Abstract
Classic and novel protein kinase C (PKC) isozymes contain two zinc finger motifs, designated “C1a” and “C1b” domains, which constitute the recognition modules for the second messenger diacylglycerol (DAG) or the phorbol esters. However, the individual contributions of these tandem C1 domains to PKC function and, reciprocally, the influence of protein context on their function remain uncertain. In the present study, we prepared PKCδ constructs in which the individual C1a and C1b domains were deleted, swapped, or substituted for one another to explore these issues. As isolated fragments, both the δC1a and δC1b domains potently bound phorbol esters, but the binding of [3H]phorbol 12,13-dibutyrate ([3H]PDBu) by the δC1a domain depended much more on the presence of phosphatidylserine than did that of the δC1b domain. In intact PKCδ, the δC1b domain played the dominant role in [3H]PDBu binding, membrane translocation, and down-regulation. A contribution from the δC1a domain was nonetheless evident, as shown by retention of [3H]PDBu binding at reduced affinity, by increased [3H]PDBu affinity upon expression of a second δC1a domain substituting for the δC1b domain, and by loss of persistent plasma membrane translocation for PKCδ expressing only the δC1b domain, but its contribution was less than predicted from the activity of the isolated domain. Switching the position of the δC1b domain to the normal position of the δC1a domain (or vice versa) had no apparent effect on the response to phorbol esters, suggesting that the specific position of the C1 domain within PKCδ was not the primary determinant of its activity.
One of the essential steps for protein kinase C (PKC)2 activation is its translocation from the cytosol to the membranes. For conventional (α, βI, βII, and γ) and novel (δ, ε, η, and θ) PKCs, this translocation is driven by interaction with the lipophilic second messenger sn-1,2-diacylglycerol (DAG), generated from phosphatidylinositol 4,5-bisphosphate upon the activation of receptor-coupled phospholipase C or indirectly from phosphatidylcholine via phospholipase D (1). A pair of zinc finger structures in the regulatory domain of the PKCs, the “C1” domains, are responsible for the recognition of the DAG signal. The DAG-C1 domain-membrane interaction is coupled to a conformational change in PKC, both causing the release of the pseudosubstrate domain from the catalytic site to activate the enzyme and triggering the translocation to the membrane (2). By regulating access to substrates, PKC translocation complements the intrinsic enzymatic specificity of PKC to determine its substrate profile.
The C1 domain is a highly conserved cysteine-rich motif (∼50 amino acids), which was first identified in PKC as the interaction site for DAG or phorbol esters (3). It possesses a globular structure with a hydrophilic binding cleft at one end surrounded by hydrophobic residues. Binding of DAG or phorbol esters to the C1 domain caps the hydrophilic cleft and forms a continuous hydrophobic surface favoring the interaction or penetration of the C1 domain into the membrane (4). In addition to the novel and classic PKCs, six other families of proteins have also been identified, some of whose members possess DAG/phorbol ester-responsive C1 domains. These are the protein kinase D (5), the chimaerin (6), the munc-13 (7), the RasGRP (guanyl nucleotide exchange factors for Ras and Rap1) (8), the DAG kinase (9), and the recently characterized MRCK (myotonic dystrophy kinase-related Cdc42-binding kinase) families (10). Of these C1 domain-containing proteins, the PKCs have been studied most extensively and are important therapeutic targets (11). Among the drug candidates in clinical trials that target PKC, a number such as bryostatin 1 and PEP005 are directed at the C1 domains of PKC rather than at its catalytic site.
Both the classic and novel PKCs contain in their N-terminal regulatory region tandem C1 domains, C1a and C1b, which bind DAG/phorbol ester (12). Multiple studies have sought to define the respective roles of these two C1 domains in PKC regulation, but the issue remains unclear. Initial in vitro binding measurements with conventional PKCs suggested that 1 mol of phorbol ester bound per mole of PKC (13-15). On the other hand, Stubbs et al., using a fluorescent phorbol ester analog, reported that PKCα bound two ligands per PKC (16). Further, site-directed mutagenesis of the C1a and C1b domains of intact PKCα indicated that the C1a and C1b domains played equivalent roles for membrane translocation in response to phorbol 12-myristate 13-acetate (PMA) and (-)octylindolactam V (17). Likewise, deletion studies indicated that the C1a and C1b domains of PKCγ bound PDBu equally with high potency (3, 18). Using a functional assay with PKCα expression in yeast, Shieh et al. (19) deleted individual C1 domains and reported that C1a and C1b were both functional and equivalent upon stimulation by PMA, with either deletion causing a similar reduction in potency of response, whereas for mezerein the response depended essentially on the C1a domain, with much weaker response if only the C1b domain was present. Using isolated C1 domains, Irie et al. (20) suggested that the C1a domain of PKCα but not those of PKCβ or PKCγ bound [3H]PDBu preferentially; different ligands showed a generally similar pattern but with different extents of selectivity. Using synthesized dimeric bisphorbols, Newton's group reported (21) that, although both C1 domains of PKCβII are oriented for potential membrane interaction, only one C1 domain bound ligand in a physiological context.
In the case of novel PKCs, many studies have been performed on PKCδ to study the equivalency of the twin C1 domains. The P11G point mutation of the C1a domain, which caused a 300-fold loss of binding potency in the isolated domain (22), had little effect on the phorbol ester-dependent translocation of PKCδ in NIH3T3 cells, whereas the same mutation of the C1b caused a 20-fold shift in phorbol ester potency for inducing translocation, suggesting a major role of the C1b domain for phorbol ester binding (23). A secondary role for the C1a domain was suggested, however, because mutation in the C1a domain as well as the C1b domain caused a further 7-fold shift in potency. Using the same mutations in the C1a and C1b domains, Bögi et al. (24) found that the binding selectivity for the C1a and C1b domains of PKCδ appeared to be ligand-dependent. Whereas PMA and the indole alkaloids indolactam and octylindolactam were selectively dependent on the C1b domain, selectivity was not observed for mezerein, the 12-deoxyphorbol 13-monoesters prostratin and 12-deoxyphorbol 13-phenylacetate, and the macrocyclic lactone bryostatin 1 (24). In in vitro studies using isolated C1a and C1b domains of PKCδ, Cho's group (25) described that the two C1 domains had opposite affinities for DAG and phorbol ester; i.e. the C1a domain showed high affinity for DAG and the C1b domain showed high affinity for phorbol ester. No such difference in selectivity was observed by Irie et al. (20).
PKC has emerged as a promising therapeutic target both for cancer and for other conditions, such as diabetic retinopathy or macular degeneration (26-30). Kinase inhibitors represent one promising approach for targeting PKC, and enzastaurin, an inhibitor with moderate selectivity for PKCβ relative to other PKC isoforms (but still with activity on some other non-PKC kinases) is currently in multiple clinical trials. An alternative strategy for drug development has been to target the regulatory C1 domains of PKC. Strong proof of principle for this approach is provided by multiple natural products, e.g. bryostatin 1 and PEP005, which are likewise in clinical trials and which are directed at the C1 domains. A potential advantage of this approach is the lesser number of homologous targets, <30 DAG-sensitive C1 domains compared with over 500 kinases, as well as further opportunities for specificity provided by the diversity of lipid environments, which form a half-site for ligand binding to the C1 domain. Because different PKC isoforms may induce antagonistic activities, inhibition of one isoform may be functionally equivalent to activation of an antagonistic isoform (31).
Along with the benzolactams (20, 32), the DAG lactones have provided a powerful synthetic platform for manipulating ligand: C1 domain interactions (31). For example, the DAG lactone derivative 130C037 displayed marked selectivity among the recombinant C1a and C1b domains of PKCα and PKCδ as well as substantial selectivity for RasGRP relative to PKCα (33). Likewise, we have shown that a modified DAG lactone (dioxolanones) can afford an additional point of contact in ligand binding to the C1b domain of PKCδ (34). Such studies provide clear examples that ligand-C1 domain interactions can be manipulated to yield novel patterns of recognition. Further selectivity might be gained with bivalent compounds, exploiting the spacing and individual characteristics of the C1a and C1b domains (35). A better understanding of the differential roles of the two C1 domains in PKC regulation is critical for the rational development of such compounds. In this study, by molecularly manipulating the C1a or C1b domains in intact PKCδ, we find that both the C1a and C1b domains play important roles in PKCδ regulation. The C1b domain is predominant for ligand binding and for membrane translocation of the whole PKCδ molecule. The C1a domain of intact PKCδ plays only a secondary role in ligand binding but stabilizes the PKCδ molecule at the plasma membrane for downstream signaling. In addition, we show that the effect of the individual C1 domains of PKCδ does not critically depend on their position within the regulatory domain.
