Abstract
The first steps of poxvirus DNA synthesis yield concatemeric arrays of covalently linked genomes. The virus-encoded Holliday junction resolvase is required to process concatemers into unit-length genomes for packaging. Previous studies of the vaccinia virus resolvase have been problematic due to poor protein solubility. We found that fowlpox virus resolvase was much more tractable. Fowlpox resolvase formed complexes with a variety of branched DNA substrates, but not linear DNA, and had the highest affinity for a Holliday junction substrate, illustrating a previously unappreciated affinity for Holliday junctions over other substrates. The cleavage activity was monitored in fixed time assays, showing that, as with vaccinia resolvase, the fowlpox enzyme could cleave a wide array of branched DNA substrates. Single turnover kinetic analysis revealed the Holliday junction substrate was cleaved 90-fold faster than a splayed duplex substrate containing a single to double strand transition. Multiple turnover kinetic analysis, however, showed that the cleavage step was not limiting for the full reaction cycle. Cleavage by resolvase was also tightly coupled at symmetrical positions across the junction, and coupling required the complete Holliday junction structure. Last, we found that cleavage of an extruded cruciform yielded a product, which after treatment with ligase, had the properties expected for covalently closed DNA hairpin ends, as is seen for poxvirus genome monomers. These findings provide a tractable poxvirus resolvase usable for the development of small molecule inhibitors.
Poxvirus DNA replication is proposed to proceed by a “rolling hairpin” mechanism to yield linear concatemers, in which genomes are arranged in mostly head-to-head and tail-to tail orientation (Fig. 1, step 1) (1). The terminal sequences at each junction form an inverted repeat, which can be extruded to form a cruciform structure (step 2) (2). Cleavage of the resulting Holliday junctions on each end frees the monomer genome from the concatemer (step 3). The nicks left behind after resolution of the Holliday junction can then be ligated, yielding the hairpin DNA ends characteristic of poxviruses (step 4).
FIGURE 1.
Role of poxvirus resolvase during viral replication. Black lines indicate single DNA strands. Half-arrows indicate repeated sequences. Small arrows indicate resolvase cleavage sites. 1) Poxvirus genome replication yields concatemers; 2) inverted repeat sequences at concatemer junctions extrude to form cruciform structures; 3) Holliday junction cleavage by resolvase at cruciform structures yields unit-length genomes with preserved hairpin ends; 4) ligase seals nicks to yield mature genome monomers.
The vaccinia virus resolvase gene, A22R, was first recognized in bioinformatic surveys to encode a member of the RNase H superfamily of polynucleotide phosphotransfer enzymes (3). These enzymes catalyze attack of a hydroxyl group on a phosphodiester bond, thereby supporting a variety of nuclease or DNA joining reactions. Garcia et al. (3) purified recombinant vaccinia resolvase and showed that it displayed cleaving activity on model Holliday junctions. They also generated a conditional A22R recombinant vaccinia virus and showed that in the absence of A22R expression, vaccinia failed to replicate and concatemer junctions accumulated, indicating that A22 resolvase indeed is required for concatemer resolution in vivo (4). Subsequent studies by Garcia et al. (5) and Culyba et al. (6) showed that vaccinia resolvase had little sequence specificity, and that cleavage yielded a 3′-hydroxyl group suitable for subsequent DNA ligation. Culyba et al. (7) also showed that several further branched DNA molecules could be cleaved by vaccinia resolvase, establishing that the enzyme could potentially process a variety of branched DNA forms expected to arise during recombination or replication, suggesting possible additional roles for poxvirus resolvase.
Progress in studying poxvirus resolvase has been limited by the poor solubility of the purified vaccinia protein. For example, in Garcia et al. (5), the vaccinia resolvase was fused to maltose-binding protein to improve solubility, but consequently the properties of the maltose-binding protein portion of the fusion must be considered in interpreting the results. Pilot studies from our laboratory showed that the insolubility and low activity of the vaccinia virus resolvase precluded its use in high-throughput screens for inhibitors (data not shown).
In an effort to identify a more tractable poxvirus resolvase protein, we attempted to clone four other poxvirus resolvase genes and purify the gene products after overexpression in bacteria. We found that the fowlpox resolvase was much more soluble and active than the others tested. Analysis of cleavage revealed that a wide range of branched DNA forms were substrates, paralleling results with vaccinia resolvase and establishing that these activities are a conserved property of poxvirus resolvases. Binding analysis on these same DNA forms also revealed a strict specificity for branched DNA, with the highest affinity binding for the Holliday junction, suggesting that DNA binding specificity is the major discriminatory mechanism for DNA cleavage activity. Kinetic analysis was feasible with fowlpox resolvase, allowing us to show that the first-order rate constant for strand cleavage under single turnover conditions is 90-fold greater for a Holliday junction substrate than for a splayed duplex substrate. However, this rate constant was not limiting for the Holliday junction under multiple turnover conditions, where the rate of strand cleavage is 1.9-fold slower for the Holliday junction than for the splayed duplex. Last, we show that fowlpox resolvase cleavage at Holliday junctions is coupled, so that nicking on one strand also promoted nicking on the strand located across the junction from it. These studies indicate that fowlpox resolvase is well suited to in vitro analysis and suggests approaches to high-throughput screening for resolvase inhibitors.
EXPERIMENTAL PROCEDURES
Overexpression and Purification of Resolvase Proteins—Yaba monkey tumor virus, Shope fibroma virus, and fowlpox virus were obtained from ATCC and resolvase genes amplified from unpurified viral DNA. The variola resolvase gene was synthesized by site-directed mutagenesis of the vaccinia gene. A vaccinia resolvase gene was also constructed to impart a 15-amino acid N-terminal truncation. Primers were designed to amplify each resolvase open reading frame from the viral genomes (see supplemental Table S1 for primer sequences). Resolvase open reading frames were cloned into pET28a using NdeI and BamHI restriction sites, which added an N-terminal His tag. The Yaba monkey tumor virus resolvase gene could be amplified by PCR, but not cloned into an expression vector, and so was not studied further. The vaccinia virus resolvase gene was amplified using primers that yielded a product containing a C-terminal His tag, which was subcloned into pCR2.1 (Invitrogen). The tagged gene was then cloned into pET29a using NdeI and BamHI restriction sites. Plasmids conferring amino acid changes to resolvase gene products were constructed using the QuikChange site-directed mutagenesis kit (Stratagene).
Proteins were induced to express in Escherichia coli BL21(DE3) Codon plus RIL cells (Stratagene) at 25 °C by addition of 200 μm isopropyl β-d-thiogalactopyranoside. Cells were lysed in Ni-NTA Lysis Buffer (50 mm NaK-phosphate, pH 8.0, 300 mm NaCl) with the addition of lysozyme. After sonication, soluble fractions were purified via nickel-nitrilotriacetate column chromatography. Purified fractions were dialyzed against Buffer A300 (20 mm HEPES, pH 8.0, 300 mm NaCl, 1 mm EDTA, 8.5 mm β-mercaptoethanol, 10% glycerol) and concentrated using a Centriprep centrifugal filter (Millipore). The Shope fibroma virus resolvase could not be identified in cell lysates after induction and in initial trials could not be readily purified, and so was not studied further. SDS-PAGE analysis showed that column chromatography enriched for the fowlpox protein more than for the vaccinia resolvase, variola resolvase, or a mutant of the vaccinia protein with a 15-amino acid N-terminal truncation. The fowlpox virus protein had a specific activity for DNA cleavage ∼100-fold greater than the vaccinia protein. Protein concentrations were quantified by Bradford assay. The 20-kDa band of the purified fowlpox resolvase comprised >90% of the total protein by SDS-PAGE and Coomassie staining.
