Abstract
Brevetoxins (polyether breve toxins; PbTx) are polyether neurotoxins produced by the marine dinoflagellate Karenia brevis, an organism associated with red tide blooms in the Gulf of Mexico and along the Atlantic coast from Florida to North Carolina. Brevetoxin-3 (PbTx-3) is a major component of the array of brevetoxins found in marine aerosols measured along red tide affected beaches. Humans exposed to aerosolized brevetoxins for short periods of time often suffer a variety of adverse health effects. It was consequently of interest to assess the potential for aerosolized brevetoxin to produce a neurotoxic response. Female BALB/c mice were exposed nose-only for 2 consecutive days to PbTx-3 aerosol, with a 2-h exposure on the first day and a 4-h exposure on the second day. The average PbTx-3 exposure concentrations on days 1 and 2 were 312 ± 113 μg brevetoxin 3/m3 and 278± 24μg brevetoxin 3/m3, respectively. The brevetoxin-containing aerosol had a mass median aerodynamic diameter of 0.92μm with a geometric standard deviation of 1.38. Coronal sections of mouse brains were evaluated for neuronal damage using both silver and Fluoro-Jade B staining to identify degenerating neuronal elements. PbTx-3 inhalation exposure produced neuronal degeneration in the posterior cingu-late/retrosplenial cortex of mice as evidenced by silver-positive degenerating neurons in this region. No staining was found in other regions of the PBTx-3-exposed mouse brains or in brains of control, sham-exposed mice. The existence of a neurotoxic insult in PbTx-3-exposed mice was confirmed using Fluoro-Jade B to label degenerating neurons. Fluro-Jade-positive neurons were observed in the retrosplenial cortex of PBTx-3 exposed, but not control, mice. These results suggest that subacute exposure to PbTx-3 for 2 days is sufficient to induce neuronal degeneration in a discrete region of the mouse cerebral cortex.
Florida red tides are produced by the dinoflagellate Karenia brevis. They occur almost annually along Florida’s west coast, and occasionally along the Atlantic coast (Kusek et al., 1999). Tides of notable severity occurred in 1971, 1973–1974, 1996, and 2005. Karenia brevis red tides have been responsible for killing millions of fish, manatees (Bossart et al., 1998), birds (Forrester et al., 1977; Kreuder et al., 2002), and bottlenose dolphins (Naar et al., 2002). These events result in significant economic losses due to decreased tourism, closing of shellfish beds, and extensive marine life mortality (Bossart et al., 1998; Kirkpatrick et al., 2004; Fleming et al, 2005a).
Karenia brevis produces a series of lipid-soluble multiring polyethers known as brevetoxins. Ten brevetoxins have been isolated and identified from field blooms and K. brevis cultures (Baden, 1989; Baden et al., 2005). Brevetoxin 2 (PbTx-2) and brevetoxin 3 (PbTx-3) are the most predominant brevetoxins produced by K. brevis. Brevetoxins 2, 3, 6, and 9 have been identified along red-tide-affected beaches (Cheng et al., 2005b), with concentrations of PbTx-3 and PbTx-2 being predominant in marine aerosols.
During red tides, onshore winds and breaking surf can incorporate brevetoxins into marine aerosols by bubble-mediated transport (Pierce et al., 1989). Recently, responses of 129 individuals recreationally exposed to brevetoxins during two separate red tide events were measured and correlated with concentrations of brevetoxins in the marine aerosols (Backer et al., 2003; Pierce et al., 2003). Exposure was characterized at three levels: low or no exposure (<10 ng m3; n = 36), moderate exposure(<10-36 ng/m3; n = 53), and high exposure posure (20–93 ng/m3;n = 40). A statistically significant number of persons in the moderate exposure group (35%) reported upper-respiratory-tract irritation, including eye or throat irritation, nasal congestion, or cough. A significant number of individuals in the high exposure group (28%) reported lower-respiratory-tract effects, including chest tightness, wheezing, or shortness of breath. Some individuals had increases in inflammatory cells in their nose or throat as measured by cytologic examination of nose and throat swabs. Further, Fleming and coworkers reported decrements in pulmonary function among asthmatic children during Florida red tides (Fleming et al., 2005b).
Studies with neuroblastoma cells and rat brain synaptosomes have shown that brevetoxins interact with neurotoxin site 5 on the alpha subunit of voltage-gated sodium channels (VGSCs) (Catterall & Gainer, 1985; Poli et al., 1986). Four distinct effects on neurons have been identified: a shift in the activation potential to more negative values, prolongation of mean channel open time, inhibition of channel inactivation, and induction of subconductance states (Jeglitsch et al., 1998). These changes in sodium channel function promote depolarization of neurons at resting membrane potentials and can account for the acute neurotoxicity of brevetoxins in vivo and may play a role in brevetoxin-induced bronchoconstriction (Asai et al., 1982).
Little is known about how brevetoxins affect the brain following inhalation. Systemically absorbed brevetoxins distribute to the brain (Tibbetts et al., 2006; Benson et al., 1999; Cattet & Geraci, 1993) and localize primarily in the cerebellum, with lower amounts in the cerebral cortex (Bourdelais et al., 2004). The effect of inhaled brevetoxins on the central nervous system (CNS) may therefore vary with brain region and cell type.
The purpose of this study was to examine the effects of inhaled brevetoxin 3 (PbTx-3) on the brain following 2 days of inhalation exposure in mice. Since people are often exposed to red-tide aerosols containing brevetoxins for short periods (e.g., recreational weekend), it is important to determine whether neuronal damage can occur following a subacute exposure. PbTx-3 was chosen for these studies inasmuch as it is a major component of the brevetoxin mixture produced by K. brevis (Baden, 1989) and of brevetoxin-containing aerosols measured along red-tide-affected beaches (Cheng et al., 2005a).
