Abstract
We have reported that expression of Sprouty 2 (Spry2) is necessary for tumor formation by HRasV12-transformed fibroblasts. We now report on the role of Spry2 in the inhibition of UV254 nm radiation-induced apoptosis in HRasV12-transformed human fibroblasts. Silencing Spry2 in this context resulted in increased apoptosis, associated with decreased Akt activation and decreased phosphorylation of HDM2 at Ser-166, which has been shown to stabilize HDM2. As a consequence, when cells with silenced Spry2 were UV-irradiated, they exhibited diminished levels of HDM2 and elevated levels of p53. In agreement with these findings, overexpression of Spry2 in the parental non-transformed fibroblasts led to increased Akt activation and to the stabilization of HDM2. It also led to diminished expression of p53 and decreased apoptosis following UV irradiation. Silencing Spry2 in HRas-transformed cells decreased Rac1 activation, but independent expression of Spry2 in the non-transformed parental cells had no effect on Rac1, suggesting a specific involvement in the activation of Rac1 by Ras. Silencing Spry2 in HRasV12-transformed cells resulted in diminished interaction between HRas and Tiam1, a Rac1-specific nucleotide exchange factor. Expression of constitutively active Rac1 in cells with silenced Spry2 partly reversed the effect of Spry2 down-regulation. Furthermore, loss of Spry2 expression in HRasV12-transformed cells augmented the cytotoxicity of the DNA-damaging, chemotherapeutic agent cisplatin, a process that was also reversed by active Rac1. Together, these data show that Spry2 inhibits apoptosis in response to DNA damage by regulating Akt, HDM2, and p53, by a process mediated partly by Rac1.
Ras is an important regulator of cellular proliferation and survival (1). In appropriate cellular contexts, oncogenic activation of Ras inhibits apoptosis in response to DNA damage caused by UV irradiation or by chemotherapeutic agents, such as cisplatin (2, 3). This effect is mediated by phosphatidyl-inositol-3-kinase (PI3K)3 and Rac1 (4). PI3K activates several effector proteins, including the serine/threonine kinase Akt, which controls survival proteins, such as the human homolog of the murine double mutant 2 (HDM2) (5–7). Rac1, a member of the Rho family of GTPases, plays an important role in the transformation of fibroblasts by Ras. Although Rac1 is mainly involved in the regulation of migration, adhesion, and cell division, a number of studies also implicate Rac1 in the regulation of apoptosis (8, 9).
Apoptosis in response to DNA damage is under the immediate control of HDM2 and p53 (10). Under physiological conditions, transcription factor p53 is maintained at a low level by the ubiquitin-protein isopeptide ligase (E3) HDM2, which ubiquitinates p53 and targets it for proteasomal degradation. p53 is activated in response to cellular stresses that induce DNA damage. Then, the ubiquitination of p53 by HDM2 is abolished, and p53 translocates to the nucleus, where it induces the transcriptional activation of genes that mediate cell cycle arrest, DNA repair, and apoptosis (11).
Sprouty (Spry) proteins have been characterized as repressors of receptor tyrosine kinase (RTK) signaling (12–15). Spry proteins inhibit growth factor-induced cellular differentiation, migration, and proliferation (16–21), and they act as tumor suppressors in a variety of cancer types (19, 20, 22, 23). The inhibitory function of Spry is directed at various levels of the RTK/Ras-mitogen activated protein kinase pathway (16, 24–26). In some cellular contexts, however, the Spry2 isoform potentiates epidermal growth factor receptor (EGFR) signaling (27–29). This results from the interaction of Spry2 with the E3 ubiquitin ligase c-Cbl and the endocytotic protein CIN85, which regulate receptor endocytosis and degradation (29, 30). We have found that Spry2 is necessary for tumor formation by Ras-transformed fibroblasts, and in this setting, Spry2 interacts with HRas and mediates a complex between HRas and c-Cbl, which sustains the level and signaling activity of EGFR (31). In recent studies, Spry2 is reported to positively or negatively regulate apoptosis and cellular survival pathways (32–34), but these functions have not been fully characterized, especially in response to DNA damage.
In the present study, we determined the role of Spry2 in the ability of Ras to inhibit UV-induced apoptosis. We found that down-regulation of Spry2 in HRasV12-transformed fibroblast cell strain PH3MT increased UV-induced apoptosis and that overexpression of Spry2 in the parental, infinite lifespan human fibroblast cell strain MSU1.1 inhibited UV-induced apoptosis. In accordance with these results, we found that Spry2 also inhibited the cytotoxic effects of cisplatin. These findings suggest that in our model system, Spry2 has an antiapoptotic function in response to DNA damage. Our data also show that this function of Spry2 is mediated by a pathway consisting of Akt, HDM2, and p53, and that in HRasV12-transformed cells, this pathway is recruited through Rac1.
EXPERIMENTAL PROCEDURES
Cells and Cell Culture—The derivation of the human fibroblast cell line MSU1.1 has been described (35, 36). The PH3MT cell strain was derived from tumors formed in athymic mice by the injection of MSU1.1 cells malignantly transformed by an overexpressed HRas oncogene (35). The cells were cultured as described previously (31).
Apoptosis Assay—Early apoptotic events were detected with annexin V-FITC (BD Biosciences) according to the manufacturer's recommendations. Briefly, cells were plated at a density of 200,000 cells/60-mm dish; 16 h later, they were irradiated with UV at a dose of 30–60 J/m2 (60–90 s) and incubated at 37 °C under normal conditions for 4–6 h. Then, the cells were collected, washed twice with annexin V binding buffer, and incubated with annexin V-FITC at room temperature for 15 min. The cells were also stained with propidium iodide to distinguish between live and dead cells. Annexin V-FITC-positive cells were determined by flow cytometry under standard conditions. Only the cells that stained positive for annexin V, and not those that were positive for both annexin V and propidium iodide, were considered apoptotic.
