Abstract
Thrombospondin-1 regulates nitric oxide (NO) signaling in vascular cells via CD47. Because CD47 binding motifs are conserved in the C-terminal signature domains of all five thrombospondins and indirect evidence has implied CD47 interactions with other family members, we compared activities of recombinant signature domains of thrombospondin-1, -2, and -4 to interact with CD47 and modulate cGMP signaling. Signature domains of thrombospondin-2 and -4 were less active than that of thrombospondin-1 for inhibiting binding of radiolabeled signature domain of thrombospondin-1 or SIRPα (signal-regulatory protein) to cells expressing CD47. Consistent with this binding selectivity, the signature domain of thrombospondin-1 was more potent than those of thrombospondin-2 or -4 for inhibiting NO-stimulated cGMP synthesis in vascular smooth muscle cells and downstream effects on cell adhesion. In contrast to thrombospondin-1- and CD47-null cells, primary vascular cells from thrombospondin-2-null mice lack enhanced basal and NO-stimulated cGMP signaling. Effects of endogenous thrombospondin-2 on NO/cGMP signaling could be detected only in thrombospondin-1-null cells. Furthermore, tissue survival of ischemic injury and acute recovery of blood flow in thrombospondin-2-nulls resembles that of wild type mice. Therefore, thrombospondin-1 is the dominant regulator of NO/cGMP signaling via CD47, and its limiting role in acute ischemic injury responses is not shared by thrombospondin-2.
Nitric oxide (NO) is a major mediator of intracellular and paracellular signal transduction. NO preserves vascular health by minimizing the adhesion of inflammatory cells to the vessel wall, limiting platelet activation, and increasing blood vessel diameter and blood flow by relaxing vascular smooth muscle cells (VSMC).3 These actions of NO are mediated by activating soluble isoforms of guanylate cyclase (sGC) to increase cGMP levels, resulting in downstream activation of cGMP-dependent protein kinases and ion channels (1).
Physiological NO/cGMP signaling is limited by several phosphodiesterases that degrade cGMP and by thrombospondin-1 (TSP). TSP1 is a secreted protein that is produced by vascular and inflammatory cells that regulates cellular behavior by engaging several cell surface receptors. Recently we reported that TSP1 potently blocks NO-stimulated prosurvival responses in endothelial and VSMC (2, 3). TSP1 also plays a role in promoting platelet thrombus formation and hemostasis by antagonizing the antithrombotic activity of NO (4). In all of these vascular cells, picomolar concentrations of TSP1 are sufficient to block NO-stimulated fluxes in cGMP by engaging its receptor CD47 (5). Nanomolar concentrations of TSP1 further inhibit the same signaling pathway by inhibiting CD36-mediated uptake of myristate into vascular cells (6). In vivo, mice lacking TSP1 demonstrate elevated basal tissue cGMP levels and greater increases in regional blood flow in response to a NO challenge than wild type controls (4). After an ischemic insult, the absence of TSP1 or CD47 in transgenic mice is associated with better maintenance of tissue perfusion and enhanced tissue survival. Similarly, targeting TSP1 or CD47 using function blocking antibodies enhances ischemic tissue perfusion and survival in wild type mice and pigs (7, 8).
TSP1 belongs to a family of five secreted glycoproteins that share an evolutionarily conserved C-terminal signature domain (9). TSP1 and TSP2 form a distinct subfamily of trimeric proteins that exhibit similar anti-angiogenic activities for endothelial cells in vitro and activities in vivo to block tumor growth. Despite their similarities in structure, TSP1 and TSP2 have markedly different expression patterns after tissue injury, with TSP1 being immediately expressed and maximal at day 3, whereas TSP2 was not expressed until day 7 and was maximal 10 days after injury (10). In addition, large amounts of TSP1 but not TSP2 are stored in platelet α-granules and released into the wound environment. Polymorphisms in TSP1 and TSP2 have been linked to altered risk of premature myocardial infarction (11, 12). A 3′-untranslated region polymorphism in TSP2 is also associated with type 2 diabetes in men (13). The molecular basis for these associations is unclear.
Less is known about the roles of the pentameric TSP3–5 in vascular cells. TSP3 and TSP5 (also known as cartilage oligomeric matrix protein) appear to serve their primary functions in bone development (14, 15). However, a polymorphism in TSP4 is associated with premature myocardial infarcts in certain populations (11, 16, 17). A proatherogenic activity for the A387P variant of TSP4 was proposed based on its differential ability to modulate proliferation of endothelial and VSMC (18). Cardiovascular functions of TSP4 may also be linked to the high expression of TSP4 in heart (19) and its altered expression in that tissue during hypertensive heart failure (20).
The C-terminal domain of TSP1 is sufficient to mediate CD47-dependent inhibition of cGMP signaling (5). Of the two CD47 binding VVM motifs identified in this domain of TSP1, the first is conserved among all five TSPs, suggesting that CD47 binding could be a universal attribute of this family (21). Based on structural evidence that the VVM motifs may not be accessible (22, 23), however, conservation of VVM motifs may not be sufficient to predict CD47 binding. Uncertainty regarding the location of the CD47 binding site in the G domain of TSP1 therefore limits interpretation of the known sequence homology to predict CD47 binding to other TSP family members.
Although CD47 recognition of other TSPs has not been demonstrated experimentally, a local deficiency of inflammation-associated T cell apoptosis shared by TSP1-, CD47-, and TSP2-null mice is consistent with this hypothesis (24). Furthermore, a 21-residue peptide from the C-terminal domain of TSP4 was found to decrease human umbilical vein endothelial cell proliferation similar to the CD47 binding peptides from TSP1, although it lacks the VVM motif and no interaction with CD47 was demonstrated (25).
To directly address whether other TSP family members can inhibit NO responses and signaling in vascular cells, we now compare binding of recombinant signature domains of TSP1, TSP2, and TSP4 to cell surface CD47 and inhibition of NO-stimulated cell responses and cGMP signaling by these domains. We also compared acute tissue blood flow and perfusion responses to ischemic challenge in TSP1 and TSP2-null mice and cGMP responses in primary cultures of vascular cells isolated from these mice. These studies clearly demonstrate that CD47 selectively interacts with TSP1 and that the signature domains of TSP2 and TSP4 are less potent inhibitors of NO signaling in vascular cells in vitro. Furthermore, we show that the role of TSP1 to acutely limit recovery from ischemic injury in vivo is not shared by TSP2.
