Abstract
The purpose of the current study was to investigate the suitability of an isobaric laparoscopic procedure, using a single port, for obtaining serial kidney and liver biopsy samples from standing steers. The samples were used in support of a pharmacokinetic tissue–fluid correlation study. Laparoscopic access was performed 3 times in each of 8 healthy Holstein steers, alternating from the right side to the left side and then to the right side again. The surgery was performed in standing stocks after the animals were given 3 doses of sulfadimethoxine sulfate intravenously and fasted for at least 18 h. Sedation and analgesia were achieved with acepromazine and xylazine. Lidocaine 2% was injected at the center of the paralumbar fossa (left or right), and an incision was made for introduction of a trocar–cannula assembly. Room air was allowed to enter the abdomen through the cannula at the time of insertion. Once the peritoneal cavity was reached, an operating endoscope was inserted. No pressurized insufflation was performed. A biopsy forceps was introduced into the operating channel of the endoscope to obtain a 100-mg kidney or liver sample. No complications were encountered. The 24 laparoscopic procedures provided 24 kidney and 16 liver samples. The results suggest that the isobaric (gasless) single-port laparoscopic technique is feasible for kidney and liver biopsy on standing steers. The procedure can be performed in a reliable and efficient manner in the sedated standing bovine.
Résumé
L’objectif de la présente étude était d’étudier la pertinence d’une procédure isobare de laparoscopie, utilisant une entrée unique, pour obtenir des biopsies rénales et hépatiques en série chez des bouvillons en position debout. La procédure de biopsie a été utilisée pour fournir des échantillons de rein et de foie dans le cadre d’une étude pharmacocinétique de corrélation tissu-fluide. L’accès par laparoscopie a été effectué trois fois chez chaque animal en alternance du côté droit au côté gauche et de retour au côté droit. La chirurgie par laparoscopie a été effectuée en position debout après que les animaux eurent reçus du sulfate de sulfadimethoxine et mis au jeûne pour au moins 18 heures. La sédation et l’analgésie se sont faites par administration d’acépromazine et de xylazine. De la lidocaïne 2 % a été injectée au centre de la fosse paralombaire (gauche ou droite) et une incision faite afin de permettre l’introduction d’un ensemble trocart-canule. Au moment de l’insertion, on permit à l’air ambiant de pénétrer dans l’abdomen à travers la canule. Une fois le péritoine atteint, l’endoscope opératoire a été inséré et aucun gonflement n’a été effectué. Un forceps à biopsie a été introduit dans le canal opératoire de l’endoscope afin d’obtenir un échantillon de 100 mg de rein ou de foie. Aucune complication n’est survenue. Vingt-quatre procédures de laparoscopie ont fourni 24 échantillons de rein et 16 échantillons de foie à partir d’un total de 8 bouvillons. L’évaluation de 24 procédures de laparoscopie sur huit bouvillons Holstein en santé suggère que la technique de laparoscopie isobare (sans gaz) avec une entrée unique est réalisable pour obtenir des biopsies de foie et de rein sur des bouvillons en position debout. La procédure peut être réalisée d’une manière fiable et efficiente chez des bovins sous sédation en position debout.
(Traduit par Docteur Serge Messier)
Introduction
Laparoscopic surgery to obtain kidney biopsy samples in support of drug residue elimination profile studies in steers has been described for gentamicin and penicillin (1,2). Pharmacokinetic correlation studies of fluids and tissues depend on simultaneous sampling of the fluids and tissues. Rapid, nontraumatic, minimally invasive sampling techniques are a fundamental necessity in the successful completion of tissue–fluid pharmacokinetic correlation studies.
Laparoscopy-guided liver biopsy with CO2 insufflation has been performed in large animals under general anesthesia (3) and in standing horses (4). Liver biopsy is widely used for obtaining liver samples from living animals, and repeated observations can be made on the same animal (5). Endoscopy-guided serial liver biopsy has been described in recumbent sheep (6). Under laparoscopy the liver can be observed directly, an optimal biopsy site can be selected, and the site can be monitored for hemorrhage (7). Obtaining liver biopsy samples by laparoscopy has been referenced in the standing horse (4) but has not been reported in the standing bovine. This article describes in detail the laparoscopic liver biopsy procedure as performed in standing steers.