DNA Cleavage Assays of Oligonucleotide Substrates—DNA substrates were constructed (see supplemental Table S1 for oligonucleotide sequences) and resolvase reactions were carried out and analyzed as previously described (7). Briefly, 44 ng (2 pmol) of fowlpox resolvase protein was added to a solution containing 0.2 pmol of substrate DNA to obtain a final volume of 20 μl. Final solution conditions were 25 mm Tris-HCl, pH 8.0, 100 mm NaCl, 5 mm MgCl2, 100 μg/ml bovine serum albumin, 1 mm dithiothreitol (DTT),4 and 5% glycerol. Reaction mixtures were incubated at 37 °C for 30 min and then stopped with EDTA. Products of the reaction were analyzed by native or denaturing polyacrylamide gel electrophoresis.
Binding Assays—The indicated amount of fowlpox resolvase protein was added to a solution containing 0.02 pmol (1 nm) of 32P-labeled probe DNA to obtain a final volume of 20 μl. The final solution conditions were 10 mm Tris-HCl, pH 7.9, 100 mm NaCl, 1 mm EDTA, and 1% glycerol. For reactions containing poly(dI-dC)·poly(dI-dC) competitor DNA, probe and competitor were mixed first before addition of protein. Binding reactions were incubated at 37 °C for 10 min and then 1/6 volume of a loading dye consisting of 15% Ficoll-400 and 0.1% bromphenol blue was added. Binding reactions were analyzed on 4% polyacrylamide gels.
To compare binding affinities between substrates, we estimated EC50 values using nonlinear regression. Bands were quantified with ImageQuant (GE Healthcare) and the fraction bound was calculated for each condition. For substrates that exhibited two shifted forms on gels, both forms were scored as bound. The normalized data were then plotted as “fraction bound” versus “log[protein]” and fit with a sigmoidal dose-response model in GraphPad Prism (GraphPad Software, Inc.).
Kinetic Assays—For single turnover experiments, fowlpox resolvase protein (≥1 μm) and substrate DNA (1 or 10 nm) were preincubated ≥10 min in the presence of 0.1 mm EDTA. Final solution conditions were 11 mm Tris-HCl, 111 mm NaCl, and 1.1 mm DTT. Under these conditions, all substrate DNA is bound by resolvase enzyme. 18 μl of the above protein-DNA solution was added to 2 μl of 150 mm MgCl2. Reactions were stopped either with 50 mm EDTA or 1.2 m HCl, with similar results obtained using both methods. HCl-quenched reactions were neutralized with NaOH and the DNA was ethanol precipitated in the presence of 1 μg of carrier DNA using standard methods. Products of the reactions were suspended in a formamide loading dye solution, heated to 90 °C, quickly cooled to 4 °C, and analyzed on 8% polyacrylamide gels containing 7 m urea and 25% formamide. To ensure the reactions were being quenched, controls were performed where the stop reagent was added before enzyme addition. We detected no product formation in the control reactions with either the EDTA or HCl stop conditions.
For multiple turnover experiments, fowlpox resolvase protein (100 nm) was added to a substrate DNA solution containing 1 μm unlabeled substrate and 10 nm of the same 32P-labeled substrate DNA in a final volume of 100 μl. Reactions were incubated at 37 °C and aliquots were stopped by 2- or 3-fold dilution into a formamide loading dye solution containing 60 mm EDTA. Reactions were analyzed on formamide containing denaturing-PAGE gels as above. Bands were quantified using ImageQuant (GE Healthcare) and linear regression of the normalized data were performed with GraphPad Prism (GraphPad Software, Inc.).
Plasmid Resolution Assays—Plasmid pUC-AT (New England BioLabs) was propagated in SURE2 E. coli cells (Stratagene) and prepared by alkaline lysis and anion exchange. Different plasmid DNA forms were then separated by agarose-gel electrophoresis and supercoiled monomer was isolated by band excision and gel extraction.
For cleavage site mapping experiments, a solution containing 30 ng/μl of plasmid DNA and either 400 nm fowlpox resolvase or the indicated restriction enzyme was incubated at 37 °C for 1 h. The amount of restriction enzyme used was as recommended by the manufacturer (New England BioLabs). Final solution conditions were 10 mm Tris-HCl, 50-65 mm NaCl, 10 mm MgCl2, 1 mm DTT, 0.5-5% glycerol, pH 7.9. Enzymes were then inactivated by incubation at 65 °C for 20 min. For sequential digestions, the indicated enzymes were added to an aliquot from the first digestion and incubated at 37 °C for 1 h. Reactions were stopped by addition of 1/5 volume of a solution containing 100 mm EDTA, 1% SDS, 200 μg/ml proteinase K, and 0.1% bromphenol blue. Products of the reactions were separated on a 1% agarose gel by electrophoresis in 1× TAE buffer containing 0.5 μg/ml ethidium bromide and visualized under UV light.
For ligation analysis, 2.6 μg of pUC-AT DNA was first treated with either 30 units of AlwNI (New England BioLabs) or 4.8 μg of fowlpox resolvase protein by incubation at 37 °C for 75 min in a final volume of 40 μl. Solution conditions were 50 mm potassium acetate, 20 mm Tris acetate, 10 mm magnesium acetate, 1 mm DTT, pH 7.9. Reactions were then heated at 70 °C for 20 min to inactivate the enzymes and then allowed to cool to room temperature. Next, 400 units of T4 DNA ligase (New England BioLabs) was added to half of each reaction and incubated at room temperature for 30 min before addition of a 1/6 volume alkaline loading dye solution consisting of 300 mm NaOH, 6 mm EDTA, 0.15% bromcresol green, 0.25% xylenecyanol, and 18% Ficoll-400. Products of the reactions were electrophoresed on 0.8% agarose gels under neutral conditions (1× TAE) or under alkaline conditions (50 mm NaOH, 1 mm EDTA). After electrophoresis, the alkaline gel was neutralized with a solution consisting of 1 m Tris-HCl, pH 7.6, and 1.5 m NaCl. DNA was stained with ethidium bromide, and visualized under UV light.
For active-site mutant experiments, a mixture containing 120 nm wild-type fowlpox resolvase protein and the indicated amount of active-site mutant protein was preincubated at either 4 or 37 °C for 15 min and then 2 μl of the mixture was added to a solution containing 400 ng of plasmid DNA to obtain a final volume of 20 μl. Final solution conditions were 40 mm Tris-HCl, pH 7.9, 40 mm NaCl, 6 mm MgCl2, 2 mm spermidine, 10 mm DTT, and 5% glycerol. Reactions were incubated at 37 °C for 12 min and stopped by addition of 1/5 volume of a solution consisting of 250 mm EDTA, 30% glycerol, 1% SDS, 0.1% bromphenol blue, and 200 μg/ml proteinase K. Products of the reactions were separated on 0.8% agarose gels by electrophoresis in 1× TAE buffer containing 0.5 μg/ml ethidium bromide and quantified by densitometry under UV light. For kinetic analysis, reactions were scaled up to 100 μl and reaction aliquots were stopped at the indicated times after enzyme addition. Linear regression of the normalized data were performed with GraphPad Prism (GraphPad Software, Inc.).