EXPERIMENTAL PROCEDURES
Cells and Reagents—Human aortic VSMC were obtained from Lonza (Switzerland) and maintained in the appropriate growth medium provided by the manufacturer. Wild type and CD47 negative (clone JinB8) Jurkat cells were obtained from Drs. Kevin Gardner and Eric Brown, respectively, and maintained in RPMI 1640 containing 10% fetal calf serum. The nitric oxide donors diethylamine/NONOate (DEA/NO) and diethylenetriamine/NONOate were provided kindly by Dr. Larry Keefer (NCI-Frederick, Maryland). Type I collagen was obtained from Inamed Biomaterials (Fremont, CA). TSP1 was prepared as previously described from fresh platelets obtained under an approved protocol from the transfusion service of the National Institutes of Health (26). Recombinant proteins derived from TSP4 were expressed in insect cells using baculovirus (27). Recombinant regions of TSP1 and TSP2 as summarized in Fig. 1A were prepared as described (28). Recombinant extracellular domain of human SIRPα including all three Ig domains fused to a modified human Fc domain was prepared as described (29). SIRPα and E123CaG1 were labeled using Na125I by the iodogen method as previously described for TSP1 (30). A CD47 monoclonal antibody (B6H12) was purified from conditioned medium of the corresponding hybridoma (American Type Culture Collection). An isotype-matched control IgG1 was obtained from Santa Cruz Biotechnology. TSP1 antibody A6.1 was obtained from Thermo Scientific (Cheshire, UK). An antisense morpholino oligonucleotide complementary to murine TSP2 mRNA (5′-GGCCAGTGCCCAGAGCATCTTGTCT-3′) and a 5-base mismatched control morpholino were obtained from Gene Tools (Philomath, OR).
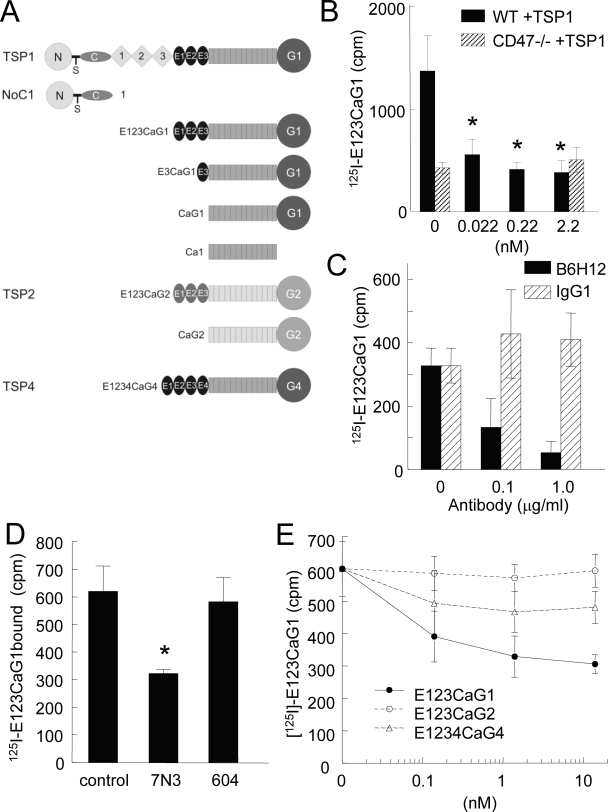
FIGURE 1.
Selective binding of the signature domain of TSP1 to CD47. A, schematic diagram of the signature domains of TSP1, TSP2, and TSP4 and other recombinant constructs used in these studies. B, wild type (WT) and JinB8 CD47-deficient (CD47-) Jurkat T cells (106 cells/tube) were treated with the indicated concentrations of TSP1 for 15 min then incubated with 125I-E123CaG1 (0.025 μg) at room temperature on a shaker for 1 h. After centrifugation through silicone oil to remove unbound ligand, the bound radioactivity was quantified and is presented with background subtracted; *, = p < 0.05. C and D, Jurkat T cells (106 cells/tube) were treated with CD47 antibody (B6H12) or an isotype-matched control IgG1 or with peptides 7N3 and 604 (1 μm), and 125I-E123CaG1 binding was quantified. E, wild type Jurkat cells were pretreated with the indicated concentrations of E123CaG1, E123CaG2, or E1234CaG4 for 15 min, and then binding of 125I-E123CaG1 was quantified.
Animals—C57BL/6 wild type and TSP1-null mice were housed in a pathogen-free environment with ad libitum access to water and rat chow on a 12-h light-dark cycle. TSP2-null male mice 12 weeks of age and matched wild type mice in a B6129sf1/J background were obtained from The Jackson Laboratory (Bar Harbor, ME). All animal studies conformed to the guidelines of the Animal Care and Use Committee of the National Cancer Institute of the NIH.
Primary Murine Vascular Cell Isolation—Using sterile technique, whole lungs were excised from 12-week-old wild type, TSP1, and TSP2-null male mice, minced into 1–2-mm fragments, and incubated in basal medium with collagenase type II (Worthington, MA). The cell suspension was then plated in standard culture flasks (Nunc) in the presence of endothelial cell growth medium (Lonza) and used at the first passage. Under such conditions staining for CD31 has demonstrated 85–95% of cells to be endothelial cells (2).
Cell Adhesion Assay—Human aortic VSMC were plated at 10,000 cells/well onto 96-well plates (Nunc Maxisorb) precoated with type I collagen (3 μg/ml). Cells were incubated for 1 h in basal medium with 0.1% BSA and no growth factors and the indicated concentrations of recombinant TSP2, -3, and -4 domains ± DEA/NO. Plates were then washed with PBS, fixed with glutaraldehyde, stained with crystal violet, and washed. Absorbed stain was solubilized with acetic acid from fixed cells and quantified using a Micro580 Elisa plate reader (Dynatech Laboratories, Alexandria, VA) at 450 nm.
Cell Proliferation Assay—Primary wild type and TSP2-null lung-derived endothelial cells were harvested as described (2) and plated at a density of 10,000 cells/well in standard endothelial cell growth medium ± NO (10 μm diethylenetriamine/NONOate) and incubated for 72 h at 37 °C and 5% CO2. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) reagent (Promega, Madison, WI) was then added, and the product was quantified at 450 nm.
Intracellular Cyclic GMP Measurement—Yucatan white hairless pig femoral artery VSMC (8) or wild type and TSP1- and TSP2-null murine endothelial cells (105 cells/well) grown overnight in 6- or 12-well culture plates (Nunc) were pretreated for 24 h with medium containing 2% fetal calf serum and weaned off serum over 48 h before treatment with DEA/NO with or without the indicated treatment agents in serum-free medium containing 0.1% BSA. Intracellular cGMP levels were determined using an enzyme immunoassay (Amersham Biosciences) as per the manufacturer's instructions.
Hindlimb Ischemia Model—Wild type and TSP2-null 12-week-old male mice underwent inhalation general anesthesia with 1.5% isoflurane. Using sterile techniques, the femoral artery at the level of the inguinal ligament was identified and under operative magnification ligated with a 5–0 nylon suture, and the skin incision was closed. Limb perfusion was then measured at 5-min intervals for the first hour postoperatively and at 72 h post-operatively via laser Doppler (Moor Instruments, Devon England). Also at 72 h post-operatively, vascular remodeling was assessed in the medial thigh musculature above and below the point of femoral artery ligation.
Laser Doppler Analysis—Dorsal cutaneous flap or hindlimb perfusion was determined in wild type and TSP2-null male mice under light general anesthesia via inhalation of 1.5% isoflurane. Core body temperature was constantly monitored and maintained at 35 °C. Pre-operative and post-operative perfusion flux levels were obtained at 5-min intervals with the following machine parameters: scan area, 1.6 × 2.5 cm; scan speed, 4 ms/pixel; scan time, 1 min 54 s; override distance, 25 cm. The override distance was 20 cm. In hindlimb studies the opposite unoperated limb served as an internal control.