Laparoscopic kidney biopsy with the use of CO2 insufflation has been described in bovines (8–10). The procedure in the standing steer has improved from the 2-port technique (8), to the 1-port technique (10), and ultimately to the isobaric (gasless), 1-port surgical technique reported here.
Insufflation of CO2 into the peritoneal cavity is a routine technique for abdominal exposure in laparoscopic surgery (11–15). The insufflation system is used to create pneumoperitoneum (distention of the peritoneal cavity with some type of gas), which improves visualization and facilitates instrumental and visceral manipulation during surgery (16). In standing laparoscopy in the bovine, gas insufflation of the peritoneal cavity can produce discomfort, restlessness, and even collapse in prolonged examination periods (14,17). Signs of colic are evident in standing cows when the intra-abdominal pressure exceeds 10 mmHg (18). If the patient moves extensively, damage to laparoscopic equipment and contamination of the abdomen due to loss of aseptic technique can result (14). During standing laparoscopic surgery, cattle frequently simply lie down when the abdomen is inflated. The discomfort produced by the elevated pressure in the peritoneal cavity may be avoided by lack of gas insufflation.
We performed isobaric (gasless) laparoscopic kidney and liver biopsy in standing steers to evaluate the utility of this approach to obtaining serial kidney and liver samples in standing steers.
Materials and methods
This study was approved by the Institutional Animal Care and Use Committee, Office of Research, Center for Veterinary Medicine, US Food and Drug Administration, and was conducted in accordance with the guidelines provided by the US Animal Care and Use of Laboratory Animals, in climate control facilities approved by the American Association for the Assessment and Accreditation of Laboratory Animal Care.
Animals
The study group comprised 8, clinically normal and drug-naïve Holstein steers, aged 4 to 6 months, and ranging in weight from 193 to 330 kg. Each animal was housed separately in a temperature-controlled barn with an automated lighting cycle. The animals were fed mixed standard feed once daily. Hay and water were available at all times.
Study design
The steers were enrolled in a pharmacokinetic study to monitor the depletion of sulfadimethoxine in tissues and biologic fluids. A complete blood profile (ruminant profile) and a clotting profile were obtained before drug administration. Values out of normal range in either profile would have excluded an animal from the study. The steers were given the drug intravenously in an approved 3-dose regimen: 55 mg/kg followed by 2 doses of 27.5 mg/kg at 12-h intervals.
In 1 group of 4 animals, kidney and liver biopsy specimens were obtained at 36, 60, and 84 h after the final dose of sulfadimethoxine. In the remaining group of 4 animals, the samples were obtained at 60, 84, and 108 h after the final dose of sulfadimethoxine. Thus, in each animal, biopsy samples were obtained 3 times, with a 24-h interval between each laparoscopy. The animals were slaughtered 6 h after the last biopsy, and necropsy was performed.
Laparoscopic procedure
The surgical procedure was performed by 1 surgeon in sterile dress with a nonsterile assistant.
Equipment
Standard laparoscopy instruments included the following: 300-W xenon light source; 4.8-mm-diameter optic light cable; 57-cm-long, 10-mm-diameter, rigid endoscope providing a 30° view; 27-cm-long, 10-mm-diameter, 0° rigid operating telescope with a 6-mm-diameter instrument channel and parallel eyepiece; 20-cm-long, 11-mm-diameter cannula with a blunt trocar; atraumatic forceps; and laparoscopic curved serrated scissors 40-cm long with a 5-mm outer diameter. Blakesley biopsy forceps 5 mm in diameter and 43 cm long were used to obtain all biopsy specimens. The endoscope contained a 6-mm-diameter working channel for insertion of 5-mm-diameter laparoscopy instruments (scissors, biopsy forceps, grasping forceps). All equipment was purchased from Karl Storz America, Goleta, California, USA.
Animal preparation and anesthesia
The steers were fasted for at least 18 h before surgery. Immediately before surgery they were haltered, led into a stock, and loosely restrained to minimize movement. An intramuscular injection of acepromazine (acepromazine maleate injection, 10 mg/mL; Boehringher Ingelheim, St. Joseph, Missouri, USA), 0.025 mg/kg, was then administered. The paralumbar fossa (either right or left) was clipped, shaved, scrubbed with povidone iodine, and rinsed with alcohol. An intravenous catheter was aseptically placed in the left jugular vein for the administration of xylazine (Sedazine, diluted 1/100 in sterile saline solution; manufactured for Fort Dodge Animal Health, Overland Park, Kansas, USA, by Phoenix Scientific, St. Joseph, Missouri, USA), 0.005 to 0.01 mg/kg. As soon as the animal was adequately sedated, it was covered with a sterile drape. Next, 12 mL of lidocaine (2% lidocaine injectable, 20 mg/mL; Bimeda Animal Health, Riverside, Missouri, USA) was infiltrated in the center of the paralumbar fossa at the proposed incision site. A 2-cm-long incision was made through the skin with a #22 surgery blade, and blunt dissection was done with Metzenbaum scissors through the muscles to facilitate insertion of the trocar cannula.
