Abstract
Nanoparticles have unique physicochemical properties which make them promising platforms for drug delivery. However, immune cells in the bloodstream (such as monocytes, platelets, leukocytes, and dendritic cells) and in tissues (such as resident phagocytes) have a propensity to engulf and eliminate certain nanoparticles. A nanoparticle’s interaction with plasma proteins (opsonins) and blood components (via hemolysis, thrombogenicity and complement activation) may influence uptake and clearance and hence potentially affect distribution and delivery to the intended target sites. Nanoparticle uptake by the immune cells is influenced by many factors. Different nanoparticles have been shown to act on different pathways, while various characteristics/properties also affect which pathway is employed for particle internalization. Nanoparticle protein binding occurs almost instantaneously once the particle enters biological medium, and the physical properties of such a particle–protein complex are often different than those of the formulated particle. These new properties can contribute to different biological responses and change nanoparticle biodistribution. Therefore, in the situation when specific delivery to immune cells is not desired, the ideal nanoparticle platform is the one whose integrity is not disturbed in the complex biological environment, which provides extended circulation in the blood to maximize delivery to the target site, is not toxic to blood cellular components, and is “invisible” to the immune cells which can remove it from circulation. This review discusses the most recent data on nanoparticle interactions with blood components and how particle size and surface charge define their hematocompatibility. This includes properties which determine particle interaction with plasma proteins and uptake by macrophages. We will also provide an overview of in vitro methods useful in identifying interactions with components of the immune system and the potential effects of such interaction on particle distribution to tissues.
Keywords: Nanoparticles, immunology, biodistribution, preclinical characterization
1. Introduction
Recently, nanoenabled drug delivery systems are gaining application in the pharmaceutical industry, since nanoparticle-based drugs may have improved solubility, pharmacokinetics, and biodistribution compared to small molecule drugs. Nanoparticle-carried drugs may also be easier to administer, have fewer side effects, and offer a market advantage to their developer. With the use of targeting, nanoparticle-based drugs can be efficacious at lower doses, which means lower costs and fewer deleterious side effects. Nanoparticle-based targeting has the potential to improve a whole host of conventional pharmaceuticals, and many nanoparticle formulations of existing drugs are already commercially available. 1 Many more will become available to patients in the near future: in 2006 alone, almost 250 nanotechnology-based products entered U.S. pharmaceutical pipelines.
However, the application of nanoparticles as intravenous drug delivery platforms may depend on avoiding rapid elimination from the systemic circulation by cells of the immune system. When nanoparticles enter the bloodstream, they immediately encounter a complex environment of plasma proteins and immune cells. Nanoparticle uptake by the immune cells may occur both in the blood stream by monocytes, platelets, leukocytes, and dendritic cells (DC) and in tissues by resident phagocytes (e.g., Kupffer cells in liver, DC in lymph nodes, macrophages and B cells in spleen). Nanoparticle uptake by immune cells may occur through various pathways2 and can be facilitated by the adsorption of opsonins (plasma proteins) to the particle’s surface. This uptake may cause a nanoparticle to be routed away from the site of its intended application and thus greatly reduce the number of nanoparticles available to reach the target site, effectively reducing the efficacy of the drug. As such, understanding nanoparticle hematocompatibility is an important step during the initial characterization of nanomaterials. There are three main categories considered relevant to nanoparticle biodistribution to the immune cells and organs; they are hemolysis, thrombogenicity and complement activation.
The term “hemolysis” is commonly used to describe damage to red blood cells (erythrocytes) leading to the leakage of the iron-containing protein hemoglobin into the bloodstream and potentially life-threatening conditions such as anemia. A nanoparticle which induces hemolysis may adsorb some quantity of the released hemoglobin and/or adhere to cell debris which in turn increases its likelihood of elimination by macrophages via scavenger receptor- and phosphatidylserine-mediated phagocytosis.3–5 Erythrocytes occupy a larger volume fraction of the blood than do mononuclear phagocytic cells, so an intravenously injected nanoparticle is likely to interact with red blood cells prior to encounters with other immune cells. This position early in the cascade of blood contact interaction, as well as the intensity of the adverse physiological outcome (severe hemolysis may lead to life threatening conditions such as anemia), makes an examination of hemolytic activity an important aspect of preclinical characterization of nanoparticles.
Thrombogenicity is the propensity of a material to induce blood clotting and partial or complete occlusion of a blood vessel by a thrombus (a mixture of red blood cells, aggregated platelets, fibrin and other cellular elements). Some early studies have indicated a relationship between nanoparticle thrombogenicity and phagocytosis by macrophages.6 Furthermore, nanoparticle-based drugs are often engineered to have longer systemic circulation times than small molecule drugs. These extended circulation times in the bloodstream increase the duration of the nanoparticle’s contact with blood components including the coagulation system, potentially amplifying activation of the coagulation cascade and blood clotting.
