Abstract
The concept of mesenchymal stem cells has gained wide popularity. Despite the rapid growth of the field, uncertainties remain with respect to the defining characteristics of these cells, including their potency and self-renewal. These uncertainties are reflected in a growing tendency to question the very use of the term. This commentary revisits the experimental origin of the concept of the population(s) referred to as mesenchymal-stem cells and the experimental framework required to assess their stemness and function.
The concept of stem cells originated at the end of the 19th century as a theoretical postulate to account for the ability of certain tissues (blood, skin, etc.) to self-renew for the lifetime of an organism even though they are comprised of short-lived cells. Many years later, identification of stem cells as discrete cellular entities followed from the development of methods for prospective isolation of stem cell candidates, in parallel with the design of rigorous bioassays to test their potency after transplantation in vivo.
The currently popular concept of mesenchymal stem cells (MSCs, a term first coined in Caplan [1991]) can be traced to classical experiments demonstrating that transplantation of bone marrow (BM) to heterotopic anatomical sites results in de novo generation of ectopic bone and marrow. Whereas examples of such studies date back to the 19th century (Goujon, 1869), the work of Tavassoli and Crosby clearly established proof of an inherent osteogenic potential associated with BM (Tavassoli and Crosby, 1968). Because these experiments were conducted with entire fragments of bone-free BM, the precise identity of any cell functioning as a progenitor of differentiated bone cells (and therefore of nonhematopoietic, mesenchymal cells) could not be delineated. It was Friedenstein and coworkers, in a series of seminal studies in the 1960s and 1970s (reviewed in Friedenstein, 1990), who demonstrated that the osteogenic potential, as revealed by heterotopic transplantation of BM cells, was associated with a minor subpopulation of BM cells. These cells were distinguishable from the majority of hematopoietic cells by their rapid adherence to tissue culture vessels and by the fibroblast-like appearance of their progeny in culture, pointing to their origin from the stromal compartment of BM. In addition to establishing BM stroma as the haystack in which to search for the proverbial needle, the work of Friedenstein and coworkers provided a second major breakthrough by showing that seeding of BM cell suspensions at clonal density results in the establishment of discrete colonies initiated by single cells (the colony-forming unit fibroblastic, CFU-Fs [Friedenstein et al., 1970]). The clonal nature of each colony was demonstrated by the linear dependence of colony formation on the number of cells explanted, the use of chromosomal markers, 3H-thymidine labeling, through time-lapse photography, and by Poisson distribution statistics (Friedenstein, 1976; Friedenstein et al., 1970, 1974; Gronthos et al., 2003). In vivo transplantation led to the recognition that multiple skeletal tissues (bone, cartilage, adipose tissue, and fibrous tissue) could be experimentally generated, in vivo, by the progeny of a single BM stromal cell (reviewed in Friedenstein, 1990). Friedenstein and Owen called this cell an osteogenic stem cell (Friedenstein et al., 1987) or a BM stromal stem cell (Owen and Friedenstein, 1988).
The implications of these discoveries were initially appreciated solely in experimental hematology and only later for their relevance to bone biology and disease. As conceptualized by the stem cell niche hypothesis proposed by Schofield (1978), the notion that hematopoietic stem cells (HSCs) are regulated by their physical association with a discrete cellular microenvironment within BM was substantiated by the seminal observations of Dexter, Allen, and colleagues (Allen, 1978; Dexter et al., 1977; Dexter and Testa, 1976). Stemming from a long-standing quest to elucidate the functional relationship between HSCs and some physical component of the bone/BM organ, the pioneering work of Tavassoli and of Friedenstein and Owen revealed that a second type of stem cell could be present in the BM and, specifically, in the hematopoiesis-supporting stroma.