Western Blotting—This was performed as described previously (31). Antibodies against pp85, p85, HDM2, p53, and HRas were purchased from Santa Cruz (Santa Cruz, CA); Spry2 was from Calbiochem; pAkt and Akt were from Cell Signaling (Danver, MA); and Ku80 was from Serotec (Raleigh, NC). Ku80 protein expression was used as a loading control. For the quantification of the Western blots, we used the image intensity tool in Adobe PhotoShop to quantify the desired band. The intensity of each band was corrected for the number of pixels in the field.
Rac1 Activation—Whole cell lysates (2 mg) were pulled down with PAK-CRIB-conjugated beads from Cytoskeleton (Denver, CO) according to the manufacturer's instructions. The pulled-down fractions were immunoblotted with a Rac1-specific antibody to determine the level of active Rac1.
Immunoprecipitations—Whole cell lysates (250–500 μg) were precleared with an appropriate IgG antibody for 30 min and then incubated with an antibody specific to HRas for 2 h followed by incubation with protein-G overnight at 4 °C. The immunoprecipitated fraction was washed several times with lysis buffer and assayed by Western blotting.
Cisplatin Cytotoxicity Assay—This was performed with the CellTiter 96® AQueous non-radioactive cell proliferation assay by Promega (Madison, WI). The cells were plated at 10,000 cells/well in a 96-well plate and allowed to grow overnight, after which they were treated for 5 h with Cisplatin at 2, 5, 10, 20, 40, and 80 μm concentration. Cisplatin-containing media were removed; the cells were washed twice with phosphate-buffered saline and then allowed to grow for 24–48 h in medium with normal serum. To determine cellular proliferation, the cells were treated with trazolium and phenazine methosulfate, and then the optical density was measured at 490 nm (A490 nm), as indicated by the manufacturer.
Statistics—Statistical analysis was performed with the Student's t test. In the determination of the p value for the change in apoptosis, independent experiments were pooled together and analyzed as one data set. A paired, two-tailed distribution was used. Also, in this determination, we corrected for the change in baseline apoptotic levels. For the determination of the significance of change in cisplatin cytotoxicity, the p value was determined from a representative independent experiment. A paired, two-tailed distribution was used, and the values were corrected for baseline proliferation differences.
RESULTS
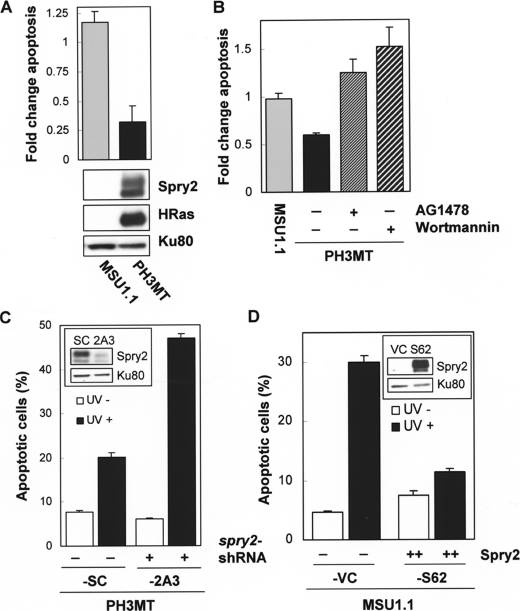
Effect of HRas Transformation in UV-induced Apoptosis—Ras protects immortalized fibroblasts from DNA damage-induced apoptosis (3). To study the role of Spry2 in this process, we used immortalized human fibroblasts (MSU1.1) and their HRas oncogene-transformed derivatives (PH3MT), which were generated in our laboratory (35, 36). To verify that the antiapoptotic function of Ras in response to UV irradiation could be reproduced in PH3MT cells, MSU1.1 and PH3MT cells in an early stage of UV-induced apoptosis were detected by using annexin V-FITC and flow cytometry. We found that the HRasV12-transformed cells exhibited a 5-fold desensitization to early apoptotic events (Fig. 1A, p < 0.05).
FIGURE 1.
Role of Spry2 on the inhibition of UV-induced apoptosis by HRas. A, normal parental non-transformed human fibroblasts (MSU1.1) and their HRas oncogene-transformed derivatives (PH3MT) were irradiated with UV (30 J/m2), incubated at 37 °C in media containing 10% serum for 4 h, and then assayed for early apoptotic events with annexin-FITC as indicated under “Experimental Procedures.” Cells that stained positive for annexin only were analyzed. The percentage of apoptotic cells following UV treatment was normalized to the percentage of cells undergoing apoptosis in the absence of treatment and is expressed in the graph as -fold change. A representative of three experiments is shown. A, MSU1.1 and PH3MT cells were treated and analyzed as described. A Western blot showing the level of Spry2 and HRas proteins is also shown. B, MSU1.1 and PH3MT cells were analyzed as described in the presence or absence of AG1478 (6 μm) and wortmannin (50 nm), as indicated. A representative of three experiments is shown. C, HRas-transformed cell lines with endogenous (PH3MT-SC) or down-regulated (PH3MT-2A3) levels of Spry2 (inset) were exposed to UV radiation (90 J/m2), and the percentage of cells undergoing apoptosis in the presence (black bars) or absence (white bars) of such treatment is shown. A representative of seven experiments is shown. D, the parental non-transformed human fibroblasts expressing an empty vector (MSU1.1-VC (VC)) or high levels of Spry2 (MSU1.1-S62 (S62)) (inset) were treated and analyzed as described. A representative of three experiments is shown.