Lastly, the complement system is a group of proteins linked to each other in a biochemical cascade which removes pathogens from the body and may also contribute to altered nanoparticle biodistribtion in the form of rapid clearance from the systemic circulation via complement receptor-mediated phagocytosis. In addition, complement activation by systemically administered drugs is responsible for hypersensitivity (allergic) reactions and anaphylaxis, a life-threatening condition. As such, nanoparticles intended for systemic administration should be tested for tendency to activate the complement system.
The above effects confer increased importance to the preclinical testing of nanoparticle interaction with the immune system. Such preclinical testing is challenged by several factors. First, there is no harmonized procedure or regulatory guidance document currently available, though both national and international efforts are being made to rectify this situation.7–12 Second, even though a few standards for selecting biological test methods for medicinal materials and devices exist,13 material biocompatibility testing itself must rapidly evolve as basic research data continually suggests new and improved methods. Third, nanoparticles are a broad class of materials intended for a broad spectrum of clinical applications (e.g., as medical devices, components of vaccine formulations, or drug carriers for therapeutic intervention of malignant, inflammatory, viral, neurodegenerative and other types of disorders). The potentially different physicochemical properties of nanoparticles pose novel methodological and regulatory challenges.
A comprehensive overview of nanoparticle interaction with, and effects on, the immune system is provided elsewhere.2 Here, we will summarize some of the available research on testing nanomaterial hematocompatibility and their interaction with macrophages. Both of these types of testing are particularly important for nanoparticle-based drugs.
2. Particle Properties That Influence Compatibility with Immune System
Internationally recognized standard ISO-10993 recommends using the following in vitro tests to examine hematocompatibility of medical devices: tests for hemolysis, thrombogenicity (this includes effects on platelets), and complement activation.14 Studies describing evaluation of these properties in nanoparticle formulations are summarized further below.
2.1. Hemolysis
Several mechanisms for drug-mediated hemolysis have been suggested. These include nonimmunogenic (e.g., via direct drug–erythrocyte membrane interactions) and immune-mediated (e.g., by a drug-specific antibody) hemolysis. Although multiple studies have looked at nanoparticle hemolytic properties, few have attempted to identify mechanisms.
For those nanoparticles which damage erythrocyte membranes directly, it is generally agreed that surface properties (especially surface charge) are important, and there are several studies which have demonstrated this. For example, among a set of similar-sized fullerenes (C60-derivatives) bearing different numbers of anionic and cationic surface moieties, those with negative surface charge were not hemolytic, and hemolytic tendency increased in proportion to the number of attached cationic surface groups (positive surface charge). Similarly, the presence of unprotected primary amines (positive charges) on the surface of polyamidoamine (PAMAM),15 carbosilane,16 polypropylene imine (PPI)17–19 and polylysine (PLL)20 dendrimers was associated with erythrocyte damage in a dose-dependent manner. In these dendrimer studies, blocking the primary amines (and by doing so effectively neutralizing the cationic surface charge) resulted in a dramatic decrease in hematotoxicity.
In the fullerene study referenced above, it was suggested that the presence of a combination of hydrophobic and hydrophilic areas on the surfaces of the cationic fullerene derivatives caused them to act as surfactants and resulted in the observed erythrocyte membrane disruption.21 Further evidence for this mechanism is presented in studies in which the addition of polyethylene glycol (PEG) to a nanoparticle surface has been shown to reduce hemolytic activity while the presence of surfactants in the formulation increases those same properties.22,23
2.2. Thrombogenicity
Certain nanoparticles intended for drug delivery applications are being engineered so as to reduce their clearance from the bloodstream to extend systemic circulation times and thus increase opportunity for delivery to a target site. When the rate of blood clearance is fast, particle interaction with blood components is minimized. An increase in the circulation time commensurately increases the duration of contact with components of the coagulation system. This may amplify adverse effects of this interaction, such as activation of the coagulation cascade, blood clotting and partial or complete occlusion of a blood vessel by thrombus. Studies evaluating nanoparticles’ propensity to induce platelet activation and/or aggregation, their effects on plasma coagulation time, and tendency to initiate vascular thrombosis are useful to assess nanoparticle thrombogenicity. An early study by Movat et al. suggested a link between nanoparticle-induced platelet aggregation and subsequent phagocytosis of the particle-containing platelets by macrophages. 6 This same study clearly demonstrated that phagocytosis of nanoparticles by platelets themselves precedes platelet activation and aggregation, thus suggesting another potential mechanism for nanoparticle removal from the circulation, i.e. redistribution to a blood clot. No comprehensive study evaluating the effects of nanoparticle size and surface properties on thrombogenic properties is currently available; however, trends observed in studies of polymer-based nanoparticles are similar in their charge-dependence to those described above for hemolysis. Specificially, Koziara et al. have shown that decreasing particle surface charge with a PEG coating decreases platelet aggregation and activation.24
The mechanisms through which nanoparticles induce platelet aggregation are largely unknown. One recent study compares five types of carbon-based materials: C60CS (a water soluble fullerene derivative), SWCT (single-walled carbon nanotubes), and MWCT (multiwalled carbon nanotubes), mixed carbon nanoparticles, and a standardized mixture of particulate matter from an urban environment. All of these particles required activation of glycoprotein integrin receptor GPIIb/IIIa in order to cause platelet aggregation. However, pathways leading to this receptor depended on the size of the particles. For instance, micronsized particles required protein kinase C (PKC), while nanoparticle-induced integrin receptor activation was PKC-independent.25 The same study suggested that, unlike classical platelet aggregation, carbon-based nanoparticle-induced platelet aggregation did not require thromboxane A2 and adenosine diphosphate (ADP) release. These data suggest that nanosized particles may induce platelet aggregation via untraditional pathways, which could render common anti-coagulant therapeutics less efficient at reducing nanoparticle-mediated platelet aggregation.