Ischemic Soft Tissue Flap Model—Under inhalation isoflurane (1.5%), animals underwent creation of a random myocutaneous (McFarlane) flap as previously described (4). Flap viability was assessed 72 h post-operatively.
Determination of Flap Survival—Clinical assessment of flap perfusion was performed with notation of color, capillary refill, and bleeding to needle-stick being recorded. Flap dimensions were then traced onto a clear plastic sheet with demarcation of viable and non-viable areas made. Weights of segments of sheeting corresponding to viable versus non-viable portions of flaps were then determined, and % survival was expressed as a percentage versus total as previously described (4).
CD47 Binding Studies—Jurkat T lymphoma cells (1 × 106 cells/well) were suspended in PBS containing divalent cations and 0.1% BSA with the indicated treatment reagents for 30 min at 37 °C. 125I-SIRPα or 125I-E123CaG1 was then added, and the cells were incubated at room temperature on a plate shaker for 1 h. Cells were separated by centrifugation through silicone oil (Nye Co, New Bedford, MA), and cell-bound radioactivity was quantified using a PerkinElmer Life Sciences gamma counter. Background counts in the absence of cells were determined for each experiment and subtracted to determine net binding.
Detection of TSP2 Secretion—A heparin-BSA capture assay was used to detect TSP2 levels in the conditioned media. A 50-μl volume of heparin-BSA conjugate diluted to 100 ng/ml was adsorbed onto 96-well plate wells overnight at 4 °C. The next day the wells were blocked with 250 μl/well 5 mm Tris, 1% BSA, 0.02 mm phenylmethylsulfonyl fluoride, 150 mm NaCl, 1 mm CaCl2, pH 7.8, for 30 min. Samples were dispensed at 50 μl per well and allowed to bind to the immobilized heparin-BSA for 2 h at 37 °C. The wells were aspirated, and 50 μl of polyclonal anti-mouse TSP2 diluted 1:1000 in 50 mm Tris and 1% BSA was added to each well. The antibody was allowed to bind to the immobilized TSP2 for 2 h at 37 °C. After aspiration, the wells were washed 3 times with 50 μl per well of Dulbecco's phosphate-buffered saline with 0.02% BSA, 0.02 mm phenylmethylsulfonyl fluoride, and 0.05% Tween 20. Peroxidase-conjugated goat anti-rabbit IgG (Kirkegaard and Perry) was diluted to 1:1000 in Dulbecco's phosphate-buffered saline with 0.05% Tween 20 and allowed to bind for 1 h at room temperature. The wells were washed 3 times with Dulbecco's phosphate-buffered saline with 0.05% Tween 20. A 50-μl volume of o-phenylenediamine dihydrochloride was dissolved in phosphate-citrate buffer with sodium perborate (Sigma #P4922), and 30 μl of hydrogen peroxide was added to each well just before use. After 10 min, 3 m sulfuric acid was added to stop the color development. Absorbance values were read at 490 nm.
Statistics—Results of vascular cell in vitro data are presented as the mean ± S.D. of at least three experiments. Significance was calculated with Student's t test or, where appropriate, one-way and two-way analysis of variance using a standard software package (Origin) with p < 0.05. In vivo tissue survival and perfusion study results represent the mean ± S.D. of five pairs of wild type and TSP2-null animals.
RESULTS
Selective Binding of the Signature Domain of TSP1 to CD47-expressing Cells—Because others have failed to detect TSP1 binding to a recombinant extracellular domain of CD47 (22), we chose to examine TSP1 binding to native CD47 using a cell binding assay. TSP1 engages multiple cell surface receptors, and high affinity binding via the N domain to heparan sulfate proteoglycans is typically dominant in such cell binding studies (31). To detect CD47 binding, therefore, we used a recombinant signature domain of TSP1 that is known to mediate CD47-dependent signaling but lacks the high affinity heparin-binding site (Fig. 1A). The only other cell surface receptors known to interact with this domain are αvβ3 integrin, which is not expressed in Jurkat T lymphoma cells (32), and β1 integrins, which interact with low affinity with the E123 domain (33). In Jurkat cells, β1 integrin interaction with this domain of TSP1 requires activating signals that were not present in these studies. Jurkat cells were also chosen for the binding studies based on their well characterized CD47-mediated responses to TSP1 (32) and the availability of a somatic mutant of the Jurkat line lacking CD47 (JinB8) (24).
125I-Labeled E123CaG1 bound at much higher levels to wild type Jurkat cells than to CD47-negative JinB8 cells (Fig. 1B). Furthermore, binding to Jurkat cells expressing CD47 was inhibited to background levels in a dose-dependent manner by the function-blocking CD47 antibody B6H12 (Fig. 1C). An isotype-matched control IgG1 did not significantly inhibit binding of E123CaG1 at the same concentrations. Binding was also significantly inhibited by the TSP1-derived CD47 binding peptide 1102FIRVVMYEGKK1112 (7N3) at 1 μm (p = 0.02) but not by the respective control peptide FIRGGMYEGKK (604, Fig. 1D).
Specific binding of 125I-E123CaG1 to Jurkat cells was inhibited in the presence of 22 pm TSP1 (Fig. 1B), consistent with the known potency of TSP1 for inhibiting NO/cGMP signaling via CD47 (5). Unlabeled E123CaG1 was similarly active for self-inhibition of labeled ligand binding to Jurkat cells (Fig. 1E). In contrast, the corresponding signature domain of TSP2 (E123CaG2) was inactive over the same concentration range, and although the signature domain of TSP4 (E1234CaG4) produced consistent inhibition, the changes did not achieve significance (Fig. 1E). Therefore, high affinity interaction with CD47 appears to be relatively specific for the signature domain of TSP1.
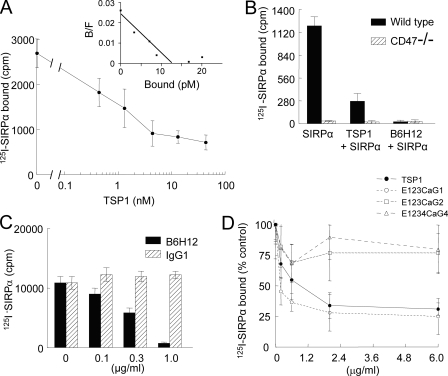
Selective Inhibition by TSP1 of SIRPα Binding to Cell Surface CD47—We were concerned that the relatively high non-displaceable fraction of E123CaG1 binding in Fig. 1 could reflect residual interactions with other TSP1 receptors. Therefore, competition with the well characterized CD47 ligand SIRPα (34) was used to further assess TSP1 binding to cell surface CD47. TSP1 potently and dose-dependently inhibited 125I-SIRPα binding to Jurkat cells (Fig. 2A). Analysis of the binding data using Scafit (Version 2.4 of Ligand software (35)) gave an apparent dissociation constant for TSP1 of 12 pm with a Bmax of ∼5000 sites/cell (Fig. 2A, inset). The latter value is consistent with the known copy numbers for CD47 on other cell lines (36). The apparent Kd is consistent with the known potency of TSP1 for inhibiting cGMP signaling, but we caution that this number is dependent on assumptions used in the analysis and does not represent an intrinsic dissociation constant.
