Abstract
Plateletworks is a screening assay used in human medicine to monitor platelet-inhibiting drugs. As arterial thromboembolism is a common complication in cats suffering from cardiomyopathy, they are often treated with anti-platelet medication. Clopidogrel (Plavix), an anti-platelet aggregation drug, has recently been evaluated in healthy cats. The purpose of this study was to determine if the Plateletworks method can detect a decrease in platelet aggregation in cats receiving clopidogrel. Nine healthy adult cats were used for this study. Platelet aggregation was measured before and after a 3-day clopidogrel treatment (18.75 mg SID). Platelet aggregation after the clopidogrel treatment was significantly lower (P < 0.01). The Plateletworks method appears to be a promising test to monitor clopidogrel therapy in cats.
Résumé
En médecine humaine, la méthode des tubes Plateletworks est utilisée afin de surveiller l’efficacité des médicaments inhibant l’agrégation plaquettaire. Chez le chat, les thromboembolismes artériels sont une complication fréquente des cardiomyopathies et ces patients sont fréquemment traités avec des inhibiteurs plaquettaires. L’efficacité du clopidogrel (Plavix), un anti-agrégant plaquettaire, a récemment été évaluée chez le chat en santé. L’objectif de cette étude était de démontrer que la méthode des tubes Plateletworks peut détecter une diminution de l’agrégation plaquettaire chez les chats recevant du clopidogrel. Neuf chats en santé ont été utilisés pour cette étude. L’agrégation plaquettaire a été mesurée avant et après 3 jours de traitement avec du clopidogrel (18,75 mg SID). L’agrégation plaquettaire était significativement diminuée (P < 0,01) après la période de traitement. La méthode des tubes Plateletworks semble prometteuse dans le suivi des chats recevant du clopidogrel.
(Traduit par les auteurs)
In cats, cardiac disease, diabetes mellitus, neoplasia, nephrotic syndrome, and some drugs may lead to a thrombophilic state predisposing the animal to thromboembolic complications (1). Cardiomyopathy is the most frequent cause of arterial thromboembolism (ATE) in cats (2–4); ATE has been reported in 48% of cats suffering from cardiomyopathy (5). A number of factors have been brought forth to explain the development of thrombi in cats with myocardial disease. Left atrial dilation causes stretching of the endocardium leading to an altered endothelium on which platelets can adhere (5). Mitral regurgitation along with an enlarged left atrium results in turbulent blood flow and stasis (2). Studies of platelet function in cats with cardiomyopathies have yielded conflicting results. In one study, cats with HCM have been shown to have platelets that are hyperaggregable, but other studies did not support these observations (6,7). The most common site for developing ATE is the aortic trifurcation (2,3). A study of 127 cats with ATE documented a survival rate of 37% following the ATE episode (4). Cats surviving the first episode have a recurrence rate that varies between 25% to 45% (2,4). Many drugs, such as aspirin and warfarin, have been used to prevent ATE in the long-term management of these patients; however, none of these treatments appear to affect the recurrence rate (8).
Thienopyridines irreversibly inhibit the binding of adenine diphosphate (ADP) to specific platelet ADP receptors (P2Y12). This ADP receptor blockade impairs platelet release reaction and ADP-mediated activation of GPIIb/IIIa, thereby reducing the aggregation response. Clopidogrel (Plavix; Sanofi-Aventis, Laval, Quebec), a thienopyridine, is readily available and has been evaluated in healthy cats. Clopidogrel, at dosages of 18.75 to 75 mg PO q24h, was well tolerated and resulted in significant anti-platelet effects (9).
Several tests are available to evaluate platelet function in patients receiving anti-platelet medication. Optical aggregometry is considered the gold standard for platelet function studies; however, several sample manipulations require a well-trained technician and specialized equipment. Plateletworks is a screening assay used to monitor platelet aggregation in human patients receiving anti-platelet therapy. It is quick, simple, inexpensive, reproducible, and only requires standard hematology equipment. This assay evaluates platelet aggregation by comparing impedance platelet counts between a sample anticoagulated with ethylenediamine tetra-acetic acid (EDTA) (baseline count) and a sample in a tube containing a platelet agonist (ADP or collagen). The agonist will stimulate platelet aggregation resulting in platelet clumping (10). This will cause a drop in platelet count as platelet aggregates are excluded in impedance platelet enumeration. The ADP tubes could be used to monitor clopidogrel therapy since platelet aggregation is inhibited by blocking the ADP receptor.