METHODS
Chemicals
Brevetoxin-3 (molecular weight [MW] 897.1) was isolated and purified from the Wilson clone of K. brevis at the Center for Marine Sciences, University of North Carolina, Wilmington. Enzyme-linked immunosorbent assay (ELISA) kits for breve-toxin analysis were obtained from Dr. Jerome Naar, University of North Carolina, Wilmington.
Animals
Female BALB/c mice were purchased from Charles River Laboratory, Wilmington, MA. The animals were approximately 8 wk old upon receipt and were quarantined for 14 d before being assigned to the study. All mice were housed in polycarboneate cages with hardwood chip bedding; the animal rooms were maintained at 20–22°C with relative humidity at 20–50% and a 12-h light cycle beginning at 0600 h. Food (Harlan Teklad rodent diet [W], Madison, WI) and water were provided ad libitum. Prior to exposure, the mice were conditioned to nose-only restraint tubes for 0.5, 2, and 4 h, over a 3-day period. Mice were randomized by weight into control and exposed groups. The study protocol was approved by the Lovelace Respiratory Research Institute Institutional Animal Care and Use Committee.
Exposure System
The exposure system consisted of two 36-port cylindrical nose-only inhalation chambers (InTox Products, Edgewood, NM), each supplied with a single nebulizer (Hospitak, Inc., Farmingdale, NY). The nebulizers were operated at 6 psi. A dessicant-containing trap was included in the aerosol transfer line to dry the aerosol. The total flow rate through the chamber was10 L/min. The exposure system was located within a glove box. Temperatures within the box were monitored continuously with an acceptable range of 18–22°C. Chamber oxygen concentration was monitored continuously with an action level at ≤18%.
Aerosol Generation and Characterization
Stock solutions containing 0.5 mg brevetoxin/ml were prepared in 100% ethanol. Generator solutions for brevetoxin exposure were prepared daily by dilution the stock solution with 0.9% saline to achieve a final PbTx-3 concentration of 0.078 mg/ml. The generator solution for the vehicle control animals consisted of 30% ethanol in saline. The total aerosol concentrations in the control and brevetoxin exposure atmospheres were determined gravimetrically at approximately half-hour intervals throughout the exposure period. Aerosol was collected from the breathing zone of the control and brevetoxin-exposed animals at aflow rate of 2 L/min onto preweighed 25-mm glass fiber filters (SKC Gulf Coast, Houston, TX). The brevetoxin aerosol concentration was estimated by multiplying the total mass collected on the filter (sodium chloride plus brevetoxin) by the fraction of the mass contributed by brevetoxin (0.1). Brevetoxin content of the mass collected on each of the filter samples was confirmed by ELISA (Benson et al., 2004; Naar et al., 2002). The stability of the total aerosol concentration during the exposure was monitored using a TSI Dust Trak real-time aerosol monitor (TSI Industries, Shoreview, MN). The particle size distributions (mass median aerodynamic diameter [MMAD] and geometric standard deviation μg) of the aerosols were determined using an eight-stage cascade impactor (InTox Products).
Experimental Design
Groups of three BALB/c mice per exposure level (control and brevetoxin-exposed) were exposed to the sodium-choride-based aerosol containing a target concentration of 300 μg PbTx-3/m3. The animals were exposed on 2 consecutive days to aerosol, with a 2-h exposure on the first day and a 4-h exposure on the second day. The exposure paradigm was chosen based on the results of a pilot study suggesting neuronal changes in brain of CBA/CaJ mice exposed to brevetoxin for 2 h on one day followed by 4 h on a second day (unpublished observations). Following each day’s exposure the animals were observed for general behavior after return to their home cage.
Perfusion
Approximately 18 h after the second exposure the animals were sacrificed. Five minutes before euthanasia, each animal was administered heparin by introperitoneal (ip) injection (200 U/kg). The animals were than euthanized by ip injection of an overdose of Euthasol (Virbac AH, Inc., Fort Worth, TX) followed by induction of pneumothorax. Fixation was by whole-body perfusion. Briefly, the chest cavity was opened and a cannula was placed in the left ventricle of the heart. Blood was removed from the vasculature using a perfusion wash (0.8 NaCl, 4% dextrose, 0.8% sucrose, 0.023% anhydrous calcium chloride, and 0.034% hydrated sodium cacodylate). When the liver had blanched and the perfusate was clear, fixation was achieved by perfusing the vasculature with fixative (4% sucrose, 4% paraformaldehyde, and 1.4% hydrated sodium cacodylate). The head was then depleted, the skull cap was removed, and the brain was fixed within the skull in a bath of fixative for 48 h before removing for analysis.
Silver Staining
Neurons undergoing degeneration become argyrophilic and may therefore be specifically detected with silver staining protocols. Whole brains were fixed by immersion in 4% paraformaldehyde/0.1 M phosphate-buffered saline (PBS) and then cryoprotected in a 30% sucrose solution for 48 h before sectioning. Coronal sections were cut on a cryostat at a thickness of 40 μm and then reimmersed in the paraformaldehyde fixative solution. The sections were then rinsed and incubated in proprietary reagents according to the manufacturer’s instructions (FD Neuro Silver Kit, FD Neurotechnologies, Inc., Baltimore, MD). This commercial kit has been demonstrated to be a sensitive approach to detect degenerating neurons in a variety of neurotoxicological and neuropathological investigations (McCormack et al., 2002; Manning-Bog et al., 2003; Lo Bianco et al., 2004). Silver-stained sections were mounted, coverslipped without dehydration in ethanol, and stored protected from light. The brain regions examined included the cerebral cortex, hippocampus, striatum, thalamus, hypothalamus, and cerebellum. Inasmuch as the PbTx-3-induced argyrophilia was restricted to the retrosplenial cortex, the total number of degenerating neurons per section was determined in five coronal sections through the posterior cingulate/retrosplenial cortices of each of three mice for both treatment groups. This cortical area was assigned with the aid of the Paxinos and Franklin (2004) atlas and is equivalent to the retrosplenial granular cortex (Corso et al., 1997).
