Summary
Introduction:
Lactoferrin, an 80-kDa basic N-glycoprotein, has been identified as a potent immune modulator. When used experimentally as an adjuvant in mice, lactoferrin can boost the efficacy of the BCG vaccine to increase delayed type hypersensitive responses and to limit subsequent pathology upon infection with virulent mycobacterium. These studies outline preliminary findings to examine the multiple mechanisms of action of lactoferrin on antigen-presenting cells which would enhance vaccination and bridge the innate and adaptive responses.
Material/Methods:
Bone marrow-derived macrophages (BMMs) and human and murine cells lines were infected with BCG in the presence or absence of bovine lactoferrin. The cells were examined for changes in uptake of BCG post infection and increases in the surface expressions of Class I or Class II molecules by flow cytometric analysis. Infected cultures were collected to examine cytokine production by ELISA.
Results:
Lactoferrin was found to significantly increase the uptake of BCG organisms during infection of BMMs and human monocyte cell lines. Lactoferrin added to BCG-infected BMMs demonstrated significantly increased surface expression of Class II (I-Ab), but no change in Class I (H-2kb) molecules. In addition, BCG-infected cells incubated in the presence of lactoferrin demonstrated a significant increase in relative IL-12 to IL-10 ratios in a dose-dependent manner.
Conclusions:
Overall, lactoferrin was able to alter BCG-infected antigen-presenting cells (APCs) in vitro in a manner consistent with the induction of responses required for successful presentation of antigen to the adaptive arm of the immune response, which would lead to the generation of strong T-helper 1 type immunity.
Keywords: Lactoferrin, immunomodulation, adjuvant, BCG
Introduction
Tuberculosis (TB) is a contagious disease caused by Mycobacterium tuberculosis (Mtb) and is responsible for nearly three million deaths every year [25]. The current vaccine against tuberculosis is a live, attenuated strain of Mycobacterium bovis Bacillus Calmette-Guerin (BCG), which was first used in humans in the 1920's. Although the BCG vaccine has proven to be successful in preventing disease in infants and children, its efficacy wanes dramatically in adulthood [2,22]. Thus there are on-going studies to improve vaccine efficacy either through developing novel antigens or by improving the existing BCG vaccine through incorporation of adjuvant materials.
Lactoferrin (LF), an 80-kDa basic N-glycoprotein, was first identified in 1939 in bovine milk and subsequently found in tears, saliva, bile, pancreatic juices and intestinal secretions, and in plasma. LF consists of two globular lobes, each with one ferric ion binding site. It is found predominantly in the secondary granules of neutrophils and has been attributed with many protective functions, e.g. antibacterial, both bacterio-static and bactericidal, antiviral, antifungal, antiparasitic, antitumor, antioxidant, and anti-inflammatory [23]. In addition, LF is identified as an immunomodulator of innate and adaptive responses. In the studies outlined here, LF was shown to function as an adjuvant to the BCG vaccine, increasing the host protective response during infection of mice with virulent Mycobacterium tuberculosis (Mtb) [10]. Addition of LF adjuvant to the BCG vaccine led to an upregulation of the delayed type hypersensitivity response (DTH, antigenic recall) and decreased deleterious pulmonary pathology.
The studies described in this report examine the role of LF in modulating the responses of BCG-infected APCs. Organism uptake by APCs, subsequent changes in surface presentation molecules, and alterations in cytokine production are described. Thus the results presented here provide, in part, a potential mechanism for LF to function as an adjuvant to augment the BCG vaccine.