Although the hypothesis was firmly established, and the supporting experimental evidence was published and widely reproduced, the concept of a nonhematopoietic stem cell in BM did not resonate worldwide until additional similar work was published in 1999 (Pittenger et al. [1999], from a commercial entity, Osiris Therapeutics, Inc). Combined with the timing of the isolation of human embryonic stem (ES) cells, the term mesenchymal stem cell (MSC), proposed previously as an alternative to “stromal” or “osteogenic” stem cell (Caplan, 1991; as applied to cells ex vivo), gained wide popularity. In the minds of many, MSCs became one kind of postnatal human stem cell with a differentiation potential that would be broader than originally envisioned or perhaps even as broad as that of ES cells. This assumption, echoed in later studies claiming transgermal potential (“plasticity”) of postnatal stem cells, including MSCs (Beltrami et al., 2007; Jiang et al., 2002; Lakshmipathy and Verfaillie, 2005; Poulsom et al., 2002), evoked attention and also generated confusion, and it remains highly controversial (Bianco, 2007; Wagers et al., 2002). The notion of the MSC evolved from the historical roots of the conceptualized nonhematopoietic stem cell present in BM. Unbeknown to the vast majority of current workers in the MSC field, these roots, together with general basic tenets of stem cell biology, set precise limits as to how the biology of MSCs should be assessed, how the stem cell concept might be applied, what their envisioned clinical applications could be, and what nomenclature would be most appropriate.
Is “Mesenchymal Stem Cell” a Proper Term?
Questions have been raised over the usage of the term “mesenchymal stem cells” (Dominici et al., 2006; Horwitz et al., 2005), but there are multiple reasons that indicate it is inappropriate. First, the original naming of this class of stem cells as mesenchymal was based on the hypothesis that multiple tissues beyond skeletal lineages could be generated by postnatal MSCs, including skeletal muscle, myocardium, smooth muscle, tendon, etc. (reviewed in Caplan, 2005). However, the nonskeletal potential of single MSCs has not been formally proven in vivo, and the point remains controversial. Second, during prenatal organogenesis, the series of tissues regarded by many as related by lineage to postnatal MSCs are generated by a system of distinct progenitors, rather than from a common ancestor. Bone and skeletal muscle arise from distinct progenitors. In fact, bone as a tissue develops from neuroectodermal progenitors (craniofacial bones) or from axial and lateral specifications of the mesoderm (reviewed in Olsen et al., 2000). In addition, although neuroectoderm gives rise to a transient embryonic population of cells with properties of MSCs (Takashima et al., 2007), postnatal progenitors (and MSCs) have a distinct origin, which has yet to be defined.
Nonetheless, the term has gained such global usage that it would perhaps be futile to suggest replacing it with another that would better adhere to the known biology of the system. Debating nomenclature always conveys a flavor of pedantry. However, debating misconceptions that accompany the popular use of any term may correct flawed experimental approaches based on mistaken assumptions, may trigger experimental advances, and may ultimately promote better understanding. The term MSC is indeed questioned, and questionable, because it conveys assumptions that were neither included in the original concept of nonhematopoietic stem cells in the BM nor supported by direct experimental evidence. These assumptions revolve around multipotency and self-renewal, the two defining characteristics of a stem cell, and also around the experimental assays relevant to both properties, as well as to additional criteria (such as, for example, clonogenicity). Furthermore, whereas the original notion of MSCs specifically referred to cells in BM (bone marrow stromal cells, BMSCs), the current notion has been extended to include cells from additional sources (such as synovium, adipose tissue, dental pulp, etc.) and, indeed, from almost every postnatal connective tissue. On the whole, uncertainty over the usage of the term MSC reflects, and arises from, imprecision in the use of a system of terms and experimental assays (Table 1).
Table 1.
