Abstract
Epigenetic information is hypothesized to be encoded in histone variants and post-translational modifications. Varied cell- and locus-specific combinations of these epigenetic marks are likely contributors to regulation of chromatin-templated transactions, including transcription, replication, recombination, and repair. Therefore, the relative abundance of histone modifications in a given cell type is a potential index of cell fate and specificity. Here, we utilize mass spectrometry techniques to characterize the relative abundance index of cell type-specific modifications on histones H3 and H4 in distinct cell types from the frog Xenopus laevis, including the sperm, the stored predeposition histones in the egg, the early embryo equivalent pronuclei, cultured somatic cells, and erythrocytes. We used collisionally associated dissociation to identify the modifications present on histone H3 in a variety of cell types, resolving 26 distinctly modified H3 peptides. We employed the electron transfer dissociation fragmentation technique in a “middle-down” approach on the H4 N-terminal tail to explore the overlap of post-translational modifications. We observed 66 discrete isoforms of the H4 1–23 fragment in four different cell types. Isolation of the stored, predeposition histone H4 from the frog egg also revealed a more varied pattern of modifications than the previously known diacetylation on Lys5 and Lys12. The developmental transitions of modifications on H3 and H4 were strikingly varied, implying a strong correlation of the histone code with cell type and fate. Our results are consistent with a histone code index for each cell type and uncover potential cross-talk between modifications on a single tail.
Histone proteins compose approximately half of the mass of chromatin, the physiological form of the genome. Two molecules each of H2A, H2B, H3, and H4 are wrapped with DNA into a nucleosome (1); nucleosomes are further compacted in larger fibers and ultimately into chromosomes. Post-translational modifications (PTMs)3 of histone proteins, including, but not limited to, acetylation, methylation, phosphorylation, ubiquitylation, sumoylation, and ribosylation, primarily on the disordered N-terminal “tails” (2), have been hypothesized to constitute part or all of the histone code (3, 4). These histone modifications are thought to participate in the regulation of the usage of the underlying genetic information. Post-translational modifications of histones have been shown to play many roles in chromatin biology, including cis-acting roles for histone PTMs such as acetylation, in which the neutralization of the positively charged lysine ε-amine alters chromatin compaction, and trans-acting roles for modifications, in which binding partners are recruited to facilitate enzymatic action (5). These varied roles for histone modifications result in downstream chromatin positive and negative regulatory events, including, but not limited to, transcription, replication, and repair.
Historically, these PTMs have been individually identified and characterized by a variety of techniques, including radioactive labeling and amino acid analysis, modification-specific antibodies, “bottom-up” mass spectrometry, and most recently by “middle-down” and “top-down” mass spectrometry. Mass spectrometry approaches have allowed the relative quantitation of individual modifications on histones. Recently, techniques have been developed to identify modifications on a single, large peptide fragment, effectively a “middle-down” approach to MS analysis. A large piece of the intact protein can be analyzed separately for accurate mass and for sequence information/PTM localization. The development of electron capture dissociation (6) and electron transfer dissociation (ETD) (7, 8) fragmentation has allowed middle-down analysis of larger proteolytic fragments of histone tails as a result of the charge state dependence of electron capture (6, 9, 10). Furthermore, relatively labile post-translational modifications, such as phosphorylation, remain intact when a peptide is subjected to electron capture dissociation or ETD fragmentation, making these techniques very useful for novel modification identification. However, we do note that the MS/MS spectra generated by these techniques are very complex, especially when generated by coeluting, isobaric parent ions, and therefore require extensive and time-intensive analysis.
In particular, the single isoform of histone H4 has been particularly amenable to middle-down analysis of its N-terminal 1–23 fragment (11–13). However, other histones have also been studied using these approaches, including H2A (14), H2B (9), and H3 (12, 15–17). ETD and electron capture dissociation have been used to explicitly link H3K4 methylation to H3 acetylation on a single N-terminal tail (15, 16), an observation that strongly supports the combinatorial, or multivalent, extension to the histone code hypothesis (18).
We have presented in the accompanying article (40) an analysis of histone modifications and histone variants present in seven distinct cell types from the anuran Xenopus laevis, including the stored, predeposition histones in the oocytes and in the eggs, sperm histones, early embryo equivalent pronuclei histones, A6 (kidney cell lineage) cultured cells, S3 (neurula cell lineage) cultured cells, and nucleated adult erythrocytes. In the accompanying article (40), we present evidence that histone modifications and variants are distinctly enriched in each cell type, providing a “histone code” index that may serve to characterize each distinct tissue.
Here, we present the mass spectrometric analysis of histone H3, using conventional MS/MS collisionally activated dissociation (CAD) fragmentation, from Xenopus sperm, early embryo equivalent pronuclei, erythrocytes, and S3 cells. We also present the mass spectrometric analysis of the histone H4 N-terminal tail, in a middle-down approach using ETD fragmentation, from sperm, stored (predeposition) egg, early embryo equivalent pronuclei, and S3 cells. These results and the similar results presented in the accompanying article (40) strongly support the histone code hypothesis, since they provide an index of modifications for each cell type and are further correlated with the particular biological phenomena of the given cell.
EXPERIMENTAL PROCEDURES
Xenopus Egg Extract Preparation, Pronuclei Assembly, and Histone Purification
Xenopus interphase egg extract was prepared as described (19) and in the accompanying article (40). Pronuclei were assembled and isolated as described in the accompanying article (40). Egg histones were purified on heparin-Sepharose, as described, whereas chromatin-bound histones from pronuclei, erythrocytes, and tissue culture cells were acid-extracted as described, all in the presence of phosphatase inhibitor mixture (Roche PhosSTOP), protease inhibitor mixture (Roche Complete), and 10 mm sodium butyrate (20). Core histones were chromatographically separated on a 4.6-μm C8 reversed phase column as described (20). Fractions containing each core histone were identified based on known retention times (20) and verified by Coomassie staining and Western blotting.