2.3. Complement Activation
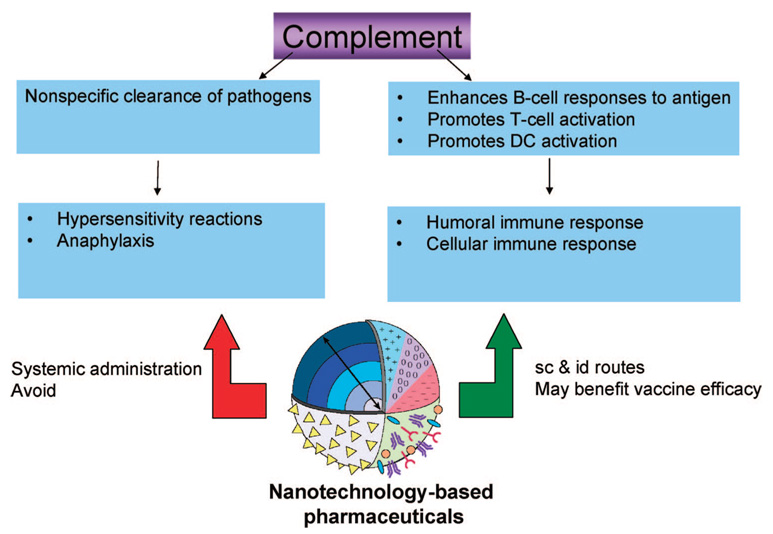
Complement is a term used to describe a group of proteins linked to each other in a biochemical cascade which removes pathogens from the body and thus “complements” cell-mediated and humoral immunity. Nanoparticle-induced complement activation may result in altered biodistribution in the form of rapid clearance from the systemic circulation via CR-mediated phagocytosis by mononuclear cells. In addition to its primary role in nonspecific pathogen clearance, complement activation was proven to be important in supporting cell-mediated immunity through enhancement of B-cell responses to an antigen and promotion of the activation of dendritic cells (DC) and T-cells.26 Complement activation by systemically administered drugs is responsible for hypersensitivity (allergic) reactions and anaphylaxis, a life-threatening condition. As such, nanoparticles intended for systemic administration should be tested for tendency to activate the complement system. If a particular nanoparticle does cause significant complement activation, its surface properties must be tuned to decrease these interactions to an acceptable level. On the other hand, local activation of complement by nanoparticles may be desirable for enhanced antigen presentation to benefit vaccine efficacy when nanoparticles are administered via other routes, e.g. subcutaneously or intradermally (Figure 1). One of the best examples of complement activation by systemically administered nanoparticles is a study by Chanan-Khan and colleagues. They followed up clinical studies reporting hypersensitivity reactions to the nanoliposome encapsulated-doxorubicin formulation Doxil, and found that complement activation was causing the observed hypersensitivity.27 Another recent study demonstrated surface-dependent complement activation occurring locally upon intradermal administration of a nanoparticle-carried vaccine. In this case, the uptake of the nanoparticles by dendritic cells, activation of T-cells, and generation of the antigen-specific immune response was dependent on complement activation by the nanoparticle.28 Thus a nanoparticle’s tendency to activate complement may be either beneficial or deleterious depending on its intended application.
Figure 1.
Complement activation: friend or foe? The complement system serves as a nonspecific pathogen clearance aid, which complements humoral and cell-mediated immunity. Undesirable activation of the complement system results in hypersensitivity reactions, and overwhelming activation may even lead to anaphylactic shock. For this reason, it is frequently desirable to design nanoparticles intended for systemic administration such as to avoid complement activation. However, in addition to its primary role in pathogen clearance, the complement system also promotes humoral and cell-mediated immunity. Local activation of complement by nanoparticles administered via subcutaneous and intradermal routes may benefit vaccine efficacy. Nanotechnology-based pharmaceuticals are frequently composed of multiple components with varying compositions, size, and surface properties which may be engineered to achieve desirable properties.