Isobaric right-flank laparoscopy (for access to the right kidney and right liver lobe)
The trocar cannula was inserted perpendicular with a rotating movement through the skin incision. When resistance was met, the trocar assembly was thrust into the peritoneal cavity, with the point directed slightly cranial and 45° to the lateral body planes. The air valve on the trocar cannula was kept open, and a rush of air was audible as the peritoneum was perforated. The blunt trocar was then replaced with the endoscope, which was inserted in the same direction as the trocar assembly had been inserted. The endoscopic field was over the great omentum. The endoscope was guided craniomediodorsally until the liver was in view on the screen. A rapid examination was performed to ensure a lack of trauma. The endoscope was then replaced by the operating telescope, which was directed to the upper right quadrant for observation of the anatomic landmarks needed for the liver biopsy: right triangular ligament, quadrate lobe, right kidney (embedded in a large amount of perirenal fat, termed the “adipose capsule”), and diaphragm (Figure 1). At the end of the procedure the endoscope was removed and the skin incision closed with a single interrupted suture (2-0 Monosof USS DG nylon polyamide; United States Surgical, a division of Tyco Healthcare Group, Norwalk, Connecticut, USA). The second laparoscopic incision on the right side was made 2.5 cm caudal to the first incision.
Figure 1.
Isobaric (gasless) laparoscopic view of the right paralumbar approach. The important anatomic landmarks for liver biopsy are: right kidney, right triangular ligament (RTL), quadrate lobe of the liver (QL), right lobe of the liver (RL), and diaphragm (D). Yellow circle — biopsy site.
Isobaric left-flank laparoscopy (for access to the left kidney)
The trocar was similarly introduced and directed caudally through the left paralumbar fossa toward the right coxofemoral joint. Once in the peritoneum (as determined by an audible rush of air), the trocar was replaced with the endoscope, which was guided cranially to the upper left quadrant. The dorsal sac of the rumen was gently pushed down with the endoscope, and the left kidney emerged on the screen (Figure 2). The anatomic structures identified were the dorsal sac of the rumen, the left kidney, and the psoas muscle. At the end of the procedure the endoscope was removed and the skin incision closed with a single interrupted suture (2-0 Monosof USS DG nylon polyamide, United States Surgical).
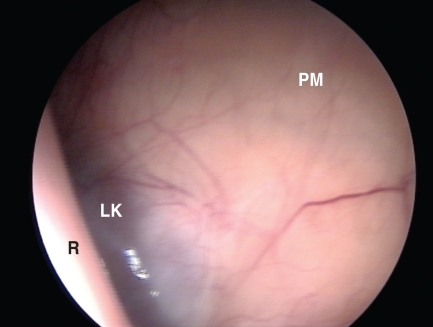
Figure 2.
Isobaric (gasless) laparoscopic view of the left paralumbar approach. R — rumen dorsal sac; LK — left kidney; PM — psoas muscle.
One-port liver biopsy technique
Two liver biopsy samples were obtained from the ventral margin of the right lobe approximately 2 cm apart. The first sample was taken ventral, the second sample (taken immediately afterwards) dorsal (Figure 3). The ventral margin was observed directly with the endoscope to monitor for hemorrhage at the biopsy site.
Figure 3.
Minimal hemorrhage is observed after liver biopsy. The longer arrow indicates the second biopsy site and the shorter arrow the first site.
One-portal kidney biopsy technique
Upon completion of the liver biopsy, the endoscope was redirected dorsally in preparation for the kidney biopsy sample. With a curved serrated scissors, introduced into the operating channel of the endoscope, the capsule of the kidney was dissected in a cross pattern by rotating the scissors 90° after the first cut, and then a biopsy sample was obtained with Blakesley biopsy forceps introduced through the operating channel into the cross incision in the capsule.