RESULTS
Branched DNA Nuclease Activity of Fowlpox Resolvase—Twelve branched and control linear DNA substrates were constructed by annealing oligonucleotides of 50 and 25 nucleotides in length (Fig. 2). Each substrate has one DNA strand 5′-end labeled with 32P to enable visualization by PAGE and autoradiography. We analyzed all of the strands in each substrate by labeling each 5′-end separately.
FIGURE 2.
DNA substrates used in this study. A, single-strand. B, duplex. C, 5′-recess. D, splayed duplex. E, bulge. F, hairpin. G, 5′-flap. H, 3′-flap. I, Y-junction. J, Holliday junction. K, three stranded Holliday-like junction. Thick lines represent single DNA strands. Thin lines represent regions of complimentary base pairing between strands. Filled in circles indicate DNA 5′-ends.
We incubated fowlpox resolvase with each substrate and analyzed the products of the reaction by both native and denaturing PAGE. As controls, we incubated the DNA with either protein storage buffer (i.e. no enzyme) or an active site mutant of fowlpox resolvase, D7N, which inactivates the nuclease activity of the enzyme while leaving its DNA binding activity intact.5 We also included chemical sequencing reactions derived from the same oligonucleotides as size markers.
Linear Substrates—Analysis of the single-stranded and 3′-recessed substrates (Fig. 2, A and C) revealed only a slight resolvase cleavage activity (<6% product). We were unable to detect any DNA cleavage activity on the duplex DNA substrate (Fig. 2B) (data not shown).
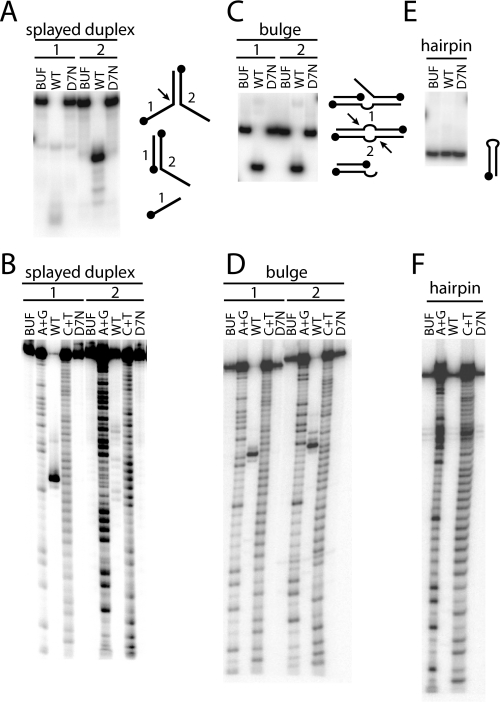
Splayed Duplex, Bulge, and Hairpin Substrates—The splayed duplex substrate consists of two 50-nt oligonucleotides that when annealed create a 25-bp region of complimentary duplex DNA that branches, or “splays,” into two non-complimentary single-stranded regions, each 25 nt in length (Fig. 2D). In contrast to the non-branched DNA substrates, the splayed duplex substrate was completely converted into product in the reaction with fowlpox resolvase (Fig. 3A, WT). We observed no product formation in the control reaction with the D7N protein (Fig. 3A, D7N), allowing us to attribute the observed activity to fowlpox resolvase. Native-PAGE analysis of the products of the reaction in which the substrate had been labeled on strand-2 revealed a faster migrating DNA (Fig. 3A, strand-2). The same analysis on the strand-1 labeled substrate revealed an even faster migrating DNA form (Fig. 3A, strand-1).
FIGURE 3.
DNA cleavage on splayed duplex, bulge, and hairpin substrates. A, native PAGE analysis of splayed duplex substrate. B, denaturing PAGE analysis of splayed duplex substrate. C, native PAGE analysis of bulge substrate. D, denaturing PAGE analysis of bulge substrate. E, native PAGE analysis of hairpin substrate. F, denaturing PAGE analysis of hairpin substrate. Contents of lanes are indicated above the gel picture. BUF, buffer (no enzyme); A+G, purine chemical sequencing reaction; WT, wild-type resolvase; C+T, pyrimidine chemical sequencing reaction; D7N, active-site mutant resolvase. Diagrams to the right of native gels indicate assignment of product bands. Filled in circles indicate DNA 5′-ends. Arrows indicate resolvase cleavage sites. The 32P-5′-end-labeled strand for each substrate is indicated above the gel picture and refers the strand numbers indicated in the diagrams.
Denaturing-PAGE analysis of the same reactions (Fig. 3B) revealed the nuclease activity was specific to strand-1 and the cut-site mapped near the branch point of the DNA junction. We conclude the fowlpox enzyme resolves the splayed duplex substrate into two DNA forms by cleaving off the single-stranded region of strand-1 (Fig. 3A, see diagrams to the right of gel picture).
The bulge substrate consists of a 10-nt bulge flanked on both sides by a region of 20-bp duplex DNA (Fig. 2E). As with the splayed duplex substrate, our native PAGE analysis of the bulge substrate showed conversion of the substrate DNA into faster migrating forms that were specific to the reaction with wild-type resolvase (Fig. 3C). We observed most of the product DNA as a faster migrating form, but also noted a small amount of slower migrating DNA.
In contrast to the splayed duplex substrate, however, denaturing PAGE analysis revealed that both strand-1 and strand-2 were cleaved on the bulge substrate and that the cut-site, for each strand, mapped adjacent to the 3′-side of the bulge (Fig. 3D). We conclude the fowlpox enzyme resolves the bulge substrate into two 5′-recessed molecules and that these represent the fast migrating major products observed in the native gel (Fig. 3C, see diagrams to the right of gel picture). The slower migrating minor form we observed is likely the product of a single DNA cut at one of the bulge branch points. This DNA form would contain a single-stranded flap that would be expected to hinder passage of the DNA through the gel.
The hairpin substrate consists of a 10-nt single-stranded loop constrained by a 20-bp duplex stem region (Fig. 2F). To prepare the hairpin substrate for resolvase reactions, we snap-cooled the DNA after boiling to favor the intra-molecular annealing reaction (hairpin formation) over the inter-molecular reaction. We observed the 50-nt hairpin substrate to migrate much faster than the 50-nt single-stranded substrate in native gels, suggesting proper hairpin formation (data not shown). We were unable to detect any product formation in the resolvase reactions (Fig. 3, E and F).
Flap and Y-junction Substrates—The 5′-flap and 3′-flap substrates are similar to the splayed duplex substrate except in each case one of the two single-stranded regions of the splayed duplex is paired with complimentary DNA (Fig. 2, G and H). For both flap substrates, we observed complete conversion of the substrate DNA into faster migrating product bands in the native gel after incubation with fowlpox resolvase (Fig. 4, A and C).
FIGURE 4.
DNA cleavage on flaps and Y-junction substrates. A, native PAGE analysis of 5′-flap substrate. B, denaturing PAGE analysis of 5′-flap substrate. C, native PAGE analysis of 3′-flap substrate. D, denaturing PAGE analysis of 3′-flap substrate. E, native PAGE analysis of Y-junction substrate. F, denaturing PAGE analysis of Y-junction substrate. Markings are as described in the legend to Fig. 3.
For the 5′-flap substrate labeled on stand-1 we observed rapidly migrating DNA as the major product, whereas the strand-2- and strand-3-labeled substrates exhibited comparatively less mobile bands as the major product. The mobility of the strand-2 and strand-3 product bands in the native gel was indistinguishable from each other and similar to a 50-bp duplex marker that was loaded on the same gel.
