Abstract
CD44 and fibrin(ogen) play critical roles in the hematogenous dissemination of tumor cells, including colon carcinomas. We recently reported that CD44 is the primary fibrin, but not fibrinogen, receptor on LS174T colon carcinomas. However, the biochemical nature of this interaction and the roles of CD44 standard (CD44s) versus CD44 variant (CD44v) isoforms in fibrin(ogen) recognition have yet to be delineated. Microspheres, coated with CD44 immunopurified from LS174T or T84 colon carcinoma cells, which express primarily CD44v, effectively bind to immobilized fibrin, but not fibrinogen, in shear flow. In contrast, CD44s from HL-60 cells binds to both immobilized fibrin and fibrinogen under flow. Use of highly specific enzymes and metabolic inhibitors reveals that LS174T CD44 binding to fibrin is dependent on O-glycosylation of CD44, whereas CD44s-fibrin(ogen) interaction has an absolute requirement for N-, but not O-, linked glycans. The presence of chondroitin and dermatan sulfate on CD44 standard and variant isoforms facilitates fibrin recognition. Use of the anti-CD44 function-blocking monoclonal antibody Hermes-1 nearly abolishes binding of LS174T CD44 to fibrin, although it has no effect on CD44s-fibrin(ogen) interaction. The CD44-binding site is localized within the N-terminal portion of the fibrin β chains, including amino acid residues (β15-66). Surface plasmon resonance experiments revealed high affinity binding of immobilized CD44 with solubilized fibrin but not fibrinogen. Collectively, these data suggest that immobilization of fibrinogen exposes a cryptic site that mediates binding to CD44s but not CD44v. Our findings may provide a rational basis for designing novel therapeutic strategies to combat metastasis.
CD44 is a multitasking protein that plays a pivotal role in a number of biological processes, including inflammation, hematopoiesis, wound healing, and cancer metastasis (1). CD44 proteins are type I transmembrane molecules encoded by a single gene, which spans ∼50 kb on human chromosome 11 and includes at least 20 exons (2). Exons 1-5, 16-18, and 20 are spliced together to form the smallest CD44 transcript known as standard form (CD44s)2 (2). The human CD44s protein is composed of 341 amino acid residues with a predicted size of 37 kDa, whereas its estimated molecular mass by SDS-PAGE is 80-95 kDa because of extensive post-translational modification resulting from the attachment of carbohydrates to N- and O-linked glycosylation sites of the extracellular domain. At least 10 exons (6-15; typically identified as v1-v10 that encode a membrane-proximal portion of the extracellular domain) can be alternatively spliced and inserted at a single site between exons 5 and 16 to give rise to multiple variant isoforms of CD44 (CD44v) with a molecular mass up to 250 kDa (1, 2). CD44s can be found in most tissues of the adult organism, with a particularly strong expression on cells of the hematopoietic system, whereas the larger variant isoforms are expressed in only a few epithelial tissues, mainly in proliferating cells, and in several cancers (1).
Several studies have disclosed the critical involvement of CD44 in the facilitation of blood-borne metastasis. In certain cases, such as with colorectal carcinomas, the expression of CD44v confers metastatic potential in vivo (3, 4) and results in poor prognosis (5). Interestingly, the up-regulation of CD44 expression appears to be an early event in colon carcinogenesis (6) and requires adenomatous polyposis coli gene inactivation (7). These observations coupled to the well established hyaluronic acid binding function of CD44 have led to the hypothesis that CD44-mediated tumor cell adhesion to hyaluronan is a dominant factor regulating metastasis (8).
Fibrinogen is a 340-kDa glycoprotein that is involved in diverse physiological and pathological processes. Structurally, fibrinogen consists of two identical disulfide-linked subunits, each of which is formed by three distinct polypeptide chains, Aα, Bβ, and γ (9). These chains assemble to form a number of independently folded domains, grouped into five structural regions as follows: the central E region, two identical terminal D regions, and two αC regions (10-12). The central region E is a dimer formed by the N-terminal portions of all six chains; the distal D regions are formed by the C-terminal portions of the Bβ and γ chains and a portion of the Aα chain, and the two αC regions are made up of the C-terminal two-thirds of the Aα chains. Proteolytic degradation of fibrin(ogen) by plasmin or some other proteases results in the D and E fragments, which correspond to the D and E regions. Because these fragments mainly preserve the structure and functional properties of the D and E regions, they are often used as models of these regions in fibrin(ogen) studies (9). In contrast, the αC regions are highly susceptible to proteolysis and degraded into smaller fragments. However, the full-length αC region can be prepared by the recombinant technique (13, 14). It should be noted that the N-terminal portions of the Bβ chains (residues ∼1-55), which form in the central E region a pair of functional BβN-domains (15), are also easily degraded upon proteolysis into a smaller (Bβ1-42) fragment (15). Thus, the proteolytically prepared E fragment, which is often called E3 fragment, is devoid of these domains while preserving other structures of the E region. The BβN-domains were also expressed in a disulfide-linked dimeric form, as the (Bβ1-66)2 fragment, which mimics the dimeric arrangement of these domains in fibrinogen (15).
Although fibrinogen is relatively inert in the circulation, upon conversion to fibrin it interacts with a variety of proteins and cells to participate in numerous (patho)physiological processes. Most notably, fibrinogen is involved in the blood clotting cascade whereby its thrombin-mediated conversion to fibrin (via cleavage of two N-terminal fibrinopeptides A and B, residues Aα(1-16) and (Bβ1-14), respectively) results in formation of insoluble fibrin clots that prevent the loss of blood upon vascular injury (9). It is now widely accepted that fibrin(ogen) plays a critical role in the hematogenous dissemination of tumor cells, including colon carcinomas. The most compelling evidence for the direct role of fibrin(ogen) in metastatic spread is the profound inhibition of experimental and spontaneous metastasis in fibrinogen-deficient mice compared with wild type controls (16-19). It is believed that fibrin(ogen) clots surrounding tumor cells may protect them from immunologic and physiologic stresses in the bloodstream, and facilitate their lodging to the pulmonary vasculature. This hypothesis is corroborated by recent in vivo data, which disclose that in mice lacking functional NK cells, fibrinogen deficiency was no longer a significant determinant of metastatic potential. It is also noteworthy that many patients with cancer, including those with disseminated colon cancer (20), also have detectable abnormalities of blood coagulation such as elevated levels of fibrinogen and fibrinopeptide A (21). Increased plasma levels of soluble fibrin have also been observed at the time of recurrence of colorectal cancer (22).
Using RNA interference, we have recently shown that CD44 is the functional fibrin, but not fibrinogen, receptor on LS174T colon carcinoma cells (23). However, detailed studies on the biochemical and biophysical characterization of CD44-fibrin(ogen) recognition have yet to be performed. Circumstantial evidence in the literature based on antibody interference assays suggests nonglycosylated CD44s supports human lung fibroblast adhesion and migration on both fibrinogen and fibrin under static conditions (24). In striking contrast, CD44s-mediated transmigration of human dermal fibroblasts into fibrin gels requires dermatan-, but not chondroitin-, sulfate proteoglycans (25). On the other hand, a CD44-related chondroitin-sulfate proteoglycan, expressed on rabbit microvascular endothelial cells, serves as a receptor for both fibrinogen and fibrin (26). Given the conflicting nature of the existing literature, we aimed to elucidate the roles of the various glycosaminoglycans and glycosylation patterns in CD44-fibrin(ogen) recognition, and to identify the CD44-binding sites on fibrin(ogen). Additionally, it is not clear whether the presence or absence of CD44 variant isoforms modulates CD44-fibrin(ogen) binding in shear flow. To this end, we have employed an integrated biophysical and biochemical approach, involving cell-free flow-based adhesion assays coupled to enzymatic and metabolic inhibition studies and surface plasmon resonance, to comprehensively characterize the molecular interaction between CD44 and fibrin(ogen).
EXPERIMENTAL PROCEDURES
Reagents and Monoclonal Antibodies—Fibrinogen (von Willebrand factor-, plasminogen-, and fibronectin-free) and plasmin were from Enzyme Research Laboratories (South Bend, IN). Thrombin, fibrin polymerization inhibitor Gly-Pro-Arg-Pro-amide (GPRP-NH2), human IgG, chondroitinase ABC (Proteus vulgaris), chondroitinase B (Flavobacterium heparinum), chondroitinase AC II (Arthrobacter aurescens), heparinase II (F. heparinum), and benzyl 2-acetamido-2-deoxy-α-d-galactopyranoside (benzyl-GalNAc), hyaluronic acid, and sodium chlorate were purchased from Sigma. The endoglycosidase H (Streptomyces plicatus) and 1-deoxymannojirimycin, hydrochloride (DMJ) were obtained from EMD Biosciences (Gibbstown, NJ). The keratanase I (Pseudomonas), keratanase II (Bacillus), and chondroitinase AC I (Flavobacterium heparinum) were from Seikagaku Corp. (Tokyo, Japan). The sialidase (Vibrio cholerae) was from Roche Applied Science. The p-nitrophenyl α-d-xylopyranoside (α-xyloside) and p-nitrophenyl β-d-xylopyranoside (β-xyloside) were purchased from Glycosynth (Warrington, UK). The PNGase F was from New England Biolabs (Ipswich, MA). The anti-human CD44 mAbs, 2C5 and 156-3C11, were obtained from R&D Systems (Minneapolis, MN) and AbD Serotec (Raleigh, NC), respectively. The unlabeled and PE-conjugated mouse anti-human CD44 (515), PE-labeled mouse IgG1 isotype control, and anti-CLA (HECA-452) were from BD Biosciences.
Cell Culture and Staining—LS174T and T84 human colon adenocarcinoma cells and HL60 human myeloid cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in the recommended medium. LS174T and T84 cells were detached from culture flasks by mild trypsinization (0.25% trypsin/EDTA for 5 min at 37 °C), and subsequently incubated at 37 °C for 2 h to regenerate surface glycoproteins, as described previously (27-29). The cells were resuspended in Dulbecco's phosphate-buffered saline (DPBS) containing Ca2+/Mg2+, and stored at 4 °C for no longer than 4 h before use in flow-based adhesion assays or flow cytometry. HL60 cells were grown in suspension and as such required no trypsinization or regeneration step.