A grasping forceps was used to control hemorrhage. The cut edges of the kidney capsule were compressed for at least 2 min. The endoscope and grasping forceps were left in situ until it was possible to verify that hemorrhage was controlled.
Data acquisition
Each laparoscopic procedure was fully recorded by means of a Sony medical image system on an individual compact disc. After completion of the procedure, the recording was reviewed by the investigators, who recorded biopsy time, number of punched “bites” per sample, and degree of hemorrhage (8). The liver biopsy time was defined from when the endoscope was placed in the peritoneum until the last liver sample was taken. The right kidney biopsy time was defined from when the endoscope reached the right kidney, until the endoscope was withdrawn from the peritoneal cavity. The left kidney biopsy time was defined from when the endoscope was placed in the peritoneum until it was withdrawn from the peritoneal cavity.
Post-operative management
The animals were transported to the barn in a trailer towed by a tractor immediately after the surgery. Once in their pen, the steers were allowed free access to hay, feed, and water. Respiratory rate, heart rate, temperature, appetite, attitude, and postural positions were evaluated and recorded 4, 8, 12, and 24 h after surgery or until the animal was slaughtered. No special care was given to any steer, and no antimicrobial or antinflammatory agent was administered. A sweet treat (100 to 200 g of Omolene, Purina Mills, St. Louis, Missouri, USA) was given at the time of each evaluation to encourage cooperation and avoid stress. The surgical incision site was inspected for swelling.
The steers were humanely killed by captive bolt stunning and exsanguination 6 h after the last biopsy. Immediately after slaughter, both kidneys and the liver were removed. The kidneys were cleaned of surrounding fat and the capsules dissected to enable inspection of the kidney surface. The biopsy areas and surrounding tissue were carefully explored in the liver and kidney.
Results
Of the 24 laparoscopic surgical procedures performed on the 8 standing steers, 16 were performed on the right side (which provided 16 kidney and 16 liver samples) and 8 (which provided 8 kidney samples) were performed on the left. Laparoscopic access was achieved in all cases, and no complications were encountered. The animals showed no signs of ataxia, distress, or discomfort during the procedures; they remained quiescent and did not interfere with placement of the laparoscopic instruments by moving. No complications were observed in any of the animals after the surgery or during transportation to the barns.
Liver results
An acceptable liver sample consisted of 2 or more biopsy bites (mean number 2.2). The mean liver biopsy time was 1.5 min. The mean sample weight was 119.2 mg. Data for the individual animals are provided in Table I. At the time of the second biopsy (48 h after the first), no significant tissue alterations were observed at first biopsy with the endoscope.
Table I.
Results of serial kidney and liver biopsy in 8 standing Holstein steers
| Number of biopsy bites per sample
|
Biopsy time (minutes)
|
Sample weight (mg)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Steer number | Procedure number | LK | RK | L | LK | RK | L | LK | RK | L |
| 1 | 1 | 2 | 2 | 5.6 | 1.5 | 122 | 111 | |||
| 2 | 3 | 3.6 | 161 | |||||||
| 3 | 2 | 3 | 5.4 | 1.2 | 129 | 123 | ||||
| 2 | 1 | 2 | 2 | 5.1 | 1.3 | 120 | 117 | |||
| 2 | 3 | 4.1 | 119 | |||||||
| 3 | 2 | 2 | 5.5 | 1.5 | 84 | 115 | ||||
| 3 | 1 | 2 | 2 | 5.5 | 1.2 | 126 | 120 | |||
| 2 | 2 | 5.5 | 126 | |||||||
| 3 | 2 | 3 | 4.5 | 1.5 | 146 | 126 | ||||
| 4 | 1 | 3 | 2 | 3.5 | 1.1 | 125 | 98 | |||
| 2 | 3 | 5.2 | 157 | |||||||
| 3 | 1 | 3 | 3.5 | 2.1 | 113 | 158 | ||||
| 5 | 1 | 2 | 2 | 4.2 | 1.6 | 101 | 95 | |||
| 2 | 2 | 3.6 | 99 | |||||||
| 3 | 2 | 2 | 4.2 | 1.2 | 119 | 125 | ||||
| 6 | 1 | 2 | 2 | 4.5 | 1.2 | 85 | 120 | |||
| 2 | 2 | 4.5 | 104 | |||||||
| 3 | 2 | 2 | 4.5 | 2.1 | 96 | 104 | ||||
| 7 | 1 | 2 | 2 | 5.4 | 1.5 | 102 | 120 | |||
| 2 | 5 | 5.1 | 128 | |||||||
| 3 | 4 | 2 | 4.1 | 2.0 | 106 | 119 | ||||
| 8 | 1 | 3 | 2 | 5.5 | 1.6 | 141 | 138 | |||
| 2 | 2 | 5.2 | 115 | |||||||
| 3 | 3 | 2 | 3.3 | 2.0 | 108 | 118 | ||||
| Mean | 2.6 | 2.2 | 2.2 | 4.6 | 4.6 | 1.5 | 126.1 | 113.9 | 119.2 | |
Right kidney results
Each tissue sample consisted of at least 2 biopsy bites (mean number 2.2). The mean biopsy time was 4.6 min. The mean sample weight was 113.9 mg. All procedures showed moderate hemorrhage at the biopsy site (8). Bleeding was controlled with the application of pressure with forceps for 2 min. All samples were classified as easy to obtain: “no complications, easily recognizable kidney surface, easy to obtain biopsy”(8).