Glossary of Terms
| Term | Definition | Term | Definition |
|---|---|---|---|
| Bone marrow stromal cells (in situ) | -Extravascular cells of nonhematopoietic, nonendothelial lineages. | Multipotent mesenchymal stromal cell (MSCs) | -An alternative name for MSCs. |
| -Highlight their multilineage potential. | |||
| -Physically associated with hematopoietic cells in BM. | -Underscores their questionable stemness due to lack of evidence for self-renewal. | ||
| -Provide cues for the homing, retention, proliferation, and differentiation of hematopoietic stem/progenitor cells. | |||
| -Include adventitial reticular cells, marrow adipocytes, developing and mature osteogenic cells, and pericytes/mural cells. | |||
| Bone marrow stromal cells (in vitro, BMSCs) | -Cultured cell strains derived from explantation of adherent, nonhematopoietic, nonendothelial BM cells. | Pericyte, mural cell | -Subendothelial, nonendothelial cell in the microvascular wall. |
| -May be initiated by one or more CFU-Fs, by stromal cells seeded at non-clonal density, or by cells sorted by phenotype. | -Express MCAM, BG5, a-smooth muscle actin, otherwise defined histologically. | ||
| -Can be derived from mesoderm or ectomesenchyme. | |||
| -Some BMSCs are CFU-Fs. | -A putative progenitor for connective tissues. | ||
| Colony (fibroblastic) | -The in vitro clonal progeny of a CFU-F. | Self-renewal | -The ability to generate cells identical in phenotype and potency to the starting cell population. |
| -Appears as a discrete colony of 50 or more fibroblast-like cells. | -Occurs when one cell divides to generate one stem cell and one nonstem cell. | ||
| -Not a CFU-F. | -Can only be assayed via in vivo transplantation of homogeneous populations. | ||
| -For connective tissues, has been shown for adventitial reticular cells and some CFU-Fs in the human bone marrow and satellite cells in murine skeletal muscle. | |||
| -Does not imply infinite or continuous cell division in vivo or in vitro. | |||
| -A defining property of postnatal stem cells. | |||
| Colony forming unit-fibroblastic (CFU-F) | -A single cell, freshly isolated from an intact tissue. | Skeletal stem cell, stromal stem cell, osteogenic stem cell | -The postnatal multipotent and self-renewing progenitor of skeletal tissues (bone, cartilage, bone marrow adipocytes, fibroblasts, and bone marrow stromal cells). |
| -Able to initiate clonal growth of fibroblastic cells at low density. | |||
| -Designated according to tissue of origin: BM-CFU-F, [any tissue]- CFU-F. | -Found in the bone marrow stroma. | ||
| -Has been assayed in vivo at clonal level. | |||
| Expansion (of stem cells) | -The absolute increase in stem cell number within a cell population. | Stroma | -Anatomical term referring to the supporting (often connective) tissue in any organ. |
| -Occurs when one cell divides to generate two identical stem cells. | -Serves a trophic and mechanical function for the specialized cell types of that organ (parenchyma). | ||
| -Expansion of a whole culture of BMSCs is not equivalent to expansion of the stem cells therein. | |||
| Mesenchymal stem cell (MSCs) | -A conceptual postnatal progenitor of most if not derivatives of mesoderm. | [Any Tissue] Stromal cells | -Fibroblastic populations established in culture from any tissue. |
| -Lineage potential includes skeletal (bone, cartilage, fat, tendon) and nonskeletal (smooth muscle, skeletal muscle, and possibly myocardium and endothelial cells) tissues. | -Likely derived from the stroma/connective tissue of the originating organ. | ||
| -Postulated to exist in BM, liver, synovium, adipose tissue, heart, and other postnatal connective tissues. | |||
| -Suggested to include a subset of pluripotent cells. | |||
| -Often used to denote cultured cells from almost any connective tissue. | |||
| Mesenchyme | -A primitive embryonic loose connective tissue. | ||
| -An embryonic subset of mesoderm-derived cells. | |||
| -Also a subset of neuroectoderm-derived cells (ectomesenchyme). |
How Should MSCs and Their Properties Be Assayed?
The current highest state of the art assay to establish stem cell function is exemplified by the capacity of a single prospectively isolated HSC to reconstitute, serially and long term, multilineage hematopoiesis in lethally irradiated recipient mice. The crucial general lessons from such stringent and rigorous assays are that (1) stemness is probed through in vivo transplantation experiments, (2) multipotency can only be probed at the singlecell level, and (3) self-renewal means reconstitution of a stem cell population identical in phenotype and function to the one originally explanted. The relative ease and efficacy of assaying HSCs derives in part from the ability of prospectively isolated HSCs to circulate and home to their permissive niche, the continuous and rapid turnover of HSCs and their progeny, and the systemic rather than localized distribution of hematopoiesis across the body. Some of these inherent biological properties of the HSC system are not necessarily duplicated either in the BMSC system or in other systems in which definitions of stemness are sought. For example, single HSCs can be transplanted in vivo via the circulation and distributed at high efficiency without ex vivo culture. On the other hand, sufficient numbers of BMSCs necessary to regenerate a skeletal defect typically need to be locally transplanted, and even prospectively isolated, single skeletal progenitors need to be cultured to generate sufficient numbers of cells prior to transplantation. Furthermore, the capacity for self-renewal undoubtedly relates to the rate of tissue turnover. Whereas skin in its entirety turns over every ~30 days, the whole skeleton turns over three to five times during adulthood. Consequently, self-renewal of stem cells capable of reforming skeletal tissues, in nature, would not be expected to involve the same number of cell divisions as for HSCs or epidermal stem cells. Assessing self-renewal and multipotency (the defining characteristics of all postnatal stem cells) of nonhematopoietic stem cells thus requires the development and use of in vivo assays based on the same rigorous principles as in HSC bioassays, but adapted to the specific biology of the system under study. How do these considerations relate to MSCs?