Material and Methods
Cell culture
Murine bone marrow macrophages (BMMs) were collected from C57BL/6 mice (six weeks, Jackson Laboratories, Bar Harbor, ME) by flushing femur bones with McCoy's medium (Cellgro, VA) supplemented with 100 μg/ml penicillin (Sigma), 50 μg/ml gentamycin sulfate (Sigma), and 2.2 g/l sodium bicarbonate. The cells were treated with AKC buffer (Cambrex Bio Sciences, East Rutherford, NJ) to lyse the erythrocytes. The remaining cells were then differentiated for a week at 1×106 cells/ml in McCoy's medium with addition of GM-CSF (10 ng/ml, Cell Sciences, Canton, MA), antibiotics as above, and 10% FBS. Media was changed on days 3 and 5. Differentiated macrophages were washed with 1×PBS and rested overnight in complete DMEM media (DMEM supplemented with 10% FBS, sodium bicarbonate, 50 mg/l HEPES and 50 mg/l L-arginine). THP1 and U937 human macrophage-like cell lines, or J774A.1 murine monocytes, (ATCC, Manassas, VA) were grown in DMEM complete media. For the apoptosis assay, human cells were activated with 10 ng/ml phorbol ester 12-tetradecanoylphorbol-13 acetate (PMA) over-night. Non-adherent cells were then removed by washing with PBS and the remaining cells were rested in complete DMEM media for 4 h prior to use.
BCG and in vitro infection
Mycobacterium bovis Calmette Guerin, Pasteur strain (TMC 1011, ATCC, Manassas, VA) was grown as described [10] in Dubos base (without addition of glycerol) with 10% supplement (5% BSA and 7.5% dextrose in saline) on an orbital shaker at 37°C for 2 weeks before use. The BCG was diluted with 1×Dulbecco's phosphate-buffered saline (PBS) (Cellgro, Herndon, VA) to the indicated MOI [multiplicity of infection] and confirmed by plating dilutions onto 7H11 agar plates (Remel, Lenesa, KS).
Bacterial uptake
BCG was stained with BacLight™ Green bacterial stain according to the manufacturer's instruction immediately before use (Molecular Probes, Invitrogen). Cells were infected with green-stained BCG in an MOI of 100:1 for 4 h at 37°C with or without LF. Lactoferrin (bovine milk LF, <1 E.U./mg endotoxin, <20% iron saturation, >95% purity) was generously provided by PharmaReview Corporation (Houston, TX). Cells were washed with PBS to eliminate free organisms and harvested by trypsin digestion and scraping, spun down at 1000 rpm for 5 min, fixed in 2% paraformaldehyde at 4°C overnight, and finally resuspended in 1% BSA (Sigma) in PBS. The cells were analyzed using Coulter FlowCentre™ (EPICS XL-MCL).
Apoptosis
U937 cells prestimulated with PMA were infected with green-stained BCG in the presence or absence of LF at 100 μg/ml. Seventy-two hours later the cells were harvested and apoptosis was measured with Vybrant Apoptosis Assay Kit #2 (Invitrogen). Briefly, the cells were spun down at 1000 rpm for 5 min, washed with cold PBS, and resuspended in annexin-binding buffer. Then Annexin V and propridium iodide were added. After 15 min of incubation at room temperature, 400 μl of annexin-binding buffer was added. The cells were put on ice and analyzed by flow cytometry.
Class I and Class II molecule surface expression
Macrophages remained uninfected or were infected with BCG (MOI: 10:1) with or without lactoferrin (100 μg/ml). At 72 hrs post-infection, the macrophages were isolated by washing with 1×PBS, then 0.25% Trypsin-EDTA (Sigma) was added to each well and incubated at 37°C for 3–5 minutes. The reaction was neutralized with an equal volume of 15% BSA.
Fluorescent Activated Cell Sorting (FACS)
Antibodies were diluted to a working concentration of 1 μg/106 cells in staining buffer (1% BSA in 1×PBS). Macrophages were stained (50 μl diluted antibody/106 cells) in the dark with anti-mouse I-Ab-FITC and H-2kb-FITC (BD Biosciences Pharmingen) on ice for 30 minutes. The macrophages were washed with staining buffer and fixed with 4% paraformaldehyde (Fisher Scientific, Fair Lawn, NJ) in 1×PBS on ice for 15 minutes. The fixed cells were washed with staining buffer and stored in staining buffer at 500 μl/106 cells at 4°C. Analysis was performed using a Coulter FlowCentreTM (EPICS XL-MCL). Graphs were generated with WinMDI 2.8.