Fluoro-Jade B Staining
Whole brains were fixed by immersion as already described. Brain sections were mounted on slides, warmed at 50°C for 1.5 h, and then immersed in a 1% sodium hydroxide solution (80% ethanol) for 5 min. Slides were placed in 70% alcohol for 2 min and then rinsed in distilled water for 2 min. The slides were transferred to a solution of 0.06% potassium permanganate for 10 min on a shaker table to ensure consistent background suppression between sections. The slides were then rinsed in distilled water for 2 min. The staining solution was prepared from a 0.01% stock solution of Fluoro-Jade B (Chemicon) that was prepared per manufacturer’s instructions. The stock solution was diluted by adding 0.1% acetic acid vehicle for staining solution, resulting in a final dye concentration of 0.0004%. This stock solution, when stored at 5°C, was stable for several months. The working stain solution was prepared within 10 min of use and was not reused. Slides were stained for 20 min and then rinsed in distilled water (3 × 1 min). Excess water removed by briefly (about 15 s) draining the slides vertically on a paper towel. The slides were then placed on a slide warmer (50°C) until they were fully dry. The dry slides were cleared by immersion in xylene for 2 min before coverslipping (Schmued et al., 2000) The total number of degenerating neurons per section was determined in five coronal sections per mouse of the retrosplenial cortex of each of three mice for both treatment groups.
Statistical Analysis
Differences among means were analyzed using one-way analysis of variance (ANOVA). Newman—Kuels post hoc analysis was employed when differences were observed in ANOVA testing ( p <.01%).
RESULTS
Brevetoxin Inhalation Exposure
The total aerosol concentrations in the brevetoxin chamber on days 1 and 2 were 30.1 ± 5.0 mg/m3 and 31.1 ± 2.4 mg/m3, respectively. Total aerosol concentrations in the control chamber due to the saline component of the vehicle were 31.9 mg/m3 and 30.8 mg/m3 on days 1 and 2, respectively. The average PbTx-3 exposure concentration on days 1 and 2 were 313± 63.8 μg PbTx-3/m3(n = 5) and 307 ± 21.5 μg PbTx-3/m3 (n = 8), calculated based on the fraction of PbTx-3 mass in the total=aerosol. Brevetoxin concentrations measured by ELISA on days 1 and 2 were 312 ± 113 μg /m3(n = 5) and 278 ± 24 μg /m3 (n = 8), respectively. The aerosol had a mass median ± aerodynamic diameter of 0.92 μm with a geometric standard deviation of 1.38, indicating the particles were highly respirable and polydisperse in size (Schlesinger, 1989).
Silver Staining
Silver staining protocols have been historically utilized to detect degenerating neuronal elements in the central nervous system, and this argyrophilia is considered a hallmark indicator of neurotoxicity (Switzer, 2002). Sections obtained from control mouse brains demonstrated virtually no silver-positive cells in the brain regions examined. The silver neurodegeneration stain did, however, detect restricted argyrophilia in animals exposed to PbTx-3. These silver-stained neurons were detected in the posterior cingulate/retrosplenial cortex of mice exposed via inhalation to PbTx-3 (Figures 1 and 2). The silver-stained neurons were scattered throughout this region of the cerebral cortex. The results of a quantitative evaluation of these brain sections depicted in Figure 3 document the PbTx-3-induced increase in the density of silver-stained neurons in the retrosplenial cortex. The PbTx-3-induced argyrophilia was, however, absent in other regions of the cerebral cortex, hippocampus, striatum, thalamus, hypothalamus, and cerebellum. The retrosplenial granular cortex of the mouse therefore appears to be uniquely vulnerable to brevetoxin-induced neuronal degeneration.
FIG. 1.
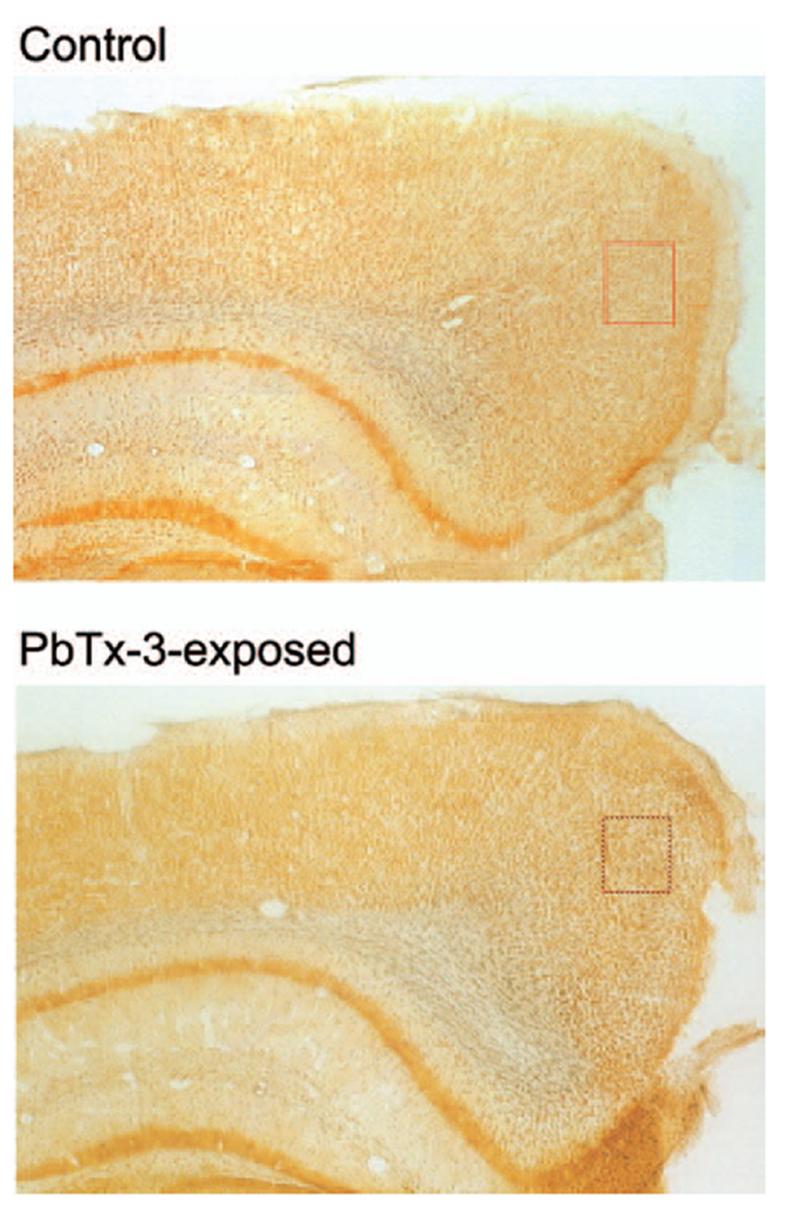
Silver-stained coronal sections of mouse brains sectioned through the retrosplenial cortex. Rectangles indicate areas shown at higher magnification in Figure 2. Depicted section is approximately 2 mm posterior to bregma. Magnification, 4×.