Whole Cell Lysis and Immunoprecipitation of CD44—LS174T, T84, or HL60 whole cell lysate was prepared by membrane disruption using 2% Nonidet P-40 followed by differential centrifugation (30-32). CD44 was immunoprecipitated from colon carcinoma cell lysate with the anti-CD44 mAb 2C5, whereas CD44s was immunopurified from HL60 human myeloid cells with the anti-CD44 mAb 515 using recombinant protein G-agarose beads (Invitrogen) (33).
Preparation of CD44-coated Microspheres—Prior to protein binding, 10-μm polystyrene microspheres (2.5 × 107 microspheres/ml; Bangs Labs, Fishers, IN) were washed three times with DPBS, followed by two times with citrate/phosphate buffer, pH 3.0. After a 1-h incubation at room temperature, the microspheres were washed once more with the citrate/phosphate buffer, three times with DPBS, and two times with binding buffer (0.2 m carbonate/bicarbonate buffer, pH 9.2). Immunoprecipitated CD44 from LS174T, T84, or HL60 whole cell lysate or human IgG was diluted to the desired concentrations with binding buffer and incubated with the microspheres overnight at 4 °C with constant rotation (23, 30-32). Microspheres were washed two times with DPBS and subsequently blocked with DPBS, 1% BSA for 1 h at room temperature. Subsequently, microspheres were resuspended (2 × 106 microspheres/ml) in DPBS, 0.1% BSA for use in flow cytometric and flow chamber assays.
Flow Cytometry—CD44 expression levels on LS174T, T84, and HL60 cells and microspheres were quantified by single-color immunofluorescence and flow cytometry (FACSCalibur; BD Biosciences) using the PE-conjugated anti-CD44 mAb 515. Background levels were determined by incubating cell or microsphere suspensions with the properly matched PE-conjugated mouse IgG isotype control antibody (30). Using flow cytometry, we previously reported that LS174T colon carcinoma cells predominantly express the v3, v5, v7, and v8 variant isoforms of CD44 (30), whereas T84 cells primarily express the v3, v5, and v7 CD44 isoforms (31).
Enzymatic Treatments—To remove terminal sialic acid residues, LS174T cells or CD44-coated microspheres were incubated with 0.1 unit/ml V. cholerae sialidase for 90 min at 37 °C (30-32). To cleave specific glycosaminoglycans (GAGs) from CD44, LS174T cells or CD44-coated microsphere suspensions were incubated for 1 h at 37 °C with either 1 unit/ml P. vulgaris chondroitinase ABC (which degrades all forms of chondroitin sulfate as well as dermatan sulfate), chondroitinase B (which digests only dermatan sulfate), A. aurescens chondroitinase AC II (which catalyzes the eliminative cleavage of N-acetylhexosaminide linkage in chondroitin sulfate), or F. heparinum chondroitinase AC I (which in addition to the ACII activity also catalyzes the cleavage of N-acetylgalactosaminide linkages to d-glucuronic acid in dermatan sulfate-chondroitin sulfate copolymers) (31, 34). To remove heparin sulfate GAGs from CD44, LS174T cells or CD44-coated microspheres were treated with 0.5 unit/ml of F. heparinum heparinase II for 24 h at 37 °C (35, 36). To digest keratan sulfate GAGs from CD44, cells or CD44-coated microspheres were treated with either 70 milliunits/ml Pseudomonas keratanase I or 5 milliunits/ml Bacillus keratanase II for 24 h at 37 °C (37). To cleave N-linked glycans from CD44, LS174T or HL60 whole cell lysates were treated with 8 units/ml PNGase F for 48 h at 37 °C (30). Site densities of CD44 adsorbed onto microspheres following enzymatic treatments were determined by flow cytometry and confirmed to be equivalent to untreated controls before use in adhesion assays.
Inhibitor Treatments—Prior to metabolic inhibitor studies, LS174T or HL60 cell suspensions (107 cells/ml) were pretreated with 0.1 unit/ml V. cholerae sialidase for 60 min at 37 °C to remove terminal sialic acid residues and to ensure de novo synthesis of newly generated HECA-452 reactive carbohydrate structures (30, 31, 33). Complete removal of sialic acid was confirmed via flow cytometry using the HECA-452 mAb that recognizes sialic acid-bearing epitopes. Subsequently, LS174T or HL60 cells were cultured for 48 h at 37 °C in medium containing either 2 mm benzyl-GalNAc to inhibit O-linked glycosylation (30, 31, 34) or 1 mm DMJ to inhibit N-linked processing of glycoproteins (30); DPBS diluent was used for control untreated cells. Cells were either used in flow-based adhesion assays or lysed to immunopurify CD44 for coating onto microspheres. To prevent coupling of chondroitin sulfate proteoglycans to the core protein, LS174T or HL60 cells were incubated at 37 °C with 1 mm of p-nitrophenyl α- or β-xyloside for 48 h (24, 26) prior to use in adhesion studies or lysis and immunoprecipitation.
Fibrinogen, Fibrin, and Their Fragments—Solubilized fibrin was prepared by mixing plasminogen-depleted fibrinogen with thrombin followed by incubation for 30 min in the presence of 5 mm Gly-Pro-Arg-Pro peptide as described earlier (38). The dimeric D-D fragment was prepared by plasmin digestion of factor XIIIa cross-linked fibrin according to the procedure described in Refs. 38, 39. The E3 fragment was prepared by plasmin digestion of fibrinogen as in Ref. 38. The dimeric (Bβ1-66)2 fragment corresponding to the N-terminal portions of the fibrinogen Bβ chains, which are missing in the E3 fragments, was expressed in Escherichia coli and subsequently purified as described elsewhere (15). The (β15-66)2 fragment lacking fibrinopeptides B was prepared by treatment of (Bβ1-66)2 with thrombin and then purified as described previously (15). The recombinant αC-fragment (residues Aα(221-610)) corresponding to the human fibrinogen αC region was produced in E. coli and purified and refolded using the previously published protocols (14). The purity of all fragments was confirmed by SDS-PAGE.
Hermes-1 Antibody Purification—Hybridoma cells expressing Hermes-1 anti-CD44 mAb were obtained from the Developmental Studies Hybridoma Bank at the University of Iowa (Iowa City) and grown in the recommended medium. Hermes-1 mAb was purified from cell supernatants using an Immunopure protein A IgG purification kit (Pierce). The fractions collected upon elution were then checked for protein concentration and purity via BCA assay and Western blotting.
Flow-based Adhesion Assays—Fibrinogen-coated surfaces were prepared by incubating 0.5 or 1 mg/ml von Willebrand factor-, plasminogen-, and fibronectin-free fibrinogen in DPBS on untreated 35-mm polystyrene suspension culture dishes overnight at 4 °C (23). Fibrin-coated surfaces were prepared by washing the immobilized fibrinogen three times with DPBS, and subsequently incubating with 2 units/ml thrombin for 2 h at 37 °C (23). Plates were then washed with DPBS and blocked with 1% BSA for 1 h prior to their use in flow-based adhesion assays. In experiments using fibrin(ogen) fragments, 100 μg/ml of each fragment in binding buffer was incubated with suspension culture dishes overnight at 4 °C. The plates were then washed three times with DPBS and used immediately in flow-based assays. In select experiments, microspheres were incubated with 20 μg/ml Hermes-1 mAb, 1 mg/ml hyaluronic acid, or DPBS for 1 h at 37 °C, followed by washing with DPBS and use in flow assays. Suspensions of cells (1 × 106/ml) or microspheres (2 × 106/ml) were perfused over fibrin- or fibrinogen-coated dishes using a parallel plate flow chamber (250 μm channel depth, 5.0 mm channel width) (30, 31, 40) for 5 min at 37 °C. The number of interacting cells was then quantified by averaging over multiple 10× fields of view for each condition.
SDS-PAGE and Western Blotting—LS174T CD44 or HL60 CD44s was immunopurified from either control cells or cells treated with metabolic inhibitors of O- or N-glycosylation as described above. In select experiments, CD44 from DMJ-treated cells was incubated with 0.12 unit/ml endoglycosidase H for 3 h at 37 °C to remove high mannose or hybrid N-linked glycans, which lack further processing. Alternatively, immunopurified CD44 was treated with PNGase F for 48 h at 37 °C to remove all N-linked glycans. The treated and control immunoprecipitates were then separated by SDS-PAGE on a 4-20% Tris-HCl Criterion gel (Bio-Rad) for 40 min using Tris/glycine/SDS running buffer. Resolved proteins were transferred to Immun-blot polyvinylidene difluoride membranes (Bio-Rad) and blocked with Starting Block (Pierce) for 10 min. The immunoblots were stained with either anti-CD44 mAbs (2C5 or 515) or a HECA-452 mAb.
Inhibition of CD44 Sulfation—LS174T or HL60 cells were cultured for 48 h at 37 °C in medium containing 60 or 20 mm sodium chlorate, respectively. DPBS diluent was used for control untreated cells. Cells were then lysed to immunopurify CD44 for coating onto microspheres. To confirm the effectiveness of sodium chlorate treatment on removal of sulfate groups from CD44, LS174T or HL60 cells were treated with 20 μCi/ml of Na35SO4 (PerkinElmer Life Sciences) for 48 h in the presence or absence of sodium chlorate. Immunoprecipitated CD44 was then separated via SDS-PAGE on a 4-12% BisTris NuPAGE gel (Invitrogen), fixed in 10% acetic acid, 50% methanol for 30 min, incubated in Amplify fluorographic reagent (GE Healthcare) for 30 min, and dried onto paper before exposure and scanning using a Typhoon 9410 Variable Mode Imager (GE Healthcare).