Identification and Expansion in Culture
Classically, a subset of BMSCs is designated as clonogenic if it is able to generate colonies of fibroblast-like cells from single cells when plated in culture. Importantly, colony growth can be observed when cells are plated at higher, nonclonal density, but in this case, colonies cannot be assumed to be clonal, and enumeration or analysis of colonies formed under nonclonal conditions is experimentally meaningless. As assessed by current CFU-F assays, clonogenicity of BMSCs reflects the ability of a cell to grow in a density-insensitive fashion. Of note, any genuine stem cell within theBMstroma would be clonogenic, but the reverse statement is not valid, as only a fraction of CFU-Fs are multipotent based on in vivo transplantation (Bianco and Robey, 2004; Gronthos et al., 2003). No markers are available to distinguish multipotent CFU-Fs from more committed ones, but the frequency of CFU-Fs does correlate with the incidence of progenitors in a given BM sample. Clonogenic BMSCs can be enriched by using surface markers such as STRO-1 (Simmons and Torok-Storb, 1991) or MCAM (Sacchetti et al., 2007). However, as long as experimentation requires the use of cultured cells, sorting clonogenic progenitors by surface phenotype or “sorting” them by plastic adherence has the same practical meaning (Sacchetti et al., 2007). Consequently, the investigative value of isolation procedures based on surface phenotype will only unfold after in vivo assays are developed for the use of uncultured clonogenic progenitors.
Adherent cells capable of density-independent growth are found in a number of nonhematopoietic tissues, such as periosteum and dental pulp, and probably in all connective tissues, and are also called CFU-Fs in the literature. In addition, it has been reported that some nonhematopoietic tissues have higher frequencies of CFU-Fs compared to BM. However, because such tissues contain very few hematopoietic cells against which BM CFU-F frequencies are calculated, they may not harbor relatively more clonogenic cells over BM.
Importantly, a primary culture of BMSCs can be established at clonal or nonclonal density (in most of the current literature, the latter is the case). In the first instance, the entire culture represents the progeny of CFU-Fs. In the second instance, the primary culture includes cells derived from nonclonogenic, adherent cells with limited but demonstrable potential for growth. Thus, primary cultures established at clonal or nonclonal density are remarkably different, but neither type of culture should be called a culture of stem cells, mesenchymal or otherwise qualified. Expansion of monoclonal or multiclonal primary cultures can yield populations that are homogeneous in the expression of certain markers, but not others. Functionally, within clonal cultures, the initially multipotent cells do self-renew (Sacchetti et al., 2007), and may even stochastically expand, to some extent. However, simultaneously and within the same culture, some of the progeny of the culture-initiating cells differentiate or even senesce. Thus, any culture of nontransformed mammalian cells is heterogeneous due to inherent kinetics, as the expansion of stem cells within the culture is neither the sole nor the predominant event. The stochastic frequency of this event with respect to commitment or senescence in culture is as yet undefined; consequently, the expansion of stem cells cannot be measured or simply inferred from growth of the whole culture.
One implication of this trait is that, although one can purchase commercial cultures of MSCs, they are more accurately described as cultures of BMSCs and may or may not include a proportion of multipotent and self-renewing cells. In addition, many modifications of the original simple culture conditions have been proposed to improve the expansion of MSCs in culture. However, measuring in vitro expansion of stem cells requires in vivo assays at the single-cell level, at least until such time that a phenotypic marker is identified that defines the stem cell pool. Therefore, to truly claim ex vivo expansion, the progeny of single original CFU-Fs isolated after expansion would need to be transplanted in vivo and comparatively evaluated for the formation of different tissues. This is admittedly demanding, and has never been done, so it remains unclear whether any proposed cocktail for BMSC expansion indeed promotes expansion of stem cells within the population, rather than simply promoting the growth of the entire BMSC population as a whole and possibly even depleting the stem cell subset contained within it.