FIG. 2.
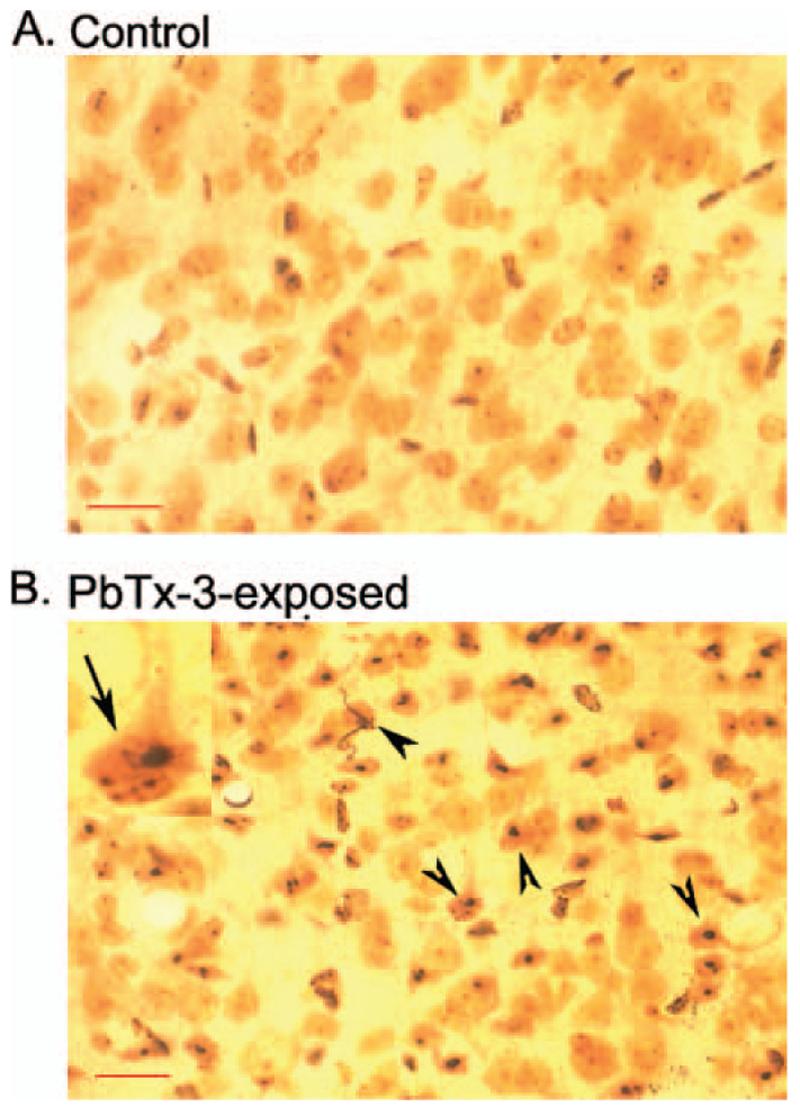
Representative silver-stained section of retrosplenial cortex demonstrating PbTx-3-induced neurodegeneration. Sections are shown at 40× magnification. (A) Section obtained from control animal. (B) Section obtained from PbTx-3-exposed mouse. The arrows in B indicate silver grain deposition in degenerating neurons, and the inset in upper left represents a higher magnification of a degenerating neuron. Scale bar, 50 μm.
FIG. 3.
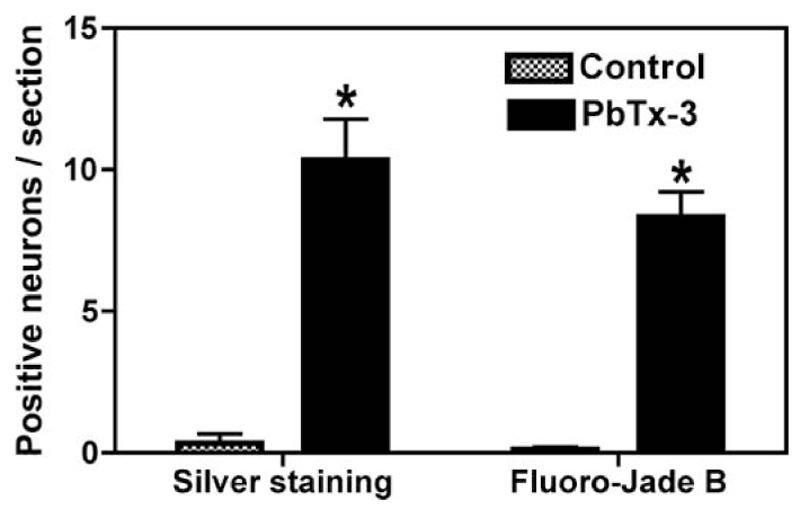
A quantitative analysis of neurodegeneration of the retrosplenial cortex in mice exposed to aerosolized PBTx-3. The values are means ± SEM (n = 15). Asterisk indicates difference significant at p <.01 compared to corresponding control.