MATERIALS AND METHODS
Materials—[20-3H]Phorbol 12,13-dibutyrate ([3H]PDBu, 17 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA). PDBu and PMA were from LC Laboratories (Woburn, MA). Brain phosphatidylserine (PS) and egg phosphatidylcholine (PC) were purchased from Avanti Polar Lipids (Alabaster, AL). Reagents for purification of glutathione S-transferase (GST) fusion proteins were obtained from Pierce Biotechnology, Inc. (Rockford, IL). Cell culture medium and reagents were obtained from Invitrogen. The LB broth and agents used for bacteria culture were from KD Medical, Inc. (Columbia, MD). The DNA primers were obtained from Invitrogen. The mouse monoclonal anti-GFP antibody was purchased from Roche Applied Science (Indianapolis, IN). The mouse monoclonal anti-β-actin antibody and anti-mouse IgG were purchased from Bio-Rad (Hercules, CA).
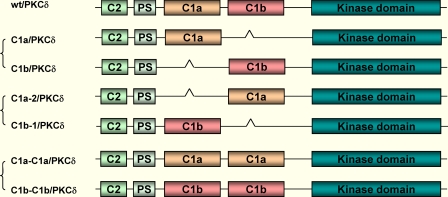
Molecular Engineering of the Wild-type PKCδ-GFP to Generate Different C1 Mutants—The murine cDNA of PKCδ was inserted into the pEGFP-N1 vector as described elsewhere (36). Three sets of mutations were performed on the plasmid DNA of PKCδ-GFP to generate different C1 mutants, as shown in the scheme of Fig. 1. Briefly, the GeneTailor™ Site-Directed Mutagenesis System (Invitrogen) was used to insert restriction sites into the PKCδ-GFP templates according to the manufacturer's protocol. The δC1a and δC1b fragments were derived from the pDNA of the wild-type PKCδ-GFP (wt/PKCδ-GFP) by PCR using the Platinum® Pfx DNA polymerase (Invitrogen). The single δC1a (C1a/PKCδ-GFP) and δC1b (C1b/PKCδ-GFP) mutants, the double δC1a (C1a-C1a/PKCδ-GFP) and δC1b (C1b-C1b/PKCδ-GFP) mutants, and the position-switched δC1a (C1a-2/PKCδ-GFP) and δC1b (C1b-1/PKCδ-GFP) mutants were generated as described in detail in the supplemental materials.
FIGURE 1.
Structures of the C1 mutants of intact PKCδ. Wild-type PKCδ was fused with GFP at the C terminus. The single δC1a or δC1b containing mutants (deleting one of the C1 domains), the position-switched C1 mutants (interchanging the positions of the δC1a and δC1b domains), and the double δC1a or δC1b mutant (replacing one of the C1 domains with the other) were prepared as described under “Materials and Methods.”
Expression and Imaging of the Fluorescent Protein-labeled PKCδ in Live Cells—LNCaP cells (obtained from ATCC, Manassas, VA) were cultured at 37 °C in RPMI 1640 medium containing 10% fetal bovine serum, penicillin (50 units/ml), and streptomycin (0.05 mg/ml) in a 5% CO2 humidified atmosphere. The plasmids of GFP-fused proteins were transfected into the cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The expression of the fluorescent protein was detected 24 h after transfection. Confocal fluorescent images were collected with a Zeiss MRC 1024 confocal scan head (Zeiss, Thornwood, NY) mounted on a Nikon microscope with a 60× planapochromat lens as described before (33).
Preparation of Cell Fractions and Western Blot Analysis of Membrane Translocation—CHO-K1 cells (obtained from ATCC, Manassas, VA) were seeded on 60-mm tissue culture dishes and transfected with PKCδ-GFP or the indicated C1 domain mutants using Lipofectamine 2000. The transfected cells were treated with the indicated drugs 24 h after transfection. After drug treatment, the cell monolayer was washed once with culture medium followed by washing once with ice-cold Dulbecco's phosphate-buffered saline (KD Medical, Inc., Columbia, MD). The cells were harvested with 400 μl of 20 mm Tris-HCl (pH 7.4) plus protease inhibitor mixture (Sigma). The cell suspension was then sonicated in an Eppendorf tube three times with a pulse of 6 s each time. 200 μl of the sonication mixture was transferred to a Beckman ultracentrifuge tube and centrifuged at 200,000 × g for 1 h in a Beckman ultracentrifuge to separate the cytosolic and membrane fractions as described before (33). Membrane translocation of PKCδ-GFP and the C1 mutants in response to ligand treatment was determined by Western blotting, and the reduction in the amount in the cytosolic fraction as a function of the concentration of the ligand was quantified.
Protein Purification by Immunoprecipitation—CHO-K1 cells were seeded on 60-mm tissue culture dishes. The plasmid DNA of PKCδ-GFP and the C1 mutants were transfected into the CHO-K1 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. 24 h after transfection, the cell monolayer was washed twice with ice-cold phosphate-buffered saline without Ca2+/Mg2+ and incubated with 400 μl of lysis buffer (1% Triton X-100 in phosphate-buffered saline plus protease inhibitors) on ice for 20 min. The cells were collected and centrifuged at 6000 rpm for 15 min at 4 °C. The supernatant was subjected to immunoprecipitation by addition of mouse monoclonal anti-GFP antibody (Roche Applied Science) and incubation at 4 °C for 3 h while being gently rotated. 30 μl of Protein A/G-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) was then added to the mixture, and incubation continued overnight at 4 °C. The beads were then washed twice with 1 ml of lysis buffer and once with 1 ml of 20 mm Tris-HCl (pH 7.5) plus 0.03% Triton X-100. Finally, the beads with the bound protein were suspended in 30 μl of 20 mm Tris-HCl plus 0.03% Triton X-100 for the following kinase and [3H]PDBu binding assays.
In Vitro Kinase Assays—The kinase activities of the purified PKCδ-GFP and the C1 mutants were analyzed using the Pep-Tag® Assay Kit for Non-Radioactive Detection of Protein Kinase C (Promega, Madison, WI) according to the manufacturer's instructions. Briefly, 5 μl of the above beads with the PKCδ-GFP proteins or C1 mutants was incubated with different concentrations of PDBu (or the PKC inhibitor GF109203X, EMD Chemicals, Gibbstown, NY), the specific peptide substrate, and the PKC reaction buffer including a PS:PC lipid mixture (200 μg/ml phosphatidylserine and 800 μg/ml phosphatidylcholine) for 30 min at 30 °C. The reaction was stopped by placing the tube in a 95 °C heating block for 10 min. The samples were then separated on a 0.8% agarose gel at 100 V for 7 min. Phosphorylated peptide migrated toward the anode (+), while nonphosphorylated peptide migrated toward the cathode (-). The intensity of the phosphorylated bands was quantified using ImageJ software (National Institutes of Health (NIH) Image).
[3H]PDBu Binding Assays—Scatchard analysis was performed in this study to determine the dissociation constant (Kd values) for [3H]PDBu binding to the purified wild-type PKCδ-GFP proteins and the C1 mutants as described elsewhere (22). Competitive binding assays were performed as described elsewhere (37) to determine the Ki values for some mutants with weak affinity for [3H]PDBu and to measure the binding affinities of mezerein and of 1,2-dioctanoylglycerol.
Western Blot Analysis of Down-regulation of PKCδ by PMA in NIH3T3 Cells—NIH3T3 cells (obtained from ATCC, Manassas, VA) were seeded on 6-well tissue culture dishes (Costar®, Corning Inc., New York, NY) at 37 °C in Dulbecco's modified Eagle's medium (ATCC, Manassas, VA) containing 10% fetal bovine serum, penicillin (50 units/ml), and streptomycin (0.05 mg/ml) in a 5% CO2 atmosphere. The plasmid DNAs of the wild-type PKCδ-GFP and the C1 mutants were transfected into the cells with Lipofectamine 2000. Levels of PKCδ-GFP and of the C1 mutants from PMA-treated NIH3T3 cells were determined by Western blotting. Briefly, 24 h after transfection, the cells were treated with PMA (0, 10, 100, 1,000, 3,000, and 10,000 nm) for 24 h at 37 °C. After treatment, cells were harvested and lysed by incubation in RIPA buffer (50 mm Tris (pH 7.5), 150 mm NaCl, 1% Triton X-100, 0.1% SDS, 10 mg/ml deoxycholic acid (sodium salt)) plus protease inhibitors for 30 min on ice followed by centrifugation. Supernatants were collected and subjected to SDS-PAGE on 4-20% polyacrylamide gels (Invitrogen) and then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). The mouse monoclonal anti-GFP antibody (Roche Applied Science) and anti-mouse IgG were used to detect the GFP-tagged PKCδ protein. The membrane was also blotted with the mouse monoclonal anti-β-actin antibody as a loading control. The immunostaining was finally visualized by ECL (Amersham Biosciences). Films were scanned and quantification was done using ImageJ software (NIH Image).