Surface Plasmon Resonance—The interaction of fibrinogen, fibrin, and their fragments with the immobilized CD44s and CD44 receptors, from HL60 and LS174T cells, respectively, was studied by surface plasmon resonance (SPR) using the BIAcore 3000 biosensor (BIAcore AB, Uppsala, Sweden), which measures the association/dissociation of proteins in real time. Immobilization of HL60 CD44s and LS174T CD44 to the CM5 sensor chip was performed using the amine coupling kit (BIAcore AB, Uppsala, Sweden) according to the recommended procedure. Briefly, HL60 CD44s or LS174T CD44, both at 25 μg/ml in 10 mm sodium acetate, pH 5.0, was injected onto the chip surface to achieve the immobilization level of ∼1000 response units. Binding experiments were performed in binding buffer, HBS-P (BIAcore AB), containing 1 mm CaCl2, at 10 μl/min flow rate. Fibrinogen, soluble fibrin, and their fragments were injected at prescribed concentrations, and the association between them and immobilized CD44 was monitored by the change in the SPR response; the dissociation was measured upon replacement of the ligand solution for the binding buffer without ligand. To regenerate the chip surface, complete dissociation of the complex was achieved by adding a solution containing 2 m NaCl in HBS-P for 1 min followed by re-equilibration with the binding buffer. Experimental data were analyzed using BIAevaluation 3.2 software supplied with the instrument. Kinetic constants for association (ka) and dissociation (kd) were estimated by global analysis of the association/dissociation curves using the 1:1 Langmurian interaction model, and the dissociation equilibrium constant (Kd) was calculated as Kd = kd/ka. The values were examined for self-consistency of the data are as described in Ref. 41.
Statistics—Data are expressed as the mean ± S.E., unless otherwise stated. Statistical significance of differences between means was determined by analysis of variance. If means were shown to be significantly different, multiple comparisons by pairs were performed by the Tukey test. Probability values of p < 0.05 was selected to be statistically significant.
RESULTS
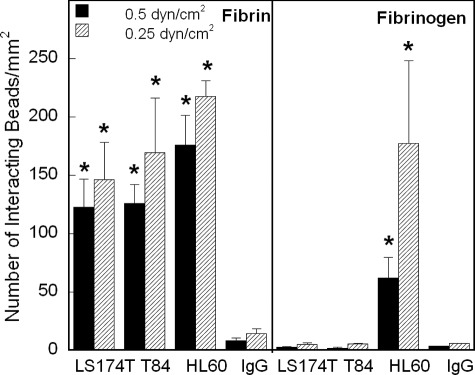
CD44 Standard and Variant Isoforms Are Fibrin Receptors, whereas Only CD44s Binds to Immobilized Fibrinogen under Shear—We recently reported that CD44 is the primary fibrin, but not fibrinogen, receptor on LS174T colon carcinoma cells (23). Here we sought to determine whether CD44 isoforms expressed on other colon carcinoma cell lines, such as T84 (31), also displayed the same capacity to bind to fibrin under controlled levels of shear stress. We also wished to examine whether the standard form of CD44, CD44s, which lacks any variant exons and represents the most prevalent form expressed on a variety of human cell types (1, 2), interacts with fibrin(ogen) in shear flow. To address these issues, we used a cell-free flow-based adhesion assay to compare the adhesion of microspheres coated with CD44 immunopurified from either colon carcinoma cells (LS174T and T84), which predominantly express CD44v (30, 31), or human myeloid cells (HL60), which only express CD44s (30), to immobilized fibrin or fibrinogen under flow. The extent of CD44 coating on microspheres was quantified by flow cytometry and was directly compared with the CD44 expression on the surface of intact cells. As shown in Table 1, the site density of CD44 on the microspheres was equivalent to that on intact cells. In cell-free flow-based adhesion assays, LS174T and T84 CD44-coated beads as well as HL60 CD44s-bearing microspheres bound extensively and avidly to immobilized fibrin in the low shear regime (Fig. 1). No significant difference was detected in the extent of CD44-coated microsphere adhesion to fibrin substrates when the shear stress increased from 0.25 to 0.5 dyne/cm2 (Fig. 1). However, the extent of CD44-coated microsphere binding to immobilized fibrin dropped to base-line levels at 1 dyne/cm2 (data not shown). The specificity of CD44-fibrin binding was confirmed through the use of nonspecific IgG-coated control microspheres, which bound minimally to immobilized fibrin in all experiments (Fig. 1). In accordance with our recent results (23), CD44 immunopurified from both LS174T and T84 colon carcinoma cells failed to bind to immobilized fibrinogen (Fig. 1). In marked contrast, HL60 CD44s is capable of binding to immobilized fibrinogen in a shear-dependent manner (Fig. 1).
TABLE 1.
Relative fluorescence intensity of CD44 on cells and protein-coated beads
CD44 expression on cells or microspheres coated with immunoprecipitated CD44 from the corresponding cell type was quantified by single-color immunofluorescence and flow cytometry using the PE-conjugated anti-CD44 mAb 515. Background levels were determined by incubating cell or microsphere suspensions with the properly matched PE-conjugated mouse IgG isotype control antibody. Values represent mean fluorescence intensity ± S.E. from n = 5 to 8 experiments.
|
Cell type
|
Mean fluorescence intensity
|
|
|---|---|---|
| Cells | Beads | |
| LS174T | 1205 ± 88 | 1191 ± 139 |
| HL60 | 626 ± 10 | 700 ± 66 |
| T84 | 654 ± 68 | 726 ± 195 |
FIGURE 1.
CD44 from colon carcinoma cells and HL60 CD44s effectively bind to fibrin under flow, but only CD44s binds to immobilized fibrinogen. Immunoprecipitated CD44 from LS174T or T84 colon carcinoma whole cell lysates or immunopurified CD44s from HL60 human myeloid cell whole cell lysate was adsorbed onto 10-μm microspheres. The CD44-coated microspheres were then perfused over 1.0 mg/ml immobilized fibrin or fibrinogen at the specified wall shear stresses in a parallel plate flow chamber. The number of beads interacting with fibrin or fibrinogen was quantified using video microscopy. Data represent the mean ± S.E. of n = 3-4 experiments. *, p < 0.05 with respect to IgG control-coated microspheres.
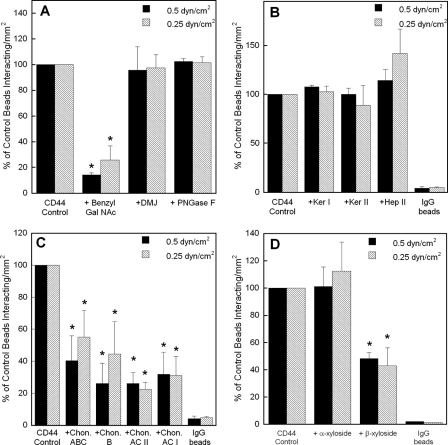
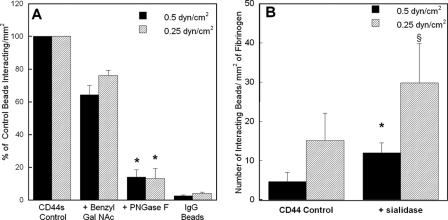
LS174T CD44 Binding to Immobilized Fibrin Requires O-, but Not N-, Linked Glycans and Is Dependent on the Presence Chondroitin and Dermatan Sulfate Glycosaminoglycans—We next aimed to characterize the fibrin binding determinants of CD44 by using glycoconjugate biosynthesis inhibitors that disrupt oligosaccharide processing on the CD44 core protein on LS174T colon carcinoma cells or highly specific enzymes that cleave GAG moieties from CD44 adsorbed on microspheres. To assess the relative contribution of O-versus N-linked glycans on LS174T CD44-fibrin binding, microspheres were generated using CD44 immunoprecipitated from LS174T cells after being cultured for 48 h in medium containing benzyl-GalNAc (2 mm) to inhibit O-glycosylation or DMJ (1 mm) to disrupt N-linked glycan processing. The CD44 site densities on microspheres from benzyl-GalNAc- or DMJ-treated LS174T cells were comparable with those from untreated controls (data not shown). CD44-coated microspheres from benzyl-GalNAc-treated LS174T cells displayed a marked reduction in their capacity to bind immobilized fibrin under shear (Fig. 2A). In contrast, CD44-bearing microspheres from DMJ-treated cells bound at levels equivalent to the control (Fig. 2A). Similarly, treatment of CD44-coated microspheres with PNGase F (8 units/ml), which enzymatically removes N-linked oligosaccharides from the CD44 core protein, failed to interfere with the extent of microsphere adhesion to immobilized fibrin relative to untreated control CD44-bearing beads (Fig. 2A).
FIGURE 2.
A, effect of O-versus N-glycosylation on the adhesion of LS174T CD44-coated microspheres to immobilized fibrin under flow. CD44-coated microspheres were generated using CD44 immunopurified from LS174T cells pretreated with either 2 mm benzyl-GalNAc or 1 mm DMJ, or from LS174T whole cell lysate treated with 8 units/ml PNGase F. B, contribution of keratan and heparan sulfates to the adhesion of LS174T CD44-coated microspheres to immobilized fibrin under flow. CD44-coated microspheres were generated using CD44 immunoprecipitated from LS174T cells, which was subsequently treated with either 70 milliunits/ml keratanase (Ker) I, 5 milliunits/ml keratanase II, or 0.5 unit/ml heparinase (Hep) II for 24 h at 37 °C prior to their use in flow-based adhesion assays. C, effect of chondroitin and dermatan sulfates on the adhesion of LS174T CD44-coated microspheres to immobilized fibrin under flow. CD44-coated microspheres were generated using CD44 immunopurified from LS174T cells, which was subsequently treated with either 1 unit/ml chondroitinase (Chon) ABC, B, AC II, or AC I for 60 min at 37 °C. D, effect of chondroitin and dermatan sulfate chain coupling to the CD44 core protein on CD44-fibrin binding. CD44-coated microspheres were generated using CD44 immunoprecipitated from LS174T cells, which was previously treated with either 1 mm p-nitrophenyl α-d-xylopyranoside or p-nitrophenyl β-xylopyranoside for 48 h at 37 °C. In all experiments (A-D), CD44-coated microspheres (2 × 106/ml) were perfused over 1 mg/ml fibrin for 5 min at the specified wall shear stresses. Data are reported as percent of untreated control beads that interacted with immobilized fibrin and represent the mean ± S.E. of n = 3-4 experiments. *, p < 0.05 with respect to untreated control microspheres.