It is recognized that nanoparticle surface charge is an important factor for activation of the complement system. Studies investigating polypropylene sulfide nanoparticles,28 lipid nanocapsules,29 cyclodextrin-containing polycation-based nanoparticles 30 and polystyrene nanospheres31 have shown that charged nanoparticles are more efficient activators of the complement system than their neutral counterparts. Furthermore, poly(ethylene glycol) (PEG) and poloxamine 908 coatings were shown to reduce complement activation by nanoparticles.29,32 However, dextran coatings were associated with an increase in nanoparticle-mediated complement activation and complement activation increased with the coating thickness.33 In this same study, dextran and chitosan coatings of the same thickness were able to either inhibit or enhance nanoparticle mediated complement activation depending on the specific polymer conformation.33 Thus complement activation is very sensitive to several aspects of nanoparticle surface coatings in that the type of polymer coating, its size, density, configuration on the surface, and accessibility to reactive groups may all play a role.
3. Interaction with Plasma Proteins and Macrophage Uptake
Physicochemical properties such as nanoparticle size, surface charge, solubility, and surface functionality influence nanoparticle uptake and clearance by macrophages. In general, larger particles are taken up more efficiently than smaller particles of the same composition and surface properties.34 Particles bearing cationic or anionic surface charges have been shown to be more attractive to phagocytes than neutral particles of the same size.35 Manipulation of nanoparticle size and charge has also proven useful to increase particle delivery to immune cells for applications such as vaccine delivery where this is desirable, and to avoid opsonization and subsequent nonspecific uptake by phagocytic cells for drug-delivery applications where the immune system is not targeted (e.g., for cancer applications or imaging). For example, for several studies with vaccine carriers, surface functionalization with chitosan and mannose was shown to deliver nanoparticles to macrophages and dendritic cells via specific phagocytic pathways and thus improve immune response to nanoparticle-bound antigens.23,36,37 In drug-delivery applications outside vaccine delivery, addition of polymer coatings such as PEG, poloxamer, and poloxamine to the nanoparticle surface is a tool for avoiding recognition by the immune cells.38–42 Of these polymers, PEG is the mostly commonly used in formulations for medical applications. Addition of this polymer was demonstrated to prolong particle circulation in the blood and significantly decrease uptake by spleen and liver resident phagocytes.41 It has been hypothesized that PEG creates a steric shield around the coated particle, effectively preventing plasma proteins from adhering to the particle surface and thus avoiding subsequent uptake by mononuclear phagocytes (Figure 2).
Figure 2.
Role of protein binding on nanoparticle distribution to immune cells. Nanoparticle surface properties largely determine particle interaction with plasma proteins. Unprotected nanoparticles, such as citrate-stabilized colloidal gold, rapidly bind plasma proteins (this can be analyzed by particle separation from bulk plasma, removal of surface bound proteins and analysis by 2D PAGE). After opsonization with human plasma, these particles are taken up by macrophages. Here, transmission electron microscopy (TEM) micrographs of macrophages demonstrate particle accumulation inside cells. In contrast, PEGylated gold particles bind less protein (as can be seen by 2D PAGE) and are not taken up by macrophages (as can be seen from TEM images).
Adsorption of plasma proteins onto the surface of a nanoparticle, also known as opsonization, can occur practically the instant a particle enters the bloodstream.43 Some opsonins have high binding affinities and thus long residence time on certain nanoparticle surfaces. The physical characteristics of the resulting nanoparticle–protein complex can be vastly different from those of the native nanoparticle, and understanding these differences is essential to understand eventual distribution, clearance, and the biological responses that occur. The binding of protein can increase the nanoparticle’s effective size and change its effective surface charge, which will then affect macrophage uptake. The mechanism by which protein adsorption occurs is not completely understood. However, the specific blood proteins which bind particular nanoparticles have been examined. Of those, the most common are immunoglobulins and components of the complement system, as well as other abundant blood serum proteins such as fibrinogen and albumin.39 These proteins are thought to be important in the clearance process.43,44 However, the complete composition of the protein coating at any given time is a function of the concentrations of all plasma proteins and their binding kinetics (equilibrium constants, on/off rates, and binding affinity).45 Because of this, the true composition of the nanoparticle–protein complex is in continual flux through the duration of its interaction with the body.