FIGURE 2.
TSP1 and not TSP2 blocks SIRPα binding to CD47-bearing cells. A, inhibition of 125I-SIRPα binding to CD47-expressing Jurkat cells by increasing concentrations of TSP1 (0.2–20 μg/ml) was quantified as in Fig. 1. Inset, data were analyzed using Ligand software and are presented as a Scatchard plot. B/F, bound/free. B and C, wild type or CD47 negative Jurkat cells (106 cells/well) were incubated in PBS plus 0.1% BSA alone or in the presence of TSP1 (10 μg/ml) or the CD47 blocking antibody B6H12 (5 μg/ml) (B) or an isotype-matched control IgG1 antibody (C), and binding of 125I-SIRPα was quantified. D, 125I-SIRPα (0.25 μg/ml) binding to Jurkat cells was quantified in the presence of the indicated concentrations of TSP1, E123CaG1, E123CaG2, and E1234CaG4 and is presented normalized to 125I-SIRPα binding in the absence of inhibitors (±S.D., n = 3).
The requirement of CD47 for this interaction was confirmed by complete inhibition of 125I-SIRPα binding to Jurkat cells in the presence of the CD47 blocking antibody B6H12 and the absence of 125I-SIRPα binding to CD47-negative JinB8 cells (Fig. 2B). The dose dependence for B6H12 inhibition of SIRPα binding paralleled that for inhibiting E123CaG1 binding (compare Figs. 2C and 1C), and an isotype-matched control IgG1 did not significantly inhibit binding of SIRPα to wild type Jurkat cells (Fig. 2C).
E123CaG1 showed a similar dose dependence for inhibiting SIRPα binding to Jurkat cells as intact TSP1, but the corresponding signature domains of TSP2 (E123CaG2) and TSP4 (E1234CaG4) were essentially inactive (Fig. 2D). This confirmed the relative specificity of CD47 for TSP1 and also established that TSP1 binding to CD47 can prevent interaction with its counter-receptor SIRPα. This has been hypothesized to occur in smooth muscle cells based on reduced co-immunoprecipitation of SIRPα with CD47 in the presence of TSP1 (37), but here is the first direct proof that such inhibition occurs at the level of SIRPα-CD47 binding.
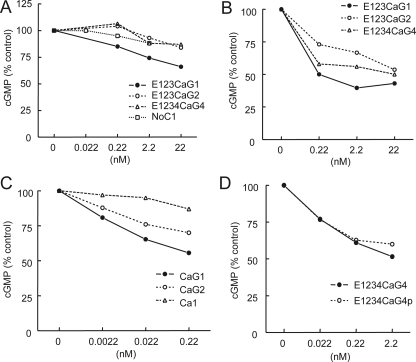
Differential Effects of TSPs on NO-driven cGMP in Porcine VSMC—As previously reported for human VSMC (5), pretreatment of porcine VSMC with the full-length signature domain of TSP1 (E123CaG1) before exposure to the rapidly releasing NO donor DEA/NO at 10 μm to activate sGC potently inhibited NO-stimulated cGMP accumulation in VSMC (Fig. 3A). The corresponding signature domains of TSP2 and TSP4 and a trimeric N-terminal region of TSP1 did not show significant inhibition under the same conditions (Fig. 3A). Decreasing the NO donor concentration to 1 μm, however, increased the sensitivity to inhibition by E123CaG1, and E123CaG2 and E1234CaG4 showed significant inhibition of cGMP levels under these conditions (Fig. 3B). The relative inhibitory potencies E123CaG1 > E1234CaG4 > E123CaG2 are consistent with their respective CD47 binding activities in Fig. 1E.
FIGURE 3.
Differential effects of TSPs on NO-driven cGMP in vascular cells. A, porcine VSMC were plated at 5 × 105 cells/well in growth medium, weaned from serum over 48 h, and treated in minimal medium containing 0.1% BSA with the indicated dosages of the indicated recombinant domains for 15 min. DEA/NO (10 μm) was then added. After 2.5 min the cells were extracted, and cGMP levels were determined by immunoassay. Results are presented as the mean of duplicate determinations normalized to NO-stimulated cGMP levels in VSMC and are representative of three or more independent experiments. B, C, and D, porcine VSMC were treated as in A using the indicated proteins before assessing NO-stimulated cGMP levels except that the concentration of DEA/NO was reduced to 1 μm. E1234CaG4p = A387P variant of the TSP4 signature domain.
A truncated construct CaG1 retained inhibitory activity, showing that the epidermal growth factor repeats of TSP1 are not required for activity (Fig. 3C). Consistent with the lesser activity of the full-length TSP2 signature domain, CaG2 retained activity but was less active than CaG1. However, the isolated calcium wire (Ca1) only marginally changed cGMP levels, consistent with our previous report that bacterially expressed recombinant G1 is sufficient to inhibit NO-stimulated cGMP synthesis (5).
Because a polymorphism in the signature domain of TSP4 is associated with premature coronary artery disease (16), we also compared signature domains containing the normal sequence and the A387P variant for inhibiting cGMP signaling in porcine VSMC (Fig. 3D). No significant difference in inhibitory activity was noted for this variant, indicating that both variants can signal through CD47.
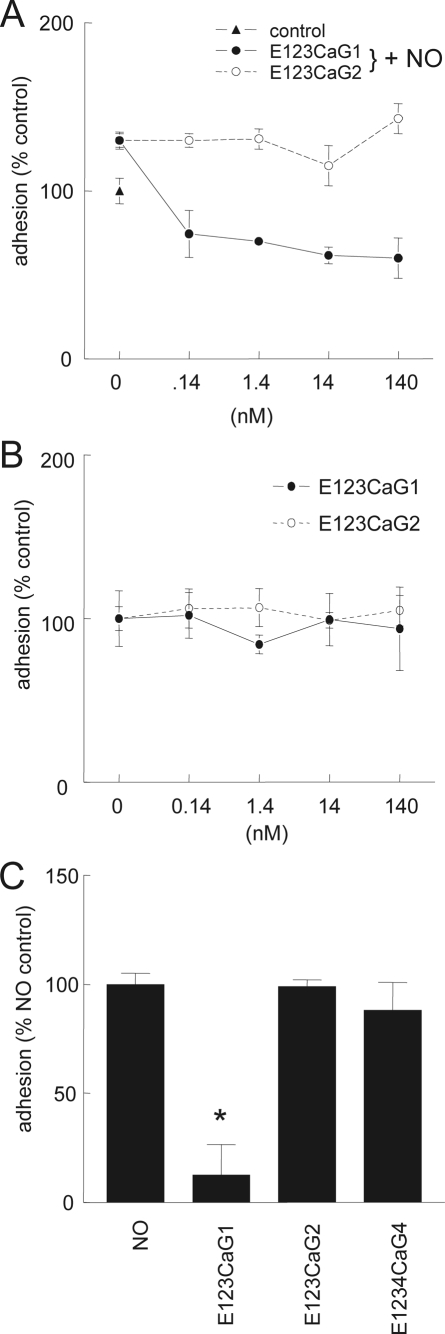
NO-stimulated VSMC Adhesion on Type I Collagen Is Selectively Blocked by the Signature Domain of TSP1—As previously reported (3), human aortic VSMC demonstrate enhanced adhesion to immobilized type I collagen in the presence of 10 μm DEA/NO, and the signature domain of TSP1 potently inhibits this stimulation (Fig. 4A). In the absence of NO, VSMC adhesion to collagen was not significantly altered by recombinant signature domains of either TSP1 or TSP2 (Fig. 4B). Similar to their effects on cGMP levels, signature domains of TSP2 and TSP4 did not significantly inhibit NO-stimulated adhesion at a concentration sufficient for the corresponding domain of TSP1 to maximally inhibit this response (Fig. 4C, p = 0.006).