Denaturing PAGE analysis of the same reactions revealed the major products resulted from strand-1 cleavage near the DNA branch point, although we also noted some minor cleavage products on strand-2 (Fig. 4B). We conclude that the fowlpox enzyme resolves the 5′-flap substrate by clipping off the single-stranded flap to leave a duplex molecule containing a 1-nt gap (Fig. 4A, see diagrams to the right of gel picture).
For the 3′-flap substrate labeled on strands-1 and strand-3 we observed a rapidly migrating band on the native gel, whereas the strand-2-labeled substrate exhibited a comparatively less mobile band as the major product (Fig. 4C). The mobility of the strand-2 product was slightly faster than a 50-bp duplex marker that was loaded on the same gel, consistent with the 5′-recessed product form discussed below.
Denaturing PAGE analysis of the same reactions revealed the major products resulted from strand-1 cleavage near the DNA branch point, although we also noted some minor cleavage products on strand-2 (Fig. 4D). We conclude the fowlpox enzyme resolves the 3′-flap in a different manner than the 5′-flap substrate. In the case of the 3′-flap, resolution is achieved by cleavage of the continuously paired strand opposite the branch point of the flap (Fig. 4C, see diagram to the right of gel picture).
The Y-junction substrate is similar to the splayed duplex substrate except both single-stranded arms of the splayed duplex are paired with complimentary DNA from a single oligonucleotide to yield a 3-way junction of duplex DNA (Fig. 2I). Previously, we found the Y-junction to be a relatively poor substrate for vaccinia resolvase, but that we could boost cleavage activity by using reaction buffer containing dimethyl sulfoxide (7). Here with fowlpox resolvase, in the absence of dimethyl sulfoxide, we observe complete conversion of the Y-junction substrate into two faster migrating bands on the native gel (Fig. 4E) and note the relative abundance of the two product bands are approximately similar for all three of the differentially 32P-labeled substrates.
Denaturing PAGE analysis of the same reactions revealed all three strands were cleaved to similar extents and the major products of each strand cleavage map near the DNA branch point, although we also note some minor cleavage products on strand-1 and strand-2 as well (Fig. 4F). We conclude the fowlpox enzyme resolves the Y-junction substrate by cutting two of the three strands to release one of the junction arms and leave behind a 50-bp nicked duplex (Fig. 4E, see diagram to the right of gel picture). Given the similar extent of cleavage on each of the three strands, we conclude the enzyme displays little sequence preference for catalyzing the resolution reaction on this substrate.
Holliday Junction and Three-stranded HJ-like Substrates—The Holliday junction substrate used here contains the same two oligonucleotides that comprised the splayed duplex substrate plus two additional oligonucleotides that allow formation of a 4-way junction of duplex DNA (Fig. 2J). The sequences of each of the four arms of the junction are unique and the branch point does not contain homologous sequences that would allow for branch migration. After incubation of each of the four Holliday junctions, labeled separately on each of the four strands, we observed complete conversion of the substrate into a single faster migrating product band on the native gel having mobility consistent with a 50-bp duplex molecule (Fig. 5A).
FIGURE 5.
DNA cleavage on Holliday junctions and Holliday junction-like substrates. A, native PAGE analysis of Holliday junction substrate. B, denaturing PAGE analysis of Holliday junction substrate. C, native PAGE analysis of 3HJ-1 substrate. D, denaturing PAGE analysis of 3HJ-1 substrate. E, native PAGE analysis of 3HJ-2 substrate. F, denaturing PAGE analysis of 3HJ-2 substrate. Markings are as described in the legend to Fig. 3.
Denaturing PAGE analysis revealed all four strands were cleaved near the branch point of the junction with a preference for strand-1 and strand-3. We also noted minor cleavage products on strand-2. We conclude the fowlpox enzyme resolves the Holliday structure into two nicked-duplex molecules, with a preference toward strand-1/3 resolution (75% strand-1/3 versus 25% strand-2/4) on this substrate.
The two Holliday junction-like substrates, 3HJ-1 and 3HJ-2, are identical to the Holliday junction substrate used above, except in each case one of the four strands was omitted from the annealing reaction (Fig. 2K). In the case of 3HJ-1, strand-4 was omitted and in the case of 3HJ-2, strand-3 was omitted. Incubation with 3HJ-1 and 3HJ-2 with fowlpox resolvase resulted in the complete conversion of substrate DNA into multiple faster migrating products on the native gel (Fig. 5, C and E).
Denaturing PAGE analysis of 3HJ-1 (Fig. 5D) revealed that strand-1 was cleaved to near completion with the major product mapping near the branch point of the junction, although we also note some minor cleavage products. In contrast, strand-2 and strand-3 were not cleaved to the same extent. We observed two major strand-2 cleavage products of nearly equal abundance, one of which maps near the junction branch point and the other 6 nt further to the 3′-end of the strand. Finally, we observed two strand-3 cleavage products that both map near the branch point separated by only 1 nt from each other.
Denaturing PAGE analysis of 3HJ-2 (Fig. 5F) revealed that, similar to 3HJ-1, strand-1 was again cleaved to near completion with the major product mapping near the branch point of the junction. Also similar to 3HJ-1, strand-2 and strand-3 were not cleaved to the same extent. We observed several strand-2 cleavage products of near equal abundance that map to the 3′-side of the branch point. Finally, we observed two major strand-3 cleavage products that both map near the branch point separated by only 1 nt from each other.
The results with the three-stranded HJ-like substrates indicate resolution of these junctions by the fowlpox enzyme is different than for the Holliday junction. For the Holliday junction, resolution is achieved via dual-strand scission across the junction branch point (i.e. either strand-1/3 or strand-2/4 cleavage) and results in nicked-duplex products. In contrast, for 3HJ-1 and 3HJ-2, some of the products are indicative of cleavage events similar to those observed for resolution of the Y-junction above, where two cleavage events result in the release of one of the duplex-arms of the junction (Fig. 5, C and E, see diagrams to the right of gel pictures). We conclude coupling of DNA cleavage across a four-armed junction by resolvase requires structural elements present in the Holliday junction, but absent from the Holliday junction-like junctions, indicating the importance of duplex arms for coupled cleavage.
Branched DNA Binding Activity of Fowlpox Resolvase—Our analysis of DNA cleavage by fowlpox resolvase above indicated that the enzyme has a strict specificity for cutting branched DNA forms. However, it remained possible that the substrate requirements for DNA binding were different than for DNA cleavage. We thus performed band-shift DNA binding assays utilizing the same panel of DNA substrates used above.
We prepared protein-DNA complexes by incubating different amounts of protein with a fixed concentration of DNA. To prevent DNA cleavage, we included 1 mm EDTA in the binding reaction buffer to sequester divalent metal ions. We also included a 500-fold excess of unlabeled linear duplex competitor DNA, poly(dI-dC)·poly(dI-dC), to minimize nonspecific interactions. Products of the binding reactions were separated by native PAGE. Our analysis of resolvase binding to the single-stranded and duplex substrates did not reveal any shifted DNA at the lowest three protein concentrations tested, indicating the protein only weakly interacts with the linear substrates (Fig. 6A).
FIGURE 6.