ELISA (Enzyme-linked immunosorbent assay)
Supernatants were assayed for IL-12 and IL-10 cytokine production using DuoSet ELISA kits (R&D Systems, Minneapolis, MN) according to manufacturer's instructions. Briefly, the capture antibody was coated onto Costar EIA/RIA 1×8 StripwellTM plates (Corning Inc, Corning, NY) in 1×PBS at 100 μl/well and incubated at room temperature overnight. The plates were washed with wash buffer (0.05% v/v Tween-20 in 1×PBS) three times. Blocking buffer (1% w/v bovine serum albumin, BSA, 5% w/v sucrose, 0.05% w/v sodium azide in 1×PBS) was added and incubated at room temperature for at least one hr. After washing, the isolated supernatants were loaded in triplicate and incubated at room temperature for two hrs and detected by the provided antibody reagent. The signal was generated after addition of Streptavidin-HRP followed by SureBlueTM TMB microwell peroxidase substrate 1-component (KPL, Gaithersburg, MD). The plates were read at 450 nm, subtracting the background at 570 nm. The concentration was calculated by generating a standard curve fit. The lower assay limits of detection were approximately 15 pg/ml.
Statistical analysis
The data obtained from the experiments were analyzed by one-way ANOVA (apoptosis and uptake) or Student's t-test (ELISA). Results were considered significant with p<0.05.
Results
LF augments the uptake of live BCG in antigen-presenting cells
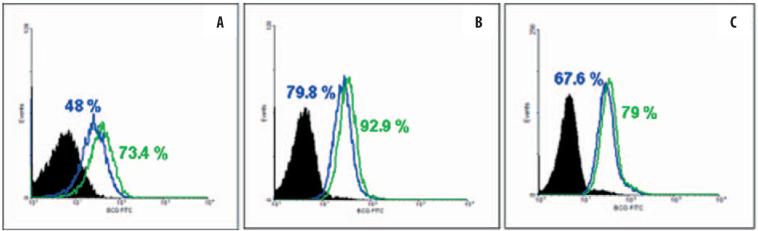
The first encounter with vaccine antigen, necessary for successful presentation to the adaptive arm of the immune response, requires uptake by professional antigen-presenting cells. Therefore, LF was first investigated for its function to augment the uptake of BCG in murine bone marrow-derived macrophages (BMM). Differentiated, cultured murine BMMs were infected with live, fluorescently labeled BCG in the presence or absence of LF at 100 μg/ml. Four hours later the cultures were examined by flow cytometric analysis. The BMM cultures significantly (p<0.01) demonstrated increased uptake of organisms (Figure 1A). The effect was also shown in the human macrophage cells lines THP-1 and U937 (Figures 1B and 1C), where significant uptake of BCG was observed (p<0.001).
Figure 1.
Increased uptake of BCG in lactoferrin-treated cells. LF (green line) at 100 μg/ml increases BCG uptake in bone marrow-derived macrophages (A), THP1 cells (B) and U937 cells (C). Comparison to untreated cells is shown (blue line); isotype controls are in black. Representative of 3–4 experiments. For all groups, difference is significant with p<0.001.
Increase in surface Class II expression in LF-treated bone marrow-derived macrophages
LF was examined for its potential to alter the surface expressions of molecules involved in antigen presentation. Specifically, murine BMMs incubated with LF (100 μg/ml) and BCG demonstrated increased surface expression of Class II I-Ab (p<0.01) (Figure 2). The increased expression was only apparent in the infected cells; LF treatment of uninfected cells did not alter the surface expression (not shown). Of interest is that there was no relative change in Class I expression (H-2kb).
Figure 2.
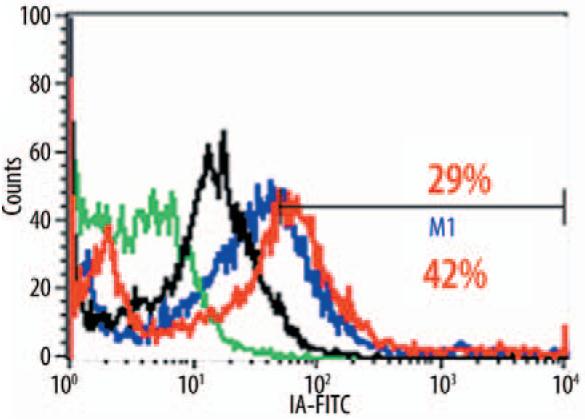
Elevated Class II expression on lactoferrin-treated BCG-infected bone marrow macrophages. BCG-infected murine BMMs were incubated with (red) or without LF (100 μg/ml) (blue) and monitored for expression of Class II I-Ab. Unstained populations (green) and isotype controls (black) are also shown. Mean intensity of fluorescence indicated, with a significant difference between the lactoferrin-treated vs. the untreated groups (p<0.01).