Enzymatic Digestion
H4—C8 reverse phase-HPLC-purified fractions corresponding to histone H4 from Xenopus sperm, egg, early embryo, or neurula (S3) cultured cells were combined (∼20 pmol of total protein) and incubated with endoproteinase AspN (Roche Applied Science) (1:20 enzyme/protein) for 7 h at 37 °C and in NH4HCO3, pH 8. The digestion was quenched with glacial acetic acid and stored at -35 °C prior to analysis.
H3—Purified H3 (∼25 pmol of total protein) from Xenopus sperm, early embryo, neurula (S3) cultured cells, and erythrocyte was reduced with dithiothreitol and alkylated with iodoacetamide (21). Following alkylation, H3 fractions were treated with propionylation reagent, digested with trypsin (Promega, Madison, WI) (1:20 enzyme/protein) for 7 h at 37 °C, and immediately retreated with propionylation reagent, as previously described (22).
Mass Spectrometry
Roughly 10–15 pmol of enzymatically digested mixtures of either H3 or H4 peptides were loaded onto self-packed microcapillary precolumns. Briefly, a fused silica precolumn (360 × 75 μm, outer diameter × inner diameter; Polymicro Technologies, Phoenix, AZ) was packed with 8–10 cm of irregular 5–20 μm C18 beads (YMC, Kyoto, Japan) and connected via a Teflon sleeve (0.060 × 0.012 inch, outer diameter × inner diameter; Zeus Industrial Products, Orangeburg, SC) to a fused silica microcapillary analytical column (360 × 50 μm, outer diameter × inner diameter) packed with 6–8 cm of regular 5 μm C18 beads (YMC) and equipped with a laser-pulled (P-2000, Sutter Instruments, Novato, CA) electrospray emitter tip (2–5 μmin diameter).
Peptides were gradient-eluted using an Agilent 1100 series HPLC (Palo Alto, CA) into either a Finnigan LTQ Quadrupole Ion Trap (Thermo Fisher Scientific, San Jose, CA) modified for ETD/proton transfer charge reduction (PTR) (6), a Thermo Electron LTQ-Orbitrap (Thermo Fisher Scientific, Bremen, Germany), or a Finnigan LTQ-FT (Thermo Fisher Scientific, Bremen, Germany) utilizing a gradient of 0–60% B in 60 min, 60–100% B in 3 min, and held at 100% B for 4 min (A = 0.1% acetic acid; B = 70% acetonitrile in 0.1% acetic acid). The LTQ-Orbitrap and LTQ-FT were operated in a data-dependent mode in which the MS1 scan was taken from m/z 300–2,000 in the Orbitrap (r = 30,000 at m/z 400) or ion cyclotron resonance (r = 100,000 at m/z 400) mass analyzer, followed by 10 CAD MS/MS scan events (normalized collision energy of 35 with a mass window of 3 atomic mass units) in the linear ion trap mass analyzer. A dynamic exclusion of 30 s was used with a repeat count of 2. The ETD/PTR-modified LTQ was operated in a data-dependent mode in which the MS1 scan was taken from m/z 300–2,000, followed by six MS/MS scans. For ETD, the radical anion of fluoranthene (m/z 202) accumulated in the linear ion trap for 2–5 ms and subsequently reacted with the parent ion from the MS1 scan for 90–120 ms. For PTR, the benzoate anion (m/z 121) accumulated in the linear ion trap for 2–3 ms and reacted with the ETD-generated fragment ions for 100–150 ms.
Data Analysis
High resolution H4 data were inspected manually using Qual Browser (Thermo Electron Corp.) software for all masses corresponding to H4 1–23 with any combination of 0–5 acetylations (Δ42.0106 Da), 0–5 methylations (Δ14.0157 Da), and 0–1 phosphorylation (Δ79.9663 Da); H3 analyses were manually inspected by accurate mass using Qual Browser for all combinations of up to six methylations and two acetylations on each the five tryptic peptides listed in Table 1. In addition, spectra were manually inspected for any additional highly abundant modifications and adducts. Masses that agreed to within 5 ppm of the theoretical mass were analyzed to determine their relative abundance with respect to all other modifications and combinations of modifications. Relative abundance information for each species was determined by taking the area under the curve of the selected ion chromatogram for the most abundant isotope for every charge state present utilizing a ±0.01 Da mass window and by comparing it with the sum of the areas for every unique species of the given peptide. For all species of H4 1–23 found in the high resolution analysis, ETD/PTR data were manually interrogated for sequence validation and for PTM localization; potential modifications observed on the peptides of H3 were localized using CAD data acquired in the linear ion trap of the LTQ-Orbitrap or LTQ-FT.
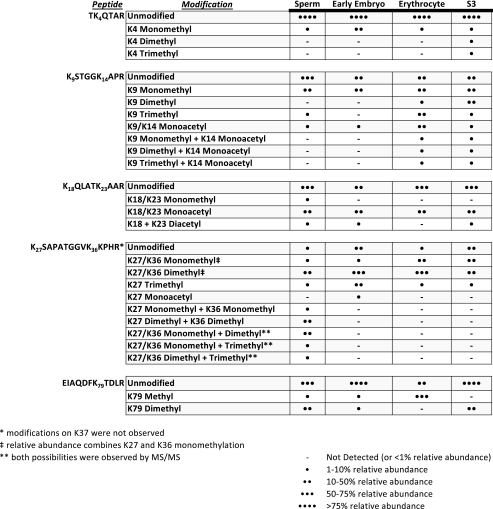
TABLE 1.