FIGURE 4.
NO-stimulated vascular cell adhesion to collagen is selectively inhibited by the signature domain of TSP1. A and B, human aortic VSMC were pretreated for 15 min with the indicated TSP recombinant domains (0.022–22 nm) and then plated at 104 cells/well in minimal medium with 0.1%BSA onto wells that were coated with collagen (3 μg/ml) in the presence (A) or absence (B) of DEA/NO (10 μm). The cells were incubated for 1 h, and adhesion was determined colorimetrically after washing the wells. C, cells were incubated with the indicated signature domains (22 nm), and then adhesion on collagen was quantified in the presence of DEA/NO (10 μm). Results are presented as the mean ± S.D. of three or more experiments; *, p < 0.05.
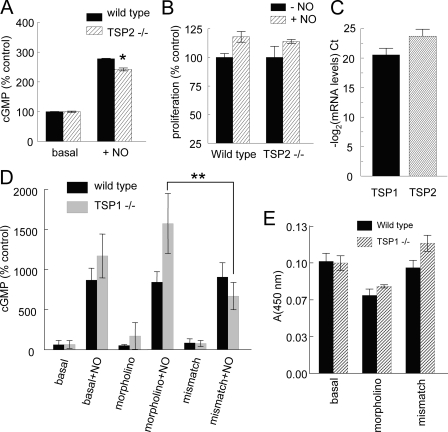
Endogenous TSP2 Does Not Limit NO-driven cGMP Flux in Vascular Cells—The absence of either TSP1 or its receptor CD47 was previously shown to elevate basal and NO-stimulated cGMP levels in primary murine vascular cells (2, 5). To assess whether endogenous TSP2 can significantly limit NO/cGMP signaling in vascular cells, wild type and TSP2-null lung-derived murine endothelial cells were treated with exogenous NO and cGMP levels assayed (Fig. 5A). In untreated cells no difference was seen between wild type and TSP2-null cell cGMP levels. After NO treatment, cGMP levels were moderately but consistently lower in TSP2-null endothelial cells (p < 0.05 in 3 independent experiments). NO/cGMP signaling also stimulates proliferation in these cells (2), but we observed no significant differences in basal or NO-stimulated proliferation between wild type and TSP2-null lung endothelial cells (Fig. 5B, p = 0.38).
FIGURE 5.
Endogenous TSP2 does not regulate NO-stimulated responses in vascular cells. A, wild type and TSP2-null lung-derived endothelial cells were plated at 5 × 105 cells/well in 12-well plates in growth medium, weaned from serum over 48 h, treated in minimal medium containing 0.1% BSA with DEA/NO (1 μm) for 2.5 min, and cGMP levels were determined. Results represent the mean ± S.D. of three separate experiments; *, = p < 0.05. B, wild type and TSP2-null lung-derived endothelial cells were plated at 1 × 104 cells/well in 96-well plates in growth medium ± diethylenetriamine/NONOate (10 μm) and incubated for 72 h, and proliferation was determined using a tetrazolium salt reduction assay. C, real time PCR analysis of TSP1 and TSP2 mRNA expression in primary lung endothelial cells from wild type mice. D, wild type and TSP1-null lung endothelial cells were plated on 6-well plates and grown to 90% confluence in standard growth medium, pretreated with a TSP2 morpholino or control (10 μm) for 48 h, and then stimulated in minimal medium with 10 μm DEA/NO for 2 min, and cGMP was measured. Results represent the mean ± S.D. Data from each type of vascular cells (wild type or TSP1-null) are presented normalized to basal cGMP in that cell type as 100%. **, p = 0.024 and 0.084 in two separate experiments. E, wild type and TSP1-null endothelial cells were plated on 6-well plates, grown to 90% confluence in growth medium, treated with a TSP2 targeting morpholino or mismatch control (10 μm) for 36 h, and the conditioned medium was assayed via a heparin-capture enzyme-linked immunosorbent assay using a murine polyclonal TSP2 antibody. Because no standard is available to quantify TSP2 concentrations, results are presented as absorbance, which is proportional to TSP1 concentration.
Although both of these results suggest that endogenous TSP2 expressed by vascular cells does not limit their sGC response to NO, it is possible that these cells do not express sufficient concentrations of TSP2 to limit this response. Quantitative real time RT-PCR confirmed expression of TSP2 mRNA in primary cultures of wild type lung endothelial cells, but the level was somewhat lower than that of TSP1 mRNA (Fig. 5C). Therefore, the endogenous TSP1 produced by these cells may mask any activity of the endogenous TSP2.
To more clearly define the ability of endogenous TSP2 to modulate cGMP signaling, we used primary TSP1-null vascular cells and employed an antisense morpholino to suppress TSP2 expression. In wild type primary cells, pretreatment with the morpholino had no significant effect on basal or NO-stimulated cGMP levels (Fig. 5D). However, pretreatment of primary TSP1-null endothelial cells with the TSP2 morpholino demonstrated increased intracellular cGMP after stimulation with exogenous NO compared with the response of cells treated with a 5-base mismatched control morpholino (Fig. 5D, p = 0.02 and 0.08 in 2 independent experiments). A heparin capture enzyme-linked immunosorbent assay confirmed significant suppression of TSP2 secretion by the morpholino-treated cells that was lacking in cells treated with the mismatched control morpholino (Fig. 5E). Because we lack purified native TSP2 as a standard, it was not possible to quantify the extent of suppression. These data establish that endogenous TSP2 can limit NO/cGMP signaling, but this activity is normally masked by the greater activity of endogenous TSP1.