DNA binding on branched and linear DNA substrates. A, band-shift analysis of single-stranded and duplex substrates. B, band-shift analysis of 5′-flap, 3′-flap, and Y-junction substrates. C, band-shift analysis of bulge and splayed duplex substrates. D, band-shift analysis of Holliday junction and Holliday junction-like substrates. Each binding reaction contained 1 nm of the 32P-labeled substrate indicated above the gel and 500-fold excess (w/w) poly[dI-dC]·poly[dI-dC] competitor DNA. From left to right the binding reactions contained 0, 0.1, 0.4, 1.1, 3.2, 9.6 μg of fowlpox resolvase protein.
In contrast to the linear substrates above, the addition of resolvase protein to eight different branched substrates resulted in the formation of discrete slower migrating bands in each case (Fig. 6, B-D). All of these shifted forms were observed at protein concentrations lower than that which produced the shifted DNA for the linear substrates. The presence of 500-fold excess competitor DNA in the binding reactions further underscores the branch-specific nature of the resolvase-DNA interactions.
Here, in the presence of competitor DNA, we found the Holliday junction substrate to have the highest binding affinity by a factor of ∼3-150 relative to the other branched DNA forms (Fig. 6 and data not shown). The relative affinities for the substrates ranked, from highest affinity to lowest affinity, as follows: Holliday junction, 3HJ-2, bulge, Y-junction, 3′-flap, 3HJ-1, 5′-flap, and splayed duplex. In the absence of competitor DNA, we estimated the equilibrium dissociation constant for fowlpox resolvase binding this Holliday junction substrate (Kd < 2 × 10-8 m, data not shown), which is consistent with other Holliday junction resolving enzymes (8-11).
We also noted the appearance of multiple slower migrating bands for the Holliday junction and the three-stranded Holliday junction-like substrates, 3HJ-1 and 3HJ-2 (Fig. 6D). For other Holliday junction resolving enzymes, the appearance of multiple shifted forms has been attributed to additional, weaker protein-binding sites located on junction arms outside the branch point (12). In the absence of competitor DNA, we also observe multiple shifted bands for the splayed duplex substrate (data not shown), which is consistent with this idea and indicates the presence of multiple complexes is not specific to Holliday-like structures.
Kinetic Analysis of Holliday Junction and Splayed Duplex Substrates—The improved in vitro properties of fowlpox resolvase allowed us to determine the first-order rate constant for DNA cleavage under single turnover conditions. We preincubated the strand-1 32P-labeled splayed duplex and Holliday junction substrates with saturating amounts of fowlpox resolvase in the presence of 0.1 mm EDTA to prevent DNA cleavage. Reactions were started by addition of MgCl2 and stopped with EDTA at various times. Product formation was assessed by denaturing PAGE and a plot of ln[total/uncut] versus time is expected to be linear with the slope of the line corresponding to the first-order rate constant.
The splayed duplex reactions were carried out at room temperature and the plot of ln[total/uncut] versus time revealed a linear relationship (Fig. 7, A and B). Decreasing the concentration of enzyme did not reduce the rate of cleavage, thus confirming single turnover conditions. Using linear regression, we estimate the first-order rate constant to be 0.0025 s-1 (95% confidence interval (CI): 0.0024, 0.0027).
FIGURE 7.
Kinetic analysis of splayed duplex and Holliday junction strand-1 cleavage. A, denaturing PAGE analysis of strand cleavage under single turnover conditions. Both substrates were 32P-labeled on strand-1. The substrate indicated above the gel lanes was pre-bound under saturating conditions and reactions were started by the addition of MgCl2. Reactions were stopped at the time (seconds) indicated above each lane. Substrate (uncut) and product (cut) bands are indicated to the right of the gel picture. Although resolution of the Holliday junction is complete by 10 s, the remaining substrate is expected because resolution also occurs via cleavage of the adjacent unlabeled strands. B, determination of first-order rate constant for cleavage of the splayed duplex substrate under single turnover conditions. Reducing the concentration of protein in the reaction did not result in a drop in rate, confirming single turnover conditions. For the 8 μm enzyme reactions, data points and error bars represent the mean ± S.E., respectively, of three independent reactions. For the 4 μm enzyme reactions, data points and error bars represent the mean ± S.E., respectively, of 1 or 2 independent reactions. For the 2 and 1 μm enzyme reactions, data points represent the value of 1 reaction. The line indicates the line of best fit for linear regression of all the data, the slope of which is an estimate of the first-order rate constant. C, determination of first-order rate constant for cleavage of the Holliday junction substrate under single turnover conditions. For the room temperature reactions (RT), data points and error bars represent the mean ± S.E., respectively, of two independent reactions. For the 4 °C reactions (4 °C), data points represent the value of 1 reaction. The dotted line indicates maximum strand-1 cleavage (75% cleaved). The thick black line indicates the line of best fit for linear regression of the room temperature reactions using the 0- and 5-s time points, the slope of which is an estimate of the first-order rate constant. The thick gray line indicates the line of best fit for linear regression of the 4 °C reactions using the 0-, 5-, and 10-s time points, the slope of which is an estimate of the first-order rate constant. D, denaturing PAGE analysis of strand cleavage under multiple turnover conditions. Both substrates were 32P-labeled on strand-1. Substrates are indicated above the gel pictures. Reactions were started by addition of 100 nm enzyme to 1 μm substrate and were stopped at the time (min) indicated above each lane. Substrate (uncut) and product (cut) bands are indicated to the right of the gel picture. E, determination of initial rate of strand cleavage under multiple turnover conditions. Data points and error bars represent the mean ± S.E., respectively, of 3 independent reactions. The thick black and gray lines indicate the lines of best fit for linear regression of the 0- and 5-min time points for the splayed duplex and Holliday junction, respectively, the slopes of which are an estimate of the initial rate of strand cleavage.
Analysis of Holliday junction strand cleavage revealed the reaction is complete within 10 s at room temperature and within 15 s at 4 °C (Fig. 7, A and C). Therefore, we used the data collected at earlier times, prior to completion of the reaction, to estimate the first-order rate constants. Using linear regression, we estimate the rate constants at room temperature and 4 °C to be 0.23 s-1 (95% CI: 0.19, 0.26) and 0.10 s-1 (95% CI: -0.16, 0.36), respectively, thus revealing that the Holliday junction rate constant is ∼90-fold greater than the splayed duplex rate constant at room temperature (p < 0.0001).
Next, we compared the two substrates under multiple turnover conditions where the cleavage rate is determined by the limiting step in the full catalytic cycle of substrate binding, catalysis, and product release. Reactions were carried out at 37 °C using 100 nm enzyme and excess substrate DNA (1 μm). Reactions were started by addition of enzyme and stopped with EDTA at various times (Fig. 7, D and E). The 0- and 5-min time points were used to estimate the initial velocities of the reactions. Using linear regression, we estimate the initial velocities for the splayed duplex and Holliday junction to be 0.38 nm s-1 (95% CI: 0.35, 0.41) and 0.20 nm s-1 (95% CI: 0.16, 0.24), respectively, thus revealing that the splayed duplex rate is ∼1.9-fold greater than the Holliday junction rate (p < 0.0001). Assuming enzyme dimers, we calculate the turnover under these conditions to be 0.0076 and 0.0040 s-1 for the splayed duplex and Holliday junction, respectively. Taken together, the kinetic data imply the rate-limiting step for the Holliday junction under multiple turnover conditions is not strand cleavage within the enzyme-substrate complex.