Summary of high resolution analyses of PTMs seen on H3 peptides from Xenopus sperm, egg, erythrocyte, and S3 cultured cells Five H3 propionylated tryptic peptides (TKQTAR; KSTGGKAPR; KQLATKAAR; KSAPATGGVKKPHR; EIAQDFKTDLR) are shown in the left column. Each apparent modification on the lysines in those peptides is highlighted in the second column. The relative abundances of each modification in each histone source are displayed in the final four columns. Note that chromatographic separation of species containing Lys27 methylation from species with Lys36 methylation was not possible in erythrocyte or in S3 cells. Therefore, relative abundance information for mono- and dimethylation are reported as a single value.
RESULTS
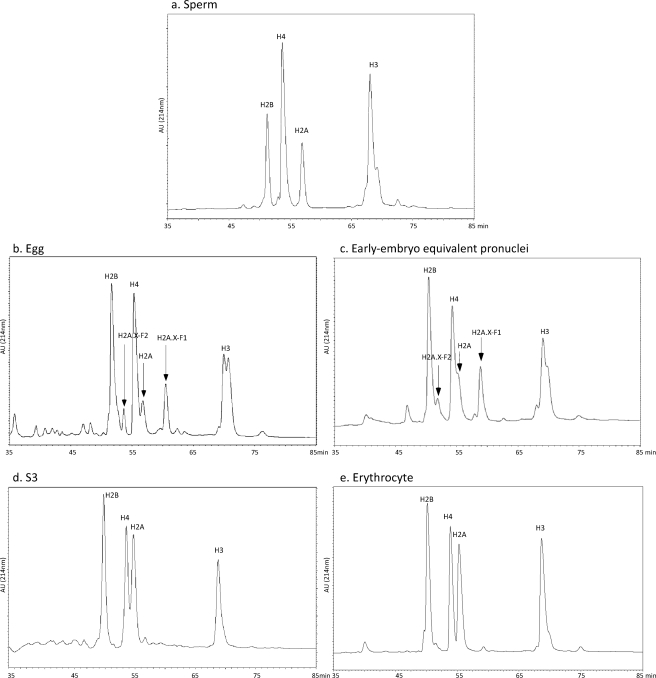
Histones were isolated from X. laevis eggs (predeposition histones complexed with chaperones), sperm, early embryo equivalent pronuclei, erythrocytes, and S3 cultured cells. The mixed histone population was further purified on a C8 reversed phase HPLC column to resolve each core histone in an individual fraction (Fig. 1). The peak fractions of histone H3 and H4 were then subjected to enzymatic proteolysis and subsequent mass spectrometry as described below.
FIGURE 1.
HPLC reversed phase chromatograms of isolated histones used for mass spectrometry analysis. Acid-extracted histones (chromatin-bound in the sperm, egg, pronuclei, S3, and erythrocyte cells) or heparin-Sepharose isolated (chaperone-bound in the egg) were applied on a reversed phase C8 HPLC column and eluted. The protein chromatogram (absorbance at 214 nm) for each column is shown from 35 to 85 min. a, sperm histones. b, stored, predeposition egg histones. c, early embryo equivalent pronuclei histones. d, S3 cultured cell histones. e, erythrocyte histones.
We propionylated histone H3 both before and after proteolysis and prior to mass spectrometric analysis. Conversion of unmodified and monomethylated lysine residues as well as protein and peptide N termini to their propionylated derivatives (an addition of 56.0262 Da per lysine and/or N termini), prevents trypsin from cleaving at lysines and also results in an increase in the overall hydrophobicity of derivatized peptides, allowing for enhanced retention of smaller H3 peptides. Propionlyation also tends to reduce the overall charge of a given peptide, allowing for efficient MS/MS analysis of the typically basic histone proteins via CAD.
Histone H3—Table 1 shows an overview of all of the post-translational modifications observed on H3 from the four cell types analyzed.
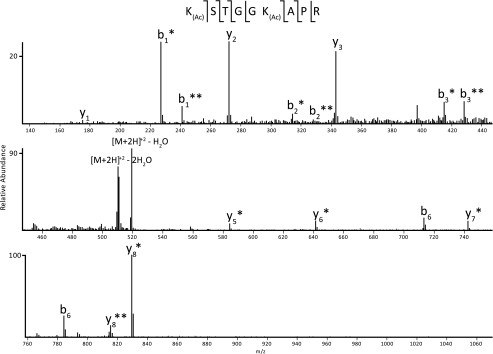
H3 species containing Lys9 and Lys14 from sperm and early embryo show Lys9 monomethylation at a level roughly equivalent to that of the nonmethylated form. This is in striking contrast to H3 from erythrocyte, in which a majority of Lys9 exists in the trimethylated state, as well as to S3 H3, which displays a preference for both mono- and dimethylated Lys9. Interestingly, although all cell types contain low to moderate levels of acetylation, only erythrocyte and S3 cells contain any combination of Lys9 methylation and Lys14 acetylation. For the mono-acetylated species observed in all cell types, analysis of various CAD MS/MS spectra shows the presence of both acetylated Lys9 and acetylated Lys14 (see Fig. 2). Although these two species are unique from one another (i.e. the modifications are not simultaneously present on the same peptide), they were not chromatographically resolved under the experimental conditions, and separate relative abundances could not be efficiently obtained. Therefore, the relative abundance noted in Table 1 for monoacetylated H3 9–17 includes the summation of both Lys9 acetylation and Lys14 acetylation.