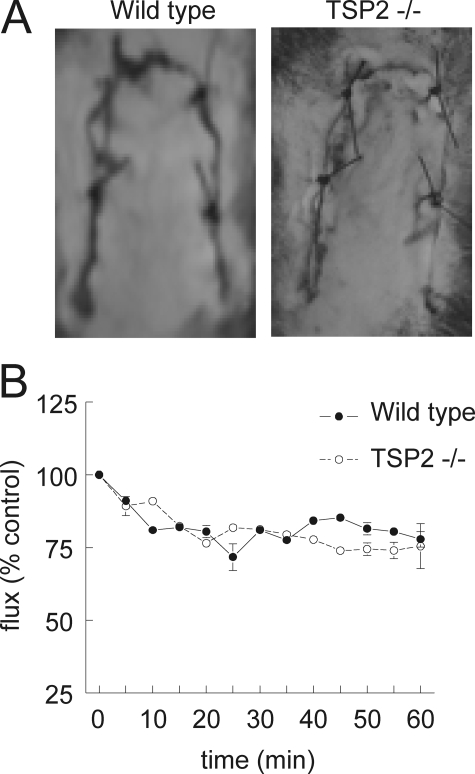
Ischemic Cutaneous Tissue Survival Is Not Limited by TSP2—Although TSP2 only weakly limited cGMP signaling in lung endothelial cells in vitro, we wanted to determine whether endogenous TSP2 has any effect in vivo under ischemic stress conditions. We previously reported that endogenous TSP1 and CD47 limit ischemic tissue survival in dorsal random skin flaps in a C57BL/6 background (4, 38). We found that wild type 129SvJ/BL6 are more resistant to the dorsal flap injury than C57BL/6 mice but observed no difference between wild type and TSP2-null animals assessed 72 h post-operatively (Fig. 6A). Furthermore, the rate of acute decrease in flow in the ischemic flaps as assessed in the first 60 min after surgery using laser Doppler imaging did not differ between TSP2-null mice and their wild type controls (Fig. 6B). Therefore, endogenous TSP2 does not acutely limit tissue perfusion under these conditions.
FIGURE 6.
TSP2 does not limit cutaneous tissue survival to ischemic challenge. A, wild type and TSP2-null 12-week-old male mice underwent random dorsal cutaneous flaps. Flap viability was determined 72 h post-operatively. Results are presented as the mean ± S.D. for five pairs of animals. B, laser Doppler analysis of cutaneous flap perfusion in wild type and TSP2-null flaps was performed during the first post-operative hour. Animals were maintained on 1.5% isoflurane and a core temperature of 35 °C.
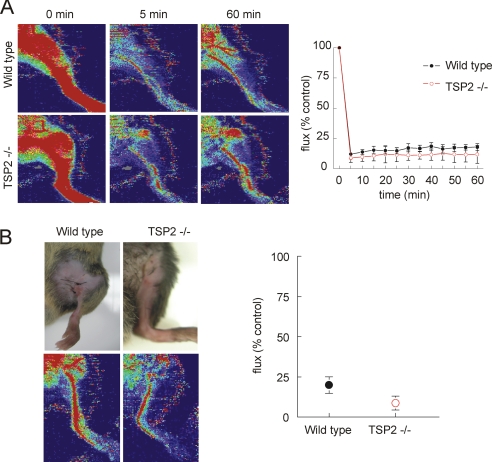
Hindlimb Perfusion under Ischemic Challenge Is Not Limited by TSP2—Because mice in this background appear to be relatively resistant to skin flap ischemia, we turned to a more severe fixed ischemic injury model using femoral artery ligation of the hindlimb. A similar although not identical hindlimb model was recently shown to have moderately improved long term restoration of flow in the TSP2-null (39). Wild type and TSP2-null 12-week-old male mice underwent ligation of the femoral artery. Laser Doppler analysis was then performed. During the first post-operative hour, both wild type and TSP2-null animals demonstrated profound decreases in hindlimb perfusion (Fig. 7A). Analysis of hindlimb perfusion 72 h post-operatively demonstrated similar persistence in the perfusion defect in wild type and TSP2-null mice (Fig. 7B), and clinical assessment indicated no acute advantage of the TSP2-null mouse in this ischemic injury model.
FIGURE 7.
TSP2 is not limiting for reperfusion after hindlimb arterial ligation. A, wild type and TSP2-null 12-week-old male mice underwent femoral artery ligation at the inguinal ligament. Hindlimb perfusion was assessed using laser Doppler imaging during the immediate post-operative interval. Representative images are shown on the left, and integrated Doppler signals from five pairs of animals are presented as the mean ± S.D. on the right panel. B, the mice were reanalyzed 72 h post-operatively.
DISCUSSION
The present results establish that the signature domain of TSP1 is a ligand for cell surface CD47 and demonstrate more avid recognition by cell surface CD47 of the signature domain of TSP1 versus those of TSP2 and TSP4. The signature domain of TSP1 binds with high affinity only to CD47-positive T cells, and its binding is inhibited by a known CD47 blocking antibody. This selectivity correlates with stronger inhibition of NO/cGMP signaling by the signature domain of TSP1 and suggests a more potent activity of TSP1 as opposed to TSP2 or TSP4 for blocking NO-stimulated vascular cell responses and tissue perfusion and survival under ischemic challenge. Despite the similar activities of TSP1 and TSP2 for inhibiting tumor growth and tumor-driven angiogenesis (40, 41), TSP1 more potently inhibits NO/cGMP signaling in vascular cells. Indeed, significant effects of endogenous TSP2 on NO/cGMP signaling in vascular cells can only be detected in TSP1-null cells. We have previously shown that TSP1 inhibits NO/cGMP signaling via CD36, which is also a TSP2 receptor (42), but only at nanomolar concentrations (6). Therefore, the selective interaction of TSP1 with CD47 rather than CD36 is critical for its potent inhibition of NO/cGMP signaling.
The differential activities of recombinant signature domains of TSP1 and TSP2 for binding to CD47 are reflected in the phenotypes of TSP1 and TSP2-null mice. Vascular cells and platelets lacking TSP1 demonstrate significant differences in both basal and NO-stimulated cGMP levels compared with cells that produce functional TSP1 (2, 3, 43). In contrast vascular cells obtained from TSP2-null animals do not demonstrate differences in cGMP accumulation under either basal or NO-stimulated conditions.
Although initial studies clearly identified CD47 as a receptor for two synthetic peptides derived from the signature domain of TSP1 (21), some recent publications have raised concerns about the relevance of such peptide binding to physiological TSP1 signaling through CD47. Independent studies have concluded that some signaling responses to such peptides can be CD47-independent (44). Although genetic evidence has established that CD47 is necessary for a number of signaling responses to TSP1 (5, 32, 44), direct binding of native TSP1 to this receptor has not previously been reported. Furthermore, surface plasmon resonance studies using a recombinant extracellular domain of CD47 that was verified to bind to its other known ligand SIRPα failed to detect TSP1 binding (22). This could be interpreted as evidence that TSP1 does not directly bind to CD47, but the recombinant CD47 domain used may simply exist in a conformation that selectively precludes TSP1 binding. Recent computational modeling also suggests that CD47 binding may require a conformation change in TSP1 (45). Our current data show that saturable binding of the signature domain of TSP1 to the surface of Jurkat cells requires CD47 and is inhibited by a function-blocking CD47 antibody. Furthermore, binding of SIRPα to CD47 on the same cells is potently inhibited by TSP1. Therefore, it is clear that platelet TSP1 exists in a conformation that can bind to CD47 and does so via its signature domain. We further established that the G module is required for this interaction.