Once internalized by the immune cells, a nanoparticle’s fate is largely determined by its composition (Figure 3). Biodegradable particles are digested and cleared from the body,46,47 while nonbiodegradable particles (e.g., metal colloids, ceramics) accumulate in cells for extended periods.48 The processing of multicomponent, multifunctional nanoparticles is more complex. How do immune cells handle the individual components of a multicomponent particle? Are they processed separately or together? These and other questions likely depend on the strengths of the attachements of the individual components as well as their individual compositions.
Figure 3.
Particle fate inside the immune cell is largely determined by its composition. Biodegradable particles are digested and cleared from the body, while nonbiodegradable particles accumulate in cells for extended periods. Processing of multicomponent, multifunctional nanoparticles is more complex, and more studies are required to answer questions regarding the fates of the individual components of these particles.
4. Assays To Study Particle Interaction with the Immune System
An important initial step in the preclinical characterization of nanoparticles is testing for sterility and pyrogenicity. Pyrogens are substances that cause fever. Two types of pyrogens exist and are subject to testing during preclinical development of medicinal products: biological pyrogens (most commonly endotoxin or lipopolysaccharide) and chemical (material-mediated pyrogens). The US FDA recommends two methods for pyrogenicity evaluation: the lumilus amebocyte lysate (LAL) based assay and the rabbit pyrogen test. The importance of the LAL test to nanoparticle characterization and challenging aspects of its application are reviewed elsewhere.49 After sterility and pyrogenicity questions have been addressed, nanoparticle formulations may be characterized in terms of their in vivo and in vitro biologic and toxic properties. Although there is no simple test (either in vitro or in vivo) which can predict a material’s immunotoxicity to human patients, subjecting a nanoparticle to a battery of in vitro immunological tests may be helpful during the early stages of preclinical development to eliminate potentially dangerous candidates from pipelines and to improve understanding of the physicochemical properties which can be tuned to decrease their immunotoxicity.
The most commonly used in vitro blood compatibility tests include hemolysis, complement activation, and thrombogenicity. In vitro tests that may be useful to predict particle uptake by macrophages and distribution to organs of the reticuloendothelial system (RES) are chemotaxis and phagocytosis. These and other methods are summarized in Table 1 and can be used to understand the properties of nanoparticles intended for systemic administration (e.g., by iv route).
Table 1.
In Vitro Assays To Test Nanoparticle Compatibility with the Immune Systema
| assay for analysis of nanoparticles | description | URL |
|---|---|---|
| hemolysis | test for nanoparticle ability to damage red blood cells | http://ncl.cancer.gov/NCL_Method_ITA-1.pdf |
| platelet aggregation | test for nanoparticle potential pro- and anticoagulant properties | http://ncl.cancer.gov/NCL_Method_ITA-2.pdf |
| plasma coagulation | used in support of platelet aggregation test | http://ncl.cancer.gov/NCL_Method_ITA-12.pdf |
| complement activation | test for nanoparticle ability to activate complement | http://ncl.cancer.gov/NCL_Method_ITA-5.pdf |
| plasma protein binding | helps understanding degree of opsonization | http://ncl.cancer.gov/NCL_Method_ITA-4.pdf |
| phagocytosis | test potential particle uptake by macrophages | http://ncl.cancer.gov/NCL_Method_ITA-9.pdf |
| CFU-GM | test for nanoparticle effects on bone marrow cells | http://ncl.cancer.gov/NCL_Method_ITA-3.pdf |
| leukocyte proliferation | identifies particle effects on leukocytes | http://ncl.cancer.gov/NCL_Method_ITA-6.pdf |
| NO- production by macrophages | test for induction of macrophage oxidative burst | http://ncl.cancer.gov/NCL_Method_ITA-7.pdf |
| chemotaxis | test for particles property to attract macrophages | http://ncl.cancer.gov/NCL_Method_ITA-8.pdf |
These assays have been established at Nanotechnology Characterization Lab and optimized to specifically suite nanoparticle studies. URLs are provided for free access to full-text protocols and instructions. The full list of NCL assays is also available at http://ncl.cancer.gov/working_assay-cascade.asp.