A22 Resolvase Activity on a Cruciform-containing Plasmid Substrate—Mapping and analysis of poxvirus resolvase cut sites on model Holliday junctions consisting of four annealed oligonucleotides revealed that: 1) resolution of the DNA junction into two nicked-duplex products occurs by dual-strand scission across the junction branch point, and 2) a DNA 3′-OH is formed as a product of the phosphodiester bond cleavage (5-7). We therefore anticipated the product of poxvirus resolvase acting on a plasmid DNA containing an extruded cruciform structure would: 1) contain hairpin ends with DNA nicks derived from cleavage of the Holliday structure, and 2) those nicks could be sealed closed by treatment with DNA ligase yielding hairpin ends resembling those formed in poxvirus genomes (Fig. 8A). We thus tested a plasmid, pUC-AT, which contains a 40-bp insert consisting of 20 consecutive AT-dinucleotides known to be extruded as a cruciform structure under negative superhelical tension (depicted schematically in Fig. 8A as supercoiled) (13, 14). We separated the products of resolvase reactions on agarose gels via electrophoresis and visualized the DNA by staining with ethidium bromide.
FIGURE 8.
Characterization of the cruciform-containing plasmid resolution product. A, schematic of ligation assay. Each line represents a single DNA strand of a double-stranded DNA plasmid. Filled in circles represent DNA 5′-ends. Supercoiled, under superhelical tension plasmid pUC-AT extrudes a cruciform structure, which at its base contains a Holliday junction that serves as a substrate for fowlpox resolvase (A22); linear, dual-strand scission across the Holliday junction preserves the hairpin termini of the cruciform structure; closed linear, the product of ligation by T4 DNA ligase has sealed nicks and is expected to migrate as a single-stranded circle under denaturing electrophoresis conditions. B, restriction enzyme mapping of the resolvase cut site on pUC-AT. pUC-AT was incubated with fowlpox resolvase and/or different restriction enzymes and the products of the reactions were analyzed by agarose gel electrophoresis. The enzyme conditions used in each reaction are indicated above the gel lanes (-, no enzyme; A22, fowlpox resolvase; A, AlwNI; N, NdeI; X, XmnI; XN, XmnI + NdeI). Gel lanes where two conditions, upper and lower, are seen indicate sequential digestions. The upper label indicates the first digestion and the lower label indicates the second digestion. 1kb, 1-kb DNA ladder standard; 100 bp, 100-bp DNA ladder standard. Numbers on the side of the gel picture indicate the size (kbp) of the DNA standards. Plasmid map: pUC-AT has a total length of 2,693 bp. Restriction sites are indicated with a line and labeled as above. The black box indicates the 40-bp AT-repeat region. The numbers in parentheses next to each feature indicates the position, in base pairs, of that feature relative to an origin common to all the features. The numbers located between features indicate the distance, in base pairs, between those features. C, analysis of ligation products via native agarose gel electrophoresis. Products were electrophoresed under neutral pH conditions to preserve base pairing. Lane contents are indicated above the gel picture. Product assignments are indicated to the right of the gel picture. The arrow indicates the expected size of a linear dimer. D, analysis of ligation products via denaturing agarose gel electrophoresis. Products were electrophoresed under alkaline pH conditions to disrupt base pairing. Lane contents are indicated above the gel picture. Product assignments are indicated to the right of the gel picture. The arrow points the unique ligation product mentioned in the text that is suspected to be migrating as a single-stranded circle. Buf, buffer-treated pUC-AT; Alw, AlwNI-treated pUC-AT; A22, fowlpox resolvase-treated pUC-AT.-lig: no subsequent treatment with T4 DNA ligase; +lig: subsequent treatment with T4 DNA ligase.
Fowlpox resolvase incubated with supercoiled pUC-AT DNA yielded a linear monomeric form of the plasmid as the predominant product (Fig. 8B, compare lane 3 to lane 2). In contrast, we observed no product formation when we incubated fowlpox resolvase with supercoiled pUC19 DNA (i.e. a plasmid lacking the AT-repeat sequence) (data not shown). Similarly, we observed no product formation in reactions with linear pUC-AT (Fig. 8B, lane 5) or pUC-AT DNA that had been relaxed with a type IB topoisomerase (data not shown). These controls indicate the observed resolvase activity is dependent upon the presence of the AT-repeat sequence as well as the topological state of the plasmid DNA, confirming the expectation that the AT-repeat sequence is present as a cruciform structure only when the plasmid DNA is sufficiently supercoiled.
To confirm that fowlpox resolvase cuts pUC-AT at the AT-repeat sequence, we used restriction enzymes to map the cut site (Fig. 8B). Each of the restriction enzymes used cuts at a unique site in pUC-AT (Fig. 8B, see plasmid map). We incubated pUC-AT that had been pretreated with fowlpox resolvase (Fig. 8B, lane 3) with the different restriction enzymes and separated the products of the reactions by agarose gel electrophoresis next to standard DNA size markers. As controls, we also incubated the restriction enzymes with supercoiled pUC-AT that had not been treated with fowlpox resolvase. As expected, the control incubations of pUC-AT with each restriction enzyme alone yielded a product with the size of a linear monomer of the plasmid (Fig. 8B, lanes 4, 7, and 9) and digestion with a combination of two enzymes yielded two products of the expected sizes (Fig. 8B, lane 11). When we used the restriction enzymes to digest pUC-AT that had been pretreated with fowlpox resolvase, we observed the formation of discrete, faster migrating bands in each case (Fig. 8B, lanes 6, 8, 10, and 12). The number and sizes of these newly formed bands confirm that the location of the major fowlpox resolvase cut site is indeed within or adjacent to the AT-repeat sequence.
To ascertain the structure of the observed cruciform resolution product, we treated the products of a pUC-AT resolvase reaction with T4 DNA ligase and then analyzed the ligation products by both native and denaturing agarose gel electrophoresis (Fig. 8, C and D, respectively). As controls, we also analyzed ligation products of pUC-AT DNA that had been previously incubated with buffer (i.e. no enzyme) or AlwNI, a restriction enzyme that cuts at a unique site in pUC-AT.
Buffer-treated pUC-AT DNA revealed no changes in mobility after the ligase incubation in either of the two electrophoresis conditions (Fig. 8C, compare lane 3 to lane 6; and D, compare lane 1 to lane 4) and AlwNI-treated pUC-AT exhibited reduced mobility after ligase incubation under both electrophoresis conditions (Fig. 8C, compare lane 4 to lane 7; and D, compare lane 2 to lane 5).
Analysis of fowlpox resolvase-treated pUC-AT by native gel electrophoresis showed the expected linear size after ligase treatment (Fig. 8C, compare lane 5 to lane 8). We observed only a slight amount of slower migrating DNA with the mobility of a linear dimer ligation product (Fig. 8C, arrow). Denaturing gel electrophoresis, however, revealed the majority of ligation product migrated more slowly than the ligation-minus control and as a discrete band (Fig. 8D, compare lane 3 to lane 6). Furthermore, the mobility of the ligation product is distinct from the mobility of the AlwNI controls, indicating formation of a different DNA form (Fig. 8D, compare lanes 2 and 5 to lane 6; arrow indicates the unique ligation product). These data are consistent with production of a linear monomer of plasmid DNA with closed hairpin termini that migrates slower after denaturation into a single-stranded circle. Thus, we infer that the product of DNA cruciform resolution by fowlpox resolvase is a linear monomer with two intact hairpins, each containing a nick on the hairpin-stem that can be sealed by T4 DNA ligase. These results reconstruct the hairpin formation pathway, as takes place in vivo, and demonstrate the presence of a 5′-PO4 after resolvase cleavage.