The weaker activity of signature domains from TSP2 and TSP4 to compete with that of TSP1 for binding to CD47 or to inhibit SIRPα binding suggests that conservation of VVM motifs is not sufficient for high affinity binding of other TSPs to CD47. Notably, we found that the CD47-binding peptide 7N3 does compete with the signature domain of TSP1 for binding to cell surface CD47, and disrupting the VVM motif in the peptide abolishes this activity. Although computational modeling suggests that the VVM could become exposed in intact TSP1 (45), other residues in this active peptide are exposed in the published crystal structure and may play more important roles in CD47 binding. We propose that mutating the VVM motif in the peptide 7N3 changes the conformation of these adjacent residues to prevent its binding to CD47.
Defining the CD47 binding site in the signature domain of TSP1 still requires further study. One necessary step will be to solve the structure of native CD47. The existing recombinant domains of CD47 may not be suitable for this purpose based on their failure to replicate the binding of TSP1 to native CD47 on the cell surface (22). Interactions with the membrane spanning domain of CD47 may alter the conformation of the extracellular domain to permit TSP1 binding. Indeed a disulfide bond connects the IgV domain to a cell surface loop of the transmembrane domain of CD47 in its native structure (46). An additional possibility is that specific interactions of CD47 with other proteins on the cell surface such as integrins may be required to enable high affinity TSP1 binding.
In vivo, endogenous TSP1 continuously limits cGMP levels in vascular cells and thereby antagonizes NO to maintain blood pressure and platelet homeostasis. Consequently, TSP1-null animals demonstrate enhanced tissue perfusion and survival to various ischemic challenges compared with wild type animals due to enhanced NO/cGMP responses (4, 38, 43). In contrast, TSP2-null animals showed no tissue survival or perfusion advantage in the same ischemia models. TSP2 may play other roles in cardiovascular pathophysiology, but it does not play a major role in ischemic injury responses. Similarly, we recently showed that TSP2-null mice do not replicate the radioresistance phenotypes of TSP1- and CD47-null mice (47).
The weak activity of TSP2 to inhibit NO/cGMP signaling via CD47 may explain why TSP2-null animals respond so poorly to ischemic challenge. Strain-specific characteristics may account for some variation in responses to ischemia as the TSP1-null is in a C57Bl/6 background, whereas the TSP2-null is in a B6129sf1/J background. Nonetheless, despite the reported overall increase in vascular density in the TSP2-null basally and after wounding (48), these animals consistently showed no acute advantage in tissue perfusion and survival in two fixed ischemia models compared with their respective controls, and a modest long term advantage that was attributed to increased arteriogenesis and matrix remodeling in a third model employing hindlimb arteriectomy (39). In contrast, TSP1-null animals always show dramatic acute increases in tissue blood flow, perfusion, and survival compared with controls in ischemic stress models (4, 7, 38, 43).
The dominant activity of TSP1 in blocking NO-driven sGC activation is particularly important as it is by sGC activation through cGMP that NO stimulates dephosphorylation of myosin light chain 2 to relax VSMC. These findings underscore the priority role TSP1 plays in regulating NO-driven responses in vascular cells and ischemic tissues. We caution that our results to not preclude roles of TSP2 and TSP4 in modulating NO/cGMP signaling via CD47 in other disease states. In tissues that express high levels of these TSPs, their weaker interactions with CD47 may be sufficient to inhibit NO/cGMP signaling. For example, TSP4 is highly expressed in heart (19), so it may significantly engage CD47 in that tissue.
Acknowledgments
We thank Dr. Larry Keefer for providing the NO donors.
This work was supported, in whole or in part, by National Institutes of Health Grants National HL54462 (to D. F. M.) and HL54390 (to W. A. F.) and by the Intramural Research Program of the NCI, National Institutes of Health, Center for Cancer Research (to D. D. R.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: VSMC, vascular smooth muscle cell(s); DEA/NO, diethylamine/NONOate; sGC, soluble guanylate cyclase; SIRP, signal-regulatory protein; TSP, thrombospondin; BSA, bovine serum albumin; PBS, phosphate-buffered saline.
References
- 1.Ignarro, L. J. (2002) J. Physiol. Pharmacol. 53 503-514 [PubMed] [Google Scholar]
- 2.Isenberg, J. S., Ridnour, L. A., Perruccio, E. M., Espey, M. G., Wink, D. A., and Roberts, D. D. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 13141-13146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isenberg, J. S., Wink, D. A., and Roberts, D. D. (2006) Cardiovasc. Res. 71 785-793 [DOI] [PubMed] [Google Scholar]
- 4.Isenberg, J. S., Hyodo, F., Matsumoto, K., Romeo, M. J., Abu-Asab, M., Tsokos, M., Kuppusamy, P., Wink, D. A., Krishna, M. C., and Roberts, D. D. (2007) Blood 109 1945-1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isenberg, J. S., Ridnour, L. A., Dimitry, J., Frazier, W. A., Wink, D. A., and Roberts, D. D. (2006) J. Biol. Chem. 281 26069-26080 [DOI] [PubMed] [Google Scholar]
- 6.Isenberg, J. S., Jia, Y., Fukuyama, J., Switzer, C. H., Wink, D. A., and Roberts, D. D. (2007) J. Biol. Chem. 282 15404-15415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isenberg, J. S., Romeo, M. J., Abu-Asab, M., Tsokos, M., Oldenborg, A., Pappan, L., Wink, D. A., Frazier, W. A., and Roberts, D. D. (2007) Circ. Res. 100 712-720 [DOI] [PubMed] [Google Scholar]
- 8.Isenberg, J. S., Romeo, M. J., Maxhimer, J. B., Smedley, J., Frazier, W. A., and Roberts, D. D. (2008) Ann. Surg. 247 860-868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson, C. B., Lawler, J., and Mosher, D. F. (2008) Cell. Mol. Life Sci. 65 672-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agah, A., Kyriakides, T. R., Lawler, J., and Bornstein, P. (2002) Am. J. Pathol. 161 831-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topol, E. J., McCarthy, J., Gabriel, S., Moliterno, D. J., Rogers, W. J., Newby, L. K., Freedman, M., Metivier, J., Cannata, R., O'Donnell, C. J., Kottke-Marchant, K., Murugesan, G., Plow, E. F., Stenina, O., and Daley, G. Q. (2001) Circulation 104 2641-2644 [DOI] [PubMed] [Google Scholar]