The 1st objective of this study was to determine if Plateletworks ADP tubes can detect a decrease in platelet aggregation in cats receiving clopidogrel. The 2nd objective was to determine the time period during which the results are stable. The ultimate goal of this study was to determine if Plateletworks ADP tubes can be used as a screening test to monitor clopidogrel therapy in cats.
Ten healthy neutered adult cats (2 males and 8 females) from the veterinary teaching colony of the Faculté de Médecine Vétérinaire, Université de Montréal were used in this prospective study. Their ages ranged from 1 to 5 y. Cats were determined to be healthy based on history and physical examination. All cats were routinely vaccinated and dewormed, and were enzyme-linked immunosorbent assay- (ELISA-) negative for Feline immunodeficiency virus (FIV) and Feline leukemia virus (FeLV). None of the cats were under treatment prior to the testing period. Animals were cared for according to guidelines provided by the Canadian Council on Animal Welfare.
On day 1, a 2-mL venous blood sample was collected from the jugular vein using a 22-gauge needle and a 3-mL syringe. Immediately after collection, 1 mL of blood was placed in the Plateletworks baseline tube (EDTA), and 1 mL of blood was placed in the Plateletworks ADP tube that had a final ADP concentration of 20 μM. Blood smears were made and used to check for platelet clumping in EDTA tubes. Each tube was mixed 15 to 20 times to ensure adequate mixing of blood with the reagent. After a 5-minute delay, EDTA tubes were analyzed using a hematology impedance cell counter (Cell Dyn 3500, Abbott Laboratories, Mississauga, Ontario) to determine the initial platelet count and to obtain a complete blood (cell) count (CBC). The ADP tubes were mixed again 3 to 5 times and run immediately in the same impedance cell counter to determine the platelet count. The platelet aggregation (PA) was then calculated according to the following formula:
Immediately following blood sampling, 18.75 mg of clopidogrel was administered orally to each cat q24h for 3 d. A complete daily physical examination was performed on each cat throughout the study. Venipuncture and blood analysis were repeated on day 4, in the same manner as on day 1. In 4 cats, PA was evaluated at times 5, 10, 15, 30, and 60 min following sampling before and after clopidogrel therapy. Statistical analysis was performed using NCSS (version 2004, NCSS and PASS, Kaysville, Utah, USA). The effect of clopidogrel treatment on PA was evaluated in 9 cats with a paired t-test using data obtained at 5 min. A 2-factor analysis of variance (ANOVA) model with repeated measures on both factors was used to evaluate the effect of time of analysis and treatment with clopidogrel on PA. Data obtained at 5, 10, 15, 30, and 60 min before and after treatment with clopidogrel obtained for 4 cats and used in this analysis. A post-hoc Tuckey-Kramer multiple-comparison test was used to detect significant differences between time points. The α level for significance was set at 0.05.
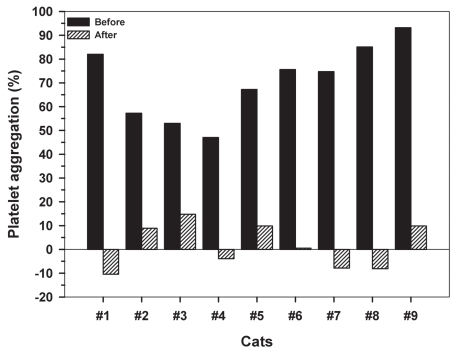
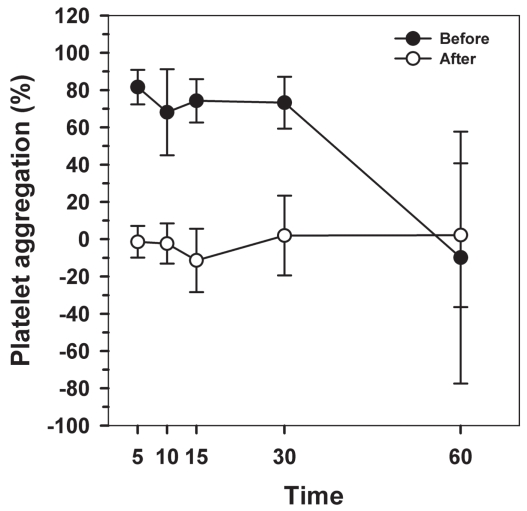
Ten cats were enrolled in this study, but only data from 9 cats were used for statistical analysis. One cat was removed from the study as he was thrombocytopenic at baseline (day 1). Clopidogrel treatment was generally well tolerated; however, 3 cats developed mild self-limiting diarrhea during the treatment period. Complete (cell) blood count results from the remaining cats were unremarkable before and after clopidogrel therapy. In EDTA, significant platelet clumping resulting in artefactual thrombocytopenia was noted in 6 samples before treatment and in 3 samples after treatment with clopidogrel (Figure 1). The PA was calculated as 70.55 ± 15.59% on day 1 [mean ± standard deviation (s)] and 1.52 ± 9.5% on day 4. Clopidogrel therapy caused a marked decrease in PA (P < 0.01) (Figure 2). The repeated measure ANOVA results indicate a significant effect of time (P = 0.028), clopidogrel treatment (P = 0.006) with a significant interaction term (P = 0.017) (Figure 3). The PA before treatment at 5, 10, and 15 min were statistically different than the PA at 60 min, with a marked drop between 30 and 60 min. This effect of time was present only in PA before treatment.