Fluoro-Jade B Staining
Fluoro-Jade B was used as an alternative marker for neuronal degeneration to confirm the brevetoxin-induced neural insult in mouse brain. Fluoro-Jade B is an anionic fluorochrome capable of selectively staining degenerating neurons in the central nervous system (CNS) (Schmued et al., 1997). Although brain sections from control animals showed no staining, Fluoro-Jade B-labeled neurons were detected in a restricted brain region in animals exposed to PbTx-3 (Figure 4). These Fluoro-Jade B-stained neurons were again detected in the posterior cingu-late/retrosplenial cortex of mice exposed via inhalation to PbTx-3, but not in any other brain region. The restricted distribution and density of degenerating neurons detected with Fluoro-Jade B in PbTx-3-exposed mice therefore closely paralleled the pattern found with silver staining. These results confirm that a subacute exposure to aerosolized PbTx-3 produced discrete neurodegeneration in the retrosplenial granular cortex of mouse brain.
FIG. 4.
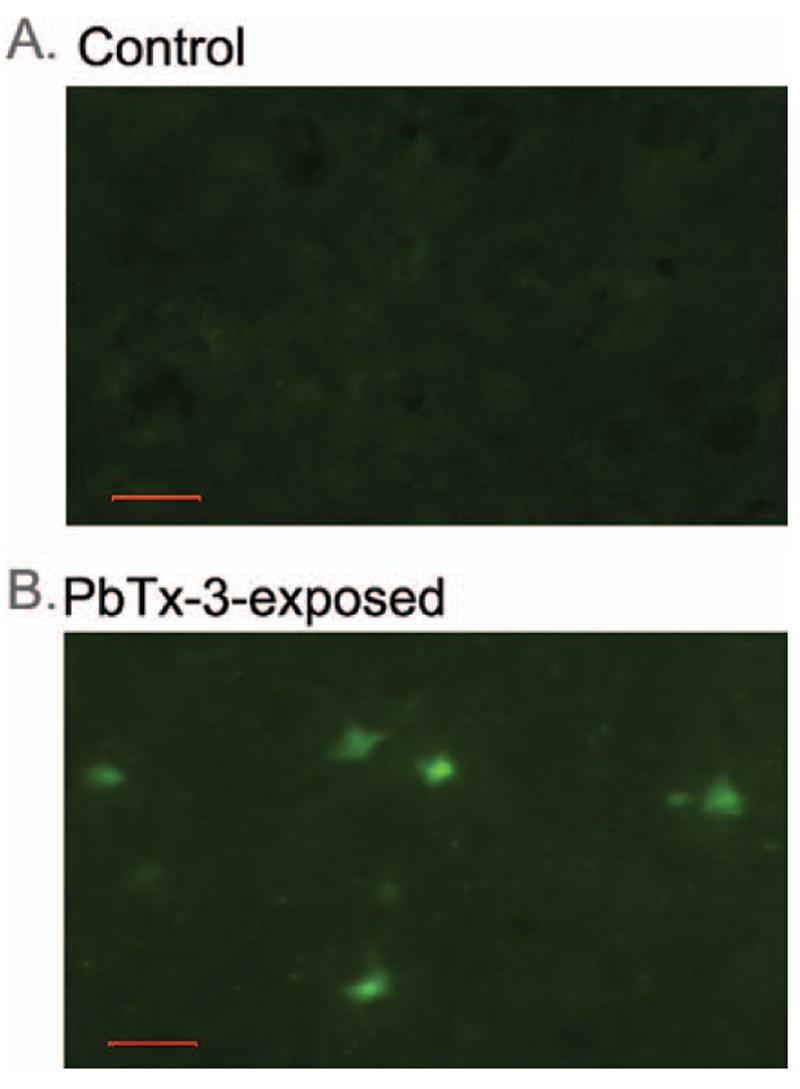
Representative Fluoro-Jade B-stained section of retrosplenial cortex demonstrating PbTx-3-induced neurodegeneration. Sections are shown at 40× magnification. (A) Section obtained from control animal. (B)Section obtained from PbTx-3-exposed mouse. Scale bar, 50 μm.
DISCUSSION AND CONCLUSIONS
Neurotoxic shellfish poisoning (NSP), caused by the ingestion of shellfish contaminated by the brevetoxin-producing red-tide dinoflagellate Karenia brevis, is characterized by gastrointestinal and neurological sequelae of the peripheral and central nervous system. The signs and symptoms of oral brevetoxin exposure in humans accidentally ingesting contaminated shell-fish include nausea; cramps; paresthesias of the lips, face, and extremities; weakness and difficulty in movement; and, if severe, paralysis, seizures, and coma. Severe cases of human intoxication, but no deaths, have been reported.