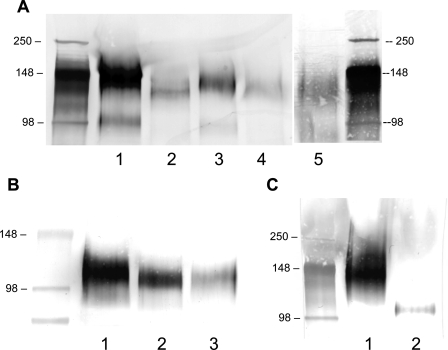
The impact of benzyl-GalNAc and DMJ treatments was apparent by the reduction in the molecular weight of CD44, as evidenced by its faster migration in the SDS-polyacrylamide gel compared with the untreated control whole cell lysates (Fig. 3A, lanes 1-3), which is consistent with an effect on structural glycosylations. Furthermore, the efficacy of DMJ treatment was confirmed by assessing the sensitivity of CD44 from DMJ-treated cells to the enzyme endoglycosidase H (Fig. 3A, lane 4), which only cleaves high mannose or hybrid N-linked glycans that lack further processing (42). The shift in the molecular weight of CD44 observed with this combined intervention matched that of CD44 treated with PNGase F (Fig. 3A, lane 5), which cleaves high mannose, hybrid, and complex N-linked glycans from the glycoprotein backbone (43).
FIGURE 3.
A, Western blots of LS174T whole cell lysates using cells pretreated with highly specific glycoconjugate biosynthesis inhibitors. CD44 was immunoprecipitated from untreated control LS174T cells (lane 1) and subjected to SDS-PAGE followed by Western blotting with the anti-CD44 mAb 2C5. Lysate was also immunoprecipitated from LS174T colon carcinoma cells cultured for 48 h in medium containing 2 mm benzyl-GalNAc (to inhibit O-linked glycosylation; lane 2) or 1 mm DMJ (to disrupt N-linked processing; lane 3). The efficacy of the DMJ treatment was verified by incubating CD44 from DMJ-treated cells with 0.12 unit/ml endoglycosidase H for 3 h at 37 °C, which cleaves high mannose and hybrid but not complex oligosaccharides from glycoproteins (lane 4). Alternatively, LS174T whole cell lysate was treated with 8 units/ml PNGase F for 48 h at 37 °C prior to immunoprecipitation (to cleave N-linked glycans from the glycoprotein core, lane 5). B, Western blots of HL60 cell lysates using cells pretreated with highly specific glycoconjugate biosynthesis inhibitors. CD44s was immunoprecipitated from untreated control HL60 cells (lane 1) and subjected to SDS-PAGE followed by Western blotting with the anti-CD44 mAb 156-3C11. Lysate was also immunopurified from HL60 cells cultured for 48 h in medium containing 2 mm benzyl-GalNAc (to inhibit O-linked glycosylation; lane 2). Alternatively, HL60 whole cell lysate was treated with 8 units/ml PNGase F for 48 h at 37 °C prior to immunoprecipitation (to remove N-linked glycans from the glycoprotein core; lane 3). C, detection of HECA-452 reactive epitopes on LS174T CD44 and HL60 CD44s proteins. CD44 immunoprecipitated from LS174T colon carcinoma cells using the anti-CD44 mAb 2C5 (lane 1) and CD44s immunopurified from HL60 cells using the anti-CD44 mAb 515 (lane 2) were separated by SDS-PAGE followed by Western blotting with the HECA-452 mAb.
To further characterize the biochemical nature of LS174T CD44-fibrin binding, CD44-coated microspheres were treated with highly selective enzymes that cleave specific carbohydrate and GAG moieties from the glycoprotein. Sialidase treatment of LS174T CD44-coated microspheres, which eliminated HECA-452 reactivity without affecting CD44 expression (31), failed to impair microsphere adhesion to immobilized fibrin under flow (data not shown). Treatment of CD44-coated microspheres with keratanase (I or II) or heparinase II, which digest keratan sulfate and heparan sulfate, respectively, had no effect on the extent of microsphere adhesion to immobilized fibrin under flow (Fig. 2B).
Prior albeit conflicting reports have suggested a potential role for dermatan sulfate or chondroitin sulfate proteoglycans in CD44-fibrin(ogen) molecular recognition (24-26). For instance, CD44s-mediated transmigration of human dermal fibroblasts into fibrin gels requires dermatan, but not chondroitin, sulfate proteoglycans (25). In contrast, a CD44-related chondroitin sulfate proteoglycan, expressed on rabbit microvascular endothelial cells, binds both fibrinogen and fibrin (26), whereas nonglycosylated CD44s supports human lung fibroblast adhesion and migration on both fibrinogen and fibrin under static conditions (24). To test the relative contributions of the aforementioned GAGs to LS174T CD44 fibrin binding, CD44-bearing microspheres were treated with a panel of highly specific enzymes, which cleave chondroitin and/or dermatan sulfate chains from CD44. Specifically, we tested chondroitinase ABC, which degrades all forms of chondroitin sulfate as well dermatan sulfate (25), chondroitinase B, which digests only dermatan sulfate (44), chondroitinase AC II, which catalyzes the eliminative cleavage of N-acetylhexosaminide linkage in chondroitin sulfate (44), and chondroitinase AC I, which in addition to the AC II activity also catalyzes the efficient cleavage of the N-acetylgalactosaminide linkages to d-glucuronic acid in dermatan sulfate-chondroitin sulfate copolymers. As shown in Fig. 2C, all these enzymatic interventions had a significant inhibitory effect on CD44-bearing microsphere adhesion to fibrin in shear flow, indicating that both chondroitin and dermatan sulfates are involved in CD44-fibrin molecular recognition. Because both chondroitin and dermatan sulfate are connected to the glycoprotein via an o-xylose linkage, we also used p-nitrophenyl β-d-xylopyranoside to competitively inhibit GAG side chain attachment to the proteoglycan core. The inactive analog of β-xyloside, p-nitrophenyl α-d-xylopyranoside, was used as a negative control. Our data reveal that β-xyloside was effective in suppressing the extent of CD44-coated microsphere binding to immobilized fibrin by greater than 50% as compared with both untreated and α-xyloside-treated control microspheres (Fig. 2D), indirectly confirming that o-xylose-linked chondroitin/dermatan sulfate chains are important fibrin binding determinants. Taken together, our data reveal that the fibrin binding determinants of LS174T CD44, which is mainly composed of CD44 variant isoforms, are presented on O- but not N-linked glycans and also require chondroitin and dermatan sulfate chains. We also confirmed the microsphere findings by performing experiments in which whole LS174T colon carcinoma cells, treated with either glycoconjugate biosynthesis inhibitors or highly specific enzymes, were perfused over immobilized fibrin in shear flow (data not shown).
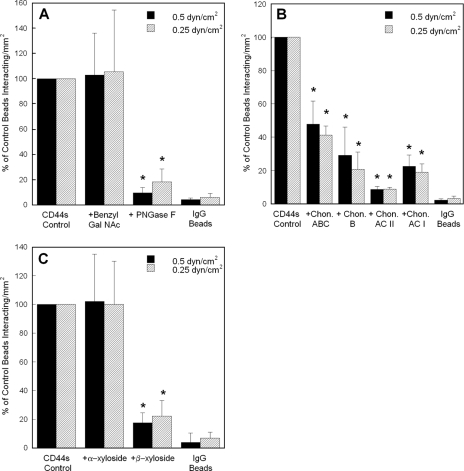
CD44s-fibrin Recognition Requires N- but Not O-Linked Glycans and Is Dependent on the Presence of Chondroitin and Dermatan Sulfate—We next wished to compare and contrast the molecular requirements that mediate binding of the standard versus variant isoforms of CD44 to immobilized fibrin under flow. To this end, microspheres were coated with CD44s immunopurified from HL60 human myeloid cells (30) as opposed to CD44 from LS174T colon carcinoma cells, which primarily express CD44v and are nearly devoid of CD44s (30, 31). In distinct contrast to the data acquired with LS174T CD44, CD44s-coated microspheres generated using CD44s immunoprecipitated from HL60 cells cultured in medium containing 2 mm benzyl-GalNAc interacted comparably to those of untreated control beads with immobilized fibrin under flow, suggesting that O-linked glycans are not involved in CD44s-fibrin molecular recognition (Fig. 4A). However, treatment of CD44s-bearing microspheres with 8 units/ml PNGase F nearly abrogated their adhesion to immobilized fibrin, suggesting that N-linked glycans displayed on CD44s are responsible for its ability to bind to fibrin (Fig. 4A). The impact of the benzyl-GalNAc and PNGase F treatments was apparent by the reduction in molecular weight of CD44s as compared with untreated CD44 controls via SDS-PAGE followed by Western blot analysis (Fig. 3B).
FIGURE 4.
A, effect of O-versus N-glycosylation on the adhesion of HL60 CD44s-coated microspheres to immobilized fibrin under flow. CD44s-coated microspheres were generated using CD44s immunoprecipitated from HL60 cells pretreated with 2 mm benzyl-GalNAc or from HL60 whole cell lysate treated with 8 units/ml PNGase F. B, effect of chondroitin and dermatan sulfates on the adhesion of HL60 CD44s-coated microspheres to immobilized fibrin under flow. CD44s-coated microspheres were generated using CD44s immunopurified from HL60 cells, which was subsequently treated with 1 unit/ml chondroitinase (Chon) ABC, B, AC II, or AC I for 60 min at 37 °C. C, effect of chondroitin and dermatan sulfate chain coupling to the CD44s core protein on CD44s-fibrin binding. CD44s-coated microspheres were generated using CD44s immunoprecipitated from HL60 cells treated with either 1 mm p-nitrophenyl α-d-xylopyranoside or p-nitrophenyl β-xylopyranoside for 48 h at 37 °C. In all experiments (A-C), CD44s-coated microspheres (2 × 106/ml) were perfused over 1 mg/ml fibrin for 5 min at the specified wall shear stresses. Data are reported as percent of untreated control beads that interacted with immobilized fibrin and represent the mean ± S.E. of n = 3-4 experiments. *, p < 0.05 with respect to untreated control microspheres.
Removal of chondroitin and dermatan sulfate via the use of a panel of highly specific enzymes suppressed CD44s-coated microsphere binding to immobilized fibrin by >50% (Fig. 4B) suggesting CD44s-fibrin recognition exhibits a dependence on the presence of both chondroitin and dermatan sulfate GAGs on CD44s. Along these lines, CD44s-coated beads, created using CD44s immunopurified from HL60 cells treated with β-xyloside which inhibits chondroitin and dermatan sulfate GAG attachment to the proteoglycan core, displayed a markedly reduced capacity (80% relative to untreated control or α-xyloside-treated microspheres) to bind to immobilized fibrin under flow (Fig. 4C).