Increased IL-12 to IL-10 ratios in LF-treated macrophages
The ability to present antigen is only one facet of successful augmentation of subsequent adaptive immune response (stimulation of cellular and humoral responses). Elicited cytokines must also accompany the upregulated presentation of antigen. Therefore, BCG-infected cultured J774A.1 cells were incubated in the presence of LF in increasing concentrations (1, 10, and 100 μg/ml) and subsequently evaluated for changes in the production of IL-12 and IL-10. Table 1 demonstrates a marked and significant (p<0.05) increase in IL-12 relative to IL-10 produced by BCG infected cells in the presence of LF, with responses occurring in a dose-dependent manner.
Table 1.
Ratio of IL-12 to IL-10 in BCG-infected J774A.1 macrophages. Cells were infected with BCG (MOI 10: 1) with or without lactoferrin. Supernatants collected at 72 hrs were analyzed by ELISA. The ratio was calculated by dividing the concentration of IL-12p40 by IL-10. Avg, average; Stdev, standard deviation
p<0.05 relative to non-lactoferrin-treated controls.
Enhanced apoptosis of BCG-infected macrophages in the presence of LF
An important component of vaccination may also require secondary antigenic presentation, thought to occur by dendritic cells' acquirement of antigen via uptake of apoptotic initial presenters. As a preliminary investigation, U937 cells were infected in the presence or absence of LF and measured for indicators of apoptotic events. Infected cells treated with LF demonstrated greater levels of Annexin V on their surface (57.6% vs. 46.1%) (Figure 3). Of interest is that LF alone (in the absence of infection) was able to lower the levels of this marker, perhaps increasing the normal cell membrane's integrity (11.2% vs. 6.18%).
Figure 3.
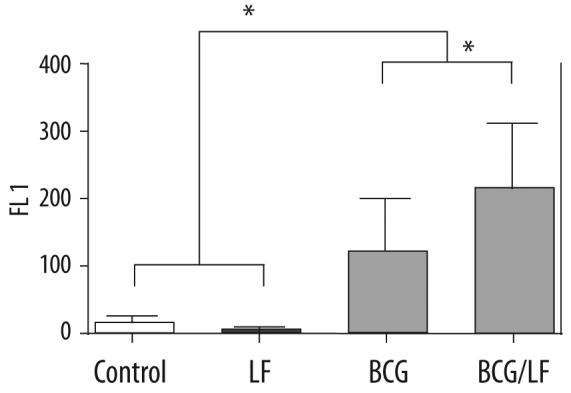
Increased apoptosis of BCG-infected cells in the presence of lactoferrin. U937 cells were BCG infected in the presence or absence of LF and measured for Annexin V on their surface. Comparisons are made with uninfected cells with or without LF. *p<0.001.
Discussion
LF is an integral part of the innate immune system [12] and an immunomodulator of leukocyte populations, including neutrophils [13], peritoneal macrophages [7], natural killer cells [5,19], T cells [6], and B cells [28]. As an immune-modulating agent, LF is capable of enhancing the T cell-mediated delayed type hypersensitivity (DTH) response, as measured by footpad swelling, to a variety of antigens, including sheep red blood cells (SRBCs), ovalbumin (OVA), and BCG [11,27]. Recent observations suggest that LF can also augment the efficacy of the BCG vaccine [10], although the mechanisms of its mode of action have not yet been determined. The results outlined in this report indicate that LF may augment BCG vaccine in multiple ways, ranging from increased organism uptake to alteration of the presentation molecules on APCs. As a whole, the findings identify possible mechanisms that would support the increased DTH (e.g. IFN-γ specific recall) observed in LF-BCG-immunized mice.