FIGURE 2.
CAD MS/MS spectrum of the H3 9–17 peptide containing one acetylation. An example spectrum from CAD fragmentation of the H3 9–17 peptide is shown. Note that this spectrum is mixed with monoacetylation present separately on Lys9 and Lys14. Fragment ions marked with a single asterisk are acetylated on Lys9; those marked with a double asterisk are acetylated on Lys14. The difference in mass of 14 Da between the fragment ions containing Lys9 acetylation and Lys14 acetylation arises from the presence of a propyl group on the nonacetylated lysine residue. Shown along the top of the figure is the primary amino acid sequence of the peptide.
Acetylation distributed over Lys18 and Lys23 appears to be a relatively major modification seen on H3 of all cell types, existing on roughly the same order of magnitude as the unmodified form of the 18–26 H3 peptide. Diacetylation over both Lys18 and Lys23 was also detected in three of the four cell types and exists at roughly an order of magnitude below the acetylated species in sperm, early embryo, and S3 cells.
MS/MS Analysis of H3 27–40 reveals the apparent mutually exclusive nature of methylation upon Lys27 and Lys36 in all cell types except for sperm; interestingly, only sperm H3 27–40 displays any combinatorial methylation marks over Lys27 and Lys36. Also of note is the observation that trimethylation was shown to exist on Lys27 in all cell types, yet no Lys36 trimethylation was seen.
MS signals for the H3 peptide containing Lys4 display a clear preference in each cell type for the unmodified form of the peptide, with the monomethylated form existing at roughly an order of magnitude below the unmodified form.
Histone H4—Following enzymatic digestion with endoproteinase AspN, a 23-residue peptide corresponding to the extreme N terminus of histone H4 was generated, providing complete coverage for the most heavily modified region of H4 and thus allowing for the comparative analysis of all combinations of N-terminal modifications of H4.
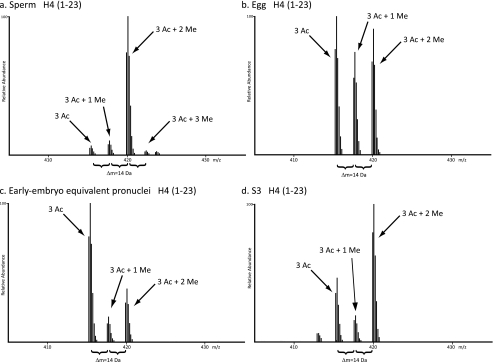
H4 peptides were subjected to C18 reversed phase HPLC, which provides separation based upon increasing numbers of acetylations (differing by 42.0106 Da mass units). High resolution MS1 scans on the LTQ-Orbitrap of the various species of H4 1–23 containing, for example, a total of three acetylations, revealed distinct differences of 14.0157/z mass units, indicative of variously methylated forms of triacetylated H4 1–23. The same mass differences were seen for mono-, di-, and tetra-acetylated H4 1–23.
Comparison of the N terminus of H4 from the four cell types under analysis revealed distinct patterns of acetylation, methylation, and phosphorylation for each biological state. Fig. 3 shows MS1 scans from the LTQ-Orbitrap, demonstrating the unique patterns of methylation for triacetylated H4 1–23 for each cell type analyzed. For example, triacetylated sperm H4 1–23 displays abundant dimethylation but very minor mono- and trimethylation. This pattern of methylation appears drastically different from the methylation states of triacetylated egg, early embryo, and S3 H4 1–23. Egg H4 1–23 shows relatively equivalent amounts of the non-methylated, monomethylated, and dimethylated forms of triacetylated H4 1–23, whereas early embryo displays fairly abundant amounts of nonmethylated, triacetylated H4 1–23 relative to the mono- and dimethylated forms. S3 also exhibits abundant amounts of dimethylation in combination with triacetylation of H4 1–23 but also contains relatively abundant amounts of nonmethylation and monomethylation.
FIGURE 3.
Example high resolution MS1 scan of H4 1–23. Shown are high resolution MS1 scans of the various methylation states seen on H4 1–23 containing two internal acetylations for all four cell types analyzed. Differences of 14 Da are noted to represent the addition of up to three methylations for each cell type. a, sperm histones. b, stored, predeposition egg histones. c, early embryo equivalent pronuclei histones. d, S3 cultured cell histones.
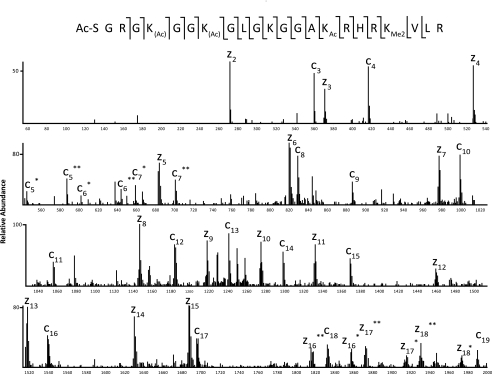
An ETD/PTR tandem mass spectrum of the [M + 6H]6+ species of H4 1–23 containing two internal acetylations and two methylations (at m/z 419.9237) from Xenopus sperm is shown in Fig. 4. Manual sequencing of the fragment ions allowed for the full characterization of every potential PTM observed in the LTQ-Orbitrap analysis. Localization of both methylations to Lys20 was facilitated by the observation of the z3 (m/z 371) and z4 (m/z 527) ions, the latter of which displays a mass shift of 28 Da, indicative of lysine dimethylation. Full acetylation of Lys16 is indicated by the shift in mass of 42 Da above that of an unmodified lysine residue, observed on both the c16 (m/z 1539) ion and the z8 (m/z 1146) ion. Full N-terminal acetylation is observed from the 42 Da shift of the first c′ ion observed (c3, m/z 360) and is consistent with the known amino acid sequence of the N terminus of H4. Localization of the third acetyl group was more difficult due to the presence of two isobaric species within the isolation window: H4 1–23, containing N-terminal, Lys5, and Lys16 acetylation in combination with Lys20 dimethylation, and H4 1–23, containing N-terminal, Lys8, and Lys16 acetylation in combination with Lys20 dimethylation. We differentiated these two peptide species by the presence of two fragment ions, separated by 42 Da, for each of the c5, c6, and c7 ions (as well as for the complementary z16, z17, and z18 ions). We note that since the Lys5 acetyl species and the Lys8 acetyl species do not separate chromatographically on a C18 column, it is not possible to obtain separate relative abundance information.