We previously reported that HRas transformation resulted in elevated EGFR levels and that intact EGFR was necessary for the ability of HRas-transformed cells to form colonies in agarose (31). Also, PI3K activity is necessary for the activation of survival pathways downstream of Ras, as well as for the transformation of human cells by Ras oncogene (1). Therefore, we determined whether EGFR and PI3K were also necessary for the resistance of PH3MT cells to UV-induced apoptosis. To accomplish this, we measured UV-induced apoptosis in the presence or absence of AG1478, a selective EGFR inhibitor, and wortmannin, an inhibitor of PI3K. As shown in Fig. 1B, both inhibitors sensitized these cells to UV-induced apoptosis (p < 0.05), suggesting that the resistance of PH3MT cells to UV-induced apoptosis is dependent on intact EGFR and PI3K activities.
Effect of Spry2 in UV-induced Apoptosis in HRas-transformed and in the Parental Non-transformed Human Fibroblasts—To determine the role of Spry2 in UV-induced apoptosis, we compared Ras-transformed cells expressing a Spry2-specific short hairpin RNA (PH3MT-2A3) with cells expressing a scrambled short hairpin RNA (PH3MT-SC). The generation of these stable cell strains has been described (31). The level of Spry1 is unaffected by the Spry2 short hairpin RNA (31). Down-regulation of Spry2 resulted in an increase in the percentage of cells undergoing apoptosis in response to UV damage (Fig. 1C, closed bars, p < 0.02), suggesting that Spry2 expression contributes to the protective effects that Ras signaling has in response to UV irradiation. To increase the number of cells undergoing early apoptotic events, we increased the dose of UV treatment for the next two experiments. To evaluate whether Spry2 had an effect on apoptosis independent of UV treatment, we also show the percentage of cells undergoing apoptosis (Fig. 1C). Down-regulation of Spry2 had no effect on apoptosis when the cells were cultured under normal conditions (Fig. 1C, open bars, p > 0.1).
To determine the role of Spry2 in the absence of HRas oncogene expression, we compared control MSU1.1 cells (MSU1.1-VC) with MSU1.1 cells stably expressing high levels of Spry2 (MSU1.1-S62) and found that high expression of Spry2 inhibited apoptosis in response to UV irradiation (Fig. 1D, p < 0.04). Together, these findings suggest that Spry2 has an antiapoptotic function when expressed at high levels and is necessary for the ability of oncogenic Ras to protect fibroblasts from UV-induced apoptosis.
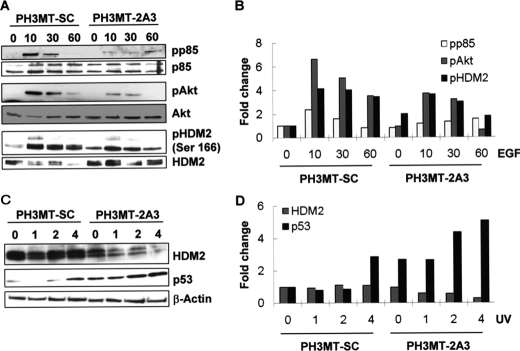
Effect of Spry2 in the Regulation of a Signaling Pathway Consisting of Akt, HDM2, and p53—We found that the ability of PH3MT cells to resist UV-induced apoptosis required intact PI3K, we hypothesized that Spry2 contributes to this resistance through PI3K signaling. The phosphorylation of its p85 subunit by RTKs, including EGFR, contributes to PI3K activation (37). Additionally, the level of phosphorylated (phospho-) p85 is proportional to the activation of PI3K (37). We chose to examine EGF-induced signaling because EGFR is necessary for the resistance of Ras-transformed fibroblasts to UV-induced apoptosis (Fig. 1B), and we found that in PH3MT cells, Spry2 sustains EGFR signaling (31). When we compared Spry2-down-regulated (PH3MT-2A3) and control (PH3MT-SC) Ras-transformed cells, we found that loss of Spry2 resulted in diminished levels of phospho-p85 in response to EGF stimulation (Fig. 2, A and B). The level of phospho-Akt was also decreased with down-regulation of Spry2 (Fig. 2, A and B). Akt phosphorylates HDM2 at Ser-166, which results in the stabilization of HDM2, and subsequently, in the degradation of its substrate, p53. We found that down-regulation of Spry2 in Ras-transformed cells led to a modest decrease in the level of Ser-166 phosphorylation, in response to EGF stimulation (Fig. 2, A and B).
FIGURE 2.
Effect of Spry2 down-regulation on the regulation of p85, Akt, HDM2, and p53. A, the indicated cell strains were deprived of serum for 12 h and then treated with EGF (100 ng/ml) for the indicated times (in min). Whole cell lysates (WCL) were prepared and assayed by Western blotting to determine the levels of the indicated proteins; pp85, phospho-85; pAkt, phospho-Akt; pHDM2, phospho-HDM2. B, bar graphs showing the quantification of the bands in the Western blot in panel A. C, the cell lines were exposed to UV radiation (90 J/m2) and allowed to grow under normal conditions for the indicated time periods (in hours) following UV treatment. WCL were prepared and then analyzed by Western blotting with the indicated antibodies. D, bar graphs showing the quantification of the bands in panel C.
Because HDM2 and p53 are critical regulators of the cellular response to UV-induced DNA damage, we next determined the levels of these proteins in response to UV irradiation. As expected, the down-regulation of Spry2 resulted in diminished levels of HDM2 and in increased levels of p53, both of which were observed even 1 h after UV irradiation (Fig. 2, B and D). These results suggest that in Ras-transformed cells, in the absence of DNA damage, Spry2 sustains the RTK-mediated activation of a survival pathway, consisting of PI3K, Akt, and HDM2, and through this pathway, Spry2 contributes to suppression of p53 levels in response to UV-induced DNA damage.
