey RM, McBride WJ, Chang CH. Multifunctional antibodies by the Dock-and-Lock method for improved cancer imaging and therapy by pretargeting. J Nucl Med. 2008;49:158–63. doi: 10.2967/jnumed.107.046185. [DOI] [PubMed] [Google Scholar]
- 28.Goldenberg DM, Sharkey RM. Novel radiolabeled antibody conjugates. Oncogene. 2007;26:3734–44. doi: 10.1038/sj.onc.1210373. [DOI] [PubMed] [Google Scholar]
- 29.Sharkey RM, Karacay H, Litwin S, et al. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin’s lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer Res. 2008;68:5282–90. doi: 10.1158/0008-5472.CAN-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]