Dual-strand Scission Is Highly Coupled through an Oligomer of A22 Resolvase—Resolvase cleavage of pUC-AT requires dual-strand scission (i.e. a DNA cut on each of the two strands of the plasmid). In contrast, a nick in only one strand would release superhelical tension in the DNA, causing both the removal of supercoils and the reabsorption of the cruciform, thus yielding a relaxed DNA circle containing a single nick (Fig. 9A, top pathway).
FIGURE 9.
Analysis of cruciform-containing plasmid resolution using wild-type and active-site mutant enzyme mixtures. A, potential pathways to observed products. Each line represents a single DNA strand of a double-stranded DNA plasmid. Filled in circles represent DNA 5′-ends. Supercoiled, under superhelical tension plasmid pUC-AT extrudes a cruciform structure, which at its base contains a Holliday junction that serves as a substrate for resolvase; nicked, a single DNA cut (one cut) releases superhelical tension allowing for removal of supercoils and reabsorption of the cruciform; linear, if both DNA cuts (one cut + second cut) precede cruciform reabsorption then the hairpin termini of the cruciform structure are retained in the final linear product. B, analysis of wild-type and active site mutant enzyme mixtures. The active site mutant enzyme (D7N) was titrated over a fixed amount of wild-type (WT) enzyme and the various enzyme mixtures were preincubated at the temperature indicated to the left of the gel picture. The mixtures were then incubated with pUC-AT and the products of the reactions were analyzed by agarose gel electrophoresis. Markers: lanes containing supercoiled (sc), linear (lin), or nicked (nk) marker DNA are indicated; D7N lane, active-site mutant only; WT lane, wild-type resolvase only; D7N/WT lanes, D7N/WT molar ratios are indicated above the gel lanes. C, quantification of wild-type and active-site mutant enzyme mixtures. Bands from ethidium bromide-stained gels, as in B, were quantified and the fraction of product nicked plotted versus the logarithm of the D7N/WT molar ratio. For the 4 °C reactions (4 °C), data points represent the value of one reaction. For the 37 °C reactions (37 °C), data points and error bars represent the mean ± S.E., respectively, of two independent reactions. D, kinetic analysis of reactions with wild-type and mutant enzyme mixtures. Wild-type enzyme or a mixture of wild-type and active-site mutant enzyme (D7N/WT ratio = 81) was added to pUC-AT and reaction aliquots were stopped at the times (min) indicated above the gel lanes. Aliquots were analyzed via agarose gel electrophoresis next to lanes containing DNA markers. Markers: lanes containing supercoiled (sc), linear (lin), or nicked (nk) marker DNA are indicated. Wild-type (WT) and wild-type and mutant enzyme mixture (mix) reactions are indicated to the left of the gel pictures. E, quantification of kinetic analysis. Bands from ethidium bromide-stained gels, as in D, were quantified and the percent of total DNA for both nicked and linear DNA species was plotted versus reaction time (min). Black squares, nicked product of WT reaction (wt-nk); black triangles, linear product of WT reaction (wt-lin); gray squares, nicked product of WT/D7N reaction (het-nk); gray triangles, linear product of WT/D7N reaction (het-lin). Data points and error bars represent the mean ± S.E., respectively, of two independent reactions. Lines connect data points as indicated in the legend.
Previous studies with other resolvases suggest both cuts are made in a highly cooperative fashion to form the observed linear-hairpin product before cruciform reabsorption can take place (15, 16). The second-strand cleavage rate is enhanced relative to the first-strand cleavage rate, thus ensuring productive resolution and limiting the amount of nicked form produced (Fig. 9A, bottom pathway) (16). Vaccinia resolvase has previously been shown to bind the Holliday structure as a dimer (5), however, the poor behavior of vaccinia resolvase in solution has hampered interrogation of the degree of dual-strand scission coupling. Thus, we took advantage of the fowlpox resolvase system to address this question.
As has been shown for other Holliday junction resolving enzymes, we reasoned that mixtures of the wild-type (WT) and active site mutant (D7N) enzymes would force accumulation of the nicked product if WT:D7N heterodimers bind and catalyze cleavage of a single DNA strand (15, 16). To test this, we preincubated WT:D7N mixtures of varying molar ratios at either 4 or 37 °C and then added the mixture to pUC-AT DNA. Reactions were stopped after 12 min and loaded on native gels next to markers for supercoiled, linear, and nicked pUC-AT DNA (Fig. 9, B and C).
For the enzyme mixtures preincubated at 37 °C, we observed greater amounts of nicked product with larger D7N/WT molar ratios (Fig. 9, B and C, 37 °C). Because we were unable to observe any nuclease activity from the control D7N-only reaction, we infer the nicking activity observed in the WT:D7N mixtures is attributable to a WT:D7N heterodimer. In contrast, for the enzyme mixtures preincubated at 4 °C, we observed no accumulation of nicked product with larger D7N/WT molar ratios (Fig. 9, C and D, 4 °C). These data indicate subunit mixing is slower than binding and catalysis under the reaction conditions used, because a preincubation step is required to observe the inferred heterodimer activities.
We compared the kinetic profiles of the WT-only and a WT:D7N mixture reaction (Fig. 9, D and E). WT resolvase, or the mixture, was preincubated at 37 °C to allow subunit mixing and then added to a solution containing pUC-AT DNA. Reaction aliquots were quenched at various times after enzyme addition and the products of the reaction were analyzed as above. We estimated initial rates of product formation using the 0- and 1-min time points. For the WT-only reaction, the rate of linear product formation was ∼14-fold greater than the rate of nicked product formation (p = 0.0003). In contrast, for the WT:D7N mixture reaction, the rate of linear product formation was ∼1.9-fold slower than the rate of nicked product formation (p = 0.03). Taken together, these data indicate dual-strand scission for fowlpox resolvase is coupled through a protein oligomer with the second cut being faster than cruciform reabsorption, therefore suggesting dual-cleavage occurs within the lifetime of the protein-DNA complex.
DISCUSSION
Previous efforts to study poxvirus resolvase have focused on the protein encoded by vaccinia virus, however, studies of the purified vaccinia protein were problematic due to the poor solubility of the enzyme. These properties have prevented a more detailed analysis of poxvirus resolvase, including its DNA binding specificity, DNA cleavage kinetics, its activity on cruciforms, and its ability to coordinate dual-strand scission across the Holliday structure. We identified fowlpox resolvase as a more biochemically tractable poxvirus resolvase and here we report our findings on these more detailed analyses. The fowlpox resolvase system has also allowed us to study the enzyme active site and the role of divalent metal-ions in catalysis.5 An alignment of the vaccinia and fowlpox resolvase sequences reveals the proteins have 43% amino acid identity (supplemental Fig. S1). Because we used similar methods to purify the two proteins, the differences we observe in solubility and activity are likely a result of the differing amino acid compositions, as opposed to an artifact of the purifications.
DNA binding and cleavage studies with a panel of different branched and linear DNA substrates revealed the relationship between DNA binding and catalysis for poxvirus resolvase. Our DNA cleavage studies demonstrated the enzyme has a strict specificity for cleaving branched DNA forms. One possibility was that DNA branches were important only to turn on catalysis within a pre-formed protein-DNA complex, but not for formation of the complex itself. However, we observed protein-DNA complexes for all the branched substrates we tested, but for none of the linear substrates. These data indicate recognition of DNA branches via protein-DNA complex formation is the mechanism that determines the catalytic specificity for branched DNA. In addition, our data revealed the enzyme had the highest affinity binding for the Holliday junction DNA, revealing a previously unappreciated specificity for the Holliday structure by this enzyme.