The increased uptake of BCG in the presence of LF was unexpected. LF receptors have been identified on monocytes and intestinal tissue [20]. Although fibroblasts have recently been identified to express a low-density lipoprotein receptor-related protein (LRP-1), identified as an endocytosis receptor involved in the cellular uptake of diverse ligands, including lactoferrin [21], it is not yet known if this same receptor is present on APCs. Regarding macrophages, LF can mediate responses via TLR-4-dependent pathways [4,14], which may provide a clue regarding the altered activation status seen with increased IL-12 to IL-10 cytokine ratios. The TLR-4 receptor can interact with the CD14 LPS co-receptor [15], which has also been identified as a ligand for LF [1], although in this instance there is no LPS associated with BCG. Regardless of this, the end result would be a more efficient availability of antigen for presentation by professional phagocytes. Studies have been completed indicating that LF-treated macrophages do not enhance the killing of ingested BCG (S-A Hwang, unpublished results); however, it is unknown if the treatment affects the trafficking of organisms through phagosome-lysosomal compartments. The findings of increased cellular apoptosis in infected cells treated with LF are complex, but they suggest another mechanism of the augmented responses seen in the vaccination experiments cited above [10], perhaps through increased “cross-priming” of T cells [24].
LF can modify surface molecule expression on APCs, allowing direct regulation of responding lymphocytes. Mycobacterium are very efficient at both limiting destruction upon entry into macrophages [16] and inhibiting the surface expression of Class II molecules after phagocytosis [9]. Mechanisms that allow sustained or increased Class II on APCs would certainly augment presentation pathways.
Finally, it appears that LF can also function through modification of the pro-inflammatory response to alter macrophage cytokine communication with adaptive cells in the local environment. Elevated IL-12 is an important step in directing the development of the T helper type 1 (TH1) response [17,18], critical for protection against Mtb infection [3,8]. The addition of LF to BCG-infected macrophages enhanced the IL-12 to IL-10 ratio from cultured macrophages infected with BCG. This suggests that LF modulates the innate function of macrophages to potentially direct the development of TH1 immunity. This again would assist in the explanation of how LF could act as an adjuvant to augment BCG vaccine efficacy against subsequent mycobacterial challenge [10].
The experiments presented here suggest that LF modulates APCs in a manner that would, potentially, promote TH1 development in vivo. The direct molecular mechanisms of LF mediation of immune activation and cytokine production still remain unresolved. However, the results presented identify LF as an immune modulator and provide possible molecular avenues to support its use to boost efficacy as an adjuvant for the BCG vaccine to promote host protective responses against virulent Mtb infection.
Acknowledgments
This study was presented in part at the Lactoferrin Minisymposium in October, 2006, at the University of Texas-Houston Health Science Center, Houston, TX, USA. The work was accomplished with support from NIH grants R41AI51050-01 and R42-AI051050-02.
Abbreviations
- BCG
Mycobacterium bovis Bacillus Calmette-Guerin
- LF
lactoferrin
- BMM
bone marrow-derived macrophage
- ELISA
enzyme-linked immunosorbent assay
- IL-
interleukin-
- TH1
T helper 1
- Mtb
Mycobacterium tuberculosis
- APC
antigen-presenting cell
References
- 1.Baveye S, Elass E, Fernig DG, Blanquart C, Mazurier J, Legrand D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 2000;68:6519–6525. doi: 10.1128/iai.68.12.6519-6525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behr MA. Correlation between BCG genomics and protective efficacy. Scand. J. Infect. Dis. 2001;33:249–252. doi: 10.1080/003655401300077180. [DOI] [PubMed] [Google Scholar]