Left kidney results
Each tissue sample consisted of at least 2 biopsy bites (mean 2.6). The mean biopsy time was 4.6 min. The mean sample weight was 126.1 mg. The kidney surface was easily detected, and all samples were classified as easy to obtain. Applying pressure with forceps for 2 min stopped hemorrhage.
Postmortem results
No adhesions, infections, or gross changes were detectable in any of the steers’ carcasses when the kidneys and the liver were harvested. The biopsy sites were clearly visible in the kidney cortex and very easy to identify in the right lobe of the liver. No hemorrhage at the biopsy bite site or in the abdominal cavity was found in any of the 8 steers.
Drug analysis
All the samples obtained by laparoscopy were of sufficient weight for sulfadimethoxine analysis.
Discussion
Laparoscopic surgery presents the surgeon with the problem of working within a confined space. In both human and animal surgery, a routine technique is to expand this working space by elevating the internal abdominal pressure. Expansion of the space improves the surgeon’s view of the internal organs and facilitates manipulation of the surgical instruments.
For most laparoscopic procedures gas insufflation to create sufficient visual and operating space is mandatory (13); however, gas insufflation, especially over prolonged examination periods, produces animal discomfort and restlessness and may even result in the animal’s collapse (17). The animal may experience some postoperative discomfort related to the use of CO2 (12), which has been reported to form carbonic acid on the moist peritoneal surface in humans (19,20).
Recent evidence suggests that the use of CO2 to create pneumoperitoneum during laparoscopy can lead to structural, metabolic, and immune derangements within the peritoneal cavity that include structural alterations in the mesothelial lining, pH disturbances, and alterations in peritoneal macrophage responsiveness (21).
The effect of CO2 insufflation has been studied in llamas (22), horses (23), ewes (24), dogs (25), and pigs (26). There are no reports that discuss the secondary effect of CO2-induced pneumoperitoneum or ambient air in cattle (27); however, signs of colic are evident in standing cows when the intra-abdominal pressure exceeds 10 mmHg. (18).
Because of the adverse physiological effects and technical disadvantages of pneumoperitoneum, alternative methods of abdominal wall lifting have been explored (28–30). It has been reported that with isobaric (gasless) laparoscopy the adverse effects and potential risk of CO2 insufflation are eliminated; post-operative convalescence is improved, and post-operative pain reduced (31).
Our investigation attempted to simplify the laparoscopic biopsy technique by avoiding pressurized CO2 insufflation. Even though our goal was not to evaluate the effect of CO2 on laparoscopic surgery, we believe that the standing steer could be a good model for comparing procedures performed with and without CO2 insufflation. The current study has refined and improved the standard 2-port and the 1-port techniques discussed previously (8,10). These improvements enable safe sampling with minimal or no postsurgical recovery time, which allows more frequent sampling to provide a greater number of samples throughout the drug residue elimination phase.
The isobaric approach is possible in the standing steer since pneumoperitoneum occurs by the incorporation of room air through the cannula.