CD44s Adhesion to Fibrinogen Is Predominantly Mediated by N-Linked Glycans Displayed on CD44s—In view of our observations showing that CD44s is also capable of binding to immobilized fibrinogen under shear flow (Fig. 1), we next aimed to delineate the relative contribution of N-versus O-glycosylation to CD44s-fibrinogen molecular interaction. To this end, we prepared microspheres using CD44s immunoprecipitated from HL60 whole cell lysates treated with PNGase F (8 units/ml) or from HL60 cells cultured in medium containing benzyl-GalNAc (2 mm). Fig. 5A shows that the molecular recognition between CD44s and immobilized fibrinogen was nearly abolished by the enzymatic removal of N-linked glycans from the CD44s core protein via the use of PNGase F. A moderate, yet not statistically significant, reduction was also noted when O-glycosylation was disrupted via benzyl-GalNAc treatment. Cumulatively, our data suggest that the binding of CD44s to immobilized fibrinogen is primarily reliant on N-linked glycans presented on CD44s.
FIGURE 5.
A, effect of O- and N-glycosylation on the adhesion of HL60 CD44s-coated microspheres to immobilized fibrinogen under flow. CD44s-coated microspheres were generated using CD44s immunoprecipitated from HL60 cells pretreated with 2 mm benzyl-GalNAc or from HL60 whole cell lysate treated with 8 units/ml PNGase F. CD44s-coated microspheres (2 × 106/ml) were perfused over 1 mg/ml fibrinogen for 5 min at the specified wall shear stresses. Data are reported as percent of untreated control beads that interacted with immobilized fibrinogen, and represent the mean ± S.E. of n = 3 experiments. *, p < 0.05 with respect to untreated control microspheres. B, impact of cleavage of HECA-452 reactive epitopes from CD44 variant isoforms on the adhesion of LS174T CD44-coated microspheres to immobilized fibrinogen under flow. CD44-coated microspheres were treated with 0.1 unit/ml sialidase for 90 min at 37 °C. CD44-coated microspheres (2 × 106/ml) were perfused over 1 mg/ml fibrinogen for 5 min at the specified wall shear stresses. Data are reported as the number of interacting microspheres per mm2, and represent the mean ± S.E. of n = 3 experiments. *, p < 0.05; §, p < 0.1 with respect to untreated control microspheres.
Sialofucosylated Structures May Mask the Binding Site of LS174T CD44 for Immobilized Fibrinogen—To determine why HL60 CD44s, but not CD44 from colon carcinoma cells, which is mainly composed of CD44v (30, 31), is capable of interacting with immobilized fibrinogen, we explored known differences in the CD44s versus CD44v protein side chain biochemistry. Based on the pronounced difference in HECA-452 reactivity in LS174T CD44 versus HL60 CD44s (Fig. 3C, lanes 1 and 2, respectively), we investigated the effect of removing terminal sialic acid residues from LS174T CD44 via sialidase treatment on binding to immobilized fibrinogen under flow. At both wall shear stress levels examined, sialidase treatment led to an increased number of interacting LS174T CD44-coated microspheres with immobilized fibrinogen (Fig. 5B). Similar data were obtained when sialidase-treated versus untreated live LS174T colon carcinoma cells were perfused over fibrinogen substrates (data not shown). In contrast, this enzymatic intervention did not alter the extent of CD44s-coated microsphere adhesion to either immobilized fibrin or fibrinogen (data not shown), a finding that is apparently consistent with the limited HECA-452 reactivity on HL60 CD44s. Together, these results suggest that terminal sialic acid residues on the variant forms of CD44 may mask a binding site for fibrinogen, which is otherwise exposed on CD44s. It is noteworthy that the extent of sialidase-treated LS174T CD44-coated microsphere binding to fibrinogen substrates is markedly lower than that of HL60 CD44s-bearing beads onto immobilized fibrinogen. Consequently, the presence of terminal sialic acid residues on LS174T CD44 does not fully account for the difference in the extent of LS174T CD44 versus HL60 CD44s binding to immobilized fibrinogen in shear flow.
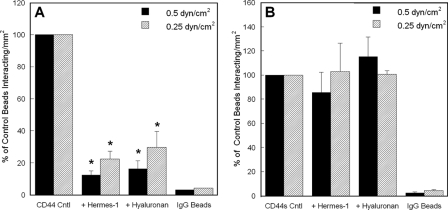
Hyaluronic Acid-binding Site on LS174T CD44 Is Responsible for Fibrin Recognition, whereas CD44s Possesses an Alternate Fibrin-binding Site—To localize the binding site on CD44 for fibrin, we incubated LS174T CD44-coated microspheres with Hermes-1, an anti-CD44 mAb, which exhibits function blocking properties via recognition of the N-terminal hyaluronate binding domain of CD44 (45). This intervention resulted in a drastic inhibition (>80%) in the extent of LS174T CD44-coated bead adhesion to immobilized fibrin in shear flow (Fig. 6A), suggesting that fibrin binds to the hyaluronic acid-binding site on LS174T CD44. This is further corroborated by observations showing that preincubation of LS174T CD44-coated microspheres with soluble hyaluronic acid reduced binding to immobilized fibrin by nearly identical levels as the Hermes-1 mAb (Fig. 6A). In distinct contrast, no inhibitory effect was detected when CD44s-coated microspheres were incubated with either the Hermes-1 mAb or soluble hyaluronic acid prior to their perfusion over immobilized fibrin, as compared with the untreated control microspheres (Fig. 6B). Along these lines, Hermes-1 mAb did not impair the extent of CD44s binding to immobilized fibrinogen in shear flow (data not shown). This finding implicates an alternate CD44s-binding site on fibrin, which may also be involved in the molecular recognition of fibrinogen.
FIGURE 6.
The anti-CD44 mAb Hermes-1 and soluble hyaluronan inhibit the adhesion of CD44-, but not CD44s-, coated microspheres to immobilized fibrin under shear. A, CD44-coated microspheres, generated using CD44 immunopurified from LS174T colon carcinoma cells, were incubated with 20 μg/ml of the anti-CD44 mAb Hermes-1 or 1 mg/ml soluble hyaluronic acid for 1 h at 37 °C. CD44-coated microspheres were subsequently washed once in DPBS and perfused at a concentration of 2 × 106 per ml over 1 mg/ml fibrin for 5 min at a wall shear stress of 0.5 or 0.25 dyne/cm2. Data are reported as percent of untreated control (Cntl) beads that interacted with immobilized fibrin, and represent the mean ± S.E. of n = 5 experiments. *, p < 0.05 with respect to untreated control microspheres. B, CD44s-coated microspheres, generated using CD44s immunoprecipitated from HL60 cells, were incubated with 20 μg/ml of the anti-CD44 mAb Hermes-1 or 1 mg/ml soluble hyaluronic acid for 1 h at 37 °C, and perfused at a concentration of 2 × 106/ml over 1 mg/ml fibrin for 5 min at the specified wall shear stresses. Data are reported as percent of untreated control beads that interacted with immobilized fibrin and represent the mean ± S.E. of n = 3 experiments. As a negative control, IgG-coated microspheres were perfused over immobilized fibrin at prescribed wall shear stresses.
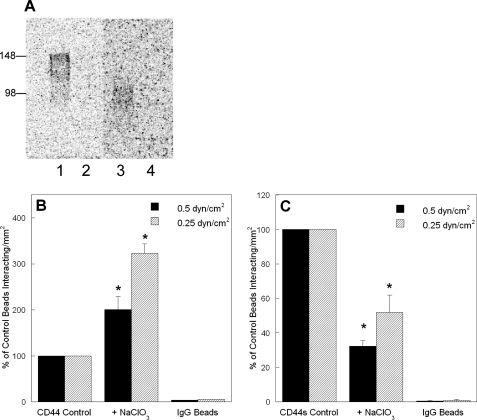
Sulfation of CD44 Differentially Modulates LS174T CD44 Versus HL60 CD44s Binding to Immobilized Fibrin under Flow—To determine the effect of CD44 sulfation on CD44-fibrin molecular recognition, LS174T or HL60 cells were cultured for 48 h at 37 °C in medium containing 60 or 20 mm sodium chlorate, respectively. These treatments completely block the incorporation of sulfate groups to LS174T CD44 and HL60 CD44s, as verified by autoradiography using Na35SO4 (Fig. 7A), without affecting cell viability. Blocking sulfation of CD44 from LS174T colon carcinoma cells resulted in a significant increase in its binding to immobilized fibrin under flow (Fig. 7B). In distinct contrast, sulfate-free CD44s displayed a markedly reduced capacity to bind fibrin (Fig. 7C). The extent of inhibition by sodium chlorate in CD44s-fibrin binding was similar to that of β-xyloside treatment, suggesting that sulfate groups on glycosaminoglycans may be involved in CD44s-fibrin molecular recognition. In contrast, the increase in LS174T CD44-fibrin binding noted following treatment of LS174T cells with sodium chlorate might be attributed to an alternate mechanism by which removal of negatively charged sulfate groups from LS174T CD44 facilitates binding to negatively charged regions of the fibrin(ogen) protein. The precise mechanisms of the sulfate-dependent changes await further characterization of the type and degree of sulfation present on LS174T CD44 versus HL60 CD44s. Nevertheless, the contrasting effects of blocking sulfation further support our findings suggesting that distinct binding sites on LS174T CD44 versus HL60 CD44s mediate fibrin(ogen) binding.
FIGURE 7.