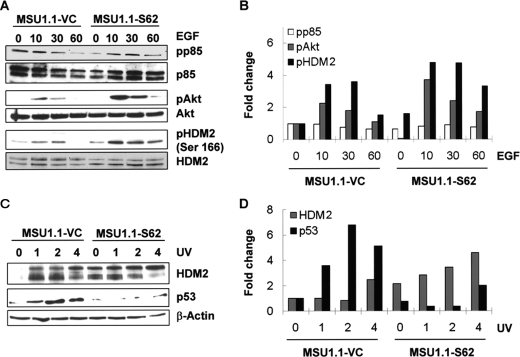
We also investigated the effect that independent expression of Spry2 in the parental fibroblasts (MSU1.1) had on this pathway. As shown in Fig. 3, A and B, expression of Spry2 in MSU1.1 resulted in elevated phospho-Akt and in elevated phospho-HDM2 levels, whereas the level of phospho-p85 remained unchanged. The unchanged level of phospho-p85 is not surprising given that the level of EGFR is unchanged by overexpression of Spry2 in MSU1.1 cells (31). Although these findings strengthen the proposed role of Spry2 in the regulation of Akt and HDM2, this effect appears to be mediated by a different mechanism in MSU1.1 cells, as compared with PH3MT cells.
FIGURE 3.
Effect of Spry2 overexpression on the activation of Akt, HDM2, and p53. A and B, a vector control cell strain, MSU1.1-VC, and a cell strain overexpressing Spry2, MSU1.1-S62, were treated with EGF and analyzed as in Fig. 2, A and B. pp85, phospho-85; pAkt, phospho-Akt; pHDM2, phospho-HDM2. C and D, the indicated cell strains were exposed to UV radiation as above and analyzed as in Fig. 2, C and D.
After exposure of the cells to UV radiation, expression of Spry2 resulted in an elevation of HDM2 (Fig. 3, B and D; 2 and 4 h; top band) as well as in a decrease in the level of p53 (Fig. 3, B and D). Because Spry2 was found to sustain a signaling pathway that promotes cellular survival through diminished p53 levels, it is plausible to conclude that the inhibition of UV-induced apoptosis by Spry2 is mediated by this mechanism.
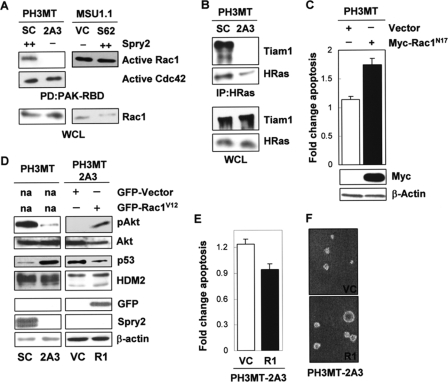
Effect of Spry2 in the Activation of Rac1 in HRas-transformed Cells—Intact Rac1 and Cdc42 activity are necessary for the malignant phenotype of PH3MT cells.4 To determine whether these were involved in antiapoptotic function of Spry2, we performed glutathione S-transferase pulldown assays with the PAK-CRIB-conjugated beads, which bind active Rac1 and Cdc42. We found that Ras-transformed cells with endogenous levels of Spry2 contained a higher level of active Rac1, as compared with cells with down-regulated Spry2 expression (Fig. 4A, left). The levels of active Cdc42 were not affected by silencing Spry2 (Fig. 4A, left). To determine whether Spry2 regulated Rac1 independently of Ras oncogene, we compared MSU1.1 cells expressing Spry2 with control MSU1.1 cells and found that expression of Spry2 did not have an effect on Rac1 activation in the absence of HRas oncogene transformation (Fig. 4A, right). This suggests that Spry2 is involved the specific regulation of Rac1 by Ras.
FIGURE 4.
Effect of Spry2 on Rac1 activation in HRas-transformed cells. A, WCL from the HRas-transformed cell strain expressing a high endogenous level of Spry2 (PH3MT-SC (SC)) or a reduced level of Spry2 (PH3MT-2A3 (2A3)) and the parental non-transformed human fibroblast cell strain expressing a low endogenous level of Spry2 (empty vector) (MSU1.1-VC (VC)) or expressing a high level of Spry2 (MSU1.1-S62 (S62)) were pulled down (PD) with PAK-CRIB-conjugated beads. The amount of Rac1 bound to the beads, as well as the Rac1 present in the WCL was determined. The amount of active Cdc42 was determined only in the PH3MT cells. B, WCL from PH3MT-SC and PH3MT-2A3 cell strains were immunoblotted or immunoprecipitated (IP) with an antibody specific to HRas and then immunoblotted to detect Tiam1 and HRas with the indicated antibodies. C, HRas-transformed fibroblasts (PH3MT) were stably transfected with an empty vector or a vector encoding a Myc-tagged, dominant negative form of Rac1 (Rac1N17). WCL from these stable clones were analyzed by Western blotting for Rac1 and β-actin expression. The same cell strains were treated and analyzed as in Fig. 1A. The average of two independent experiments is shown. D, HRas-transformed cells with down-regulated Spry2 (PH3MT-2A3) but stably expressing GFP-Rac1V12 (2A3-R1) or GFP alone (2A3-VC) were analyzed by Western blotting for pAkt; Akt; p53; HDM2; GFP; Spry2; or β-actin. E, the PH3MT-2A3 VC and PH3MT-2A3 R1 cell strains were UV-irradiated and analyzed as in Fig. 1A. The average of three independent experiments is shown. F, the indicated cell strains (5,000 cells/dish) were grown in agarose in a culture medium containing 2.5% serum for 3 weeks, as described in Ref. 31. Representative pictures of the colonies that formed in agarose are shown. R1, PH3MT-2A3-R1.
