Abstract
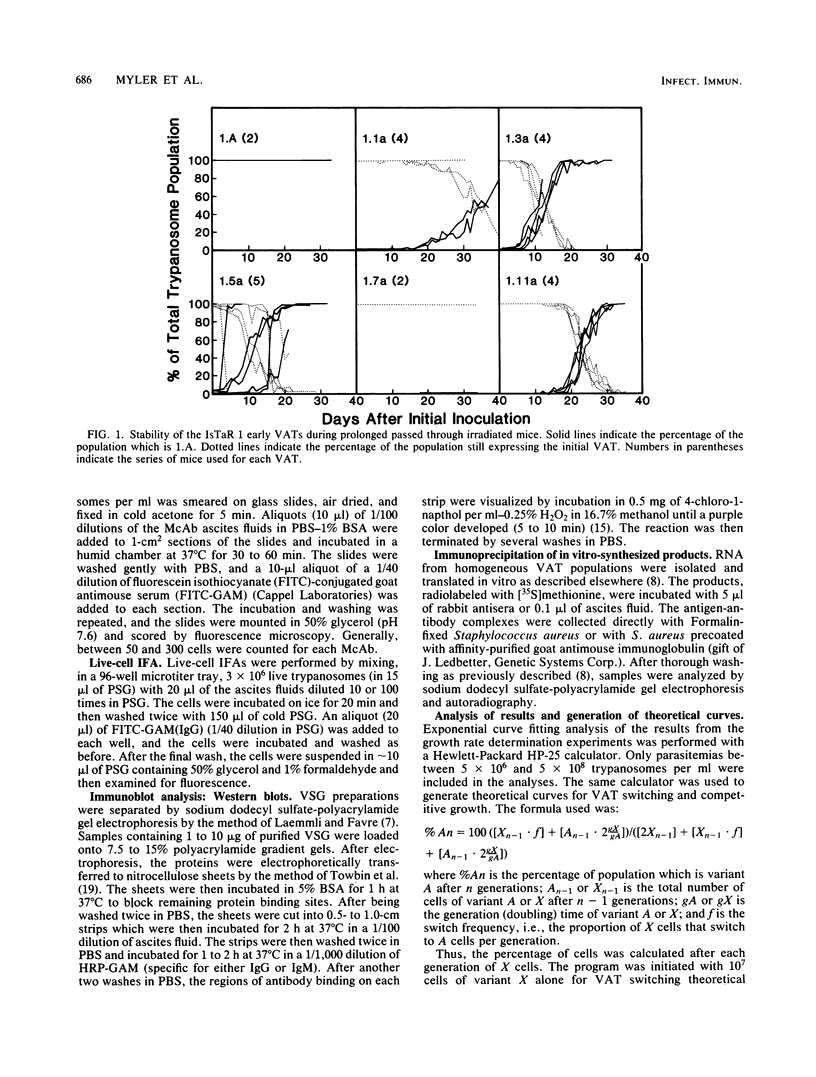
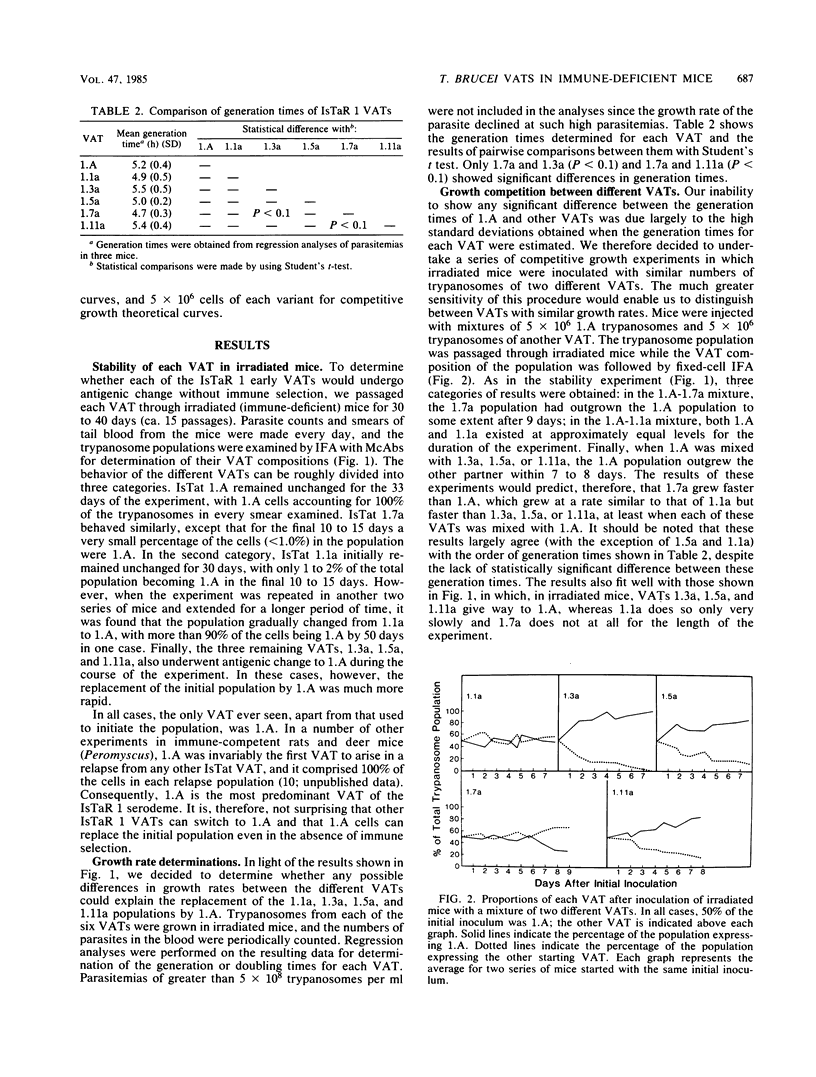
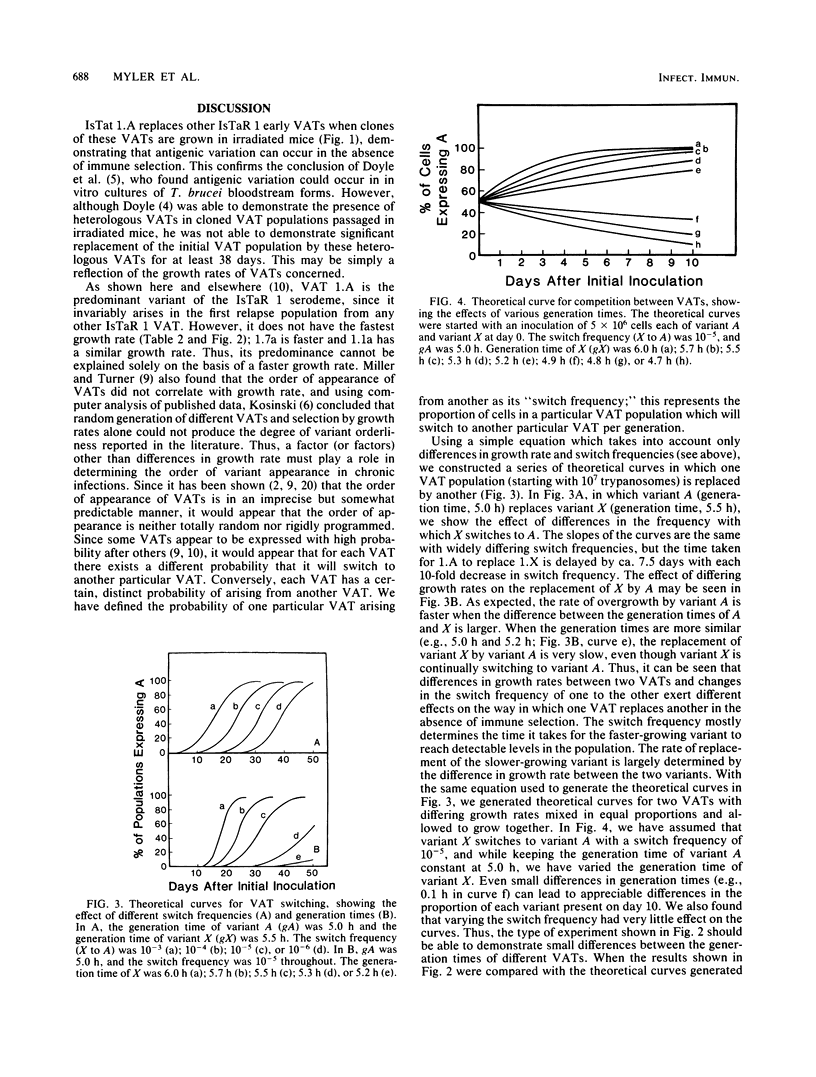
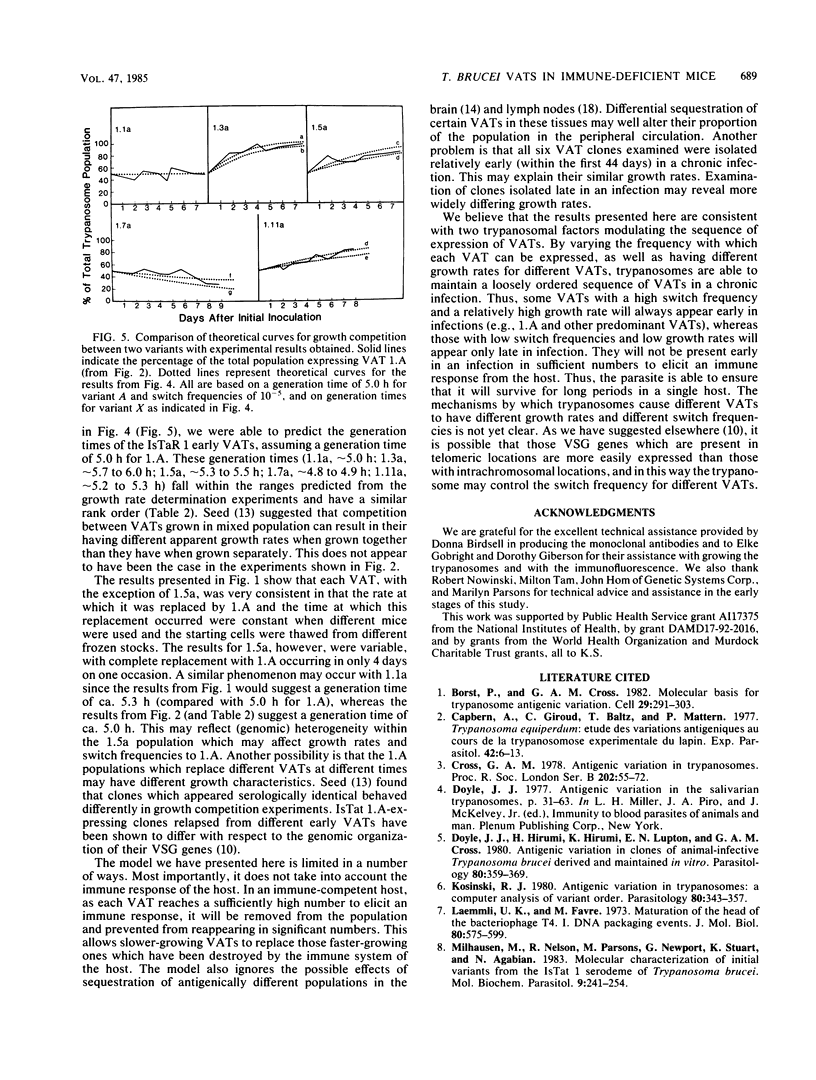
We have produced monoclonal antibodies against six variant surface glycoproteins from early variant antigen types (VATs) of the IsTaR 1 serodeme of Trypanosoma brucei brucei. We have used these in fixed cell immunofluorescence assays to follow the VAT composition of populations of each early VAT when passaged through irradiated mice. The IsTat 1.A and 1.7a populations were stable for more than 30 days (approximately 150 generations), but 1.1a, 1.3a, 1.5a, and 1.11a all changed to 1.A within this time. The time and rate of this antigenic switch were characteristic for each VAT. Growth rates of the VATs were determined when they were both grown separately and grown with 1.A. It appeared that the order of growth rates was 1.7a greater than 1.A = 1.1a greater than 1.11a greater than 1.5a greater than 1.3a. We have generated theoretical curves for the replacement of one VAT by another based on differences in their growth rates and the rate at which one VAT switches to another (switch