RESULTS
Phorbol Ester Binding of the Isolated C1a and C1b Domains of PKCδ
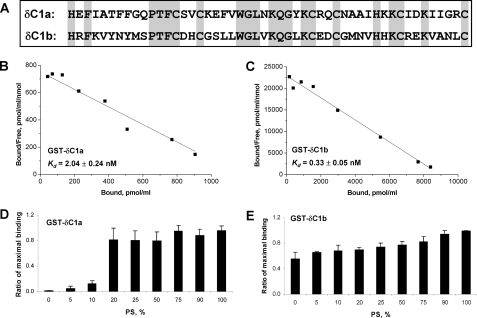
The twin C1 domains (namely C1a and C1b) of PKCδ have 44% sequence homology (Fig. 2A). They are separated in the regulatory region of PKCδ with a linker of 22 amino acids. Both of the C1 domains contain the same essential binding residues, which form a favorable tertiary structure for ligand insertion, implying they may bind ligands equally. A complication, however, is that the function of the C1 domains may be influenced by the context within which they reside in PKC. The relative importance of these two factors, C1 domain structure and context, remains unclear. In addition, efforts to define the respective roles of the C1a and C1b domains in ligand recognition and function within the intact PKC have yielded conflicting answers, reflecting the complexities of PKC regulation (21, 23, 25). These issues have great relevance to strategies for the design of therapeutics targeted to C1 domains (38).
FIGURE 2.
Characterization of the isolated C1a and C1b domains of PKCδ in vitro. A, comparison of the sequences of the δC1a and δC1b domains. B and C, Scatchard plots of [3H]PDBu binding to the purified and isolated δC1a and δC1b domains, respectively. The GST-tagged δC1a and δC1b domains were incubated with increasing concentrations of [3H]PDBu for 10 min at 18 °C in the presence of 100 μg/ml PS. Binding was measured using the polyethylene glycol precipitation assay as described under “Materials and Methods.” Points represent the mean ± S.E. of triplicate determinations in a single, representative experiment. Two additional experiments gave similar results. The Kd was expressed as mean ± S.E. (n = 3 independent experiments). D and E, Dependence of [3H]PDBu binding to the GST-tagged δC1a and δC1b domains on the composition of phospholipid in the assay. In these assays, instead of 100 μg/ml PS in the assay as in B and C, the assay contained 100 μg/ml of total phospholipid, comprising mixtures of PS:PC, where the values of weight % of PS in the PS:PC mixture are indicated. The specific binding of [3H]PDBu (2-5 nm) to the C1 domains in the presence of different ratios of PS:PC (0, 5, 10, 20, 25, 50, 75, 90, and 100% of PS) was measured and plotted as the percentage of the maximal binding in each assay. The bars in each diagram represent the average from three independent experiments. Error bars, ±S.E.
To shed further light on these questions, we first compared the potencies of the two C1 domains as isolated fragments for phorbol ester binding in vitro. The individual C1 domains, pulled out from full-length PKCδ by PCR, were tagged with GST and purified from BL-21 (DE3) Escherichia coli as described elsewhere (33). Scatchard assays were performed to measure the binding affinities (Kd values) of the individual δC1a and δC1b domains for [3H]PDBu. Assays were carried out in the presence of 100 μg/ml of phosphatidylserine, which is our usual assay condition. Both δC1a and δC1b showed high binding affinity for [3H]PDBu, with binding by the δC1a domain (Kd = 2.04 ± 0.24 nm) modestly weaker (6-fold) than that by the δC1b domain (Kd = 0.33 ± 0.05 nm) (Fig. 2, B and C).
In contrast to the limited difference in binding affinities, there was a marked difference in the PS requirement for [3H]PDBu binding by the C1a and C1b domains of PKCδ. For these experiments, [3H]PDBu binding was assayed at a fixed (100 μg/ml) concentration of total phospholipid, with variable percentages of PS and the remainder of the lipid as PC. Binding was expressed relative to the maximal binding in the assay. As shown in Fig. 2D, the δC1a domain showed marked dependence on PS, with <20% of maximal binding when the percentage of the total phospholipid that was PS was below 20% and hardly any binding when PS was absent and all the phospholipid was PC. On the contrary, the δC1b domain showed much less dependence on PS for [3H]PDBu binding with >50% of maximal binding in the absence of PS, where all the phospholipid was PC (Fig. 2E). PS is preferentially localized in the inner layer of the plasma membrane. The different dependence of δC1a and δC1b on PS for [3H]PDBu binding implies that the two C1 domains may favor different subcellular locations for ligand interaction.
Translocation of the C1a and C1b Domains of PKCδ in Response to Phorbol Ester
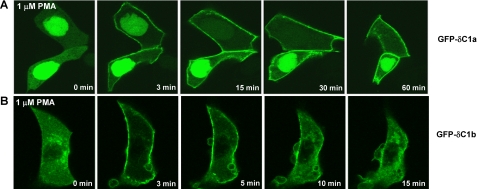
We next compared the behavior of the individual C1 domains of PKCδ in intact cells. The isolated δC1a and δC1b domains were fused with GFP. The GFP-tagged constructs were then expressed in LNCaP cells, and their intracellular distribution and response to the phorbol ester PMA were visualized by confocal microscopy (Fig. 3). Similar levels of the two constructs were expressed, and they were visualized under identical conditions. The two C1 domains showed very different intracellular distributions in the absence of phorbol ester. δC1a was mainly localized in the nucleus (Fig. 3A), whereas δC1b was mainly distributed in the cytosol with some associated with the internal membranes (Fig. 3B). Both C1 domains manifested quick (within 3 min) plasma membrane translocation in response to 1 μm PMA, but the stability of the pattern of translocation over time showed a dramatic difference. The δC1a domain translocation to the plasma membrane was persistent after the application of PMA (Fig. 3A), whereas the δC1b domain remained at the plasma membrane only transiently (Fig. 3B). As PMA equilibrated within the cell, the δC1b domain rapidly shifted from the plasma membrane to a patchy distribution within the cell. Because the cells were being continuously perfused with the PMA containing medium, breakdown or loss of the PMA with time cannot account for the transient plasma membrane translocation. Likewise, over this time course phorbol ester causes no breakdown of the GFP constructs. By itself, GFP does not respond to PMA.
FIGURE 3.
Translocation of the GFP-tagged C1a (A) and C1b (B) domains of PKCδ in response to PMA in living LNCaP cells as a function of time. Cells expressing the GFP-tagged C1a or C1b domains were treated with 1 μm PMA. The redistribution of the fluorescent proteins was monitored with a Zeiss MRC 1024 confocal microscope as a function of time after the addition of PMA. Images were captured every 15 or 30 s. Each panel represents a typical example from three independent experiments.
The dynamic difference in translocation can probably be explained by the different dependence of the two C1 domains on PS for ligand interaction, as described above in the in vitro binding assays (Fig. 2, D and E). Upon addition, the phorbol ester does not immediately equilibrate within the cell. Rather, the phorbol ester first partitions into the plasma membrane and only subsequently distributes to the internal membranes as it continues to equilibrate (39). Because the robust binding of δC1a with phorbol ester requires the presence of PS, the δC1a will move to the plasma membrane in the presence of the PMA. After the PMA has further equilibrated, however, so that PMA is now present both in the plasma membrane and in the internal membranes, the δC1a will not re-equilibrate to the internal membranes because the internal membranes lack sufficient PS. Because the binding of δC1b is much less PS-dependent, the δC1b will shift to mirror the temporally changing distribution of PMA in the cellular membranes.
Characterization of the Differential Roles of the C1a and C1b Domains in Intact PKCδ
Results with the isolated C1 fragments demonstrated that both the C1a and C1b domains of PKCδ have potent binding affinities for phorbol esters. In the following experiments, we were interested in probing the roles of the two C1 domains in intact PKCδ.