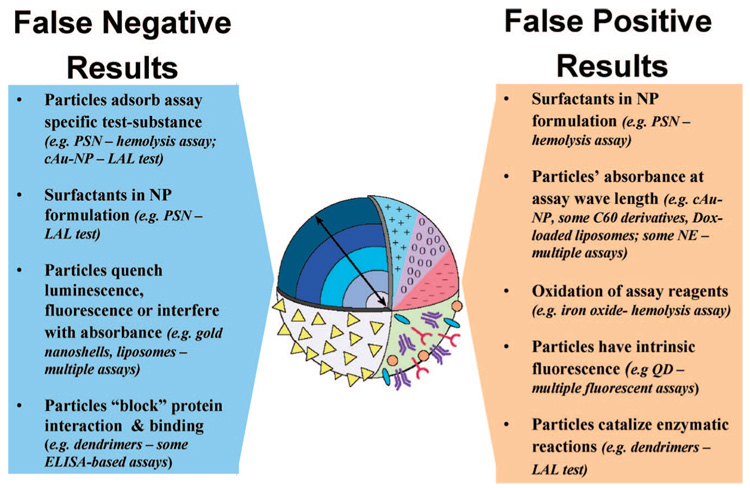
Challenges in the application of traditional in vitro tests to nanoparticle-induced hemolysis, thrombogenicity, complement activation, phagocytosis and other in vitro tests have been reviewed before.49 Here we summarize the most common mechanisms of interference (Figure 4). One of the most frequently encountered types of interference is caused by the optical properties of nanoparticles (e.g., absorbance at or near the detection wavelength of the assay). This may result in a false-positive result. Examples of nanoparticles with optical properties that interfere with commonly used absorbance-based pharmacological tests (with detection wavelengths between 405 and 540 nm) include some nanoemulsions, water-soluble fullerene derivatives, and gold-containing nanoparticles. Other sources of false-positive results are toxic surfactants or synthesis byproducts within nanoparticle formulations (e.g., some polystyrene nanoparticle formulations contain Triton X-100 as anticaking agent, and gold nanoparticles stabilized with cetyl trimethylammonium bromide (CTAB) may contain traces of this reagent. False-positive results may also arise from nanoparticle catalytic properties, intrinsic fluorescence, or activation of luminescent reagents. Nanoparticles may also adsorb assay-specific test substances causing them to escape quantification (e.g., gold nanoparticles adsorb endotoxin), quench luminescence or fluorescence, and block protein binding leading to false-negative results.
Figure 4.
Points to consider during assay development. Nanoparticles differ in size, composition, and surface characteristics. These differences may cause spurious experimental outcomes if the properties of the particle are poorly understood. The main challenge in nanoparticle characterization using traditional techniques is identifying potential nanoparticle interferences (and then overcoming them) to avoid false-positive and false-negative results. The particle properties responsible for false-positive and false-negative results are summarized in this diagram. Examples are given as type of nanoparticle - assay in which they cause interference. Abbreviations: PSN, polystyrene nanoparticle; cAU-NP, citrate-stabilized gold nanoparticles (colloidal gold); Dox, doxorubicin; QD, quantum dots; LAL, limulus amoebocyte lysate; ELISA, enzyme linked immunosorbent assay; NP, nanoparticle; NE, nanoemulsion.
All of these interferences underscore that a thorough physicochemical characterization prior to in vitro tests is critical for accurate conclusions. If a nanoparticle formulation is well understood (i.e., if its optical properties, formulation, catalytic properties, interaction with assay reagents, etc. are well-known), it is easier to predict potential types of interference and thus avoid them.
Standardization efforts in the field of nanotechnology are underway at the national and international levels. Recently, two methods related to nanoparticle interaction with the immune system have been approved as standard practices by American Society for Testing and Materials (ASTM) International.50,51 A standard for detection of nanoparticle contamination by bacterial endotoxins is being developed by International Organization for Standardization (ISO).12 Standardization of other in vitro methods is also underway to better assess the exposure and thus possible risks posed by nanomaterials.7–11,52
5. Conclusions
Understanding the mechanisms of nanoparticle interaction with the immune system is as important as analyzing potential toxic effects. It is well-recognized that physicochemical properties such as size and surface charge are critical to elements of this interaction. More experimental evidence is required to determine whether in vitro data are relevant to effects observed in vivo, and how to translate these data into a predicted response in human patients. As more mechanistic studies are published describing the interactions of various classes of nanoparticles with the immune system, a more complete picture can be proposed.
Acknowledgment
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. We are grateful to Allen Kane for the help in illustration preparation.
References
- 1.Powers M. Nanomedicine, Device & Diagnostics Report. Nanomedicine and nano device pipeline surges 68% NanoBiotech News. 2006:1–69. 2006. [Google Scholar]
- 2.Dobrovolskaia MA, McNeil SE. Immunological Properties of engineered nanomaterials. Nat. Nanotechnol. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JJ. Apoptosis: the physiologic pathway of cell death. Hosp. Pract. (Off. Ed.) 1993;28(12):35–43. doi: 10.1080/21548331.1993.11442887. [DOI] [PubMed] [Google Scholar]
- 4.Schaer DJ, Alayash AI, Buehler PW. Gating the radical hemoglobin to macrophages: the anti-inflammatory role of CD163, a scavenger receptor. Antioxid. Redox Signaling. 2007;9(7):991–999. doi: 10.1089/ars.2007.1576. [DOI] [PubMed] [Google Scholar]
- 5.Schroit AJ, Madsen JW, Tanaka Y. In vivo recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J. Biol. Chem. 1985;260(8):5131–5138. [PubMed] [Google Scholar]
- 6.Movat HZ, Weiser WJ, Glynn MF, Mustard JF. Platelet phagocytosis and aggregation. J. Cell Biol. 1965;27(3):531–543. doi: 10.1083/jcb.27.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. http://astm.org/cgi-bin/SoftCart.exe/COMMIT/COMMITTEE/E56.htm?L+mystore+vdgw9357.