frequency). These curves closely match those derived experimentally. We postulate that the differences in growth rates between VATs and the different switch frequencies for VATs may be sufficient to generate the loosely defined sequence of VATs seen in chronic infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. Trypanosoma equiperdum: etude des variations antigéniques au cours de la trypanosomose experimentale du lapin. Exp Parasitol. 1977 Jun;42(1):6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Antigenic variation in trypanosomes. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):55–72. doi: 10.1098/rspb.1978.0057. [DOI] [PubMed] [Google Scholar]
- Doyle J. J. Antigenic variation in the salivarian trypanosomes. Adv Exp Med Biol. 1977;93:31–63. doi: 10.1007/978-1-4615-8855-9_4. [DOI] [PubMed] [Google Scholar]
- Doyle J. J., Hirumi H., Hirumi K., Lupton E. N., Cross G. A. Antigenic variation in clones of animal-infective Trypanosoma brucei derived and maintained in vitro. Parasitology. 1980 Apr;80(2):359–369. doi: 10.1017/s0031182000000810. [DOI] [PubMed] [Google Scholar]
- Kosinski R. J. Antigenic variation in trypanosomes: a computer analysis of variant order. Parasitology. 1980 Apr;80(2):343–357. doi: 10.1017/s0031182000000809. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Milhausen M., Nelson R. G., Parsons M., Newport G., Stuart K., Agabian N. Molecular characterization of initial variants from the IsTat I serodeme of Trypanosoma brucei. Mol Biochem Parasitol. 1983 Nov;9(3):241–254. doi: 10.1016/0166-6851(83)90100-7. [DOI] [PubMed] [Google Scholar]
- Miller E. N., Turner M. J. Analysis of antigenic types appearing in first relapse populations of clones of Trypanosoma brucei. Parasitology. 1981 Feb;82(1):63–80. doi: 10.1017/s0031182000041871. [DOI] [PubMed] [Google Scholar]
- Myler P., Nelson R. G., Agabian N., Stuart K. Two mechanisms of expression of a predominant variant antigen gene of Trypanosoma brucei. Nature. 1984 May 17;309(5965):282–284. doi: 10.1038/309282a0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Seed J. R. Competition among serologically different clones of Trypanosoma brucei gambiense in vivo. J Protozool. 1978 Nov;25(4):526–529. doi: 10.1111/j.1550-7408.1978.tb04179.x. [DOI] [PubMed] [Google Scholar]