With this in mind, we focused our attention at the level of Ras-Tiam1 interaction. Tiam1, a Rac1-specific guanine nucleotide-releasing factor, interacts with Ras, leading to Rac1 activation (38). To determine the role of Spry2 in this interaction, we performed co-immunoprecipitation experiments and found that the amount of endogenous Tiam1 that co-immunoprecipitated with overexpressed HRas was reduced in cells with down-regulated Spry2, as compared with control cells (Fig. 4B). Together, these findings propose that in Ras-transformed cells, Spry2 contributes to the activation of Rac1 by Ras through Tiam1.
Rac1 has been shown to have a protective effect against UV-induced apoptosis. With this in mind, we determined whether Rac1 activity was necessary for the inhibition of UV-induced apoptosis in HRas-transformed cells (PH3MT). PH3MT cells expressing dominant negative Rac1 (Rac1N17, PH3MT-RC1) or vector alone (PH3MT-VC) were analyzed for induction of apoptosis as above. The cells expressing Rac1N17 displayed a modest increase in apoptosis as compared with the control cells (Fig. 4C, p < 0.03), suggesting that Rac1 plays a role in the resistance of Ras-transformed cells to UV-induced apoptosis.
To determine whether Rac1 mediates the effect of Spry2 in the activation of survival pathways and in the inhibition of apoptosis, we stably expressed the GFP-tagged, constitutively active form of Rac1 (Rac1V12) in HRas-transformed cells with down-regulated Spry2 (PH3MT-2A3-R1). This resulted in an increase in phospho-Akt and in a decrease in the level of p53 (Fig. 4D), reversing the effect caused by Spry2 down-regulation (Fig. 3D). The level of HDM2 was not altered when cells were grown under normal conditions.
We also determined whether expression of constitutively active Rac1 had an effect in apoptosis in response to UV treatment. As shown in Fig. 4E, a small decrease in UV-induced apoptosis was observed in the cells expressing Rac1V12 (p < 0.04). It should be noted that the GFP tag emits fluorescence in the same spectrum as does annexin V-FITC, which was used to measure early apoptotic events, and perhaps this is a reason why only a small change was observed. In the absence of treatment, there was no significant difference between GFP- and Rac1-expressing cells (p > 0.2). Adding further support, expression of RacV12 in cells with down-regulated Spry2 resulted in an increase in the size of colonies (Fig. 3F), partially reversing another phenotype caused by Spry2 down-regulation (31). Together, these data indicate that the effect of Spry2 on the activation of survival pathways and the evasion of apoptosis in Ras-transformed, PH3MT cells is mediated at least in part through Rac1.
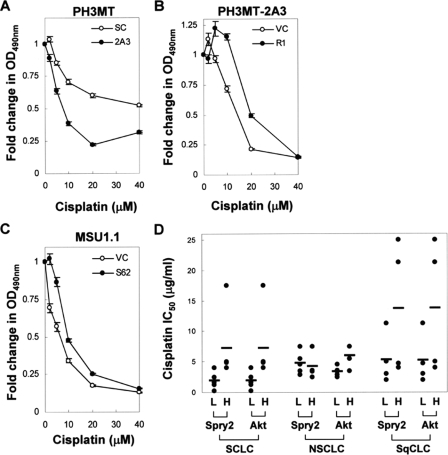
Effect of Spry2 in Cisplatin Cytotoxicity in HRas-transformed and in the Parental Non-transformed Human Fibroblasts—Cisplatin is a widely used chemotherapeutic agent that causes DNA damage, leading to cytotoxicity and decreased cellular proliferation. The finding that Spry2 inhibited apoptosis in response to UV-induced DNA damage led to the hypothesis that Spry2 reduces cisplatin cytotoxicity. To test this hypothesis, we used the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide non-radioactive proliferation assay, which has been used to assess cisplatin cytotoxicity. As shown in Fig. 5A, loss of Spry2 augmented cisplatin-induced cytotoxicity in Ras-transformed cells (p < 0.04). Similar to our previous findings, expression of Rac1 in the cells with down-regulated Spry2 diminished cisplatin cytotoxicity especially at doses less than 40 μm (Fig. 5B, p < 0.04). This adds further credence that Rac1 mediates in part the effect of Spry2 in the evasion of DNA damage-induced apoptosis in Ras-transformed cells. Furthermore, expression of Spry2 in the parental fibroblasts resulted in a decrease in the effect of cisplatin (Fig. 5C, p < 0.04). These findings show that Spry2 has the potential to decrease cisplatin cytotoxicity and is necessary for the ability of Ras-transformed fibroblasts to resist cisplatin cytotoxicity.
FIGURE 5.
Effect of Spry2 in cisplatin cytotoxicity. A–C, the PH3MT, PH3MT-2A3, and MSU1.1 cell strains were treated with cisplatin at a concentration of 0, 2, 5, 10, 20, and 40 μm concentrate for 6 h, allowed to grow under normal conditions for 24–48 h, and assayed for cisplatin cytotoxicity as described under “Experimental Procedures.” The number of cells is proportional to the optical density at 490 nm (A490 nm). The -fold decrease in A490 nm, relative to the untreated samples, is shown. For each panel, a representative of three independent experiments (in each n = 4) is shown. Where error bars are not shown, they are within the data point. D, a public data set from a previous study measuring genetic changes associated with cisplatin resistance in lung cancer was mined to determine the association between Spry2 expression and increased cisplatin IC50. Cell lines derived from patients with small cell (SCLC), squamous cell (SqCLC), or non-small cell (NSCLC) lung cancer were sorted according to their relative Spry2 expression. For each cell line, the expression of Spry2 and Akt is reported as a dichotomous variable, i.e. either high (H) or low (L), depending on whether the level of these genes in that cell line was greater or less than their median expression in the entire subset. Each circle represents a cell line, whereas the bar represents the average IC50 for that group. A similar analysis was performed for Akt expression.