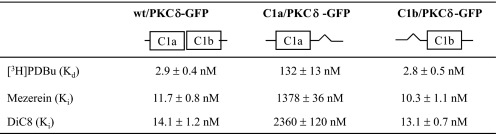
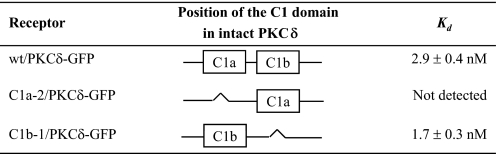
In Intact PKCδ, the Deletion of Single C1 Domains Indicates That the δC1b Domain Confers Much Higher Potency for Phorbol Ester Binding in Vitro Than Does the δC1a Domain—Using the PKCδ-GFP plasmid DNA as template, we first deleted either the δC1a or the δC1b domain to investigate the effect of the deletion on ligand binding and membrane translocation of PKCδ-GFP (Fig. 1). The wild-type PKCδ-GFP protein and the single δC1a and δC1b mutants (designated as C1a/PKCδ-GFP and C1b/PKCδ-GFP to indicate the retained C1 domain) were expressed in CHO-K1 cells and purified using immunoprecipitation with anti-GFP monoclonal antibody as described under “Materials and Methods.” The binding affinities for [3H]PDBu of the purified proteins were then determined. The single δC1a and δC1b mutants showed a dramatic difference in their binding potencies for [3H]PDBu (Table 1). When the δC1b domain was deleted, with the δC1a domain remaining, PKCδ showed a significant decrease (45-fold) in the binding potency for [3H]PDBu (Kd = 132 ± 13 nm), compared with the wild-type (Kd = 2.9 ± 0.4 nm). In contrast, deleting the δC1a domain did not affect the binding potency at all; the single δC1b mutant showed a Kd value (2.8 ± 0.5 nm) very similar to that of the wild type. This result is quite different from that with the isolated δC1a and δC1b domains, which showed only a 6-fold difference in their binding potencies for [3H]PDBu (Fig. 2, B and C).
TABLE 1.
Comparison of in vitro binding affinities (K values) of the wild-type PKCδ-GFP and the single C1a and C1b mutants for different ligands
The GFP-tagged constructs were expressed in CHO-K1 cells. The proteins were then prepared by immunoprecipitation with anti-GFP antibody. Scatchard assay (for Kd values) and competitive binding assay (for Ki values) were performed to measure the binding potencies of these proteins for ligands. Values are expressed as the mean ± S.E. for three independent experiments.
To determine the degree to which the results might reflect the behavior of the specific ligand examined, we measured the binding affinities of the single δC1a and δC1b mutants for two other ligands, mezerein and DiC8. It had been reported previously that mezerein showed no selectivity between the C1a and C1b domains of PKCδ for induction of translocation of PKCδ in NIH 3T3 cells (24). Likewise, it has been reported that DAG may have a preference for the C1a domain, contrasting with a preference for the C1b domain by the phorbol esters (16, 40). In contrast to these reports, our C1 domain deletion mutants showed the same selectivity of mezerein or of the DAG derivative DiC8 for the δC1a and δC1b domains as did PDBu. Both mezerein and DiC8 showed much weaker binding affinities for the single δC1a mutant (118-fold and 167-fold decrease in Ki values, respectively) compared with the wild-type, whereas their binding potencies remained unchanged for the single δC1b mutant (Table 1).
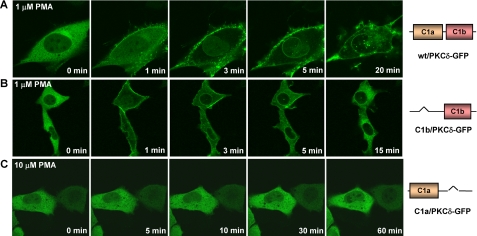
PKCδ Failed to Translocate to the Membrane in Response to PMA When the δC1b Domain Was Deleted—To further investigate the role of the C1a and C1b domains in ligand responsiveness in intact PKCδ, we assessed in vivo translocation of the single δC1a and δC1b deletion mutants of PKCδ-GFP. Plasmids encoding the C1a or C1b domain-deleted mutants of PKCδ-GFP were expressed in LNCaP cells. The intracellular redistribution of the mutants in response to PMA was then monitored by confocal microcopy. All cells were visualized under similar conditions, and individual cells were examined that showed similar levels of construct expression. The levels of overall expression of the constructs in the transient transfection did not differ by >20% (see supplemental Fig. S4). The C1 domain-deletions showed a marked differential effect on membrane translocation of PKCδ by PMA. In the absence of the δC1a domain, δC1b/PKCδ-GFP (with only the δC1b domain) translocated first to the plasma membrane and then to nuclear membrane in response to 1 μm PMA (Fig. 4B). However, its plasma membrane translocation was quite transient, unlike that of the wild-type PKCδ-GFP (Fig. 4A). It stayed at the plasma membrane for no more than 10 min and then shifted back to the interior of the cell. These translocation dynamics were very similar to those of the isolated δC1b domain (Fig. 3B). On the other hand, in contrast to the isolated C1a domain, in the absence of the δC1b domain the single δC1a mutant (C1a/PKCδ-GFP) did not show any membrane translocation up to a PMA concentration of 10 μm (Fig. 4C). Moreover, this lack of in vivo response was not ligand-dependent. Similar dependence on the C1b domain for translocation was seen with both DiC8 and mezerein-treated cells (supplemental Figs. S1 and S2).
FIGURE 4.
Translocation of the wild-type PKCδ-GFP and the single C1 domain mutants in response to PMA in living LNCaP cells. Cells expressing the GFP-tagged wild-type or mutant PKCδ were treated with PMA. The redistribution of the fluorescent proteins was monitored with a Zeiss MRC 1024 confocal microscope as a function of time after the addition of PMA. Images were captured every 30 s. A, time series images of wild-type PKCδ-GFP after the addition of 1 μm PMA. B, time series images of the single δC1b mutant of PKCδ-GFP (with δC1a domain deleted) after the addition of 1 μm PMA. C, time series images of the single δC1a mutant of PKCδ-GFP (with δC1b domain deleted) after the addition of 10 μm PMA. Each panel represents a typical example from three independent experiments.
The predominant role of the C1b domain in ligand recognition, as demonstrated with the C1a and C1b deletion mutants, is entirely consistent with earlier published experiments of ours (34) in which we introduced a mutation into position 27 of the C1a or C1b domains of PKCδ, changing a Gln into a Glu. Although this mutation caused little (C1a) or no (C1b) change in the overall structure of the C1 domain, for the isolated C1a domain it caused marked loss of phorbol ester or DAG binding affinity. For the isolated C1b domain of PKCδ, this mutation differentially caused marked loss of affinity for dioxolanones (a DAG derivative that has an additional binding interaction with the amide of the Gln side chain) but had much less effect on phorbol ester or DAG binding. Mutation of the C1a domain had no effect on translocation, other than that there was a modest shift in favor of translocation to internal membranes. Conversely, mutation of the C1b domain blocked translocation in response to the dioxolanone, whereas translocation in response to phorbol ester or DAG was retained, providing a strong control that the overall function of the PKC had not been disrupted by the mutation.
The Position of the C1a or C1b Domain in Intact PKCδ Did Not Affect Its Binding Activity for Ligand—Deleting one of the C1 domains may of course cause conformational changes of the intact protein, which might contribute to the different response to ligand of the remaining δC1a or δC1b domain because of its altered position in the intact protein. Our previous mutational study, cited above, provides one approach to address this question.
To explore the relation between position of the C1 domain and function, we investigated the effect of the position of the C1 domains on ligand binding by PKC. For this purpose, we switched the position of the δC1a or δC1b domain in the single-C1 domain-deletion mutants of PKCδ-GFP (C1a/PKCδ-GFP or C1b/PKCδ-GFP) to the positions where the δC1b or δC1a domains, respectively, would have been (Fig. 1 and Table 2) and then measured their binding affinities for [3H]PDBu in vitro. As shown in Table 2, the change of the position qualitatively had little effect on the binding potencies. We still detected very high binding affinity of the position-switched δC1b mutant (C1b-1/PKCδ-GFP) (Kd = 1.7 ± 0.3 nm), which is comparable to that of the wild-type (Kd = 2.9 ± 0.4 nm). However, we were unable to detect any binding activity of the position-switched δC1a mutant over the ligand range examined.
TABLE 2.
In vitro binding affinities of the position-switched C1 mutants of PKCδ-GFP for [3H]PDBu in comparison with that of the wild-type
Values are expressed as the mean ± S.E. for three independent experiments.