- Seed J. R., Effron H. G. Simultaneous presence of different antigenic populations of Trypanosoma brucei gambiense in Microtus montanus. Parasitology. 1973 Apr;66(2):269–278. doi: 10.1017/s0031182000045200. [DOI] [PubMed] [Google Scholar]
- Smit J., Agabian N. Cell surface patterning and morphogenesis: biogenesis of a periodic surface array during Caulobacter development. J Cell Biol. 1982 Oct;95(1):41–49. doi: 10.1083/jcb.95.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J. E., Mancini P. E., Patton C. L. Trypanosoma brucei brucei: isolation of the major surface coat glycoprotein by lectin affinity chromatography. Exp Parasitol. 1978 Dec;46(2):262–276. doi: 10.1016/0014-4894(78)90140-6. [DOI] [PubMed] [Google Scholar]
- Tanner M., Jenni L., Hecker H., Brun R. Characterization of Trypanosoma brucei isolated from lymph nodes of rats. Parasitology. 1980 Apr;80(2):383–391. doi: 10.1017/s0031182000000834. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meirvenne N., Janssens P. G., Magnus E. Antigenic variation in syringe passaged populations of Trypanosoma (Trypanozoon) brucei. 1. Rationalization of the experimental approach. Ann Soc Belg Med Trop. 1975;55(1):1–23. [PubMed] [Google Scholar]