FIGURE 4.
ETD/PTR MS/MS spectrum of H4 1–23. ETD/PTR MS/MS spectrum of H4 1–23 containing two internal acetylations and two methylations showing the presence of both Lys5/Lys16 diacetylation and Lys8/Lys16 diacetylation in a single scan. Fragment ions containing a single asterisk are acetylated at Lys5; those containing a double asterisk are acetylated at Lys8. Localization of both methylations to Lys20 is made by the z3 ion, which is shifted higher by 28 Da from the mass of the unmodified ion. Shown along the top of the figure is the primary amino acid sequence of the peptide.
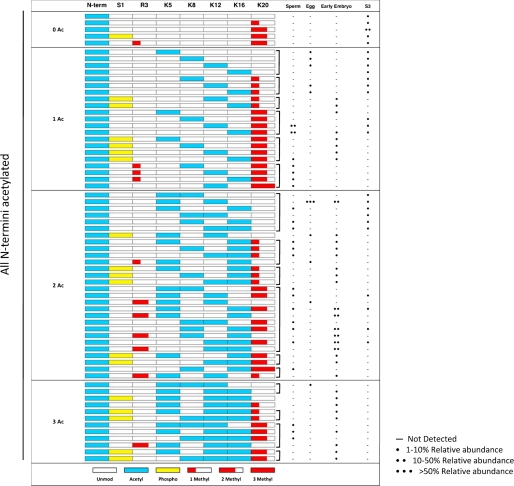
A summary of all combinations of PTMs observed on H4 1–23 from each cell type is shown in Table 2. Full N-terminal acetylation is observed in every combination in all cell types and therefore will be disregarded when the specific sites of modification are discussed.
TABLE 2.
Representation of every PTM combination observed on the N terminus of H4 from Xenopus sperm, egg, early embryo, and S3 cultured cells Each row in the table represents a single discrete H4 1–23 peptide species. The left columns indicate the potentially modified residues on each peptide; empty boxes indicate that the residue is unmodified on that species, whereas colored boxes, as annotated in the key at the bottom, show the presence of a particular modification on that species. All observed peptides were acetylated on the N terminus. The relative abundances of each modified peptide species in each histone source are displayed in the final four columns. Isobaric species that do not separate chromatographically are grouped by brackets. Relative abundances between the isobaric species could not be adequately obtained; thus, each unique species within a bracketed group that was observed via ETD/PTR MS/MS is reported as existing at equivalent relative abundance.
As seen in Table 2, analysis of H4 1–23 from transcriptionally silent sperm chromatin displays a large amount of Lys20 dimethylation, a known silencing mark, in combination with anywhere from two to four acetylations, with the majority of Lys20 dimethylation occurring in combination with acetylation over either Lys12 or Lys16. Additionally, sperm H4 uniquely contains slight amounts of Lys20 trimethylation as well as low level Arg3 monomethylation. A distinct absence of Ser1 phosphorylation is also apparent.
H4 1–23 from stored egg histones shows Lys5 and Lys12 diacetylation present at >50% relative abundance. In addition, little methylation and phosphorylation is observed, and the total number of combinations is drastically reduced compared with the other cell types analyzed; specifically, only 10 unique combinations of modifications were observed on stored egg H4 1–23.
A dramatic increase in the overall number of combinations of modifications is observed upon progression from the gametogenic cell stages (sperm and egg) to the early embryo cell type. Specifically, 34 unique combinations of modifications are observed, with a majority containing dimethylation over either Arg3 or Lys20. Interestingly, these two marks do not exist on the same N-terminal tail and thus appear to be mutually exclusive to one another. Also notable is the massive increase in Ser1 phosphorylation in the early embryo, a known mark of mitosis and DNA replication (23), which is observed in multiple different combinations. Additionally, diacetylation over Lys5, Lys8, Lys12, and Lys16 is present to a significant extent, some of which may be remnants of the massive amounts of Lys5 and Lys12 diacetylation seen in stored egg histones.
An apparent loss of overall acetylation on S3 histone H4 1–23 is seen in Table 2, with a substantial amount of the protein existing with a single, N-terminal acetylation. A loss of both Ser1 phosphorylation and Arg3 dimethylation from the early embryo stage is clear, and an increase in Lys20 dimethylation is evident.