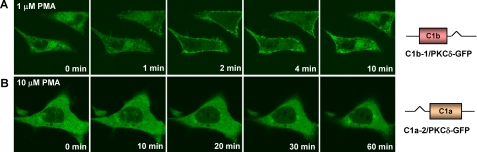
The in vivo translocation assay confirmed that the position of the δC1 domains had no major effect on their sensitivities for the phorbol ester. All cells were visualized under similar conditions, and individual cells were examined that showed similar levels of construct expression. The levels of overall expression of the position-switched single C1 mutants in the transient transfection did not differ by >15% (average of duplicate experiments, data not shown). Like the previous single δC1b mutant (C1b/PKCδ-GFP), the position-switched single δC1b mutant (C1b-1/PKCδ-GFP) still manifested quick but transient plasma membrane translocation in response to 1 μm PMA (Fig. 5A), whereas the position-switched single δC1a mutant (C1a-2/PKCδ-GFP) still did not show any response even when the concentration of PMA was increased to 10 μm. To further explore this point, we also generated a C1a/C1b position reversed full-length PKCδ mutant (C1b-C1a/PKCδ-GFP) to look at the effect of the position of the C1 domains on their function. We found that reversing the position of the C1a and C1b in the intact PKCδ molecule did not affect at all the potencies of PKCδ for phorbol esters both in vitro and in vivo (data not shown). In conclusion, our results demonstrated that, in either position, with the δC1a domain alone PKCδ could not be translocated to membranes by phorbol ester under our conditions. With δC1b alone in either position, PKCδ-GFP could be translocated but was not stabilized at the plasma membrane.
FIGURE 5.
Translocation of the position-switched C1 mutants of PKCδ in response to PMA in living LNCaP cells. Cells expressing the position-switched C1 mutants of PKCδ-GFP were treated with PMA. The redistribution of the fluorescent proteins was monitored with a Zeiss MRC 1024 confocal microscope as a function of time after the addition of PMA. Images were captured every 30 s. A, time series images of position-switched single δC1b mutant (with δC1b at the normal position for δC1a) after the addition of 1 μm PMA. B, time series images of position-switched single δC1a mutant (with δC1a at the normal position for δC1b) after the addition of 10 μm PMA. Each panel represents a typical example from three independent experiments. Controls with the wild-type PKCδ-GFP (data not shown) were similar to those in Fig. 4A.
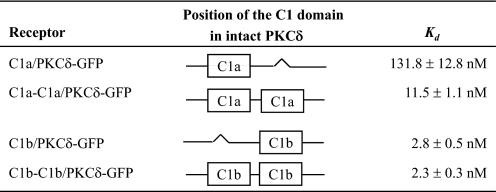
Effects on Phorbol Ester Sensitivity of PKCδ-GFP When Both C1 Domains Have Either the δC1a or δC1b Structure—In addition to generating the C1-deletion mutants to study the role of the C1a and C1b domains in PKCδ activation, we also generated double C1a (C1a-C1a/PKCδ-GFP) and double C1b (C1b-C1b/PKCδ-GFP) mutants (Fig. 1) to investigate the effect of replacing one of the C1 domains with the other on the sensitivity of the intact PKCδ protein for ligand interaction. We did in vitro binding assays as usual with these double δC1a or δC1b mutants to compare their binding affinities with the corresponding single δC1a and δC1b mutants. The results turned out to be quite interesting. When the δC1a domain was replaced with the δC1b domain (yielding dual δC1b domains), the binding affinity of the intact PKCδ-GFP remained the same (Kd = 2.3 ± 0.3 nm) as those of the wild-type (Kd = 2.9 ± 0.4 nm) and the single δC1b mutant (Kd = 2.8 ± 0.5 nm) (Table 3). Interestingly, although the sensitivity of the double δC1a mutant (with δC1b replaced by δC1a) to PDBu still remained lower (Kd = 11.5 ± 1.1 nm) than that of the wild-type PKCδ (Kd = 2.9 ± 0.4 nm), its potency was appreciably (10-fold) greater than that of the single δC1a mutant (Kd = 131.8 ± 12.8 nm) (Table 3) or of the single C1a mutant in the position normally occupied by the C1b domain (Table 2). This increased sensitivity of the double δC1a mutant was also displayed in the in vivo translocation assays (Fig. 6). All cells were visualized under similar conditions, and individual cells were examined that showed similar levels of construct expression. The levels of overall expression of the constructs in the transient transfection did not differ by more than 1.8-fold (average of two experiments, data not shown). Whereas the single δC1a mutant did not show any membrane translocation in response to 10 μm PMA (Fig. 6A), the double δC1a mutant showed limited plasma membrane translocation in response to 10 μm PMA, although the membrane redistribution was somewhat slow and of reduced extent (Fig. 6B). This result confirms that the δC1a domain in intact PKCδ does maintain the structure for ligand binding and may contribute to ligand binding under certain circumstances.
TABLE 3.
Comparison of the in vitro binding affinities of the single C1a (or C1b) and the double C1a (or C1b) mutants of PKCδ-GFP for [3H]PDBu
Values are expressed as the mean ± S.E. for three independent experiments.
FIGURE 6.
Translocation of the single C1a mutant and the double C1a mutant of PKCδ-GFP to PMA in living LNCaP cells. Cells expressing the single δC1a (with only one δC1a domain) and the double δC1a (with two δC1a domain) mutants of PKCδ-GFP were treated with PMA. The redistribution of the fluorescent proteins was monitored with a Zeiss MRC 1024 confocal microscope as a function of time after the addition of PMA. Images were captured every 30 s. A, time series images of the single δC1a mutant after the addition of 10 μm PMA. B, time series images of the double δC1a mutant after the addition of 10 μm PMA. Each panel represents a typical example of three independent experiments.
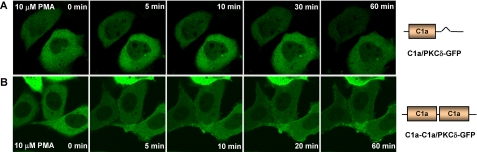
Although the double C1b mutant manifested similar binding potency for phorbol esters in vitro compared with that of the wild-type and the single C1b mutant, it showed several differences in vivo. First, the double C1b mutant showed very prominent internal membrane distribution in LNCaP cells in the absence of PMA (see supplemental Fig. S3). Second, the double C1b mutant showed persistent plasma membrane translocation in response to 1 μm PMA in LNCaP cells (supplemental Fig. S3), whereas that of the single C1b mutant was transient.
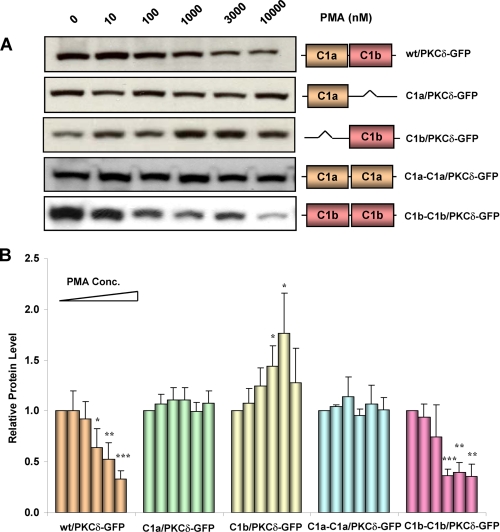
Role of the δC1a and δC1b Domains in Mediating the Down-regulation of PKCδ by PMA—Both the assays for in vitro binding and for in vivo translocation demonstrated that the δC1b domain was the primary site for ligand recognition in intact PKCδ. We wanted to determine whether this was still the case with regard to downstream functional consequences. PKCδ will undergo down-regulation in cells in response to long term (24 h) treatment of PMA (41). We therefore examined the role of the δC1a and δC1b domains in PMA-induced down-regulation of PKCδ-GFP. The wild-type PKCδ-GFP and the single and double C1 mutants were expressed in NIH3T3 cells for 24 h. The cells were then treated with different concentrations of PMA for 24 h, and the total cell lysates were subjected to Western blot analysis with anti-GFP antibody. Representative results are shown in Fig. 7A. Quantification of the intensities of the bands is shown in the diagram of Fig. 7B. As expected, the wild-type PKCδ-GFP showed dose-dependent down-regulation after PMA treatment. Both single δC1a (C1a/PKCδ-GFP) and double δC1a (C1a-C1a/PKCδ-GFP) mutants did not show any change in protein levels after treatment with PMA. This is in agreement with our previous results that, without the δC1b domain, the single δC1a and double δC1a mutants of PKCδ-GFP are not sensitive to PMA or markedly reduced in sensitivity, respectively. Consistent with its potent sensitivity for PMA, the double δC1b (C1b-C1b/PKCδ-GFP) mutant also showed clear dose dependent down-regulation in response to PMA. These results suggest again that δC1b plays the major role in driving the ligand interaction and activation of PKCδ. The single δC1b mutant (C1b/PKCδ-GFP) did not show down-regulation in response to phorbol ester. On the contrary, it showed enhanced expression at PMA concentrations of 1 μm and 3 μm. We had checked the expression level of the single δC1b mutant at 24 and 48 h after transfection in the absence of PMA and found that the expression of this mutant was quite unstable. Compared with the wild-type and single δC1a mutant, the level of the single δC1b mutant was clearly decreased 48 h after transfection (supplemental Fig. S4). We had also checked the expression of the double δC1b mutant. Its expression level was quite stable over this time period (data not shown). It seems plausible that the apparent enhanced expression of the single C1b mutant of PKCδ thus represents stabilization in the presence of phorbol ester.