Animal studies have demonstrated that brevetoxins distribute to the rat and mouse brain following acute administration by several routes (Cattet & Geraci, 1993; Tibbetts et al., 2006). These brain concentrations of brevetoxins range from 2.12 nM (Benson et al., 1999) to 6.8 nM (Cattet & Geraci, 1993) and are therefore sufficient to produce significant fractional occupancy (42–70%) of VGSCs ([3H]PbTx-3 KD = 2.9 nM for site 5 of VGSC; Poli et al., 1986). In vitro, brevetoxins cause acute neurotoxicity in primary cultures of rat cerebellar granule neurons with a rank order of potency of PbTx-1 > PbTx-3 > PbTx-2 > PbTx-6 (Berman & Murray, 1999). The cerebellar granule cell cultures represent a >90% pure culture of glutamatergic neurons. The cerebellar granule cell toxicity produced by brevetoxins is mediated by N-methyl-D-aspartate (NMDA) receptors activated secondarily to brevetoxin-induced sodium channel activation and resultant release of glutamate. In contrast, PbTx-2 is not acutely toxic in the much more heterogenous embryonic murine neocortical neuron culture, but rather induces an activation of extracellular signal-regulated kinases 1/2 (ERK1/2), increases cAMP responsive element binding protein (CREB) phosphorylation, and upregulates gene expression of brain-derived neurotrophic factor (Dravid et al., 2004). The effect of inhaled brevetoxins on the central nervous system (CNS) may therefore vary with brain region and cell type. The purpose of this investigation was to evaluate the possible neurotoxic response associated with sub-acute inhalation of PbTx-3, one of the major brevetoxins produced by K. brevis. Inasmuch as the occurrence and distribution of K. brevis-induced red tide have increased over time, there may be increased risk from brevetoxin-induced toxic effects, especially among individuals working and living along beaches affected by red tides (Benson et al., 2005). We therefore examined the neurotoxic effects of subacute exposure of PbTx-3 via inhalation in a mouse model.
Although the target PbTx-3 aerosol concentration of 300 μg/m3 is approximately 3 orders of magnitude higher than total brevetoxin concentrations measured to date along Florida beaches during red tides of mild to moderate intensity (Cheng et al., 2005a; Backer et al., 2003; Pierce et al., 2003), this exposure did not produce clinical signs of toxicity subsequent to the inhalation procedure. The 2-day subacute exposure protocol did, however, roduce neuronal degeneration in a restricted area of the cerebral cortex, namely, the retrosplenial granular cortex of the mouse. This brevetoxin-induced neural insult was detected with two sensitive markers of neuronal degeneration, silver staining and Fluoro-Jade B staining. The recent reports of Benkovic et al. (2004, 2006) have demonstrated that both silver staining and Fluoro-Jade B staining are sensitive indicators of neuronal degeneration induced by kainic acid, inasmuch as they were able to reveal considerable neuropathology that was not observed with traditional histological stains (Nissl, and hematoxylin and eosin). In the present study both of these sensitive indicators of neural degeneration revealed identical patterns of neural insult restricted to the retrosplenial granular cortex of the mouse. We have observed a similar restricted pattern of PbTx-3-induced neural degeneration in the posterior cingulate/retrosplenial cortices of F344 rats (data not shown). The primary limitation of this study is that the restricted neural insult was observed after only one exposure protocol, a 2-day subacute exposure. It will therefore be of interest to examine the response after a more prolonged PbTx-3 inhalation exposure in the future to determine whether or not the restricted neurotoxicity generalizes to other patterns of inhalation exposure.
The restricted pattern of PbTx-3-induced neuropathology observed herein is reminiscent of the pattern produced in rodents by noncompetitive antagonists of NMDA receptors. Olney and coworkers have shown that NMDA receptor antagonists such as MK-801, phencyclidine (PCP), and ketamine produce irreversible degeneration of neurons in the posterior cingu-late/retrosplenial cortex of the rat when administered at doses sufficient to cause a prolonged block of NMDA receptors (Fix et al., 1993). Based on an elegant series of studies, Olney’s laboratory has proposed that the mechanism underlying this neural insult is indirect and involves antagonism of NMDA receptors located on GABAergic neurons, which results in the inactivation of tonic inhibitory control of excitatory pathways converging on neurons within the retrosplenial cortex (Corso et al., 1997). Thus, the disinhibition of excitatory pathways (glutamatergic and cholinergic) that innervate the retrosplenial cortex results in hyperactivation and an excitotoxic stimulation of retrosplenial neurons. The glutamate receptor involved in this excitotoxic neuronal degeneration is presumably a non-NMDA receptor sub-type, inasmuch as the NMDA antagonists would block NMDA receptors in the retrosplenial cortex (Corso et al., 1997). This hypothesis has recently been supported by the demonstration of MK-801-induced disinhibition manifested as a decrease in IPSC frequency in slices of the posterior cingulate/retrosplenial cortex of rat brain (Li et al., 2002). This action of MK-801 was much more pronounced in the cingulate/retrosplenial cortex than in the parietal cortex, suggesting that this regional difference in disinhibition may contribute to the regional difference in the vulnerability to NMDA-antagonist neurotoxicity.
The ability of PbTx-3 to mimic this neurotoxic response may be related to the excitotoxic actions of brevetoxins (Berman & Murray, 1999). Brevetoxin-induced neurotoxicity in cultured rat cerebellar granule neurons has been shown to be mediated by brevetoxin-induced depolarization and attendant release of glutamate. The stimulation of glutamate release leads to the activation of NMDA receptors that in turn contribute to the neurotoxic response. Brevetoxins also produce a rapid and concentration-dependent increase in neuronal [Ca2+]i with potencies that correlate well with those for neurotoxicity (Berman & Murray, 2000). Brevetoxin mimicry of NMDA antagonist-induced disinhibition of excitatory pathways with attendant neuronal degeneration may therefore be related to brevetoxin-induced glutamate release and excitotoxicity in the retrosplenial cortex. The combination of brevetoxin-induced glutamate release and depolarization of neurons in the retrosplenial cortex may therefore mimic the disinhibition produced by NMDA receptor antagonists.