Effect of sulfation on the adhesion of CD44-coated microspheres to immobilized fibrin under flow. A, effectiveness of sodium chlorate treatment in inhibiting sulfation of CD44. CD44 immunoprecipitated from untreated (control) LS174T colon carcinoma cells (lane 1) or LS174T cells treated with 60 mm sodium chlorate (lane 2) both cultured in the presence of 20 μCi/ml Na35SO4 for 48 h was subjected to SDS-PAGE. CD44s immunoprecipitated from untreated (control) HL60 cells (lane 3) or HL60 cells treated with 20 mm sodium chlorate (lane 4) both cultured in the presence of 20 μCi/ml Na35SO4 for 48 h was subjected to SDS-PAGE. B, removal of sulfate groups increases LS174T CD44 binding to immobilized fibrin. CD44-coated microspheres were generated using CD44 immunopurified from LS174T colon carcinoma cells cultured in the presence or absence of 60 mm sodium chlorate for 48 h. C, inhibition of sulfation diminishes HL60 CD44s-fibrin binding under flow. CD44s-coated microspheres were generated using CD44s immunopurified from HL60 cells cultured in the presence or absence of 20 mm sodium chlorate for 48 h. In all experiments, CD44-coated microspheres (2 × 106/ml) were perfused over 1 mg/ml fibrin for 5 min at the specified wall shear stresses. Data are reported as percent of untreated control beads that interacted with immobilized fibrin and represent the mean ± S.E. of n = 3 experiments. *, p < 0.05 with respect to untreated control microspheres.
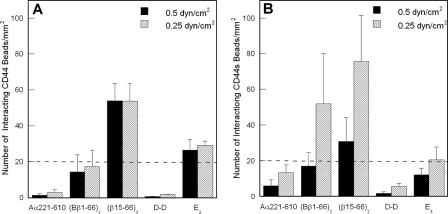
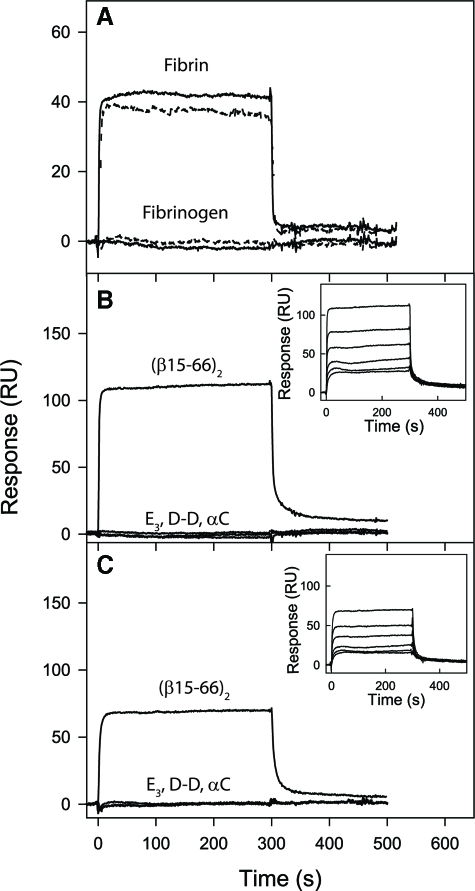
Localization of the CD44-binding Site on Fibrin(ogen)—To delineate the binding site for CD44 on fibrin(ogen), we studied the interaction of CD44 with various fibrin(ogen) fragments, which together cover practically the entire structure of the molecule, by two independent methods, cell-free flow-based adhesion assays and surface plasmon resonance (SPR). The fragments include fibrin-derived D-D dimer and E3, the recombinant Aα(221-610) αC-fragment mimicking the fibrin(ogen) D, E, and αC regions, respectively, and the recombinant disulfide-linked (Bβ1-66)2 dimer mimicking the dimeric arrangement of the BβN-domains in fibrinogen (14, 15, 38, 39). Additionally, (Bβ1-66)2 was treated with thrombin to remove fibrinopeptide B and generate a (β15-66)2 dimer mimicking the dimeric arrangement of these chains in fibrin. Using flow-based adhesion assays, we evaluated the adhesive capabilities of CD44-coated microspheres to the various immobilized fibrin(ogen) fragments. In these experiments, LS174T CD44- and HL60 CD44s-coated microspheres bound extensively and avidly to the (β15-66)2 fragment under flow (Fig. 8, A and B). In the case of LS174T CD44, none of the other fragments tested displayed a significant capacity to capture LS174T CD44-coated microspheres in shear flow (Fig. 8A). In contrast, CD44s-coated microspheres also bound to the (Bβ1-66)2 dimer in a shear-dependent manner, although to a lesser extent than to (β15-66)2 (Fig. 8B). The shear-dependent adhesion of CD44s-coated beads to (Bβ1-66)2 is also qualitatively similar to that on immobilized fibrinogen (Fig. 1). Together, these findings indicate that the binding site for CD44 is located in the N-terminal portions of the fibrin(ogen) Bβ chain (residues 15-66) forming the βN-domains. They also suggest that the mechanism of CD44s-fibrin(ogen) binding is distinct from that of LS174T CD44-fibrin interaction.
FIGURE 8.
Analysis of CD44- versus CD44s-coated microsphere binding to immobilized fibrin(ogen) fragments under flow. A, LS174T CD44 effectively binds to the (β15-66)2 fibrin fragment. CD44-coated microspheres, generated using CD44 immunopurified from LS174T colon carcinoma cells, were perfused for 5 min over fibrin(ogen) fragments (100 μg/ml) immobilized onto polystyrene dishes at prescribed wall shear stresses. B, HL60 CD44s binds to the (β15-66)2 and (Bβ1-66)2 fibrin(ogen) fragments. CD44s-coated microspheres, generated using CD44s immunopurified from HL60 human myeloid cells, were perfused for 5 min over fibrin(ogen) fragments (100 μg/ml) immobilized onto polystyrene dishes at the specified wall shear stresses. A and B, data are reported as the number of interacting beads per mm2 and represent the mean ± S.E. of n = 3 experiments. The dashed lines in each graph represent base-line microsphere binding.
Surface Plasmon Resonance-detected Interaction of CD44 with Fibrin(ogen) and Its Fragments—To further characterize the interaction of CD44 with fibrin(ogen) and its fragments, immunopurified CD44 from LS174T colon carcinoma cells or HL60 myeloid cells was immobilized on a BIAcore sensor chip, and association/disassociation of injected proteins and fragments was monitored in real time using SPR. We first examined the binding of intact purified fibrinogen and GPRP-solubilized fibrin, both at 1 μm, to immobilized LS174T CD44 and HL60 CD44s. These experiments revealed a well expressed binding of solubilized fibrin, but not fibrinogen, to immobilized LS174T CD44 and HL60 CD44s (Fig. 9A). These data are in concert with the flow-based adhesion findings, which disclose that both LS174T CD44 and HL60 CD44s are fibrin receptors (Fig. 1). The lack of binding of soluble fibrinogen to CD44s in SPR (Fig. 9A) coupled with our observations showing CD44s-coated microsphere adhesion to immobilized fibrinogen (Fig. 1) suggests that a cryptic binding site is exposed upon immobilizing fibrinogen on the polystyrene surface, and that this site can mediate binding to the standard, but not variant, isoforms of CD44. As suggested from the results of Fig. 5B, this selective ability of CD44s, but not LS174T CD44, to bind to immobilized fibrinogen is possibly because of masking of the fibrinogen-binding site by terminal sialic acid residues on CD44 variant isoforms.
FIGURE 9.
Analysis of binding of soluble fibrin, fibrinogen, and their fragments to the immobilized LS174T CD44 and HL60 CD44s receptors by surface plasmon resonance. A, fibrinogen or fibrin solubilized with GPRP, both at 1 μm, were added to immobilized LS174T CD44 (solid curves) or HL60 CD44s (dashed curves), and their association/dissociation was monitored in real time while registering the resonance signal (response). The E3, D-D, and recombinant (β15-66)2 and αC fragments, all at 1 μm, were added to immobilized LS174T CD44 (B) or HL60 CD44s (C), and their association/dissociation was monitored in real time while registering the resonance signal (response). The curves for E3, D-D, and αC in B and C essentially coincide. The insets show dose-dependent binding of the (β15-66)2 fragment added at 25, 50, 100, 250, 500, and 1000 nm to LS174T CD44 (B) or HL60 CD44s (C). The determined Kd values are listed in Table 2. Note that GPRP-solubilized fibrin was monomeric at 1 μm but formed polymers at higher concentration precluding its dose-dependent binding study.
We next injected the same panel of fibrin(ogen) fragments used in the flow-based adhesion assays to quantify their binding capacity to immobilized LS174T CD44 and HL60 CD44s by SPR. Both LS174T CD44 (Fig. 9B) and CD44s (Fig. 9C) interacted strongly with (β15-66)2, but failed to interact with soluble D-D, E3, or αC fragments even when used at concentrations up to 10 μm. Studies of a dose-dependent binding of (β15-66)2 to LS174T CD44 (Fig. 9B, inset) and HL60 CD44s (Fig. 9C, inset) were conducted to determine dissociation equilibrium constants (Kd). The analysis of binding data, performed as described under “Experimental Procedures,” revealed that the (β15-66)2 fragment bound with high affinity to both LS174T CD44 (Kd = 97 nm) and HL60 CD44s (Kd = 243 nm) (Table 2). Our experiments also revealed that both standard and variant isoforms of CD44 interacted with the (Bβ1-66)2 fragment in a dose-dependent manner (not shown); however, these interactions occurred with much lower affinities (Table 2). Together, these data suggest that removal of fibrinopeptides B upon thrombin-mediated conversion of fibrinogen to fibrin is pivotal for the exposure of high affinity CD44-binding sites. The lack of interaction between immobilized LS174T CD44 or HL60 CD44s and soluble E3 fragment, which does not contain fibrinopeptides A and B but is also devoid of the (β15-54) region (46), suggests that CD44-binding site is likely localized in this region.
TABLE 2.