DISCUSSION
Here we presented the mass spectrometry analysis of histones H3 and H4 from the eggs, sperm, early embryo, erythrocytes, and S3 cultured cells of the frog X. laevis. Along with our accompanying article (40), this is the first reported “life span” analysis of global histone modifications from gametic, embryonic, and somatic cell types. Our mass spectrometry analysis is mostly consistent with and complementary to the immunoblotting reported in the accompanying article (40). It also provides a thorough and more quantitative analysis of the H3 modifications than does immunoblotting alone. Furthermore, our use of ETD fragmentation of histone H4 from the sperm, egg, early embryo, and S3 cells allowed us to track H4 species with multiple modifications on a single N-terminal tail, providing a unique picture of the modification transitions in developmental time and space.
The analysis of histones from these varied sources in the frog has an additional advantage over the more typically studied cultured cells: the sperm, early embryo, and erythrocytes are all transcriptionally competent, whereas the S3 cultured cells are exponentially growing, transcriptionally active cells. Other histone modification analyses have been performed on transcriptionally active samples (24, 25). Ongoing transcription is tightly correlated with changes in histone modification (26), temporally both before and after transcription. Therefore, our analysis of permanently silenced (erythrocytes), transcriptionally “poised” (sperm and egg), and transcriptionally active (S3 cells) provides a snapshot of distinct chromatin states. For instance, purified egg histones are unique in that the combinations of modifications observed do not connote a specific transcriptional state, but rather are so-called “predeposition marks.” These marks are indicative of nonchromatin, chaperone-bound localization of histones in preparation for nuclear remodeling of sperm chromatin upon fertilization and the subsequent rapid cell cycles during early development.
Our analysis of histone H3 modifications revealed some intriguing global patterns of modification. We note that K4me1 was apparent in all cell types, regardless of transcriptional competence, whereas only the S3 cells had appreciable levels of K4me2 and K4me3, consistent with the known role of higher states of Lys4 methylation and transcriptional activation (27). Also noteworthy is the distribution of K9me1, highly abundant across all four cell types and probably indicative of a variety of cellular processes, including ongoing transcription and heterochromatin formation (28). Intriguingly, K9me3 was present in all of the cell types, but most abundant in erythrocytes, consistent with the permanent silencing of the erythrocyte nuclei; despite this enriched Lys9 methylation and heterochromatin formation, Xenopus erythrocytes appear not to have HP1 protein (29).
We also observed a disparate range of histone H3 Lys27 and Lys36 methylation patterns. K27me has been correlated with silencing mediated by Polycomb group effector proteins (30), yet it has also been shown to be part of a so-called “bivalent” mark when coexisting on a chromatin locus with K4me (31). In this bivalent context, the chromatin is thought to be poised for a developmental switch between activation and repression. Intriguingly, the sperm and the early embryo have lower total levels of Lys27 methylation than do the somatic erythrocyte and S3 cells, perhaps consistent with a developmentally specified lineage commitment and concomitant increase in Lys27 methylation. In the absence of a top- or middle-down analysis of the H3 tail in these samples, we cannot determine to what extent the Lys4 and Lys27 methyl marks constitute a bivalent domain. These domains are specified in a locus-specific fashion, so a global analysis of this sort is a crude tool for parsing these differences.
Intriguingly, we only observed an overlap of Lys27 and Lys36 methylation on the H3 27–40 tryptic peptide on the sperm H3. Lys36 methylation is highly correlated with ongoing transcription (28, 32), whereas Lys27 methylation is correlated with transcriptional silencing (30). We have previously observed an overlap of Lys27 and Lys36 methylation (25) in somatic HeLa and 293 cultured cells. The curious overlap between two modifications that have putatively opposite biological effects may suggest that these two modifications may encode a “bivalent” domain, similar to that between K4me and K27me (31). Clearly, future work will be necessary to determine the functional role of the simultaneous presence of these modifications. It would also be interesting to ask whether K4me, K27me, and K36me all simultaneously exist on the same H3 tail. We do also note that the relative abundances of the individual H3 Lys27 and Lys36 methylation species represented in Table 1 are pooled, since they are isobaric and cannot be chromatographically separated. Therefore, we cannot make direct comparisons with the slightly different pattern of enrichment of H32K27me species in the immunoblotting in the accompanying article (40), since the abundances presented here may be more due to the presence of K36me.
Analysis of the H3 peptide containing Lys79 reveals an interesting trend in that both sperm and early embryo contain relatively significant amounts of both mono- and dimethylation, whereas erythrocyte H3 is highly enriched in monomethylation but not dimethylation and S3 H3 contains only dimethylation and no monomethylation. The significance of these observations is unclear.
Finally, we note that although we observed H3S10 phosphorylation in the accompanying article (40) by immunoblotting of the oocyte, egg, and early embryo histones, thorough searching of the mass spectra did not reveal an appreciable quantity of S10ph. Although our immunoblotting results are consistent with the known appearance of H3S10ph during oocyte meiotic maturation (33), the discrepancy with the mass spectrometry analysis is probably explained by the greater sensitivity of chemiluminescent detection.
Our analysis of histone H4 using ETD uncovered a number of patterns of modification coexistence and negative cross-talk. The most striking observation is the relative dearth of modifications on the somatic and transcriptionally active S3 cell histones. The bulk of S3 histone H4 proteins only contain one or two total modifications on the 1–23 tail. In contrast, the early embryo histone H4 contains three, four, and even five total modifications on the 1–23 tail. We did not observe any preference for particular sites of H4 tail lysine acetylation, except for a marked enrichment of Lys5 and Lys12 acetylation in the egg, in contrast to the explicit preference observed in Drosophila (for Lys12) and in Tetrahymena (for Lys11, the equivalent residue to Lys12 in metazoans) (34). This difference is probably due to the higher sensitivity of mass spectrometry employed in our study.