The retrosplenial cortex has been demonstrated to play a fundamental role in learning and memory, having connections with the hippocampus, neocortex, and thalamus (van Groen & Wyss, 1990, 2003). MK-801 treatment that produces a modest neuronal insult in the retrosplenial agranular cortex of the mouse brain results in chronic acquisition impairment in spatial learning (Wozniak et al., 1996). Hypometabolism in the retrospenial cortex in humans has moreover been documented in patients with early Alzheimer’s disease, and this is thought to contribute to memory impairment (Nestor et al., 2003). The present results documenting brevetoxin-induced neuronal degeneration in this brain area raise concerns regarding human exposure to this marine neurotoxicant. While the aerosol concentration of PbTx-3 used in this study was approximately three orders of magnitude higher than that measured on Florida beaches during red tides, the existence of vulnerable human populations such as those with existing neurodegenerative diseases may render brevetoxin-induced neural insult from inhaled red tide aerosol a concern.
Acknowledgments
This research was supported in part by NIEHS grant PO1 ES10594 to D. G. Baden and J. M. Benson, and a grant from the Marine Freshwater Biomedical Sciences Center at Oregon State University (ES 03850) to T. F. Murray.
Footnotes
Publisher's Disclaimer: Copyright of Inhalation Toxicology is the property of Taylor & Francis Ltd. and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder’s express written permission. However, users may print, download, or email articles for individual use.
REFERENCES
- Asai S, Krzanowski JJ, Anderson WH, Martin DF, Polson JB, Lockey RF, Bukantz SC, Szentivanyi A. Effects of toxin of red tide, Ptychodiscus brevis, on canine tracheal smooth muscle: A possible new asthma-triggering mechanism. J. Allergy Clin. Immunol. 1982;69:418–428. doi: 10.1016/0091-6749(82)90116-6. [DOI] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Baden DG. Brevetoxins: Unique polyether dinoflagellate toxins. FASEB J. 1989;3:1807–1817. doi: 10.1096/fasebj.3.7.2565840. [DOI] [PubMed] [Google Scholar]
- Baden DG, Bourdelais AJ, Jacocks H, Michelliza S, Naar J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environ. Health Perspect. 2005;113(5):621–625. doi: 10.1289/ehp.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkovic SA, O’Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic—clonic seizures. Brain Res. 2004;1024:59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Benkovic SA, O’Callaghan JP, Miller DB. Regional neuropathology following kainic acid intoxication in adult and aged C57BL/6J mice. Brain Res. 2006;1070:215–231. doi: 10.1016/j.brainres.2005.11.065. [DOI] [PubMed] [Google Scholar]
- Benson JM, Tischler DL, Baden DG. Uptake, tissue distribution, and excretion of brevetoxin 3 administered to rats by intratracheal instillation. J. Toxicol. Environ. Health A. 1999;57:345–355. doi: 10.1080/009841099157656. [DOI] [PubMed] [Google Scholar]
- Benson JJ, Hahn FF, Tibbetts BM, Bowen LE, March TH, Sopori MS, Seagrave JC, Gomez A, Bourdelais A, Naar J, Bossart G, Baden D. Inhalation toxicity of brevetoxin-3 in rats exposed for five days. J. Toxicol. Environ Health A. 2004;67:1443–1456. doi: 10.1080/15287390490483809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Hahn FF, March TH, McDonald JD, Gomez AP, Sopori MJ, Bourdelais AJ, Naar J, Zaias J, Bossart GD, Baden DG. Inhalation toxicity of brevetoxin 3 in rats exposed for twenty-two days. Environ. Health Perspect. 2005;113:626–631. doi: 10.1289/ehp.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman FW, Murray TF. Brevetoxins cause acute excitotoxicity in primary cultures of rat cerebellar granule neurons. J. Pharmacol. Exp. Ther. 1999;290:439–444. [PubMed] [Google Scholar]
- Berman FW, Murray TF. Brevetoxin-induced autocrine excitotoxicity is associated with manifold routes of Ca2+ influx. J. Neurochem. 2000;74:1443–1451. doi: 10.1046/j.1471-4159.2000.0741443.x. [DOI] [PubMed] [Google Scholar]
- Bianco CL, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, Aebischer P. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an α-synuclein rat model of Parkinson’s disease. Proc. Nat. Acad. Sci. USA. 2004;101:17510–17515. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart GD, Baden DG, Ewing RY, Roberts B, Wright SD. Brevetoxicosis in manatees (Trichechus manatus latirostris) from the 1996 epizootic: Gross, histologic, and immunohistochemical features. Toxicol. Pathol. 1998;26:276–282. doi: 10.1177/019262339802600214. [DOI] [PubMed] [Google Scholar]
- Bourdelais AJ, Campbell S, Jacocks H, Naar J, Wright JLC, Carsi J, Baden DG. Brevenal is a natural inhibitor of brevetoxin action in sodium channel receptor binding assays. Cell. Mol. Neurobiol. 2004;24:553–563. doi: 10.1023/B:CEMN.0000023629.81595.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Gainer M. Interaction of brevetoxin A with anew receptor site on the sodium channel. Toxicon. 1985;23:497–504. doi: 10.1016/0041-0101(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Cattet M, Geraci JR. Distribution and elimination of ingested brevetoxin (PbTx-3) in rats. Toxicon. 1993;31:1483–1486. doi: 10.1016/0041-0101(93)90214-4. [DOI] [PubMed] [Google Scholar]