Mapping of DNA cut sites on a variety of branched DNA substrates revealed the relationship between cleavage site selection by the fowlpox enzyme and the DNA branch point. For all of the substrates, the DNA cleavage site is located 1-nt from the branch point toward the 3′-end of the scissile strand and has a distinct polarity. This polarity is best illustrated by inspection of the cleavage site for the splayed duplex substrate, but was consistent among all the branched DNAs studied. Cleavage of the splayed duplex occurs only on one of the two strands that comprises the substrate (i.e. cleavage is polarized), the strand with the single-stranded 5′-end. Furthermore, this polarity is not altered by adding complimentary sequence to either of the single-stranded regions of the splayed duplex, as illustrated with the 5′-flap and 3′-flap substrates, where we continue to observe cleavage exclusively on the same DNA strand as we observed with the splayed duplex substrate. These findings parallel exactly our previous results with vaccinia resolvase (7), indicating that all these features are conserved among poxvirus resolvases. We conclude the splayed duplex motif present within any given branched DNA substrate is sufficient to determine the location of the cleavage site within the substrate.
The fowlpox system allowed kinetic analysis of DNA cleavage, which revealed differences among the branched DNA substrates. We observed a 90-fold higher rate of strand cleavage for the Holliday junction than for the splayed duplex under single turnover conditions. These data suggest specific contacts to the Holliday junction by the enzyme within the protein-DNA complex act to enhance the catalytic rate. Under multiple turnover conditions, however, we found the rate of strand-cleavage to be 1.9-fold higher for the splayed duplex than for the Holliday junction. These data imply that DNA cleavage within the enzyme-substrate complex is not rate-limiting for the Holliday junction under conditions mandating a full catalytic cycle of substrate binding, cleavage, and product release. Nevertheless, cleavage of Holliday junction or splayed duplex was rapid compared with the time required for poxvirus DNA replication. This implies the many types of branched DNAs likely to arise during replication and recombination of viral DNA may be substrates for poxvirus resolvase in vivo, where the enzyme could play a general DNA-debranching role.
The fowlpox system also allowed studies of a cruciform-containing plasmid substrate and revealed dual-strand scission across the Holliday structure is tightly coupled through an oligomer of poxvirus resolvase. Incubation of fowlpox resolvase with the cruciform substrate resulted in a predominantly linear product, indicating that both DNA strands must be cut before the cruciform is reabsorbed. This indicates that dual-strand scission by fowlpox resolvase occurs within the lifetime of the protein-DNA complex. Consistent with dual-strand scission occurring across the Holliday structure, our ligation analysis confirmed the linear product had intact hairpin ends. Furthermore, we observed that mixtures of wild-type and an active-site mutant resolvase resulted in increased nicking of the plasmid substrate and that this heterodimer complementation required preincubation of the enzyme mixture. This indicates that an oligomer of resolvase is acting at the Holliday structure and that subunit exchange is slow compared with binding and catalysis. Garcia et al. (5) found that a vaccinia resolvase binds to Holliday junctions as a dimer, suggesting the tight coupling we observe is occurring through a dimer of resolvase. These findings parallel the results of similar experiments with other Holliday junction resolvases, such as bacterial RuvC (16).
The poxvirus Holliday junction resolving enzyme offers an attractive drug target. It is conserved among all poxviruses and is essential for viral replication. Furthermore, poxvirus resolvase is a member of the same superfamily of enzymes as HIV integrase, and a small molecule inhibitor targeting integrase was recently FDA approved for the treatment of HIV infection (17). We find the purified fowlpox protein to be well suited for high-throughput screening5 and expect our characterization of substrate binding and catalysis will help design screening assays.
Supplementary Material
Acknowledgments
We are grateful to members of the Bushman laboratory for help and suggestions.
This work was supported, in whole or in part, by National Institutes of Health NIAID Mid-Atlantic Regional Center of Excellence for Biodefense Research Grant U54 AI057168. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Fig. S1.
Footnotes
The abbreviations used are: DTT, dithiothreitol; nt, nucleotide(s); CI, confidence interval; WT, wild type; HIV, human immunodeficiency virus.
M. J. Culyba, Y. Hwang, N. Minkah, and F. D. Bushman, unpublished data.
References
- 1.Moss, B. (2001) in Virology (Fields, B. N., ed) pp. 2637-2672, Lippincott-Raven, Philadelphia
- 2.Dickie, P., Morgan, A. R., and McFadden, G. (1987) J. Mol. Biol. 196 541-558 [DOI] [PubMed] [Google Scholar]
- 3.Garcia, A. D., Aravind, L., Koonin, E. V., and Moss, B. (2000) Proc. Natl. Acad. Sci. U. S. A 97 8926-8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia, A. D., and Moss, B. (2001) J. Virol. 75 6460-6471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia, A. D., Otero, J., Lebowitz, J., Schuck, P., and Moss, B. (2006) J. Biol. Chem. 281 11618-11626 [DOI] [PubMed] [Google Scholar]
- 6.Culyba, M. J., Harrison, J. E., Hwang, Y., and Bushman, F. D. (2006) Virology 352 466-476 [DOI] [PubMed] [Google Scholar]
- 7.Culyba, M. J., Minkah, N., Hwang, Y., Benhamou, O. M., and Bushman, F. D. (2007) J. Biol. Chem. 282 34644-34652 [DOI] [PubMed] [Google Scholar]
- 8.Bennett, R. J., Dunderdale, H. J., and West, S. C. (1993) Cell 74 1021-1031 [DOI] [PubMed] [Google Scholar]
- 9.White, M. F., and Lilley, D. M. (1997) J. Mol. Biol. 266 122-134 [DOI] [PubMed] [Google Scholar]
- 10.Giraud-Panis, M. J., and Lilley, D. M. (1996) J. Biol. Chem. 271 33148-33155 [DOI] [PubMed] [Google Scholar]
- 11.Giraud-Panis, M. J., and Lilley, D. M. (1998) J. Mol. Biol. 278 117-133 [DOI] [PubMed] [Google Scholar]
- 12.White, M. F., and Lilley, D. M. (1996) J. Mol. Biol. 257 330-341 [DOI] [PubMed] [Google Scholar]
- 13.Greaves, D. R., Patient, R. K., and Lilley, D. M. (1985) J. Mol. Biol. 185 461-478 [DOI] [PubMed] [Google Scholar]
- 14.Parkinson, M. J., and Lilley, D. M. (1997) J. Mol. Biol. 270 169-178 [DOI] [PubMed] [Google Scholar]
- 15.Fogg, J. M., Schofield, M. J., Declais, A. C., and Lilley, D. M. (2000) Biochemistry 39 4082-4089 [DOI] [PubMed] [Google Scholar]
- 16.Fogg, J. M., and Lilley, D. M. (2000) Biochemistry 39 16125-16134 [DOI] [PubMed] [Google Scholar]
- 17.Summa, V., Petrocchi, A., Bonelli, F., Crescenzi, B., Donghi, M., Ferrara, M., Fiore, F., Gardelli, C., Gonzalez Paz, O., Hazuda, D. J., Jones, P., Kinzel, O., Laufer, R., Monteagudo, E., Muraglia, E., Nizi, E., Orvieto, F., Pace, P., Pescatore, G., Scarpelli, R., Stillmock, K., Witmer, M. V., and Rowley, M. (2008) J. Med. Chem. 51 5843-5855 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.