H4 Lys5 and Lys12 acetylation are known to be predeposition modifications, highly enriched prior to chromatin assembly during S-phase (35, 36), yet no known molecular function has been ascribed to these modifications. Tellingly, mutation of all four of the lysine residues in the yeast H4 N-terminal tail resulted in lethality, whereas no single mutation had a pronounced effect (37), consistent with a combinatorial role for lysine modifications. Indeed, a later study identified the crucial residues for chromatin assembly and viability as Lys5, Lys8, and Lys12 (38). Furthermore, our observation of many more predeposition H4 isoforms in the egg, including lysine-acetylated (on each of Lys5, Lys8, and Lys12), lysine-triacetylated (including on Lys5, Lys8, and Lys12), serine (Ser1)-phosphorylated, and arginine (Arg3) methylated (See Table 2), suggest an even more complex predeposition modification pattern than suspected. These observations were only possible due to our use of ETD fragmentation to analyze the combinatorial pattern of modifications on a single histone tail.
Finally, we note the entirely negative correlation between H4 arginine 3 methylation and serine 1 phosphorylation. We did not see a single species with both Ser1 phosphorylation and Arg3 methylation modifications. The significance of this observation is unclear. However, we do know that a potent arginine methyltransferase activity in Xenopus eggs methylates Arg3 and is inhibited by S1ph,4 suggesting that S1ph may serve a negative regulatory function for subsequent histone modifications.
Our observations strongly support the histone code hypothesis (3), especially in light of our recent extension of the hypothesis to explicitly include the tremendous benefits of a multivalent readout of combinatorial modifications by effector proteins (18). However, we are aware that these observations do not imply causality between relative abundances of histone modifications and histone variants and molecular and cellular phenotypes. We therefore interpret our observation of such striking differences in global enrichments of histone modifications as, at minimum, support for a syntactic definition of the histone code, in which histone modifications provide the syntax (or context) in which chromatin-mediated DNA transactions occur. We anticipate that such histone modification indexing, both on a global cell-specific scale as we have presented here and using ChIP-Seq (39) and other techniques, will have wide ranging significance for the study of epigenetic phenomena.
Acknowledgments
We are grateful to Hiro Funabiki and members of the Funabiki laboratory for generously sharing the frog colony and Jim Maller for Xenopus S3 cells. We thank Sandra Hake and Laura Banaszynski for constructive comments on the manuscript. We also thank Scott Ficarro for instruction on making Lithisil frits.
This work was supported, in whole or in part, by National Institutes of Health Grant National Research Service Award/Kirschstein Fellowship GM075486 (to D. S.), Grant GM37537 (to D. F. H.), and Grant GM53512 (to C. D. A.). This work was also supported by Rockefeller University. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PTM, post-translational modification; MS, mass spectrometry; HPLC, high pressure liquid chromatography; ETD, electron transfer dissociation; PTR, proton transfer charge reduction; FT, Fourier transform; CAD, collisionally activated dissociation.
D. Shechter, C. D. Allis, manuscript in preparation.
References
- 1.Luger, K., and Richmond, T. J. (1998) Curr. Opin. Struct. Biol. 8 33-40 [DOI] [PubMed] [Google Scholar]
- 2.Kouzarides, T. (2007) Cell 128 693-705 [DOI] [PubMed] [Google Scholar]
- 3.Strahl, B. D., and Allis, C. D. (2000) Nature 403 41-45 [DOI] [PubMed] [Google Scholar]
- 4.Jenuwein, T., and Allis, C. D. (2001) Science 293 1074-1080 [DOI] [PubMed] [Google Scholar]
- 5.Taverna, S., Li, H., Ruthenburg, A., Allis, C., and Patel, D. (2007) Nat. Struct. Mol. Biol. 14 1025-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zubarev, R. A., Horn, D. M., Fridriksson, E. K., Kelleher, N. L., Kruger, N. A., Lewis, M. A., Carpenter, B. K., and McLafferty, F. W. (2000) Anal. Chem. 72 563-573 [DOI] [PubMed] [Google Scholar]
- 7.Coon, J., Ueberheide, B., Syka, J., Dryhurst, D., Ausio, J., Shabanowitz, J., and Hunt, D. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 9463-9468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syka, J. E., Coon, J. J., Schroeder, M. J., Shabanowitz, J., and Hunt, D. F. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 9528-9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medzihradszky, K. F., Zhang, X., Chalkley, R. J., Guan, S., McFarland, M. A., Chalmers, M. J., Marshall, A. G., Diaz, R. L., Allis, C. D., and Burlingame, A. L. (2004) Mol. Cell Proteomics 3 872-886 [DOI] [PubMed] [Google Scholar]
- 10.Mackay, C. L., Ramsahoye, B., Burgess, K., Cook, K., Weidt, S., Creanor, J., Harrison, D., Langridge-Smith, P., Hupp, T., and Hayward, L. (2008) Anal. Chem. 80 4147-4153 [DOI] [PubMed] [Google Scholar]