Differentiation Potential
It is solidly established by the work of Friedenstein and others that a subset of single BMSCs is multipotent and therefore displays one property commonly found in stem cells. However, the same studies indicate that this subset is limited to differentiation into skeletal cell types found at different developmental stages as well as at specific anatomical sites. These include osteoblasts (bone), chondrocytes (cartilage), adipocytes (BM stroma), fibroblasts (periosteum), and adventitial reticular cells (BM stroma). Although the claim that BMSCs can also give rise to additional cell types of mesodermal origin (skeletal muscle, smooth muscle, cardiac muscle, endothelial cells, etc.) is commonplace, this claim is not rooted in equally solid experimental evidence with heterotopic transplantation of the progeny of a single cell and thus remains controversial. Even greater controversy exists over the claims of transgermal potential of either BMSCs or subsets thereof (Beltrami et al., 2007; Jiang et al., 2002). It is for these reasons that it would be appropriate to use the term “skeletal stem cells” for BM-derived, multipotent stromal cells capable of generating skeletal cell types in vivo (Bianco and Robey, 2004).
Beyond the BM, adherent cells capable of density-independent growth are found in a number of nonhematopoietic connective tissues, such as periosteum and dental pulp, and are also called CFU-Fs in the literature. However, the potency of CFUFs from nonhematopoietic tissues and BM has not been compared systematically by in vivo assays, and prevailing evidence suggests that CFU-Fs from different tissues are not the same. For example, when grown and transplanted in vivo under conditions identical to those used for BMSCs, CFU-Fs from dental pulp form dentin rather than bone (Gronthos et al., 2002). Thus, rather than a uniform, single class of ubiquitous MSCs, the evidence points to a varied class of clonogenic progenitors found in different tissues but endowed with tissue-specific potency.
No matter what the source of the stromal population being examined, multipotency of MSCs is commonly believed to be assessable by in vitro differentiation assays. However, these assays correlate poorly with results of in vivo differentiation assays, even when conducted in parallel on the same cell strain (reviewed in Bianco et al., 2006). Furthermore, multipotency (a property of a single cell) cannot be determined based on assays conducted on nonclonal cell strains in culture. In vitro generation of alizarin red deposits (osteogenesis), oil red O-stainable cells (adipogenesis), and alcian blue-stainable matrix (chondrogenesis) in parallel cultures of nonclonal strains of BM stromal cells, or any strain of cells, does not predict multipotency (Bianco et al., 2006; Gronthos et al., 2002), as commonly assumed in a copious literature, and therefore does not identify any cell culture as a culture of stem cells, no matter how further qualified by any name of choice.
Assaying Self-Renewal
In addition to the significant misconceptions of multipotency and of in vitro assays to probe it, even greater ambiguities persist concerning the generally assumed self-renewal of stem cells within BM stroma or within any other connective tissue. In most studies, self-renewal is equated to sustained growth in culture or, in some scenarios, is assumed based on retention of in vitro differentiation after multiple population doublings. However, the only system for which stem cell self-renewal is considered to be solidly proven is the hematopoietic system, based on the ability of phenotypically defined HSCs to serially reconstitute hematopoiesis for the lifetime of lethally irradiated mice (Osawa et al., 1996; Spangrude et al., 1988; Weissman, 2000). Notably, this property is associated with no ex vivo proliferation. Demonstration of self-renewal postulates the reconstitution in vivo of a stem cell compartment with phenotype and properties identical to the starting population. Evidence for self-renewal of progenitors within the BMSC population has only very recently started to emerge (Sacchetti et al., 2007) and does indeed support the concept that such cells include a bona fide stem cell, and it also addresses the previous lack of direct experimental evidence for the ability of stromal cells to self-renew (Dominici et al., 2006; Horwitz et al., 2005). Whether this trait is shared by CFU-F-forming populations isolated from other connective tissues remains to be seen.
A Putative Marker
It is often assumed that expression of a certain broad set of markers defines various types of cell cultures as MSCs, but in reality, most of these markers are expressed by cultures of fibroblastic cells from any tissue. In addition, most if not all such markers are highly modulated in culture, which underscores the futility of efforts to characterize stromal cell cultures per se. In contrast, the original quest for markers of the putative nonhematopoietic BM stem cell focused on those that could identify the CFU-F among uncultured BM cells. Along with the classical STRO-1 epitope, a number of other markers can assist in enriching CFU-Fs (e.g., MCAM, CD105). However, in addition to markers of uncultured CFU-Fs, markers of cells in situ are particularly needed (1) when seeking the in situ counterpart of CFU-Fs and (2) to follow the fate of cells transplanted in vivo, particularly when aiming for evidence of self-renewal. MCAM appears to be one such marker.