In the standing steer, the posterior portion of the rib cage and the dorsal ligaments form a semisolid structure from which the kidneys and the liver are suspended. The suspension and the aspiration of some room air provide space between the organs and allow the organs to be approached laterally, so that biopsy may be done without the usual CO2 insufflation to create additional space. The natural “tenting” effect is probably only possible in the sedated large standing animal. However, a similar effect may be possible with proper positioning of the anesthetized patient. The lack of discomfort and the rapid recovery after surgery may be attributed to the lack of gas insufflation throughout this study.
Isobaric (gasless) laparoscopy allows the use of conventional laparotomy instruments and reduces operative time and cost (32). No gas seal is necessary to maintain the intra-abdominal pressure, and no technical assistance is needed to control CO2 flow throughout the procedure. The cost of CO2 insufflation and the associated equipment is eliminated. The current isobaric, 1-port procedure can be performed by a single surgeon.
It has been reported that care should be taken before performing laparoscopy in an animal with gastrointestinal distention (33). In the bovine, a 24-h fast is necessary to decrease ruminal and other visceral volume sufficiently to allow successful laparoscopy (14). In the current study, an 18-h fast was enough to decrease visceral volume and allow access.
In human medicine, renal biopsy is considered the diagnostic procedure of choice for many patients in whom renal disease is suspected (34). Ultrasound-guided percutaneous biopsy has recently been described in cattle (35). The authors stated that the risk of serious complications was very low. The reported approach provided access to only the right kidney; the left kidney could not undergo biopsy during ultrasound visualization.
Hemorrhage after ultrasound-guided biopsy of the kidneys has been reported in small animals (36). Laparoscopy provides excellent visual control for positioning the biopsy forceps, provides for the application of pressure to the biopsy site, and allows monitoring for hemorrhage after the biopsy (37). The learning curve for the ultrasound-guided procedure was steeper than that for laparoscopic biopsy (38).
Hemorrhage from liver biopsy is a particular concern in animals with liver disease because hepatic dysfunction may cause concurrent coagulopathy (7). No prolonged hemorrhage was observed in the healthy animals in the current study.
When kidney and/or liver samples are collected in support of pharmacokinetic studies, repeated sampling of the same animal at various times provides greater consistency than single-time sampling of a greater number of animals. The reduction of animal-to-animal variation through the use of serial sampling to monitor drug residue depletion in only a few animals has been discussed previously (1,2) and can be maintained with the current isobaric procedure.
We performed the single-port isobaric procedures with the purpose of obtaining serial liver and kidney samples with minimal discomfort to the animal. The results indicate that laparoscopic biopsy performed through a single port without pressure insufflation is both safe and cost-effective. Stress to the animal is kept at a minimum, allowing multiple samples to be obtained from the same animal within short intervals without distressing the animal. Serial sampling of kidney and liver tissue in the same animal provides ideal support for pharmacokinetic drug distribution and elimination studies.
In clinical practice, isobaric liver or kidney biopsy could be useful when a diagnostic procedure is needed to determine the severity of a lesion and to formulate an optimal treatment plan.
Acknowledgments
The authors acknowledge the assistance of Ken Godden and Ashley Yancik.
References