- Cheng YS, McDonald JD, Kracko D, Irvin CM, Zhou Y, Pierce RH, Henry MS, Bourdelaisa A, Naar J, Baden DG. Concentration and particle size of airborne toxic algae (brevetoxin) derived from ocean red tide events. Environ. Sci. Technol. 2005a;39:3443–3449. doi: 10.1021/es048680j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YS, Zhou Y, Irvin CM, Pierce RH, Naar J, Backer LC, Fleming LE, Kirkpatrick B, Baden DG. Characterization of marine aerosol for assessment of human exposure to brevetoxins. Environ. Health Perspect. 2005b;113:638–643. doi: 10.1289/ehp.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso TD, Sesma MA, Tenkova TI, Der TC, Wozniak DF, Farber NB, Olney JW. Multifocal brain damage induced by phencyclidine is augmented by pilocarpine. Brain Res. 1997;752:1–14. doi: 10.1016/s0006-8993(96)01347-9. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Baden DG, Murray TF. Brevetoxin activation of voltage-gated sodium channels regulates Ca2+ dynamics and ERK1/2 phosphorylation in murine neocortical neurons. J. Neurochem. 2004;89:739–749. doi: 10.1111/j.1471-4159.2004.02407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix AS, Horn JW, Wightman KA, Johnson CA, Long GG, Storts RW, Farber N, Wozniak DF, Olney JW. Neuronal vacuolization and necrosis induced by the noncompetitive N-methyl-D-aspartate (NMDA) antagonist MK(+)801 (dizocilpine maleate): A light and electron microscopic evaluation + of the rat retrosplenial cortex. Exp. Neurol. 1993;123:204–215. doi: 10.1006/exnr.1993.1153. [DOI] [PubMed] [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Dalpra D, Tamer R, Zaias J, Cheng YS, Pierce R, Naar J, Abraham W, Clark R, Zhou Y, Henry MS, Johnson D, Van De BG, Bossart GD, Harrington M, Baden DG. Initial evaluation of the effects of aerosolized Florida red tide toxins (brevetoxins) in persons with asthma. Environ. Health Perspect. 2005a;113:650–657. doi: 10.1289/ehp.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Backer LC, Baden DG. Overview of aerosolized Florida red tide toxins: exposures and effects. Environ. Health Perspect. 2005b;113:618–620. doi: 10.1289/ehp.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester DJ, Gaskin JM, White FH, Thompson NP, Quick JA, Henderson GE, Woodard JC, Robertson WD. Epizootic of waterfowl associated with a red tide episode in Florida. J. Wildl. Dis. 1977;13:160–167. doi: 10.7589/0090-3558-13.2.160. [DOI] [PubMed] [Google Scholar]
- Jeglitsch G, Rein K, Baden DG, Adams DJ. Brevetoxin-3 (PbTx-3) and its derivatives modulate single tetrodotoxin-sensitive sodium channels in rat sensory neurons. J. Pharmacol. Exp. Ther. 1988;284:516–525. [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, Benson J, Cheng YS, Johnson D, Pierce R, Zaias J, Bossart GD, Baden DG. Literature review of Florida red tide: Implications for human health effects. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuder C, Mazet JAK, Bossart GD, Carpenter TE, Holyoak M, Elie MS, Wright SD. Clinicopathologic features of suspected brevetoxicosis in double-crested cormorants (Phalacrocorax auritus) along the Florida Gulf Coast. J. Zoo Wildl. Med. 2002;33:8–15. doi: 10.1638/1042-7260(2002)033[0008:CFOSBI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kusec KM, Vargo G, Steidinger K. Gymnodinium breve in the field, in the lab, and in the newspaper—A scientific and journalistic analysis of Florida red tides. Contrib. Mar. Sci. 1999;34:1–229. [Google Scholar]
- Li Q, Clark S, Lewis DV, Wilson WA. NMDA receptor antagonists disinhibit rat posterior cingulate and retrosplenial cortices: A potential mechanism of neurotoxicity. J. Neurosci. 2002;22:3070–3080. doi: 10.1523/JNEUROSCI.22-08-03070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, Aebischer P. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2004;101:17510–17515. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. α-Synuclein overexpression protects against paraquat-induced neurodegeneration. J. Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol. Dise. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney PL, Flewelling L, Steidinger K, Lancaster J, Baden DG. A competitive ELISA to detect brevetoxins from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ. Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease) Eur. J. Neurosci. 2003;18:2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Frankin KBJ. The mouse brain in stereotaxic coordinates. Elsevier Academic Press; New York: 2004. [Google Scholar]
- Pierce RH, Brown RC, Hardman KR, Henry MS, Palmer CLP, Miller TW, Wichterman G. Fate and toxicity of temephos applied to an intertidal mangrove community. J. Am. Mosquito Control Assoc. 1989;5:569–578. [PubMed] [Google Scholar]
- Pierce RH, Henry MS, Proffitt S, Hasbrouck PA. Red tide toxin (brevetoxin) enrichment in marine aerosol. In: Graneli E, Sundstorm B, Edler I, Anderson DM, editors. Toxic Marine Phytoplankton. Elsevier Science; New York: 1989. pp. 397–402. [Google Scholar]
- Poli MA, Mende TJ, Baden DG. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol. Pharmacol. 1986;30:129–135. [PubMed] [Google Scholar]
- Schlesinger RB. Deposition and clearance of inhaled particles. In: McClellan RO, Henderson RF, editors. Concepts in inhalation toxicology. Taylor & Francis; Washington, DC: 1989. pp. 191–217. [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr. Fluoro-Jade: A novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Tibbetts BM, Baden DG, Benson JM. Uptake, tissue distribution and excretion of brevetoxin 3 administered to mice by intratracheal instillation. J. Toxicol. Environ. Health A. 2006;69:1325–1335. doi: 10.1080/15287390500360091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. J. Comp Neurol. 1990;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular cortex in the rat. J. Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Brosnan-Watters G, Nardi A, McEwen M, Corso TD, Olney JW, Fix AS. MK-801 neurotoxicity in male mice: Histologic effects and chronic impairment in spatial learning. Brain Res. 1996;707:165–179. doi: 10.1016/0006-8993(95)01230-3. [DOI] [PubMed] [Google Scholar]