In human BM, MCAM marks adventitial reticular cells (Sacchetti et al., 2007), a classically known stromal cell type residing in a subendothelial position over the abluminal surface of BM sinusoids (Westen and Bainton, 1979). In other tissues, MCAM is expressed by pericytes (Li et al., 2003), an elusive cell type recognized by their anatomy and position rather than by any precisely defined phenotype. Like BM adventitial reticular cells, pericytes reside on the abluminal surface of endothelial cells in the microvasculature of every connective tissue. Given this broad distribution, and that pericytes may represent the in situ counterpart of BM CFU-Fs, one could hastily conclude that pericytes are the MSCs found in different tissues. Using MCAM to identify the pericyte population will aid in determining whether CFU-Fs from nonhematopoietic tissues are indeed pericytes. If so, it will be possible to test the hypothesis that MSCs exist in all connective tissues, and to determine whether their capacity to function in clonogenic and in vivo assays is consistent regardless of their tissue of origin. In this regard, pericytes isolated from skeletal muscle are spontaneously myogenic in vitro (Dellavalle et al., 2007) and nonosteogenic in vivo, in sharp contrast with BM CFU-Fs and with BM subendothelial cells despite their coincident anatomical identity. Thus, as with the potency of CFU-Fs derived from different tissues, current evidence regarding pericytes in different tissues seems to reflect a system of organ-specific progenitors with organ-specific potency. Nonetheless, studies of pericyte biology may provide clues as to the developmental origin of postnatal progenitor/stem cells in nonhematopoietic tissues.
Composing Diversity into a Unifying Model
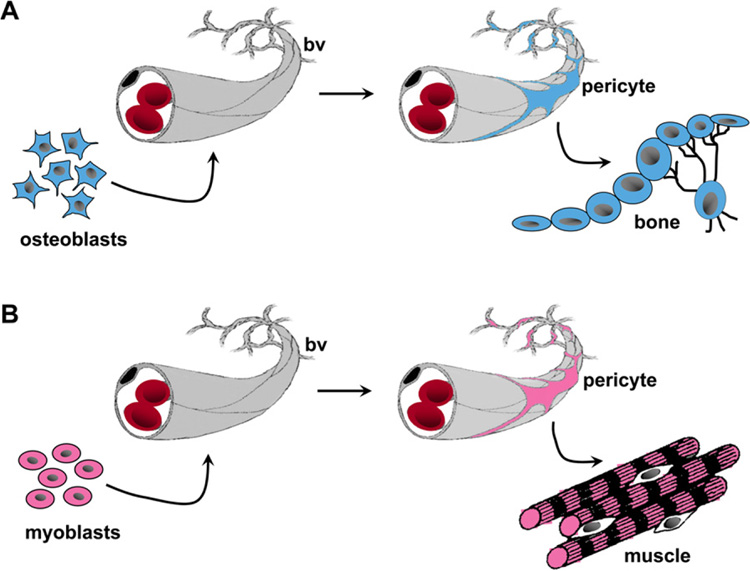
The assumed existence of a homogeneous stromal stem cell population present in multiple mesenchyme-derived tissues remains open to question. Using the example of pericytes as a model, a hypothesis can be generated such that the seeding of definitive postnatal progenitors within connective tissues may be rooted in the general mechanisms whereby pericytes are recruited to the nascent microvascular wall during development and postnatal growth (Figure 1). In this view, stemness of these cells could be interpreted as a byproduct of a general developmental mechanism, whereby local cells committed to an organ-specific fate (e.g., myogenic in muscle, skeletogenic in BM) are recruited to nascent microvascular walls during development and postnatal growth (Jain, 2003). As a result, these cells would be retained in a growth-arrested state until triggered to resume proliferation and differentiation, either in response to physiological cues, as in tissue turnover or repair, or experimentally, when explanted in vitro as CFU-Fs. Subendothelial osteoprogenitors are recruited to become stem cells in a somewhat accidental way, and they incidentally serve specific functions characteristic of pericytes in different postnatal tissues. Or, as an alternative to differentiation, tissue-specific stem cells may function to support the regeneration of other local cell types, as seen in BM.