A common problem with the use of cisplatin is the emergence of cisplatin resistance in tumor cells. Recently, Gemma et al. (39) performed an analysis of genetic changes associated with cisplatin resistance, as determined by the level of cisplatin IC50 in 19 cell lines derived from patients with small cell lung cancer, squamous cell lung cancer, or non-small cell lung cancer. We mined this public data set (National Center for Biotechnology Information (NCBI) Geo: GDS1688) to determine whether the expression Spry2 and Akt correlated with increased cisplatin IC50 (Fig. 4D). We found that in the small cell lung cancer subset, high Spry2 expression correlated with higher cisplatin IC50 (p < 0.04). In the squamous cell lung cancer subset, high expression of Spry2 was associated with higher cisplatin IC50, but this was not statistically significant (p < 0.1). In all three subsets, high Akt expression was associated with a higher cisplatin IC50 (p < 0.07).
DISCUSSION
In this study, we report the novel ability of Spry2 to inhibit apoptosis in response to UV irradiation. Spry2 expression was required if Ras-transformed cells were to evade apoptosis, and Spry2 was able to inhibit UV-induced apoptosis independently of Ras oncogene. Similarly, Spry2 inhibited the antiproliferative effect of the DNA-damaging chemotherapeutic agent cisplatin, an effect that was most pronounced in Ras-transformed fibroblasts. These data suggest that Spry2 provides an antiapoptotic signal in response to DNA-damaging agents and are consistent with a recent report showing that Spry2 inhibits the apoptotic effects of serum deprivation (32). Perhaps reflecting a context-specific role, Spry2 has also been shown to induce neuronal cell death in response to brain-derived neurotrophic factor stimulation (40) and to modulate cellular senescence in primary fibroblasts upon oncogene activation (41). In the latter setting, Spry2 participates in a global negative feedback mechanism that promotes senescence by inhibiting the Ras/PI3K pathway, which can impact the senescence machinery through HDM2 and FOXO, i.e. forkhead homeobox type O (41).
Without excluding alternative possibilities, the antiapoptotic function of Spry2 in our model system appears to be mediated by the Akt/HDM2/p53 pathway. In support of these findings, Edwin and Patel (32) similarly found that silencing Spry2 leads to diminished EGF-induced Akt activation and diminished activation of the Akt effector BAD, i.e. BCL2-associated agonist of cell death. Further support is provided by findings that independent activation of Akt, HDM2, or p53 prevents DNA damage-induced apoptosis and diminishes cisplatin cytotoxicity (42–44).
In Ras-transformed PH3MT cells, Spry2 regulates the Akt/HDM2/p53 pathway through Rac1 GTP-ase. Rac1 was necessary, not only for the inhibition of UV-induced apoptosis by Spry2 but also for the ability of Spry2 to diminish cisplatin cytotoxicity. It is important to note that Spry2 has been reported to inhibit Rac1 during wound healing, a property that is necessary for the inhibition of cellular migration by Spry2 (45). However, down-regulation of Spry2 in PH3MT cells enhanced stress fiber formation, a process that is consistent with loss of Rac1 activity (data not shown). In addition, Rac1 contributes to vascular endothelial growth factor and urokinase-type plasminogen activator secretion in PH3MT cells,4 as well as in other Ras-transformed cells (46, 47). Down-regulation of Spry2 in PH3MT cells resulted in a decrease in the amount of vascular endothelial growth factor and urokinase-type plasminogen activator secreted by these cells (data not shown). These findings also support the conclusion that Spry2 contributes to Rac1 activation in PH3MT cells.
Spry2 appears to regulate the specific activation of Rac1 by Ras. In the context of Ras transformation, this regulation is directed at the level of HRas-Tiam1 interaction, a step that is critical for the direct activation of Rac1 by Ras. Alternatively, Spry2 may contribute to the activation of Rac1 indirectly, through PI3K, which activates Rac1 in a process that is also mediated by Tiam1 (4).
The contribution of Spry2 to Akt activation may also be dependent on EGFR because Spry2 sustains EGFR in PH3MT cells (31), and EGFR enhances the activation of PI3K (48). Moreover, Edwin and Patel (32) found that Spry2 leads to Akt activation through c-Cbl and EGFR. Finally, active EGFR reduces UV-induced apoptosis, in a PI3K/Akt-dependent pathway (49, 50), findings that are also consistent with our conclusions.
Cancer treatment with radiation and chemotherapy relies strongly in the induction of apoptosis. Inactivation of proapoptotic pathways in cancer cells, as observed during the transformation of cells by Ras oncogene (2), compromises the efficacy of such treatments. Through its ability to sustain the activation of Ras effector pathways that mediate cellular survival, Spry2 appears crucial for the resistance of RasV12-transformed fibroblasts to DNA damage-induced apoptosis. Also, elevated expression of Spry2 appears to correlate with diminished cisplatin cytotoxicity, and therefore, Spry2 may contribute to the emergence of cisplatin resistance in certain cancer types, such as small cell lung cancer.