- 1.Chiesa OA, von Bredow J, Heller D, et al. Use of tissue-fluid correlations to estimate gentamicin residues in kidney tissue of Holstein steers. J Vet Pharmacol Ther. 2006;29:99–106. doi: 10.1111/j.1365-2885.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 2.Chiesa OA, von Bredow J, Smith M, Heller D, Condon R, Thomas MH. Bovine kidney tissue/biological fluid correlation for penicillin. J Vet Pharmcol Ther. 2006;29:299–306. doi: 10.1111/j.1365-2885.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 3.Pearce SG, Firth EC, Grace ND, Fennessy PF. Liver biopsy techniques for adult horses and neonatal foals to assess copper status. Aust Vet J. 1997;75:194–198. doi: 10.1111/j.1751-0813.1997.tb10065.x. [DOI] [PubMed] [Google Scholar]
- 4.Fischer AT., Jr Standing laparoscopic surgery. Vet Clin North Am Equine Pract. 1991;7:641–647. doi: 10.1016/s0749-0739(17)30491-1. [DOI] [PubMed] [Google Scholar]
- 5.Rawlings CA, Van Lue S, King C, et al. Serial laparoscopic biopsies of liver and spleen from Schistosoma-infected baboons (Papio spp.) Comp Med. 2000;50:551–555. [PubMed] [Google Scholar]
- 6.Hidiroglou M, Ivan M. Liver biopsy in sheep. Vet Res. 1993;24:260–265. [PubMed] [Google Scholar]
- 7.Vasanjee SC, Bubenik LJ, Hosgood G, Bauer R. Evaluation of hemorrhage, sample size, and collateral damage for five hepatic biopsy methods in dogs. Vet Surg. 2006;35:86–93. doi: 10.1111/j.1532-950X.2005.00117.x. [DOI] [PubMed] [Google Scholar]
- 8.Chiesa OA, Cullison R, Anderson DE, Moulton K, Galuppo LD, von Bredow J. Development of a technique for serial bilateral renal biopsy in steers. Can J Vet Res. 2006;70:87–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Naoi M, Kokue E, Takahashi Y, Kido Y. Laparoscopic-assisted serial biopsy of the bovine kidney. Am J Vet Res. 1985;46:699–702. [PubMed] [Google Scholar]
- 10.Chiesa OA, von Bredow J, Heller DN, Smith M, Thomas M. Res Vet Sci. 2008. One-port video assisted laparoscopic kidney biopsy in standing steers. [DOI] [PubMed] [Google Scholar]
- 11.Freeman LJ. Operating room, setup, equipment, and instrumentation. In: Freeman LJ, editor. Veterinary Endosurgery. St Louis, Missouri: Mosby; 1999. pp. 2–23. [Google Scholar]
- 12.Fischer AT. Equine Diagnostic and Surgical Laparoscopy. Philadelphia, Pennsylvania: Saunders; 2002. Basic laparoscopic techniques and training. [Google Scholar]
- 13.Boure L. General principles of laparoscopy. Vet Clin North Am Food Anim Pract. 2005;21:227–249. doi: 10.1016/j.cvfa.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Traub-Dargatz J. Laparoscopy, endoscopy, and surgical biopsy in large animals. In: Anderson NV, editor. Veterinary Gastroenterology. Malvern, Pennsylvania: Lea & Febiger; 1992. pp. 61–69. [Google Scholar]
- 15.Maxwell DP, Kraemer DC. Laparoscopy in cattle. In: Harrison RM, Wildt DE, editors. Animal Laparoscopy. Baltimore, Maryland: Williams & Wilkins; 1980. pp. 133–157. [Google Scholar]
- 16.Latimer FG, Eades SC, Pettifer G, Tetens J, Hosgood G, Moore RM. Cardiopulmonary, blood and peritoneal fluid alterations associated with abdominal insufflation of carbon dioxide in standing horses. Equine Vet J. 2003;35:283–290. doi: 10.2746/042516403776148273. [DOI] [PubMed] [Google Scholar]
- 17.Seeger K. Laparoscopic investigation of the bovine ovary. 1977. Vet Med Small Anim Clin. 1977;72:1037–1044. [PubMed] [Google Scholar]
- 18.Bleul U, Hollenstein K, Kähn W. Laparoscopic ovariectomy in standing cows. Anim Reprod Sci. 2005;90:193–200. doi: 10.1016/j.anireprosci.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Holthausen UH, Nagelschmidt M, Troidl H. CO2 pneumoperitoneum: What we know and what we need to know. World J Surg. 1999;23:794–800. doi: 10.1007/s002689900582. [DOI] [PubMed] [Google Scholar]
- 20.Van Glabeke E, Mandron F, Desrez G, Chartier-Kastler E, Conort P, Richard F. Review on the use of CO2 in laparoscopic surgery. Prog Urol. 1998;8:586–589. [PubMed] [Google Scholar]
- 21.Neuhaus SJ, Watson DI. Pneumoperitoneum and peritoneal surface changes. Surg Endosc. 2004;18:1316–1322. doi: 10.1007/s00464-003-8238-2. [DOI] [PubMed] [Google Scholar]