- 12.Boekholdt, S. M., Trip, M. D., Peters, R. J., Engelen, M., Boer, J. M., Feskens, E. J., Zwinderman, A. H., Kastelein, J. J., and Reitsma, P. H. (2002) Arterioscler. Thromb. Vasc. Biol. 22 24-27 [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi, S., Yamada, Y., Matsuo, H., Segawa, T., Watanabe, S., Kato, K., Yokoi, K., Ichihara, S., Metoki, N., Yoshida, H., Satoh, K., and Nozawa, Y. (2007) Int. J. Mol. Med. 19 631-637 [PubMed] [Google Scholar]
- 14.Hankenson, K. D., Hormuzdi, S. G., Meganck, J. A., and Bornstein, P. (2005) Mol. Cell. Biol. 25 5599-5606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posey, K. L., Hayes, E., Haynes, R., and Hecht, J. T. (2004) Int. J. Biochem. Cell Biol. 36 1005-1012 [DOI] [PubMed] [Google Scholar]
- 16.Wessel, J., Topol, E. J., Ji, M., Meyer, J., and McCarthy, J. J. (2004) Am. Heart J. 147 905-909 [DOI] [PubMed] [Google Scholar]
- 17.Cui, J., Randell, E., Renouf, J., Sun, G., Green, R., Han, F. Y., and Xie, Y. G. (2006) Am. Heart J. 152 543.e1-543.e5 [DOI] [PubMed] [Google Scholar]
- 18.Stenina, O. I., Desai, S. Y., Krukovets, I., Kight, K., Janigro, D., Topol, E. J., and Plow, E. F. (2003) Circulation 108 1514-1519 [DOI] [PubMed] [Google Scholar]
- 19.Lawler, J., Duquette, M., Whittaker, C. A., Adams, J. C., McHenry, K., and DeSimone, D. W. (1993) J. Cell Biol. 120 1059-1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rysa, J., Leskinen, H., Ilves, M., and Ruskoaho, H. (2005) Hypertension 45 927-933 [DOI] [PubMed] [Google Scholar]
- 21.Gao, A. G., Lindberg, F. P., Finn, M. B., Blystone, S. D., Brown, E. J., and Frazier, W. A. (1996) J. Biol. Chem. 271 21-24 [DOI] [PubMed] [Google Scholar]
- 22.Adams, J. C., Bentley, A. A., Kvansakul, M., Hatherley, D., and Hohenester, E. (2008) J. Cell Sci. 121 784-795 [DOI] [PubMed] [Google Scholar]
- 23.Kvansakul, M., Adams, J. C., and Hohenester, E. (2004) EMBO J. 23 1223-1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ticchioni, M., Raimondi, V., Lamy, L., Wijdenes, J., Lindberg, F. P., Brown, E. J., and Bernard, A. (2001) FASEB J. 15 341-350 [DOI] [PubMed] [Google Scholar]
- 25.Congote, L. F., and Temmel, N. (2004) FEBS Lett. 576 343-347 [DOI] [PubMed] [Google Scholar]
- 26.Roberts, D. D., Cashel, J., and Guo, N. (1994) J. Tissue Cult. Methods 16 217-222 [Google Scholar]
- 27.Misenheimer, T. M., and Mosher, D. F. (2005) J. Biol. Chem. 280 41229-41235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annis, D. S., Gunderson, K. A., and Mosher, D. F. (2007) J. Biol. Chem. 282 27067-27075 [DOI] [PubMed] [Google Scholar]
- 29.Piccio, L., Vermi, W., Boles, K. S., Fuchs, A., Strader, C. A., Facchetti, F., Cella, M., and Colonna, M. (2005) Blood 105 2421-2427 [DOI] [PubMed] [Google Scholar]
- 30.Guo, N. H., Krutzsch, H. C., Negre, E., Vogel, T., Blake, D. A., and Roberts, D. D. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 3040-3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandrasekaran, S., Guo, N. H., Rodrigues, R. G., Kaiser, J., and Roberts, D. D. (1999) J. Biol. Chem. 274 11408-11416 [DOI] [PubMed] [Google Scholar]
- 32.Li, Z., Calzada, M. J., Sipes, J. M., Cashel, J. A., Krutzsch, H. C., Annis, D. S., Mosher, D. F., and Roberts, D. D. (2002) J. Cell Biol. 157 509-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calzada, M. J., Annis, D. S., Zeng, B., Marcinkiewicz, C., Banas, B., Lawler, J., Mosher, D. F., and Roberts, D. D. (2004) J. Biol. Chem. 279 41734-41743 [DOI] [PubMed] [Google Scholar]
- 34.Brown, E. J., and Frazier, W. A. (2001) Trends Cell Biol. 11 130-135 [DOI] [PubMed] [Google Scholar]
- 35.Munson, P. J., and Rodbard, D. (1980) Anal. Biochem. 107 220-239 [DOI] [PubMed] [Google Scholar]
- 36.Subramanian, S., Parthasarathy, R., Sen, S., Boder, E. T., and Discher, D. E. (2006) Blood 107 2548-2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maile, L. A., and Clemmons, D. R. (2003) Circ. Res. 93 925-931 [DOI] [PubMed] [Google Scholar]
- 38.Isenberg, J. S., Hyodo, F., Pappan, L. K., Abu-Asab, M., Tsokos, M., Krishna, M. C., Frazier, W. A., and Roberts, D. D. (2007) Arterioscler. Thromb. Vasc. Biol. 27 2582-2588 [DOI] [PubMed] [Google Scholar]
- 39.Krady, M. M., Zeng, J., Yu, J., MacLauchlan, S., Skokos, E. A., Tian, W., Bornstein, P., Sessa, W. C., and Kyriakides, T. R. (2008) Am. J. Pathol. 173 879-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Streit, M., Riccardi, L., Velasco, P., Brown, L. F., Hawighorst, T., Bornstein, P., and Detmar, M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 14888-14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawler, J., and Detmar, M. (2004) Int. J. Biochem. Cell Biol. 36 1038-1045 [DOI] [PubMed] [Google Scholar]
- 42.Simantov, R., Febbraio, M., and Silverstein, R. L. (2005) Matrix Biol. 24 27-34 [DOI] [PubMed] [Google Scholar]
- 43.Isenberg, J. S., Pappan, L. K., Romeo, M. J., Abu-Asab, M., Tsokos, M., Wink, D. A., Frazier, W. A., and Roberts, D. D. (2008) Ann. Surg. 247 180-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barazi, H. O., Li, Z., Cashel, J. A., Krutzsch, H. C., Annis, D. S., Mosher, D. F., and Roberts, D. D. (2002) J. Biol. Chem. 277 42859-42866 [DOI] [PubMed] [Google Scholar]
- 45.Floquet, N., Dedieu, S., Martiny, L., Dauchez, M., and Perahia, D. (2008) Arch. Biochem. Biophys. 478 103-109 [DOI] [PubMed] [Google Scholar]
- 46.Rebres, R. A., Vaz, L. E., Green, J. M., and Brown, E. J. (2001) J. Biol. Chem. 276 34607-34616 [DOI] [PubMed] [Google Scholar]
- 47.Isenberg, J. S., Maxhimer, J. B., Hyodo, F., Pendrak, M. L., Ridnour, L. A., DeGraff, W. G., Tsokos, M., Wink, D. A., and Roberts, D. D. (2008) Am. J. Pathol. 173 1100-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kyriakides, T. R., Zhu, Y. H., Smith, L. T., Bain, S. D., Yang, Z., Lin, M. T., Danielson, K. G., Iozzo, R. V., LaMarca, M., McKinney, C. E., Ginns, E. I., and Bornstein, P. (1998) J. Cell Biol. 140 419-430 [DOI] [PMC free article] [PubMed] [Google Scholar]