- 11.Phanstiel, D., Brumbaugh, J., Berggren, W. T., Conard, K., Feng, X., Levenstein, M. E., McAlister, G. C., Thomson, J. A., and Coon, J. J. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 4093-4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia, B. A., Pesavento, J. J., Mizzen, C. A., and Kelleher, N. L. (2007) Nat. Methods 4 487-489 [DOI] [PubMed] [Google Scholar]
- 13.Pesavento, J. J., Bullock, C. R., LeDuc, R. D., Mizzen, C. A., and Kelleher, N. L. (2008) J. Biol. Chem. 283 14927-14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyne, M. T., II, Pesavento, J. J., Mizzen, C. A., and Kelleher, N. L. (2006) J. Proteome Res. 5 248-253 [DOI] [PubMed] [Google Scholar]
- 15.Taverna, S. D., Ueberheide, B. M., Liu, Y., Tackett, A. J., Diaz, R. L., Shabanowitz, J., Chait, B. T., Hunt, D. F., and Allis, C. D. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 2086-2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, L., Smith, J. N., Anderson, S. L., Ma, P., Mizzen, C. A., and Kelleher, N. L. (2007) J. Biol. Chem. 282 27923-27934 [DOI] [PubMed] [Google Scholar]
- 17.Garcia, B. A., Thomas, C. E., Kelleher, N. L., and Mizzen, C. A. (2008) J. Proteome Res. 7 4225-4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruthenburg, A., Li, H., Patel, D., and Allis, C. (2007) Nat. Rev. Mol. Cell. Biol. 8 983-994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shechter, D., Costanzo, V., and Gautier, J. (2004) Nat. Cell Biol. 6 648-655 [DOI] [PubMed] [Google Scholar]
- 20.Shechter, D., Dormann, H., Allis, C., and Hake, S. (2007) Nat. Protoc. 2 1445-1457 [DOI] [PubMed] [Google Scholar]
- 21.Schroeder, M. J., Shabanowitz, J., Schwartz, J. C., Hunt, D. F., and Coon, J. J. (2004) Anal. Chem. 76 3590-3598 [DOI] [PubMed] [Google Scholar]
- 22.Garcia, B. A., Busby, S. A., Shabanowitz, J., Hunt, D. F., and Mishra, N. (2005) J. Proteome Res. 4 2032-2042 [DOI] [PubMed] [Google Scholar]
- 23.Barber, C. M., Turner, F. B., Wang, Y., Hagstrom, K., Taverna, S. D., Mollah, S., Ueberheide, B., Meyer, B. J., Hunt, D. F., Cheung, P., and Allis, C. D. (2004) Chromosoma 112 360-371 [DOI] [PubMed] [Google Scholar]
- 24.Garcia, B. A., Hake, S. B., Diaz, R. L., Kauer, M., Morris, S. A., Recht, J., Shabanowitz, J., Mishra, N., Strahl, B. D., Allis, C. D., and Hunt, D. F. (2007) J. Biol. Chem. 282 7641-7655 [DOI] [PubMed] [Google Scholar]
- 25.Hake, S. B., Garcia, B. A., Duncan, E. M., Kauer, M., Dellaire, G., Shabanowitz, J., Bazett-Jones, D. P., Allis, C. D., and Hunt, D. F. (2006) J. Biol. Chem. 281 559-568 [DOI] [PubMed] [Google Scholar]
- 26.Berger, S. L. (2007) Nature 447 407-412 [DOI] [PubMed] [Google Scholar]
- 27.Ruthenburg, A., Allis, C., and Wysocka, J. (2007) Mol. Cell 25 15-30 [DOI] [PubMed] [Google Scholar]
- 28.Kim, A., Kiefer, C. M., and Dean, A. (2007) Mol. Cell. Biol. 27 1271-1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert, N., Boyle, S., Sutherland, H., de Las Heras, J., Allan, J., Jenuwein, T., and Bickmore, W. A. (2003) EMBO J. 22 5540-5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao, R., Wang, L., Wang, H., Xia, L., Erdjument-Bromage, H., Tempst, P., Jones, R. S., and Zhang, Y. (2002) Science 298 1039-1043 [DOI] [PubMed] [Google Scholar]
- 31.Bernstein, B. E., Mikkelsen, T. S., Xie, X., Kamal, M., Huebert, D. J., Cuff, J., Fry, B., Meissner, A., Wernig, M., Plath, K., Jaenisch, R., Wagschal, A., Feil, R., Schreiber, S. L., and Lander, E. S. (2006) Cell 125 315-326 [DOI] [PubMed] [Google Scholar]
- 32.Morris, S. A., Shibata, Y., Noma, K., Tsukamoto, Y., Warren, E., Temple, B., Grewal, S. I., and Strahl, B. D. (2005) Eukaryot. Cell 4 1446-1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt, A., Gutierrez, G. J., Lenart, P., Ellenberg, J., and Nebreda, A. R. (2002) FEBS Lett. 518 23-28 [DOI] [PubMed] [Google Scholar]
- 34.Sobel, R. E., Cook, R. G., and Allis, C. D. (1994) J. Biol. Chem. 269 18576-18582 [PubMed] [Google Scholar]
- 35.Chicoine, L. G., Schulman, I. G., Richman, R., Cook, R. G., and Allis, C. D. (1986) J. Biol. Chem. 261 1071-1076 [PubMed] [Google Scholar]
- 36.Allis, C. D., Chicoine, L. G., Richman, R., and Schulman, I. G. (1985) Proc. Natl. Acad. Sci. U. S. A. 82 8048-8052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Megee, P. C., Morgan, B. A., Mittman, B. A., and Smith, M. M. (1990) Science 247 841-845 [DOI] [PubMed] [Google Scholar]
- 38.Ma, X. J., Wu, J., Altheim, B. A., Schultz, M. C., and Grunstein, M. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 6693-6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barski, A., Cuddapah, S., Cui, K., Roh, T. Y., Schones, D. E., Wang, Z., Wei, G., Chepelev, I., and Zhao, K. (2007) Cell 129 823-837 [DOI] [PubMed] [Google Scholar]
- 40.Shechter, D., Nicklay, J. J., Chitta, R. K., Shabanowitz, J., Hunt, D. F., and Allis, C. D. (2009) J. Biol. Chem. 284 1064-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]