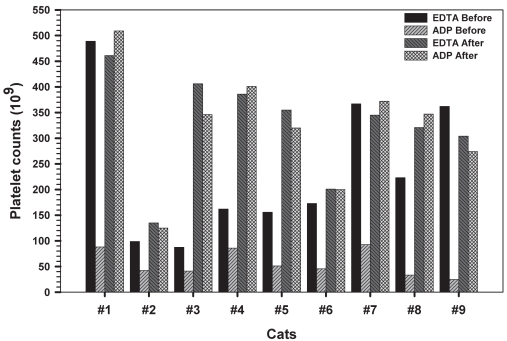
Figure 1.
Platelet counts in EDTA and ADP tubes before and after clopidogrel therapy obtained in 9 healthy cats using an impedance hematology analyzer. In EDTA tubes, decreased impedance platelet count secondary to platelet clumping was present in cats 2, 3, 4, 5, 6, and 8 before treatment and in cats 2, 3, and 6 after treatment.
Figure 2.
Platelet aggregation (PA) in 9 healthy cats before and after a 3-day clopidogrel treatment of 18.75 mg q24h.
Figure 3.
Platelet aggregation (PA) measured over time after blood collection in 4 cats before and after treatment with clopidogrel. Data represent the mean and the standard deviation.
This study clearly showed that Plateletworks® ADP tubes can be used to detect decreased PA after a 3-day clopidogrel treatment in healthy cats. Presence of pseudothrombocytopenia secondary to in vitro platelet clumping in EDTA samples, however, is a significant limitation of using this assay in cats. Artefactually decreased platelet counts in EDTA samples would significantly underestimate platelet aggregation. However, despite this limitation, significant differences in PA before and after clopidogrel treatment were noted, even in cats with significant platelet clumping. This study also demonstrated that PA remains stable until 30 minutes after blood collection. This is important, as the manufacturer recommends conducting the test within 10 min of sampling. This time frame is one of the major limitations of this test; having an on-site impedance cell counter machine is mandatory.
Before treatment, decreased PA after 30 min resulted from the combined effect of decreased platelet count in EDTA tubes, because of in vitro platelet clumping, and increased platelet count in ADP tubes (data not shown). Increased platelet counts in ADP tubes could have been secondary to reversible aggregation, a phenomenon occasionally observed in optical aggregometry when low concentrations of ADP are used to induce aggregation (11). These results suggest that tube analysis more than 30 min following blood sampling might affect the PA results. For use in veterinary practice, determination of the exact time after which the tubes should not be analyzed would be useful. Further investigations with a greater number of cats must be done to confirm these tendencies.
Mild, self-limiting diarrhea, not previously reported in cats receiving clopidogrel, was noted in 3 cats. In humans, diarrhea has been reported in 4% of patients receiving clopidogrel (12). It was not possible for us to ascertain whether the diarrhea was a direct side effect of the medication or if it was caused by other factors such as stress. Diarrhea resolved in all 3 cats by the following week. No clinical bleeding was noted during the study.
A negative PA was noted in 4 cats post clopidogrel therapy because of lower platelet counts in the EDTA than in the ADP tubes. This result could have been explained by in vitro EDTA-induced platelet aggregation causing decreased impedance platelet counts in baseline tubes (13). However, in these cases, no significant number of platelet clumps was noted on the blood smears of EDTA samples. Inter-assay variation of impedance platelet counts between ADP and EDTA samples could also be a possible explanation.