This work was supported, in whole or in part, by National Institutes of Health Grant CA098305 from the Department of Health and Human Services (to J. J. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PI3K, phosphatidylinositol 3-kinase; Akt, v-akt murine thymoma viral oncogene human homolog; Spry, Sprouty; RTK, receptor tyrosine kinase; EGF, epidermal growth factor; EGFR, EGF receptor; FITC, fluorescein isothiocyanate; PAK-CRIB, p21 activated kinase-Cdc42/Rac1 interaction domain; GFP, green fluorescent protein; WCL, whole cell lysates.
D. M. Appledorn, K-H. T. Dao, S. O'Reilly, V. M. Maher, and J. J. McCormick, manuscript in preparation.
References
- 1.Downward, J. (2003) Nat. Rev. Cancer 3 11–22 [DOI] [PubMed] [Google Scholar]
- 2.Messina, S., Leonetti, C., De Gregorio, G., Affatigato, V., Ragona, G., Frati, L., Zupi, G., Santoni, A., and Porcellini, A. (2004) Biochem. Biophys. Res. Commun. 320 493–500 [DOI] [PubMed] [Google Scholar]
- 3.Ries, S., Biederer, C., Woods, D., Shifman, O., Shirasawa, S., Sasazuki, T., McMahon, M., Oren, M., and McCormick, F. (2000) Cell 103 321–330 [DOI] [PubMed] [Google Scholar]
- 4.Campbell, S. L., Khosravi-Far, R., Rossman, K. L., Clark, G. J., and Der, C. J. (1998) Oncogene 17 1395–1413 [DOI] [PubMed] [Google Scholar]
- 5.Datta, S. R., Brunet, A., and Greenberg, M. E. (1999) Genes Dev. 13 2905–2927 [DOI] [PubMed] [Google Scholar]
- 6.Mayo, L. D., and Donner, D. B. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 11598–11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayo, L. D., Dixon, J. E., Durden, D. L., Tonks, N. K., and Donner, D. B. (2002) J. Biol. Chem. 277 5484–5489 [DOI] [PubMed] [Google Scholar]
- 8.Mayr, M., Hu, Y., Hainaut, H., and Xu, Q. (2002) FASEB J. 16 1423–1425 [DOI] [PubMed] [Google Scholar]
- 9.Eom, Y. W., Yoo, M. H., Woo, C. H., Hwang, K. C., Song, W. K., Yoo, Y. J., Chun, J. S., and Kim, J. H. (2001) Biochem. Biophys. Res. Commun. 285 825–829 [DOI] [PubMed] [Google Scholar]
- 10.Stiewe, T. (2007) Nat. Rev. Cancer 7 165–168 [DOI] [PubMed] [Google Scholar]
- 11.Vousden, K. H., and Lu, X. (2002) Nat. Rev. Cancer 2 594–604 [DOI] [PubMed] [Google Scholar]
- 12.Cabrita, M. A., and Christofori, G. (2008) Angiogenesis 11 53–62 [DOI] [PubMed] [Google Scholar]
- 13.Guy, G. R., Wong, E. S., Yusoff, P., Chandramouli, S., Lo, T. L., Lim, J., and Fong, C. W. (2003) J. Cell Sci. 116 3061–3068 [DOI] [PubMed] [Google Scholar]
- 14.Kim, H. J., and Bar-Sagi, D. (2004) Nat Rev Mol. Cell. Biol. 5 441–450 [DOI] [PubMed] [Google Scholar]
- 15.Mason, J. M., Morrison, D. J., Basson, M. A., and Licht, J. D. (2006) Trends Cell Biol. 16 45–54 [DOI] [PubMed] [Google Scholar]
- 16.Gross, I., Bassit, B., Benezra, M., and Licht, J. D. (2001) J. Biol. Chem. 276 46460–46468 [DOI] [PubMed] [Google Scholar]
- 17.Lee, C. C., Putnam, A. J., Miranti, C. K., Gustafson, M., Wang, L. M., Vande Woude, G. F., and Gao, C. F. (2004) Oncogene 23 5193–5202 [DOI] [PubMed] [Google Scholar]
- 18.Yigzaw, Y., Cartin, L., Pierre, S., Scholich, K., and Patel, T. B. (2001) J. Biol. Chem. 276 22742–22747 [DOI] [PubMed] [Google Scholar]
- 19.Sutterluty, H., Mayer, C. E., Setinek, U., Attems, J., Ovtcharov, S., Mikula, M., Mikulits, W., Micksche, M., and Berger, W. (2007) Mol. Cancer Res. 5 509–520 [DOI] [PubMed] [Google Scholar]
- 20.Shaw, A. T., Meissner, A., Dowdle, J. A., Crowley, D., Magendantz, M., Ouyang, C., Parisi, T., Rajagopal, J., Blank, L. J., Bronson, R. T., Stone, J. R., Tuveson, D. A., Jaenisch, R., and Jacks, T. (2007) Genes Dev. 21 694–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandramouli, S., Yu, C. Y., Yusoff, P., Lao, D. H., Leong, H. F., Mizuno, K., and Guy, G. R. (2008) J. Biol. Chem. 283 1679–1691 [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. A., Ho, C., Roy, R., Kosinski, C., Patil, M. A., Tward, A. D., Fridlyand, J., and Chen, X. (2007) Hepatology 47 1200–1210 [DOI] [PubMed] [Google Scholar]
- 23.Wang, J., Thompson, B., Ren, C., Ittmann, M., and Kwabi-Addo, B. (2006) Prostate 66 613–624 [DOI] [PubMed] [Google Scholar]
- 24.Hanafusa, H., Torii, S., Yasunaga, T., and Nishida, E. (2002) Nat Cell Biol. 4 850–858 [DOI] [PubMed] [Google Scholar]