- 8. http://es.epa.gov/ncer/nano/
- 9. http://icon.rice.edu/
- 10. http://www.cdc.gov/niosh/topics/nanotech/default.html.
- 11. http://www.fda.gov/nanotechnology/
- 12. http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=45640.
- 13.Standard Practice for selecting generic biological test methods for materials and devices. ASTM International. 1998:F748–F798. [Google Scholar]
- 14.ANSI/AAMI/ISO. 10993-4: Biological Evaluation of Medical Devices. Part 4: Selection of Tests for Interaction with Blood. 2002 [Google Scholar]
- 15.Domanski DM, Klajnert B, Bryszewska M. Influence of PAMAM dendrimers on human red blood cells. Bioelectrochemistry. 2004;63(1–2):189–191. doi: 10.1016/j.bioelechem.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Bermejo JF, Ortega P, Chonco L, Eritja R, Samaniego R, Mullner M, de Jesus E, de la Mata FJ, Flores JC, Gomez R, Munoz-Fernandez A. Water-soluble carbosilane dendrimers: synthesis biocompatibility and complexation with oligonucleotides; evaluation for medical applications. Chemistry. 2007;13(2):483–495. doi: 10.1002/chem.200600594. [DOI] [PubMed] [Google Scholar]
- 17.Agashe HB, Dutta T, Garg M, Jain NK. Investigations on the toxicological profile of functionalized fifth-generation poly (propylene imine) dendrimer. J. Pharm. Pharmacol. 2006;58(11):1491–1498. doi: 10.1211/jpp.58.11.0010. [DOI] [PubMed] [Google Scholar]
- 18.Dutta T, Agashe HB, Garg M, Balakrishnan P, Kabra M, Jain NK. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro. J. Drug Targeting. 2007;15(1):89–98. doi: 10.1080/10611860600965914. [DOI] [PubMed] [Google Scholar]
- 19.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Controlled Release. 2000;65(1–2):133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 20.Shah DS, Sakthivel T;, Toth I, Florence AT, Wilderspin AF. DNA transfection and transfected cell viability using amphipathic asymmetric dendrimers. Int. J. Pharm. 2000;208(1–2):41–48. doi: 10.1016/s0378-5173(00)00534-2. [DOI] [PubMed] [Google Scholar]
- 21.Bosi S, Feruglio L, Da Ros T, Spalluto G, Gregoretti B, Terdoslavich M, Decorti G, Passamonti S, Moro S, Prato M. Hemolytic effects of water-soluble fullerene derivatives. J. Med. Chem. 2004;47(27):6711–6715. doi: 10.1021/jm0497489. [DOI] [PubMed] [Google Scholar]
- 22.Kim D, El-Shall H, Dennis D, Morey T. Interaction of PLGA nanoparticles with human blood constituents. Colloids Surf., B: Biointerfaces. 2005;40(2):83–91. doi: 10.1016/j.colsurfb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Kim TH, Nah JW, Cho MH, Park TG, Cho CS. Receptor-mediated gene delivery into antigen presenting cells using mannosylated chitosan/DNA nanoparticles. J. Nanosci. Nanotechnol. 2006;6(9–10):2796–2803. doi: 10.1166/jnn.2006.434. [DOI] [PubMed] [Google Scholar]
- 24.Koziara JM, Oh JJ, Akers WS, Ferraris SP, Mumper RJ. Blood compatibility of cetyl alcohol/polysorbate-based nanoparticles. Pharm. Res. 2005;22(11):1821–1828. doi: 10.1007/s11095-005-7547-7. [DOI] [PubMed] [Google Scholar]
- 25.Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005;146(6):882–893. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knopf PM, Rivera DS, Hai SH, McMurry J, Martin W, De Groot AS. Novel function of complement C3d as an autologous helper T-cell target. Immunol. Cell Biol. doi: 10.1038/sj.icb.7100147. in press. [DOI] [PubMed] [Google Scholar]
- 27.Chanan-Khan A, Szebeni J, Savay S, Liebes L, Rafique NM, Alving CR, Muggia FM. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann. Oncol. 2003;14(9):1430–1437. doi: 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- 28.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 29.Vonarbourg A, Passirani C, Saulnier P, Simard P, Leroux JC, Benoit JP. Evaluation of pegylated lipid nanocapsules versus complement system activation and macrophage uptake. J. Biomed. Mater. Res. A. 2006;78(3):620–628. doi: 10.1002/jbm.a.30711. [DOI] [PubMed] [Google Scholar]