FIGURE 7.
Western blot analysis of the down-regulation of the wild-type PKCδ-GFP and its single and double C1a and C1b mutants in response to PMA in NIH3T3 cells. Cells overexpressing wild-type PKCδ-GFP, and the single and double C1 mutants were incubated with increasing concentrations (0 nm, 10 nm, 100 nm, 1 μm, 3 μm, and 10 μm) of PMA for 24 h. The cells were then harvested, lysed with RIPA buffer, and subjected to Western blotting. For each construct, equal amounts of protein in the control and treated samples were applied. A, results are from a single, representative experiment. Each experiment was performed four or five times with similar results. B, quantification of the change in the protein levels as a function of the PMA concentration. The films were scanned and the relative band intensities were determined using ImageJ. The intensities of the non-treated bands were normalized to 1. Each column in the diagram represents the average of four or five experiments. Statistical significance (comparison with the value of the control sample in each group) by Student t test: *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
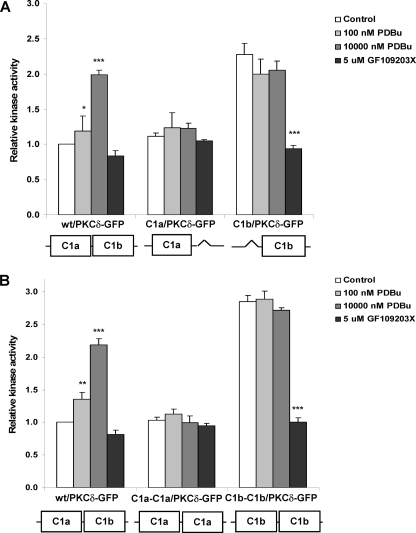
In Vitro Kinase Assay of the Single and Double C1a or C1b Mutants of PKCδ-GFP—We performed in vitro kinase assays with the C1 mutants to further investigate the functional role of the C1a and C1b domains in PKCδ activation. The single and double C1a or C1b mutants of PKCδ-GFP were expressed and purified in CHO-K1 cells using immunoprecipitation with anti-GFP antibody. The purified proteins were then evaluated for in vitro kinase activity induced in response to PDBu using the Promega kit (PepTag® Assay for Non-Radioactive Detection of Protein Kinase C). The results are summarized in Fig. 8; the basal activity of the wild-type PKCδ-GFP was normalized to 1.0. The kinase activity of the wild-type PKCδ-GFP was stimulated by the application of PDBu in a dose-dependent fashion. Neither the single δC1a (C1a/PKCδ-GFP) (Fig. 8A) nor the double δC1a (C1a-C1a/PKCδ-GFP) (Fig. 8B) mutants showed enhanced kinase activity in response to PDBu. This lack of response further confirmed that without the δC1b domain the δC1a domain alone could not recognize the ligand and mediate the activation of the PKCδ enzyme.
FIGURE 8.
In vitro kinase assay of the wild-type PKCδ-GFP and the single and double δC1a and δC1b mutants. Wild-type PKCδ-GFP and the C1 mutants were expressed in CHO-K1 cells for 24 h, yielding similar levels of expression. The proteins were isolated using immunoprecipitation with anti-GFP antibody, and then equal amounts were incubated with different concentrations of PDBu for 30 min at 30 °C. The in vitro kinase activity was assayed using the Promega Protein Kinase C Assay Kit according to the manufacturer's protocol. The kinase activities shown in the diagram were calculated relative to the basal activity of the wild-type PKCδ-GFP. A, comparison of the in vitro kinase activity of the wild-type PKCδ-GFP and the single δC1a and δC1b mutants as a function of the PDBu concentration and the effect of the PKC inhibitor GF109203X on the basal kinase activity of the constructs. B, comparison of the in vitro kinase activity of the wild-type PKCδ-GFP and the double δC1a and δC1b mutants as a function of the PDBu concentration and the effect of the PKC inhibitor GF109203X on the basal kinase activity of the constructs. The bars in each diagram represent the average from three independent experiments. Error bars, ±S.E. Statistical significance (comparison with the value of the control sample in each group) by Student t test: *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
Contrary to the results for translocation, PDBu did not stimulate further kinase activity of either the single δC1b (C1b/PKCδ-GFP) (Fig. 8A) or the double δC1b (C1b-C1b/PKCδ-GFP) (Fig. 8B) mutants. Instead, in the absence of PDBu the basal activity of the single δC1b and double δC1b mutants had already attained a similar level to that of the stimulated wt/PKCδ. We confirmed that the high basal kinase activity could be significantly reduced by the PKC inhibitor GF109203X (as shown in Fig. 8), indicating that this high basal kinase activity was not from other contaminating kinases that copurified with the PKC. These results imply that, without the δC1a domain, PKCδ is acting in vitro as if it were phorbol ester stimulated.
DISCUSSION
Targeting the C1 domains of PKC is a complementary and promising strategy to obtain selectivity for PKC isozymes among the numerous serine/threonine kinases with homologous catalytic sites (31). Using DAG analogues, the Marquez group has already developed compounds with binding affinities approaching those of the phorbol esters (31) and with selectivity among C1 domains (33). The current study seeks to explore two related issues underlying rational design of C1 targeted ligands. First, what are the differential roles of the C1a and C1b domains of PKCδ in ligand response? The existence of two C1 domains affords opportunities for complex modulation of PKC activity through bivalent ligands (35). However, although the performance of some of these ligands has shown improvement over that of monomers, it is inconsistent with expectations for cooperative binding to both C1 domains (21, 35). Second, what are the relative contributions to ligand response of the C1 domain structure per se versus the position of the C1 domain within PKCδ? Irie and co-workers have suggested that synthetic C1 domains could represent a robust platform for discovery of selective ligands (20). On the other hand, we have shown, for example, that the relative selectivity of 130C037 for the C1a and C1b domains of PKCα and PKCδ is not reflected in its selectivity for the intact PKCs (33).
In the present study, by molecular engineering of the C1 domains in intact PKCδ, we examined the roles of the twin δC1a and δC1b domains in ligand binding, membrane translocation and enzyme activation of PKCδ. The sequences of the C1a and C1b domains of PKCδ are 44% identical, and the zinc-binding residues and residues essential for ligand binding are almost completely conserved (Fig. 2A). This suggests that the backbone structures of these two C1 domains are very similar, and our previous computer modeling of the static structures of δC1a and δC1b did not reveal large structural differences between these two domains (34). The binding cleft of δC1a was wider by ∼1 Å, and the δC1a domain did not penetrate as far into the membrane as did the δC1b domain (34). Our results from the isolated δC1a and δC1b domains in the present study are quite consistent with these computer modeling results. As isolated fragments, both the δC1a and δC1b domains demonstrated similar potency for [3H]PDBu binding in vitro (Fig. 2, B and C) and PMA-induced plasma membrane translocation in vivo (Fig. 3). However, we also found in the in vitro binding assays that the binding of the δC1a domain to [3H]PDBu was absolutely PS-dependent, whereas the binding of the δC1b domain was largely retained in the absence of PS (Fig. 2, D and E). This may explain our in vivo results where the GFP-δC1b was only transiently translocated to the plasma membrane by PMA. Lipophilic phorbol esters like PMA first accumulate at the plasma membrane and subsequently re-equilibrate into the internal membranes (39). Because the δC1b domain can bind to the phorbol esters in the absence of PS, the GFP-δC1b would be expected to reflect the changing distribution of PMA with time and would move to the internal membranes as PMA equilibrated there from the plasma membrane. On the other hand, because the binding of δC1a to the phorbol esters depends on PS (which is predominantly located on the plasma membrane), the GFP-δC1a would thus be restricted to the plasma membrane where PS is present as well as the PMA (Fig. 3A).