- 25.Lee, M. (2006) Biochem. Biophys. Res. Commun. 350 450–456 [DOI] [PubMed] [Google Scholar]
- 26.Sasaki, A., Taketomi, T., Kato, R., Saeki, K., Nonami, A., Sasaki, M., Kuriyama, M., Saito, N., Shibuya, M., and Yoshimura, A. (2003) Nat. Cell Biol. 5 427–432 [DOI] [PubMed] [Google Scholar]
- 27.Rubin, C., Litvak, V., Medvedovsky, H., Zwang, Y., Lev, S., and Yarden, Y. (2003) Curr. Biol. 13 297–307 [DOI] [PubMed] [Google Scholar]
- 28.Wong, E. S., Fong, C. W., Lim, J., Yusoff, P., Low, B. C., Langdon, W. Y., and Guy, G. R. (2002) EMBO J. 21 4796–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong, E. S., Lim, J., Low, B. C., Chen, Q., and Guy, G. R. (2001) J. Biol. Chem. 276 5866–5875 [DOI] [PubMed] [Google Scholar]
- 30.Haglund, K., Schmidt, M. H., Wong, E. S., Guy, G. R., and Dikic, I. (2005) EMBO Rep. 6 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lito, P., Mets, B. D., Kleff, S., O'Reilly, S., Maher, V. M., and McCormick, J. J. (2008) J. Biol. Chem. 283 2002–2009 [DOI] [PubMed] [Google Scholar]
- 32.Edwin, F., and Patel, T. B. (2008) J. Biol. Chem. 283 3181–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwin, F., Singh, R., Endersby, R., Baker, S. J., and Patel, T. B. (2006) J. Biol. Chem. 281 4816–4822 [DOI] [PubMed] [Google Scholar]
- 34.de Alvaro, C., Martinez, N., Rojas, J. M., and Lorenzo, M. (2005) Mol. Biol. Cell 16 4454–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurlin, P. J., Maher, V. M., and McCormick, J. J. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan, T. L., Yang, D. J., Fry, D. G., Hurlin, P. J., Kohler, S. K., Maher, V. M., and McCormick, J. J. (1991) Exp. Cell Res. 197 125–136 [DOI] [PubMed] [Google Scholar]
- 37.Okkenhaug, K., and Vanhaesebroeck, B. (2001) Science's STKE 2001, PE1. [DOI] [PubMed]
- 38.Lambert, J. M., Lambert, Q. T., Reuther, G. W., Malliri, A., Siderovski, D. P., Sondek, J., Collard, J. G., and Der, C. J. (2002) Nat. Cell Biol. 4 621–625 [DOI] [PubMed] [Google Scholar]
- 39.Gemma, A., Li, C., Sugiyama, Y., Matsuda, K., Seike, Y., Kosaihira, S., Minegishi, Y., Noro, R., Nara, M., Seike, M., Yoshimura, A., Shionoya, A., Kawakami, A., Ogawa, N., Uesaka, H., and Kudoh, S. (2006) BMC Cancer 6 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross, I., Armant, O., Benosman, S., de Aguilar, J. L., Freund, J. N., Kedinger, M., Licht, J. D., Gaiddon, C., and Loeffler, J. P. (2007) Cell Death Differ. 14 1802–1812 [DOI] [PubMed] [Google Scholar]
- 41.Courtois-Cox, S., Genther Williams, S. M., Reczek, E. E., Johnson, B. W., McGillicuddy, L. T., Johannessen, C. M., Hollstein, P. E., MacCollin, M., and Cichowski, K. (2006) Cancer Cell 10 459–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, X., Fraser, M., Moll, U. M., Basak, A., and Tsang, B. K. (2006) Cancer Res. 66 3126–3136 [DOI] [PubMed] [Google Scholar]
- 43.Kondo, S., Barnett, G. H., Hara, H., Morimura, T., and Takeuchi, J. (1995) Oncogene 10 2001–2006 [PubMed] [Google Scholar]
- 44.Yip, H. T., Chopra, R., Chakrabarti, R., Veena, M. S., Ramamurthy, B., Srivatsan, E. S., and Wang, M. B. (2006) Arch. Otolaryngol. Head Neck Surg. 132 317–326 [DOI] [PubMed] [Google Scholar]
- 45.Poppleton, H. M., Edwin, F., Jaggar, L., Ray, R., Johnson, L. R., and Patel, T. B. (2004) Biochem. Biophys. Res. Commun. 323 98–103 [DOI] [PubMed] [Google Scholar]
- 46.Saniger, M. L., Oya, R., Macias, D., Dominguez, J. N., Aranega, A., and Luque, F. (2006) J. Cell. Biochem. 98 650–660 [DOI] [PubMed] [Google Scholar]
- 47.Han, Q., Leng, J., Bian, D., Mahanivong, C., Carpenter, K. A., Pan, Z. K., Han, J., and Huang, S. (2002) J. Biol. Chem. 277 48379–48385 [DOI] [PubMed] [Google Scholar]
- 48.Engelman, J. A., Janne, P. A., Mermel, C., Pearlberg, J., Mukohara, T., Fleet, C., Cichowski, K., Johnson, B. E., and Cantley, L. C. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 3788–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, H. Q., Quan, T., He, T., Franke, T. F., Voorhees, J. J., and Fisher, G. J. (2003) J. Biol. Chem. 278 45737–45745 [DOI] [PubMed] [Google Scholar]
- 50.Zhou, B. P., Liao, Y., Xia, W., Zou, Y., Spohn, B., and Hung, M. C. (2001) Nat. Cell Biol. 3 973–982 [DOI] [PubMed] [Google Scholar]