- 22.Lin HC, Baird AN, Pugh DG, Anderson DE, Gaughan EM. Effects of carbon dioxide insufflation combined with changes in body position on blood gas and acid-base status in anesthetized llamas (Llama glama) Vet Surg. 1997;26:444–450. doi: 10.1111/j.1532-950x.1997.tb01703.x. [DOI] [PubMed] [Google Scholar]
- 23.Cruz AM, Kerr CL, Bouré LP, Sears WC. Cardiovascular effects of insufflation of the abdomen with carbon dioxide in standing horses sedated with detomidine. Am J Vet Res. 2004;65:357–362. doi: 10.2460/ajvr.2004.65.357. [DOI] [PubMed] [Google Scholar]
- 24.Cruz AM, Southerland LC, Duke T, Townsend HG, Ferguson JG, Crone LA. Intraabdominal carbon dioxide insufflation in the pregnant ewe. Uterine blood flow, intraamniotic pressure, and cardiopulmonary effects. Anesthesiology. 1996;85:1395–1402. doi: 10.1097/00000542-199612000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Duke T, Steinacher SL, Remedios AM. Cardiopulmonary effects of using carbon dioxide for laparoscopic surgery in dogs. Vet Surg. 1996;25:77–82. doi: 10.1111/j.1532-950x.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 26.Clary EM, Brusch SM, Laul CL, et al. Effects of pneumoperitoneum on hemodynamic and systemic immunologic responses to peritonitis in pigs. J Sur Res. 2002;108:32–8. doi: 10.1006/jsre.2002.6520. [DOI] [PubMed] [Google Scholar]
- 27.Babkine M, Desrochers A. Laparoscopic surgery in adult cattle. Vet Clin North Am Food Anim Pract. 2005;21:251–279. doi: 10.1016/j.cvfa.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Gutt CN, Daume J, Schaeff B, Paolucci V. Systems and instruments for laparoscopic surgery without pneumoperitoneum. Surg Endosc. 1997;11:868–874. doi: 10.1007/s004649900474. [DOI] [PubMed] [Google Scholar]
- 29.Sesti F, Melgrati L, Damiani A, Piccione E. Isobaric (gasless, laparoscopic uterine myomectomy. An overview. Eur J Obstet Gynecol Reprod Biol. 2006;129:9–14. doi: 10.1016/j.ejogrb.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Udwadia TE, Kathrani BK, Bernie W, Gadgil US, Chariar VM. Vacuum-assisted abdominal wall lift for minimal-access surgery: A porcine model study. Surg Endosc. 2005;19:1113–1119. doi: 10.1007/s00464-004-2131-5. [DOI] [PubMed] [Google Scholar]
- 31.Damiani A, Melgrati L, Franzoni G, Stepanyan M, Bonifacio S, Sesti F. Isobaric gasless laparoscopic myomectomy for removal of large uterine leiomyomas. Surg Endosc. 2006;20:1406–1409. doi: 10.1007/s00464-004-9078-4. [DOI] [PubMed] [Google Scholar]
- 32.Mahajan NN. Comment on isobaric (gasless) laparoscopic uterine myomectomy. An overview. Eur J Obstet Gynecol Reprod Biol. 2006;2007;129132:9–14. 133–134. doi: 10.1016/j.ejogrb.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DE, Gaughan EM, St-Jean G. Normal laparoscopic anatomy of the bovine abdomen. Am J Vet Res. 1993;54:1170–1176. [PubMed] [Google Scholar]
- 34.Hergesell O, Felten H, Andrassy K, Kühn K, Ritz E. Safety of ultrasound-guided percutaneous renal biopsy-retrospective analysis of 1090 consecutive cases. Nephrol Dial Transplant. 1998;13:975–977. doi: 10.1093/ndt/13.4.975. [DOI] [PubMed] [Google Scholar]
- 35.Mohamed T, Oikawa S. Efficacy and safety of ultrasoundcutaneous biopsy of the right kidney in cattle. J Vet Med Sci. 2008;70:175–179. doi: 10.1292/jvms.70.175. [DOI] [PubMed] [Google Scholar]
- 36.Léveillé R, Partington BP, Biller DS, Miyabayashi T. Complications after ultrasound-guided biopsy of abdominal structures in dog and cats: 246 cases (1984–1991) J Am Vet Med Assoc. 1993;203:413–415. [PubMed] [Google Scholar]
- 37.Rawlings CA, Howerth EW. Obtaining quality biopsies of the liver and kidney. J Am Anim Hosp Assoc. 2004;40:352–358. doi: 10.5326/0400352. [DOI] [PubMed] [Google Scholar]
- 38.Cole TL, Center SA, Flood SN, et al. Diagnostic comparison of needle and wedge biopsy specimens of the liver in dogs and cats. J Am Vet Med Assoc. 2002;220:1483–1490. doi: 10.2460/javma.2002.220.1483. [DOI] [PubMed] [Google Scholar]