Figure 1. Model Proposing that Pericytes Serve as a Reservoir of Tissue-Specific Progenitors.
The recruitment of local tissue progenitors as mural cells/pericytes in different tissues such as bone marrow and skeletal muscle is depicted.
(A) During development, local osteogenic cells (osteoblasts, left side) in the BM associate with the vascular wall as subendothelial mural cells/ pericytes (adventitial reticular cells in sinusoids). In the postnatal organ, these cells can be explanted and assayed as clonogenic skeletal progenitors (bone, right side).
(B) Blood vessels assoc iate with local myogenic progenitors in developing muscle, which are recruited into being pericytes (myocytes, left side). Consequently, pericytes isolated from the microvasculature of skeletal muscle will exhibit myogenic potential (muscle, right side). Therefore, this model predicts that although pericytes in different connective tissues may arise by a common developmental pathway and share anatomic identity, their differentiation capacity is likely to be tissue specific.
In BM, BMSCs serve two functions. One is the classically recognized function of providing a supportive microenvironment for hematopoiesis. The other is related to the development, stabilization, and maintenance of the sinusoidal network (Sacchetti et al., 2007), consistent with their subendothelial localization. In the bone/BM organ, the two functions are closely intertwined. Hematopoiesis requires establishment of a sinusoidal network, and HSCs localize to sinusoidal walls (Kiel and Morrison, 2006), in addition to endosteal surfaces (Haylock et al., 2007). BMSCs also localize to sinusoidal walls, and when hematopoietic development is modeled in vivo, they do so prior to the establishment of hematopoiesis (Sacchetti et al., 2007). In addition, BMSCs rank as committed, but not differentiated, osteogenic progenitors. In view of the emerging role of osteogenic cells in providing a niche for HSCs (Arai et al., 2004; Calvi et al., 2003; Zhang et al., 2003), these data portray an appealing avenue for research into a unique, dual system of stem/progenitor cells that functionally interact in the regulation of hematopoiesis and bone physiology. Furthermore, beyond the BM, studies over the past 10 years have attempted to demonstrate that transplantation of BMSCs into nonskeletal (unorthodox) sites would result in repair of myocardium, brain, and more (reviewed in Barry, 2003). However, although evidence for the ability of transplanted BMSCs to generate differentiated nonskeletal tissue cell types (e.g., cardiomyocytes, neurons, etc.) has been controversial, a beneficial effect on the function of target organs has often been observed (reviewed in Phinney and Prockop, 2007; Picinich et al., 2007). It may be that BMSC transplantation consists of exporting the inherent biological function(s) that BMSCs exert in BM to unorthodox sites. That is, if one function is to nurture HSCs and their progeny, then it is possible that nonhematopoietic cells may also benefit from a “nursing” effect conveyed by direct interaction with BMSCs and/or by paracrine stimuli (Caplan and Dennis, 2006). The immune-modulatory activity of BMSCs, supported by a number of studies (e.g., Ren et al., 2008), may be seen as part of the pleiotropic influence of BM stromal cells on cells of hematopoietic lineage. Likewise, the functional effect of BMSCs on vascular structure, integrity, stability, or regeneration may also be retained in unorthodox BMSC transplantation, resulting in a serendipitous benefit for organ function.
What’s in a Name—or Many?
Although the concept that a bona fide stem cell can be found in BM stroma has withstood the test of time and has actually gained momentum from more recent experimentation, what should such a cell be called? There are multiple dimensions, such as function, assays used, or surface phenotype and anatomy, by which to develop appropriate terminology. Using the functional dimension, a postnatal stem cell is usually defined by the types of cells that it generates. Until direct, single-cell, in vivo evidence is provided that indicates BMSCs can generate any tissue other than skeletal cell types, we propose that Friedenstein’s BM-derived “osteogenic stem cell” should be called a “skeletal stem cell,” consistent with the nomenclature used by hematologists. Furthermore, “CFU-F” should be used to denote a cell that is assayed as clonogenic in culture. This term clearly indicates the experimental dimension from which the name arises and once applied can be extended to reflect the origin of the population in question. That is, as CFU-Fs exist in tissues beyond BM, and likely in every postnatal connective tissue, isolation of CFU-Fs from specific tissues could be denoted with prefixes such as BM-CFU-F for bone marrow, AT-CFU-F for adipose tissues, etc., pending a rigorous and comparative in vivo definition of the function of populations resident in and isolated from other anatomical locations. Using the phenotype dimension, markers suited to identify and enrich uncultured CFU-Fs, rather than cultured cells, will over time define subsets of cells to be functionally probed in vivo, linking together the function, the assay, and the phenotype. Ultimately, it would be desirable that the function, the assay, and the phenotype all be traced back to an anatomically recognizable cell type in situ. In this respect, the pericyte population in BM and other tissues represents an emerging candidate.