Dissociation constants (Kd) for the interaction of E3, (β-15-66)2, and (Bβ-1-66)2 with LS174T CD44 and HL60 CD44s obtained by SPR
Fibrinogen fragments were injected at different concentrations, and their association with immobilized LS174T CD44 and HL60 CD44s was monitored by the change in the SPR response. NB indicates no binding was detected at up to 10 μm of E3.
|
Dissociation constants (Kd)
|
|||
|---|---|---|---|
| E3 | (β15-66)2 | (Bβ1-66)2 | |
| nm | μm | ||
| CD44 | NB | 97 | 1.8 |
| CD44s | NB | 243 | 3.1 |
DISCUSSION
CD44 is a multifunctional protein involved in cell signaling, organization of the cortical actin cytoskeleton, and cell adhesion/migration by functioning as a ligand-binding receptor (1). These types of molecular action facilitate its involvement in a diverse array of pathophysiological processes ranging from hematopoiesis to wound healing and metastasis (1). The altered expression of CD44 via alternative splicing of up to 10 variable exons into the stem region confers metastatic potential in vivo (4, 47) and results in a poor prognosis (5). Most of the work in the cancer research area has focused on the interactions of CD44 with hyaluronic acid (1, 8) and more recently with selectins (23, 30, 31). Fibrin(ogen) has also been reported to facilitate blood-borne metastasis by mediating the sustained adhesion and survival of tumor cells in the high shear environment of target organs (16, 18). We recently reported that CD44 is a functional fibrin, but not fibrinogen, receptor on LS174T colon carcinoma cells (23). However it was not clear whether the fibrin binding activity of CD44 was specific to the variant isoforms expressed on LS174T colon carcinoma cells or shared by other metastatic cells as well as normal human cell types expressing the standard form of CD44. Moreover, prior work did not offer any insights into the mechanism of CD44-fibrin molecular recognition (23). By utilizing an integrated biophysical and biochemical approach, involving cell-free flow-based adhesion assays coupled to enzymatic and metabolic inhibition studies and surface plasmon resonance, we systematically characterized the biomolecular interaction of CD44 and fibrin(ogen).
By immunopurifying CD44 from LS174T and T84 metastatic colon carcinoma cells, which express primarily CD44 variant isoforms (30, 31), as well as CD44s from HL60 human myeloid cells, we show that both variant and standard isoforms of CD44 are capable of binding to immobilized fibrin with high affinity, as evidenced by the firmly adherent nature of the CD44-coated microsphere binding to fibrin substrates. This adhesion mode is in clear contrast to the transient tethering/rolling interactions involved in CD44-selectin molecular recognition (30, 31, 33). LS174T and T84 CD44 and HL60 CD44s can effectively mediate binding to immobilized fibrin in the low shear regime (up to 0.5 dyne/cm2), whereas base-line binding was detected at wall shear stresses ≥1 dyne/cm2, levels at which CD44-selectin binding can occur efficiently (30, 31, 33). Interestingly, we discovered that although microspheres coated with CD44 from colon carcinoma cells do not bind to fibrinogen substrates in shear flow, CD44s immunopurified from HL60 cells displayed a significant capacity to bind to immobilized fibrinogen in a shear-dependent manner. These findings suggest that the presence of the variant exons in the stem region of CD44 interferes with its ability to bind to a distinct site on immobilized fibrinogen.
To characterize the structural linkage-bearing fibrin(ogen)-binding determinants on CD44, we used a panel of glycoconjugate biosynthesis inhibitors and highly specific enzymes. These interventions disclosed that distinct molecular requirements mediate the binding of standard and variant isoforms of CD44 to immobilized fibrin under flow. More specifically, the fibrin binding determinants of LS174T CD44 are displayed on O- but not N-linked glycans, whereas this scenario is completely reversed for HL60 CD44s-fibrin(ogen) recognition. Strikingly, these contrasting findings are similar to those reported for CD44-selectin binding, where O-linked glycans are the primary structural elements that display selectin-binding sites on LS174T CD44 (30, 31), whereas CD44s-selectin binding has an absolute requirement for N-linked glycans (48). In contrast to CD44-selectin recognition, which is dependent on the presence of HECA-452-reactive epitopes displayed on O- or N-linked glycans (CD44v or CD44s, respectively), HECA-452 reactivity appears to negatively regulate LS174T CD44 binding to immobilized fibrinogen, but not fibrin, in shear flow. The lack of any effect of sialidase on CD44s-fibrin(ogen) interaction may be attributed to the rather limited HECA-452 reactivity on HL60 CD44s (30). These findings suggest that the terminal sialic acid epitopes, which are prevalent on CD44 variant isoforms, may mask the fibrinogen-binding site on CD44v. Cumulatively, these data suggest that the structural biology of CD44 is such that inclusion of variant regions and/or differential glycosylation patterns dramatically impact the selectin- and fibrin(ogen)-binding attributes. In contrast to CD44-selectin binding (31), both chondroitin and dermatan sulfate are involved in the molecular recognition of both the standard and variant isoforms of CD44 and fibrin. Although there is ample evidence in the literature indicating that CD44 variant isoforms, especially v3-v10, are covalently modified by chondroitin and dermatan sulfate chains, the presence of these GAGs on CD44s has been reported, among others, in epidermal melanocytes (49), murine L-fibroblast cells (50), and platelet-derived growth factor-stimulated human adult dermal fibroblasts (25). Mutation studies have further localized the site for chondroitin sulfate addition in CD44s specifically to serine 180 (50), a common region of the stem domain.
Use of the anti-CD44 function-blocking monoclonal antibody Hermes-1, which is known to interfere with the molecular recognition of hyaluronic acid (51) as well as soluble hyaluronic acid, enabled us to characterize the fibrin-binding site on CD44. The hyaluronan-binding motifs are found in the N-terminal domain of CD44, specifically amino acid residues 32-123, which are common to both standard and variant isoforms of CD44 (1). Interestingly, both Hermes-1 and soluble hyaluronic acid drastically diminished LS174T CD44-coated microsphere adhesion to immobilized fibrin under flow, even though they had no effect on the CD44s-fibrin(ogen) interaction. These results further support the existence of an alternate fibrin(ogen)-binding site on CD44s, which is not located in the hyaluronan-binding motif. Additionally, these data may offer an explanation for the selective interaction between CD44s, but not CD44v, and immobilized fibrinogen. We could speculate that the insertion of variant exons in the CD44 stem region may disrupt the three-dimensional structure of the fibrinogen-binding pocket on LS174T and T84 CD44.
To localize the CD44-binding site on fibrin(ogen), we immobilized on a polystyrene dish several fibrin(ogen) fragments, which together encompass practically the entire molecule. They include the D-D dimer and Aα(221-610) fragment corresponding to the fibrin(ogen) D and αC regions, respectively, the E3 and dimeric (Bβ1)-(15-66)2 fragments that together are tantamount to the remaining E region. We discovered that the (β15-66)2 dimer mimicking fibrin βN-domains supported pronounced adhesion of LS174T CD44- and HL60 CD44s-coated microsphere under flow. However, only CD44s bound, in a shear-dependent manner, to the immobilized (Bβ1-66)2 dimer, which contains fibrinopeptides B and mimics fibrinogen βN-domains. The absence of significant CD44 (and CD44s) binding to the E3, D-D, and αC fragments, which was also confirmed by SPR, eliminates the possibility of high affinity interactions between CD44 and aforementioned regions of fibrin(ogen). It is noteworthy that the E3 fragment, which does not contain fibrinopeptides A and B and is also devoid of the (β15-54) region (46), did not support CD44 binding. Taken together, these data suggest that the CD44-binding site on fibrin is localized in the (β15-66) regions forming fibrin βN-domains.
In contrast to the significant CD44s binding to immobilized fibrinogen detected in the cell-free flow-based adhesion assays, no binding was noted between soluble fibrinogen and immobilized CD44s or CD44 in SPR assays. These observations suggest that fibrinogen immobilization results in the exposure of a cryptic binding site capable of mediating CD44s but not CD44v binding. Support for this concept is also provided by our observations showing CD44s-coated, but not LS174T CD44-coated, microspheres interact efficiently with the (Bβ1-66)2 fragment, which mimics fibrinogen, at the low shear stress level of 0.25 dyne/cm2.
Although the binding constants of CD44-fibrin were not determined because of fibrin polymerization at higher concentrations, our SPR assays revealed high affinity binding between soluble (β15-66)2, which mimics fibrin, and immobilized LS174T CD44 (Kd = 97 nm) or CD44s (Kd = 243 nm). These Kd values are similar to the binding constants of fibrin for heparin (66 nm) (38), which appears to selectively bind to fibrin but not soluble fibrinogen.
Accumulating evidence suggests that CD44 is aberrantly expressed in many human tumors (1). In certain tumors, such as colorectal carcinomas, the expression of CD44 confers metastatic potential in vivo (4, 5, 47). Knocking down CD44 expression in colon carcinoma cells drastically reduces their metastatic capacity in mouse models (3). These observations coupled with the hyaluronan and selectin binding function of CD44 have led to the hypothesis that CD44-mediated tumor cell adhesion to P-selectin (31) and hyaluronan (1) are the dominant factors regulating metastasis. However, our data revealing the fibrin(ogen) binding function of CD44 bring another dimension to the aforementioned hypothesis, and offer a novel unifying perspective on the apparent metastatic potential associated with CD44 overexpression on colon carcinoma cells and the critical role of fibrin(ogen) in metastatic spread. Taken together with the well documented roles of CD44-selectin-mediated tumor cell tethering and CD44-hyaluronan-dependent cell transmigration, our findings that CD44 mediates firm adhesion to fibrin(ogen) substrates complement the cascade of events leading to tumor cell extravasation and establishment of secondary tumor foci. CD44-fibrin(ogen) binding may also facilitate blood-borne metastasis by mediating the sustained adhesion and survival of tumor cells in the high shear environment of target organs (16, 18). Our findings support further research into the possibility of fibrin(ogen) and CD44 as potential therapeutic targets to combat metastatic spread.
Acknowledgments
We thank Dr. Ronald L. Schnaar (The Johns Hopkins University School of Medicine) for helpful discussions and Meredith Bauman for help with autoradiography.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 CA101135 (NCI) (to K. K.) and by National Institute of Health Grant HL-56051 (to L. M.). This work was also supported by a predoctoral fellowship from the American Heart Association (to C. S. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: CD44s, CD44 standard isoform; CD44v, CD44 variant isoform; GPRP-NH2, Gly-Pro-Arg-Pro-amide; DPBS, Dulbecco's phosphate-buffered saline; mAb, monoclonal antibody; DMJ, 1-deoxymannojirimycin, hydrochloride; PNGase, peptide:N-glycosidase; BisTris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; PE, phycoerythrin; BSA, bovine serum albumin; SPR, surface plasmon resonance; GAG, glycosaminoglycan.