There are 2 limitations in this study. First, only 9 cats were enrolled in this study, and although significant differences between platelet aggregation before and after clopidogrel therapy were found because of a strong treatment effect, more cats would have allowed a better evaluation of the variation in clopidogrel response at the population level. Secondly, the results of Plateletworks were not compared with a gold standard such as platelet aggregometry or closure time using PFA-100 methodology. However, it has already been demonstrated that clopidogrel therapy at the dosage used in this study (18.75 mg/d) has a significant effect on platelet aggregation measured in whole blood using ADP and collagen (9).
In conclusion, Plateletworks appears to be a promising screening test for monitoring clopidogrel therapy in cats. Plateletworks is a practical and affordable test, requiring a small amount of blood (2 mL); it can be easily performed in a clinical setting if access to a hematology impedance analyzer is possible. Further investigations are necessary to validate this method and compare it to optic or whole blood aggregometry. Furthermore, evaluation of different anti-platelet drugs such as aspirin using Plateletworks with collagen as an agonist should be explored.
Acknowledgments
The authors thank John P. Docherty at Ryan Medical for his technical support, Dr. Jocelyn Dubuc for the statistical analysis, and Maxim Moreau for his help during the preparation of the manuscript.
References
- 1.Good LI, Manning AM. Thromboembolic disease: Predispositions and clinical management. Comp Cont Edu. 2003;25:660–674. [Google Scholar]
- 2.Laste NJ, Harpster NK. A retrospective study of 100 cases of feline distal aortic thromboembolism: 1977–1993. J Am Anim Hosp Assoc. 1995;31:492–500. doi: 10.5326/15473317-31-6-492. [DOI] [PubMed] [Google Scholar]
- 3.Schoeman JP. Feline distal aortic thromboembolism: A review of 44 cases (1990–1998) J Feline Med Surg. 1999;1:221–231. doi: 10.1053/jfms.1999.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SA, Tobias AH, Jacob KA, Fine DM, Grumbles PL. Arterial thromboembolism in cats: Acute crisis in 127 cases (1992–2001) and long-term management with low-dose aspirin in 24 cases. J Vet Intern Med. 2003;17:73–83. doi: 10.1892/0891-6640(2003)017<0073:aticac>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Fox PR, Sisson D, Moïse NS. In: Textbook of canine and feline cardiology: principles and clinical practice. Saunders, editor. Philadelphia: Montreal; 1999. p. 955. [Google Scholar]
- 6.Helenski CA, Ross JN., Jr Platelet aggregation in feline cardiomyopathy. J Vet Intern Med. 1987;1:24–28. doi: 10.1111/j.1939-1676.1987.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 7.Welles EG, Boudreaux MK, Crager CS, Tyler JW. Platelet function and antithrombin, plasminogen, and fibrinolytic activities in cats with heart disease. Am J Vet Res. 1994;55:619–627. [PubMed] [Google Scholar]
- 8.August JR, Hogan DF. Consultations in feline internal medicine. 5th ed. St Louis: Elsevier Saunders; 2006. Prevention and management of thromboembolism; pp. 331–342. [Google Scholar]
- 9.Hogan DF, Andrews DA, Green HW, Talbott KK, Ward MP, Calloway BM. Antiplatelet effects and pharmacodynamics of clopidogrel in cats. J Am Vet Med Assoc. 2004;225:1406–1411. doi: 10.2460/javma.2004.225.1406. [DOI] [PubMed] [Google Scholar]
- 10.Michelson AD, Frelinger AL, 3rd, Furman MI. Current options in platelet function testing. Am J Cardiol. 2006;98:4N–10N. doi: 10.1016/j.amjcard.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Sirridge MSaSR. Evaluation of Platelets. In: LaFebiger, editor. Laboratory Evaluation of Hemostasis and Thrombosis. 3rd ed. Philadelphia: Royal Society of Medicine Press; 1983. pp. 75–98. [Google Scholar]
- 12.Plavix: clopidogrel bisulfate tablets monography. Sanofisynthelabo.
- 13.Zandecki M, Genevieve F, Gerard J, Godon A. Spurious counts and spurious results on haematology analysers: A review. Part I: Platelets. Clin Lab Haematol. 2007;29:4–20. doi: 10.1111/j.1365-2257.2006.00870.x. [DOI] [PubMed] [Google Scholar]