The same explanation applies to the behavior of the δC1a-deleted mutant of PKCδ-GFP (yielding the single δC1b mutant). Similar transient plasma membrane translocation was observed in intact PKCδ when the δC1a domain was missing, and this response was not dependent on the specific ligand. The single C1b mutant of PKCδ showed a similar pattern of transient translocation in response to the phorbol ester PMA (Fig. 4B), to the DAG analogue DiC8 (Fig. S1) and to the daphnane analogue mezerein (Fig. S2). This suggests that one of the important functions of the δC1a domain may be to stabilize the enzyme at the plasma membrane where it can access plasma membrane localized substrates.
In our study, deletion of the δC1b domain (yielding the single δC1a mutant) caused a dramatic loss of the sensitivity of the PKCδ-GFP to ligands (Table 1, Fig. 4, and supplemental Figs. S1 and S2), consistent with the δC1b domain playing the major role in binding ligand and driving the membrane translocation of the whole PKCδ molecule. This result is in accord with other findings. For example, by mutation of the proline residue at position 11 into glycine (P11G) in either the C1a or C1b domain of intact PKCδ, Szallasi et al. found that the δC1b domain played the predominant role in translocation of PKCδ in response to PMA (23). Confirmation of this conclusion was provided using a dioxolanone as a ligand (34). For this class of DAG derivatives, a Q185E mutation in PKCδ caused the loss of affinity of the C1b domain to the dioxolanone but not to phorbol ester or DAG; similarly, translocation of the Q185E mutant of PKCδ was lost in response to the dioxolanone but not to phorbol ester or DAG. The corresponding mutation in the C1a domain of PKCδ, in contrast, failed to block translocation in response to any of the ligands. The particular strength of the dioxolanone-Q185E system was that binding activity for PMA and DAG was retained, providing a positive control that loss of binding by the dioxolanone rather than a more global change in function of the C1b domain was responsible for the loss of response.
Using bisfunctional (dimeric) phorbol derivatives and PKCδ with mutations at proline 11 in either the C1a or C1b domains, Newton's group also demonstrated that the δC1b domain played the major role in ligand binding (21). However, with the same mutations in the δC1a or δC1b domains, Bogi et al. described that the C1a and C1b domain selectivity was ligand-dependent, at least for translocation (24). Whereas the PMA and the indole alkaloids indolactam and octylindolactam were selectively dependent on the C1b domain, selectivity was not observed for mezerein, for the 12-deoxyphorbol 13-monoesters prostratin or 12-deoxyphorbol 13-phenylacetate, or for the macrocyclic lactone bryostatin 1 (24). Stahelin et al. also suggested that the C1a and C1b domains of PKCδ have opposite affinities for DAG and phorbol ester; i.e. the δC1a domain was preferentially selective for DAG and the δC1b domain showed preference for phorbol ester (25). One factor contributing to different patterns of response in different situations may be differences in the lipid environment supporting the ligand interaction as well as the variable contribution of other co-activators. Thus, the relative selectivity of the same ligands, PMA versus bryostatin 1, for the same PKC isoforms, PKCα versus PKCδ, showed marked differences between mouse keratinocytes (42) and mouse 3T3 cells (43). In any case, we did not observe ligand dependence in the selectivity of the δC1b domain in the present study, using the approach of deletion of one or the other C1 domains. This might imply that the unliganded C1b domain still is contributing to membrane interaction.
It should be noted that functions other than ligand binding have also been proposed for the C1a domain. For example, recent studies using fragments of PKCβ reveal that the C1a domain of PKCβ may interact with a novel E3 ligase RINCK (RING-Finger protein that interacts with ckinase), which promotes the down-regulation of PKC (44). Likewise, PKCδ is subject to regulation by tyrosine phosphorylation, and one of these regulatory tyrosine residues, at position 187, is located in the C1a domain (45).
The different sensitivities of the single δC1a and δC1b mutants (C1a/PKCδ-GFP and C1b/PKCδ-GFP) for ligand binding probably cannot be explained by a structural change of the protein due to the deletion, because the double δC1a and δC1b mutants (C1a-C1a/PKCδ-GFP and C1b-C1b/PKCδ-GFP) mirrored their behavior (Table 3). Furthermore, the characteristics and sensitivities for the phorbol esters again remained the same for the single C1 domain mutants, whether the single C1 domain was in its normal position or swapped into the position of the other C1 domain (Table 2 and Fig. 5). We conclude that the C1 domain itself, not the specific position in PKCδ, is the predominant factor determining its activity.
The predominant role of the C1b domain in PKCδ regulation was also apparent for the functional measures of down-regulation and kinase activity. In the absence of the δC1b domain, regardless of whether one or two C1a domains were present in PKCδ, the enzyme could not undergo down-regulation by PMA in vivo (Fig. 7) or activation by PDBu in vitro (Fig. 8). The performance of the single δC1b mutant (C1b/PKCδ-GFP) in the down-regulation assay was unique. Instead of being down-regulated, the protein level of the single δC1b mutant increased as a function of PMA dose (Fig. 7). This may reflect the lower level of expression of this construct in the absence of PMA, perhaps reflecting that it is already in a conformation making it susceptible to degradation (supplemental Fig. S4). It emphasizes the complexity of the processing of PKC isoforms, reflecting both effects of ligand on the PKC isoform itself as well as its interaction with other elements within the cell (46). Our kinase assay demonstrated that both the purified single and double δC1b mutants (C1b/PKCδ-GFP and C1b-C1b/PKCδ-GFP) manifested considerably higher basal kinase activity in vitro (Fig. 8). This suggests that the δC1a domain contributes to maintaining PKC in an inactive state, presumably by stabilizing the pseudosubstrate domain in the catalytic site. In any case, we did not observe effects such as ERK phosphorylation or apoptosis in response to overexpression of the single δC1b or double δC1b mutants in cells.
An observation in this study was that the double δC1a mutant (C1a-C1a/PKCδ-GFP) showed improved (although still very weak) sensitivity for the phorbol ester compared with the single δC1a mutant (C1a/PKCδ-GFP) (Table 3 and Fig. 6). This demonstrates that the δC1a domain maintains the structure for ligand binding in intact PKCδ. However, because the interaction of δC1a with ligand requires the presence of PS on the plasma membrane (Fig. 2D), it must first be brought to the plasma membrane for high affinity interaction. δC1b may play the role in bringing the C1a domain closer to the plasma membrane and the ligand. The interaction with the C1a domain may then strengthen the localization of the PKCδ molecule at the plasma membrane.
The approach of using a bivalent ligand to exploit differences between C1 domain-containing targets would seek to exploit the presence of two C1 domains in the classic and novel PKCs and the PKDs, in contrast to single C1 domains in the other targets, as well as different spacing between C1 domains in the classic PKCs, the novel PKCs, and the PKDs. A prerequisite for the approach, of course, is that both C1 domains can recognize ligand. Our evidence here, which indicates that the C1a domain of PKCδ is active, albeit less potent, provides support for the bivalent ligand approach. Moreover, the indications that the relative roles of the twin C1 domains may be different in different PKC isoforms, although beyond the scope of the present paper, suggests further opportunities for selectivity.
Supplementary Material
This work was supported, in whole or in part, by the National Institutes of Health Intramural Research Program, NCI, Center for Cancer Research. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental text and Figs. S1-S4.
Footnotes
The abbreviations used are: PKC, protein kinase C; PDBu, phorbol 12,13-dibutyrate; PMA, phorbol 12-myristate 13-acetate; DiC8, 1,2-dioctanoyl glycerol; GFP, green fluorescent protein; CHO, Chinese hamster ovary; PS, phosphatidylserine; PC, phosphatidylcholine; GST, glutathione S-transferase; DAG, diacylglycerol; E3, ubiquitin-protein isopeptide ligase.