References
- 1.Ponta, H., Sherman, L., and Herrlich, P. A. (2003) Nat. Rev. Mol. Cell Biol. 4 33-45 [DOI] [PubMed] [Google Scholar]
- 2.Martin, T. A., Harrison, G., Mansel, R. E., and Jiang, W. G. (2003) Crit. Rev. Oncol. Hematol. 46 165-186 [DOI] [PubMed] [Google Scholar]
- 3.Harada, N., Mizoi, T., Kinouchi, M., Hoshi, K., Ishii, S., Shiiba, K., Sasaki, I., and Matsuno, S. (2001) Int. J. Cancer 91 67-75 [DOI] [PubMed] [Google Scholar]
- 4.Hofmann, M., Rudy, W., Zoller, M., Tolg, C., Ponta, H., Herrlich, P., and Gunthert, U. (1991) Cancer Res. 51 5292-5297 [PubMed] [Google Scholar]
- 5.Wielenga, V. J., Heider, K. H., Offerhaus, G. J., Adolf, G. R., van den Berg, F. M., Ponta, H., Herrlich, P., and Pals, S. T. (1993) Cancer Res. 53 4754-4756 [PubMed] [Google Scholar]
- 6.Kim, H., Yang, X. L., Rosada, C., Hamilton, S. R., and August, J. T. (1994) Arch. Biochem. Biophys. 310 504-507 [DOI] [PubMed] [Google Scholar]
- 7.Wielenga, V. J., Smits, R., Korinek, V., Smit, L., Kielman, M., Fodde, R., Clevers, H., and Pals, S. T. (1999) Am. J. Pathol. 154 515-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, H. R., Wheeler, M. A., Wilson, C. M., Iida, J., Eng, D., Simpson, M. A., McCarthy, J. B., and Bullard, K. M. (2004) Cancer Res. 64 4569-4576 [DOI] [PubMed] [Google Scholar]
- 9.Weisel, J. W. (2005) Adv. Protein Chem. 70 247-299 [DOI] [PubMed] [Google Scholar]
- 10.Madrazo, J., Brown, J. H., Litvinovich, S., Dominguez, R., Yakovlev, S., Medved, L., and Cohen, C. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 11967-11972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medved, L. V., Gorkun, O. V., and Privalov, P. L. (1983) FEBS Lett. 160 291-295 [DOI] [PubMed] [Google Scholar]
- 12.Privalov, P. L., and Medved, L. V. (1982) J. Mol. Biol. 159 665-683 [DOI] [PubMed] [Google Scholar]
- 13.Matsuka, Y. V., Medved, L. V., Migliorini, M. M., and Ingham, K. C. (1996) Biochemistry 35 5810-5816 [DOI] [PubMed] [Google Scholar]
- 14.Tsurupa, G., Tsonev, L., and Medved, L. (2002) Biochemistry 41 6449-6459 [DOI] [PubMed] [Google Scholar]
- 15.Gorlatov, S., and Medved, L. (2002) Biochemistry 41 4107-4116 [DOI] [PubMed] [Google Scholar]
- 16.Palumbo, J. S., Kombrinck, K. W., Drew, A. F., Grimes, T. S., Kiser, J. H., Degen, J. L., and Bugge, T. H. (2000) Blood 96 3302-3309 [PubMed] [Google Scholar]
- 17.Palumbo, J. S., Potter, J. M., Kaplan, L. S., Talmage, K., Jackson, D. G., and Degen, J. L. (2002) Cancer Res. 62 6966-6972 [PubMed] [Google Scholar]
- 18.Palumbo, J. S., Talmage, K. E., Massari, J. V., La Jeunesse, C. M., Flick, M. J., Kombrinck, K. W., Jirouskova, M., and Degen, J. L. (2005) Blood 105 178-185 [DOI] [PubMed] [Google Scholar]
- 19.Camerer, E., Qazi, A. A., Duong, D. N., Cornelissen, I., Advincula, R., and Coughlin, S. R. (2004) Blood 104 397-401 [DOI] [PubMed] [Google Scholar]
- 20.Wojtukiewicz, M. Z., Zacharski, L. R., Moritz, T. E., Hur, K., Edwards, R. L., and Rickles, F. R. (1992) Blood Coagul. Fibrinolysis 3 429-437 [PubMed] [Google Scholar]
- 21.Luzzatto, G., and Schafer, A. I. (1990) Semin. Oncol. 17 147-159 [PubMed] [Google Scholar]
- 22.Iversen, L. H., and Thorlacius-Ussing, O. (2003) Thromb. Haemostasis 89 726-734 [PubMed] [Google Scholar]
- 23.Alves, C. S., Burdick, M. M., Thomas, S. N., Pawar, P., and Konstantopoulos, K. (2008) Am. J. Physiol. 294 C9-7-C916 [DOI] [PubMed] [Google Scholar]
- 24.Svee, K., White, J., Vaillant, P., Jessurun, J., Roongta, U., Krumwiede, M., Johnson, D., and Henke, C. (1996) J. Clin. Investig. 98 1713-1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark, R. A., Lin, F., Greiling, D., An, J., and Couchman, J. R. (2004) J. Investig. Dermatol. 122 266-277 [DOI] [PubMed] [Google Scholar]
- 26.Henke, C. A., Roongta, U., Mickelson, D. J., Knutson, J. R., and McCarthy, J. B. (1996) J. Clin. Investig. 97 2541-2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burdick, M. M., and Konstantopoulos, K. (2004) Am. J. Physiol. 287 C539-C547 [DOI] [PubMed] [Google Scholar]
- 28.Jadhav, S., and Konstantopoulos, K. (2002) Am. J. Physiol. 283 C1133-C1143 [DOI] [PubMed] [Google Scholar]
- 29.Mannori, G., Crottet, P., Cecconi, O., Hanasaki, K., Aruffo, A., Nelson, R. M., Varki, A., and Bevilacqua, M. P. (1995) Cancer Res. 55 4425-4431 [PubMed] [Google Scholar]
- 30.Hanley, W. D., Napier, S. L., Burdick, M. M., Schnaar, R. L., Sackstein, R., and Konstantopoulos, K. (2006) FASEB J. 20 337-339 [DOI] [PubMed] [Google Scholar]
- 31.Napier, S. L., Healy, Z. R., Schnaar, R. L., and Konstantopoulos, K. (2007) J. Biol. Chem. 282 3433-3441 [DOI] [PubMed] [Google Scholar]
- 32.Thomas, S. N., Zhu, F., Schnaar, R. L., Alves, C. S., and Konstantopoulos, K. (2008) J. Biol. Chem. 283 15647-15655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanley, W. D., Burdick, M. M., Konstantopoulos, K., and Sackstein, R. (2005) Cancer Res. 65 5812-5817 [DOI] [PubMed] [Google Scholar]
- 34.Burdick, M. M., McCaffery, J. M., Kim, Y. S., Bochner, B. S., and Konstantopoulos, K. (2003) Am. J. Physiol. 284 C977-C987 [DOI] [PubMed] [Google Scholar]
- 35.Nader, H. B., Porcionatto, M. A., Tersariol, I. L., Pinhal, M. A., Oliveira, F. W., Moraes, C. T., and Dietrich, C. P. (1990) J. Biol. Chem. 265 16807-16813 [PubMed] [Google Scholar]
- 36.Wei, Z., Lyon, M., and Gallagher, J. T. (2005) J. Biol. Chem. 280 15742-15748 [DOI] [PubMed] [Google Scholar]
- 37.Plaas, A. H., West, L. A., and Midura, R. J. (2001) Glycobiology 11 779-790 [DOI] [PubMed] [Google Scholar]
- 38.Yakovlev, S., Gorlatov, S., Ingham, K., and Medved, L. (2003) Biochemistry 42 7709-7716 [DOI] [PubMed] [Google Scholar]
- 39.Yakovlev, S., Makogonenko, E., Kurochkina, N., Nieuwenhuizen, W., Ingham, K., and Medved, L. (2000) Biochemistry 39 15730-15741 [DOI] [PubMed] [Google Scholar]
- 40.McCarty, O. J., Mousa, S. A., Bray, P. F., and Konstantopoulos, K. (2000) Blood 96 1789-1797 [PubMed] [Google Scholar]
- 41.Schuck, P., and Minton, A. P. (1996) Trends Biochem. Sci. 21 458-460 [DOI] [PubMed] [Google Scholar]
- 42.Trimble, R. B., and Tarentino, A. L. (1991) J. Biol. Chem. 266 1646-1651 [PubMed] [Google Scholar]
- 43.Tarentino, A. L., Gomez, C. M., and Plummer, T. H., Jr. (1985) Biochemistry 24 4665-4671 [DOI] [PubMed] [Google Scholar]
- 44.Michelacci, Y. M., and Dietrich, C. P. (1975) Biochem. J. 151 121-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishii, S., Ford, R., Thomas, P., Nachman, A., Steele, G., Jr., and Jessup, J. M. (1993) Surg. Oncol. 2 255-264 [DOI] [PubMed] [Google Scholar]
- 46.Tsurupa, G., Yakovlev, S., Pechik, I., Lamanuzzi, L. B., Angles-Cano, E., and Medved, L. (2006) Biochemistry 45 10624-10632 [DOI] [PubMed] [Google Scholar]
- 47.Gunthert, U., Hofmann, M., Rudy, W., Reber, S., Zoller, M., Haussmann, I., Matzku, S., Wenzel, A., Ponta, H., and Herrlich, P. (1991) Cell 65 13-24 [DOI] [PubMed] [Google Scholar]
- 48.Dimitroff, C. J., Lee, J. Y., Fuhlbrigge, R. C., and Sackstein, R. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 13841-13846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbold, K. W., Zhou, J., Haggerty, J. G., and Milstone, L. M. (1996) J. Investig. Dermatol. 106 1230-1235 [DOI] [PubMed] [Google Scholar]
- 50.Ruffell, B., and Johnson, P. (2005) Biochem. Biophys. Res. Commun. 334 306-312 [DOI] [PubMed] [Google Scholar]
- 51.Skelton, T. P., Zeng, C., Nocks, A., and Stamenkovic, I. (1998) J. Cell Biol. 140 431-446 [DOI] [PMC free article] [PubMed] [Google Scholar]