Overall, 40 plus years of work on BMSCs and the unavoidable swing of hypes and hopes have not taken away from the novel biological flavor of these cells. Simultaneously functioning as stem cells in their own right and as cells that provide the microenvironment for other stem cells, BMSCs embody properties of both the “seed” and “soil.” As expectations linked to BMSC plasticity are on the wane, these unique properties of BMSCs wax back to challenge both biology and medicine, in a quite remarkable fashion.
ACKNOWLEDGMENTS
This work was supported by AIRC, Telethon, MIUR, and Genostem (LHSB-CT-2003-503161) of Italy (to P.B.), by the DIR, NIDCR of the IRP, NIH, and DHHS (to P.G.R.), and by the Australian Stem Cell Center (to P.J.S.).
REFERENCES
- Allen TD. Ultrastructural aspects of in vitro haemopoiesis. In: Lord BI, Potten C, Cole D, editors. The Second Symposium of the British Society for Cell Biology on Stem Cells and Tissue Homeostasis. Cambridge, UK: Cambridge University Press; 1978. p. 217. [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Barry FP. Birth Defects Res C Embryo Today. 2003;69:250–256. doi: 10.1002/bdrc.10021. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D'Aurizio F, Verardo R, Piazza S, Pignatelli A, et al. Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- Bianco P. Blood. 2007;110:3090. [Google Scholar]
- Bianco P, Robey PG. Skeletal stem cells. In: Lanza RP, editor. Handbook of Adult and Fetal Stem Cells. San Diego, CA: Academic Press; 2004. pp. 415–424. [Google Scholar]
- Bianco P, Kuznetsov SA, Riminucci M, Gehron Robey P. Methods Enzymol. 2006;419:117–148. doi: 10.1016/S0076-6879(06)19006-0. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Caplan AI. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. J. Cell. Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Nat. Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dexter TM, Testa NG. Methods Cell Biol. 1976;14:387–405. doi: 10.1016/s0091-679x(08)60498-7. [DOI] [PubMed] [Google Scholar]
- Dexter TM, Allen TD, Lajtha LG. J. Cell. Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ. Int. Rev. Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ. Osteogenic stem cells in bone marrow. In: Heersche JNM, Kanis JA, editors. Bone and Mineral Research. Amsterdam: Elsevier; 1990. pp. 243–272. [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Goujon E. J de L'Anat et de La Physiol. 1869;6:399–412. [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. J. Dent. Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. J. Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- Haylock DN, Williams B, Johnston HM, Liu MC, Rutherford KE, Whitty GA, Simmons PJ, Bertoncello I, Nilsson SK. Stem Cells. 2007;25:1062–1069. doi: 10.1634/stemcells.2006-0528. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- Jain RK. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Exp. Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Immunity. 2006;25:862–864. doi: 10.1016/j.immuni.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Lakshmipathy U, Verfaillie C. Blood Rev. 2005;19:29–38. doi: 10.1016/j.blre.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Li Q, Yu Y, Bischoff J, Mulliken JB, Olsen BR. J. Pathol. 2003;201:296–302. doi: 10.1002/path.1443. [DOI] [PubMed] [Google Scholar]
- Olsen BR, Reginato AM, Wang W. Annu. Rev. Cell Dev. Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. Ciba Found. Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Picinich SC, Mishra PJ, Glod J, Banerjee D. Expert Opin. Biol. Ther. 2007;7:965–973. doi: 10.1517/14712598.7.7.965. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Alison MR, Forbes SJ, Wright NA. J. Pathol. 2002;197:441–456. doi: 10.1002/path.1176. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Schofield R. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Tavassoli M, Crosby WH. Science. 1968;161:54–56. doi: 10.1126/science.161.3836.54. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- Westen H, Bainton DF. J. Exp. Med. 1979;150:919–937. doi: 10.1084/jem.150.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]