References
- 1.Nishizuka, Y. (1992) Science 258 607-614 [DOI] [PubMed] [Google Scholar]
- 2.Ron, D., and Kazanietz, M. G. (1999) FASEB J. 13 1658-1676 [PubMed] [Google Scholar]
- 3.Ono, Y., Fujii, T., Igarashi, K., Kuno, T., Tanaka, C., Kikkawa, U., and Nishizuka, Y. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 4868-4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang, G., Kazanietz, M. G., Blumberg, P. M., and Hurley, J. H. (1995) Cell 81 917-924 [DOI] [PubMed] [Google Scholar]
- 5.Rozengurt, E., Sinnett-Smith, J., and Zugaza, J. L. (1997) Biochem. Soc. Trans. 25 565-571 [DOI] [PubMed] [Google Scholar]
- 6.Ahmed, S., Lee, J., Kozma, R., Best, A., Monfries, C., and Lim, L. (1993) J. Biol. Chem. 268 10709-10712 [PubMed] [Google Scholar]
- 7.Brose, N., Rosenmund, C., and Rettig, J. (2000) Curr. Opin. Neurobiol. 10 303-311 [DOI] [PubMed] [Google Scholar]
- 8.Ebinu, J. O., Bottorff, D. A., Chan, E. Y., Stang, S. L., Dunn, R. J., and Stone, J. C. (1998) Science 280 1082-1086 [DOI] [PubMed] [Google Scholar]
- 9.Topham, M. K. (2006) J. Cell. Biochem. 97 474-484 [DOI] [PubMed] [Google Scholar]
- 10.Choi, S. H., Czifra, G., Kedei, N., Lewin, N. E., Lazar, J., Pu, Y., Marquez, V. E., and Blumberg, P. M. (2008) J. Biol. Chem. 283 10543-10549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann, J. (2004) Curr. Cancer Drug Targets 4 125-146 [DOI] [PubMed] [Google Scholar]
- 12.Newton, A. C. (2001) Chem. Rev. 101 2353-2364 [DOI] [PubMed] [Google Scholar]
- 13.Hannun, Y. A., and Bell, R. M. (1986) J. Biol. Chem. 261 9341-9347 [PubMed] [Google Scholar]
- 14.Kikkawa, U., Takai, Y., Tanaka, Y., Miyake, R., and Nishizuka, Y. (1983) J. Biol. Chem. 258 11442-11445 [PubMed] [Google Scholar]
- 15.Mosior, M., and Newton, A. C. (1996) Biochemistry 35 1612-1623 [DOI] [PubMed] [Google Scholar]
- 16.Slater, S. J., Ho, C., Kelly, M. B., Larkin, J. D., Taddeo, F. J., Yeager, M. D., and Stubbs, C. D. (1996) J. Biol. Chem. 271 4627-4631 [DOI] [PubMed] [Google Scholar]
- 17.Bogi, K., Lorenzo, P. S., Acs, P., Szallasi, Z., Wagner, G. S., and Blumberg, P. M. (1999) FEBS Lett. 456 27-30 [DOI] [PubMed] [Google Scholar]
- 18.Burns, D. J., and Bell, R. M. (1991) J. Biol. Chem. 266 18330-18338 [PubMed] [Google Scholar]
- 19.Shieh, H. L., Hansen, H., Zhu, J., and Riedel, H. (1995) Mol. Carcinogen. 12 166-176 [DOI] [PubMed] [Google Scholar]
- 20.Irie, K., Nakagawa, Y., and Ohigashi, H. (2004) Curr. Pharm. Des. 10 1371-1385 [DOI] [PubMed] [Google Scholar]
- 21.Giorgione, J., Hysell, M., Harvey, D. F., and Newton, A. C. (2003) Biochemistry 42 11194-11202 [DOI] [PubMed] [Google Scholar]
- 22.Kazanietz, M. G., Wang, S., Milne, G. W., Lewin, N. E., Liu, H. L., and Blumberg, P. M. (1995) J. Biol. Chem. 270 21852-21859 [DOI] [PubMed] [Google Scholar]
- 23.Szallasi, Z., Bogi, K., Gohari, S., Biro, T., Acs, P., and Blumberg, P. M. (1996) J. Biol. Chem. 271 18299-18301 [DOI] [PubMed] [Google Scholar]
- 24.Bogi, K., Lorenzo, P. S., Szallasi, Z., Acs, P., Wagner, G. S., and Blumberg, P. M. (1998) Cancer Res. 58 1423-1428 [PubMed] [Google Scholar]
- 25.Stahelin, R. V., Digman, M. A., Medkova, M., Ananthanarayanan, B., Rafter, J. D., Melowic, H. R., and Cho, W. (2004) J. Biol. Chem. 279 29501-29512 [DOI] [PubMed] [Google Scholar]
- 26.Teicher, B. A. (2006) Clin. Cancer Res. 12 5336-5345 [DOI] [PubMed] [Google Scholar]
- 27.Griner, E. M., and Kazanietz, M. G. (2007) Nat. Rev. Cancer 7 281-294 [DOI] [PubMed] [Google Scholar]
- 28.Martiny-Baron, G., and Fabbro, D. (2007) Pharmacol. Res. 55 477-486 [DOI] [PubMed] [Google Scholar]
- 29.Fields, A. P., and Gustafson, W. C. (2003) Methods Mol. Biol. 233 519-537 [DOI] [PubMed] [Google Scholar]
- 30.Anderson, P. W., McGill, J. B., and Tuttle, K. R. (2007) Curr. Opin. Nephrol. Hypertens. 16 397-402 [DOI] [PubMed] [Google Scholar]
- 31.Marquez, V. E., and Blumberg, P. M. (2003) Acc. Chem. Res. 36 434-443 [DOI] [PubMed] [Google Scholar]
- 32.Mach, U. R., Lewin, N. E., Blumberg, P. M., and Kozikowski, A. P. (2006) Chem. Med. Chem. 1 307-314 [DOI] [PubMed] [Google Scholar]
- 33.Pu, Y., Perry, N. A., Yang, D., Lewin, N. E., Kedei, N., Braun, D. C., Choi, S. H., Blumberg, P. M., Garfield, S. H., Stone, J. C., Duan, D., and Marquez, V. E. (2005) J. Biol. Chem. 280 27329-27338 [DOI] [PubMed] [Google Scholar]
- 34.Choi, Y., Pu, Y., Peach, M. L., Kang, J. H., Lewin, N. E., Sigano, D. M., Garfield, S. H., Blumberg, P. M., and Marquez, V. E. (2007) J. Med. Chem. 50 3465-3481 [DOI] [PubMed] [Google Scholar]
- 35.Nowak, I. (2007) Curr. Topics Med. Chem. 7 355-362 [DOI] [PubMed] [Google Scholar]
- 36.Wang, Q. J., Bhattacharyya, D., Garfield, S., Nacro, K., Marquez, V. E., and Blumberg, P. M. (1999) J. Biol. Chem. 274 37233-37239 [DOI] [PubMed] [Google Scholar]
- 37.Wang, Q. J., Fang, T. W., Nacro, K., Marquez, V. E., Wang, S., and Blumberg, P. M. (2001) J. Biol. Chem. 276 19580-19587 [DOI] [PubMed] [Google Scholar]
- 38.Blumberg, P. M., Kedei, N., Lewin, N. E., Czifra, G., Pu, Y., Yang, D., and Marquez, V. E. (2008) Curr. Drug Targets 9 641-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braun, D. C., Cao, Y., Wang, S., Garfield, S., Hur, G. M., and Blumberg, P. M. (2005) Mol. Cancer Ther. 4 141-150 [PubMed] [Google Scholar]
- 40.Slater, S. J., Kelly, M. B., Taddeo, F. J., Rubin, E., and Stubbs, C. D. (1994) J. Biol. Chem. 269 17160-17165 [PubMed] [Google Scholar]
- 41.Choi, S. H., Hyman, T., and Blumberg, P. M. (2006) Cancer Res. 66 7261-7269 [DOI] [PubMed] [Google Scholar]
- 42.Szallasi, Z., Denning, M. F., Smith, C. B., Dlugosz, A. A., Yuspa, S. H., Pettit, G. R., and Blumberg, P. M. (1994) Mol. Pharmacol. 46 840-850 [PubMed] [Google Scholar]
- 43.Szallasi, Z., Smith, C. B., Pettit, G. R., and Blumberg, P. M. (1994) J. Biol. Chem. 269 2118-2124 [PubMed] [Google Scholar]
- 44.Chen, D., Gould, C., Garza, R., Gao, T., Hampton, R. Y., and Newton, A. C. (2007) J. Biol. Chem. 282 33776-33787 [DOI] [PubMed] [Google Scholar]
- 45.Brodie, C., and Blumberg, P. M. (2003) Apoptosis 8 19-27 [DOI] [PubMed] [Google Scholar]
- 46.Gould, C. M., and Newton, A. C. (2008) Curr. Drug Targets 9 614-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.