- 30.Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjugate Chem. 2007;18(2):456–468. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagayama S, Ogawara K, Fukuoka Y, Higaki `K, Kimura T. Time-dependent changes in opsonin amount associated on nanoparticles alter their hepatic uptake characteristics. Int. J. Pharm. 2007;342(1–2):215–221. doi: 10.1016/j.ijpharm.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hanbali O, Rutt KJ, Sarker DK, Hunter AC, Moghimi SM. Concentration dependent structural ordering of poloxamine 908 on polystyrene nanoparticles and their modulatory role on complement consumption. J. Nanosci. Nanotechnol. 2006;6(9–10):3126–3133. doi: 10.1166/jnn.2006.406. [DOI] [PubMed] [Google Scholar]
- 33.Bertholon I, Vauthier C, Labarre D. Complement activation by core-shell poly(isobutylcyanoacrylate)-polysaccharide nanoparticles: influences of surface morphology, length, and type of polysaccharide. Pharm. Res. 2006;23(6):1313–1323. doi: 10.1007/s11095-006-0069-0. [DOI] [PubMed] [Google Scholar]
- 34.Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur. J. Pharm. Sci. 2006;27(1):27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol) Langmuir. 2006;22(19):8178–8185. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- 36.Cui Z, Mumper RJ. Coating of cationized protein on engineered nanoparticles results in enhanced immune responses. Int. J. Pharm. 2002;238(1–2):229–239. doi: 10.1016/s0378-5173(02)00079-0. [DOI] [PubMed] [Google Scholar]
- 37.Cuna M, Alonso-Sandel M, Remunan-Lopez C, Pivel JP, Alonso-Lebrero JL, Alonso MJ. Development of phosphorylated glucomannan-coated chitosan nanoparticles as nanocarriers for protein delivery. J. Nanosci. Nanotechnol. 2006;6(9–10):2887–2895. doi: 10.1166/jnn.2006.435. [DOI] [PubMed] [Google Scholar]
- 38.Csaba N, Sanchez A, Alonso MJ. PLGA:poloxamer and PLGA:poloxamine blend nanostructures as carriers for nasal gene delivery. J. Controlled Release. 2006;113(2):164–172. doi: 10.1016/j.jconrel.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Goppert TM, Muller RH. Protein adsorption patterns on poloxamer- and poloxamine-stabilized solid lipid nanoparticles (SLN) Eur. J. Pharm. Biopharm. 2005;60(3):361–372. doi: 10.1016/j.ejpb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Muller RH, Maassen S, Weyhers H, Mehnert W. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. J. Drug Targeting. 1996;4(3):161–170. doi: 10.3109/10611869609015973. [DOI] [PubMed] [Google Scholar]
- 41.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Delivery. 2004;11(3):169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 42.Redhead HM, Davis SS, Illum L. Drug delivery in poly(lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J. Controlled Release. 2001;70(3):353–363. doi: 10.1016/s0168-3659(00)00367-9. [DOI] [PubMed] [Google Scholar]
- 43.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Frank MM, Fries LF. The role of complement in inflammation and phagocytosis. Immunol. Today. 1991;12(9):322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 45.Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA. The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv. Colloid Interface Sci. 2007;134–135:167–174. doi: 10.1016/j.cis.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 46.Chellat F, Grandjean-Laquerriere A, Le Naour R, Fernandes J, Yahia L, Guenounou M, Laurent-Maquin D. Metalloproteinase and cytokine production by THP-1 macrophages following exposure to chitosan-DNA nanoparticles. Biomaterials. 2005;26(9):961–970. doi: 10.1016/j.biomaterials.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Ljubimova JY, Fujita M, Khazenzon NM, Lee BS, Wachsmann-Hogiu S, Farkas DL, Black KL, Holler E. Nanoconjugate based on polymalic acid for tumor targeting. Chem. Biol. Interact. 2008;171(2):195–203. doi: 10.1016/j.cbi.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Controlled Release. 2006;114(3):343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Hall JB, Dobrovolskaia MA, Patri AK, McNeil SE. Characterization of nanoparticles for therapeutics. Nanomedicine. 2007;2(6):789–803. doi: 10.2217/17435889.2.6.789. [DOI] [PubMed] [Google Scholar]
- 50.Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles. ASTM International. 2008 E2524-08. [Google Scholar]
- 51.Standard Test Method for Evaluation of the Effect of Nanoparticulate Materials on the Formation of Mouse Granulocyte-Macrophage Colonies. ASTM International. 2008 E2525-08. [Google Scholar]
- 52. http://www.iso.org/iso/standards_development/technical_committees/list_of_iso_technical_committees/iso_technical_committee.htm?commid=381983.