- 3.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curran CS, Demick KP, Mansfield JM. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell. Immunol. 2006;242:23–30. doi: 10.1016/j.cellimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Damiens E, Mazurier J, el Yazidi I, Masson M, Duthille I, Spik G, Boilly-Marer Y. Effects of human lactoferrin on NK cell cytotoxicity against haematopoietic and epithelial tumour cells. Biochim. Biophys. Acta. 1998;1402:277–287. doi: 10.1016/s0167-4889(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 6.Dhennin-Duthille I, Masson M, Damiens E, Fillebeen C, Spik G, Mazurier J. Lactoferrin upregulates the expression of CD4 antigen through the stimulation of the mitogen-activated protein kinase in the human lymphoblastic T Jurkat cell line. J. Cell. Biochem. 2000;79:583–593. [PubMed] [Google Scholar]
- 7.Edde L, Hipolito RB, Hwang FF, Headon DR, Shalwitz RA, Sherman MP. Lactoferrin protects neonatal rats from gut-related systemic infection. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1140–G1150. doi: 10.1152/ajpgi.2001.281.5.G1140. [DOI] [PubMed] [Google Scholar]
- 8.Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 9.Fulton SA, Reba SM, Pai RK, Pennini M, Torres M, Harding CV, Boom WH. Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein. Infect. Immun. 2004;72:2101–2110. doi: 10.1128/IAI.72.4.2101-2110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int. Immunopharmacol. 2005;5:591–599. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Kocieba M, Zimecki M, Kruzel M, Actor JK. The adjuvant activity of lactoferrin in the generation of DTH to ovalbumin can be inhibited by bovine serum albumin bearing alpha-D-mannopyranosyl residues. Cell. Mol. Biol. Lett. 2002;7:1131–1136. [PubMed] [Google Scholar]
- 12.Kruzel ML, Zimecki M. Lactoferrin and immunologic dissonance: clinical implications. Arch. Immunol. Ther. Exp. 2002;50:399–410. [PubMed] [Google Scholar]
- 13.Miyauchi HS, Hashimoto M, Nakajima I, Shinoda Y, Fukuwatari H, Hayasawa H. Bovine lactoferrin stimulates the phagocytic activity of human neutrophils: identification of its active domain. Cell. Immunol. 1998;187:34–37. doi: 10.1006/cimm.1997.1246. [DOI] [PubMed] [Google Scholar]
- 14.Na YJ, Han SB, Kang JS, Yoon YD, Park SK, Kim HM, Yang KH, Joe CO. Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int. Immunopharmacol. 2004;4:1187–1199. doi: 10.1016/j.intimp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Plotz SG, Lentschat A, Behrendt H, Plotz W, Hamann L, Ring J, Rietschel ET, Flad HD, Ulmer AJ. The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood. 2001;97:235–241. doi: 10.1182/blood.v97.1.235. [DOI] [PubMed] [Google Scholar]
- 16.Radzioch D, Kramnik I, Skamene E. Molecular mechanisms of natural resistance to mycobacterial infections. Circ. Shock. 1994;44:115–120. [PubMed] [Google Scholar]
- 17.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur. J. Immunol. 1994;24:793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 18.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shau H, Kim A, Golub SH. Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. J. Leukoc. Biol. 1992;51:343–349. [PubMed] [Google Scholar]
- 20.Suzuki YA, Lopez V, Lonnerdal B. Mammalian lactoferrin receptors: structure and function. Cell. Mol. Life. Sci. 2005;62:2560–2575. doi: 10.1007/s00018-005-5371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama Y, Takezawa T. Lactoferrin promotes collagen gel contractile activity of fibroblasts mediated by lipoprotein receptors. Biochem. Cell. Biol. 2006;84:268–274. doi: 10.1139/o06-041. [DOI] [PubMed] [Google Scholar]
- 22.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 23.Valenti P, Berlutti F, Conte MP, Longhi C, Seganti L. Lactoferrin functions: current status and perspectives. J. Clin. Gastroenterol. 2004;38:S127–S129. doi: 10.1097/01.mcg.0000128941.46881.33. [DOI] [PubMed] [Google Scholar]
- 24.Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization Tuberculosis Fact sheet No 104, 2006. http://www.who.int/mediacentre/factsheets/who104/en/print.html (04.04.2007)
- 26.Zimecki M, Kocieba M, Kruzel ML. Immunoregulatory activities of lactoferrin in the delayed type hypersensitivity in mice are mediated by a receptor with affinity to mannose. Immunobiology. 2002;205:120–131. doi: 10.1078/0171-2985-00115. [DOI] [PubMed] [Google Scholar]
- 27.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol. Lett. 2000;74:183–188. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 28.Zimecki M, Mazurier J, Spik G, Kapp JA. Human lactoferrin induces phenotypic and functional changes in murine splenic B cells. Immunology. 1995;86:122–127. [PMC free article] [PubMed] [Google Scholar]