Abstract
Convective delivery of nutrients is important to enhance mass transport within tissue engineered (TE) products. Depending on the target tissue, an ideal TE product will have an integrated microvasculature that will eliminate mass transport limitations that can occur during product growth in vitro and integration in vivo. A synthetic approach to develop microvasculature involves development of network designs with efficient mass transfer characteristics. In this paper, utilizing a planar bifurcating network as a basis, we develop an approach to design optimal flow networks that have maximum mass transport efficiency for a given pressure drop. We formulated the optimization problem for a TE skin product, incorporating two types of duct flow, rectangular and square, and solved using a generalized reduced gradient algorithm. Under the conditions of this study, we found that rectangular ducts have superior mass transport characteristics than square ducts. Microvascular area per volume values obtained in this work are significantly greater than those recorded in the literature. We discuss the effect of network variables such as porosity and generations on the optimal designs. This research forms the engineering basis for the rational development of TE products with built-in microvasculature and will pave the way to design complex flow networks with optimal mass transfer characteristics.
Keywords: Optimization, Mass Transport, Nutrient Transport, Tissue Engineering, Microfabrication, Vascular Engineering, Microvascular Engineering, Flow Networks, Duct Flow, Mathematical Modeling
1. Introduction
Tissue engineered (TE) products have the potential to revolutionize health care. Tissue engineering strategies include[19] application of cells directly to the site of injury, for example, injection of chondrocytes to treat cartilage defects[2], use of cells in matrices, for example, use of hepatocytes in collagen gel to treat fulminant hepatic failure[6], and tissue regeneration templates such as collagen-GAG in the treatment of burn injuries[5,7]. Currently, a number of tissues and organs in the human body such as cartilage, bones, heart valves, blood vessels, skin and liver are investigated as targets for tissue engineering.
Despite the potential and advances in tissue engineering, only a handful of TE products such as TE skin are currently in clinical use. In addition, almost all TE products currently in use or undergoing clinical trials are either targeted at avascular tissues (e.g. heart valves and cartilage), or are not vascularized before their use in the clinics (e.g. skin substitutes). A major reason for this is the absence of a built-in microvascular system for convective delivery of nutrients and removal of metabolic waste products[12,14]. To address the problem of nutrient limitations in TE products, porous scaffolds have been employed instead of solid matrices to enhance nutrient transfer in TE products. These are usually made of biodegradable synthetic polymers and have found extensive use in the development of TE connective tissues such as cartilage and ligaments[3,21,28,37]. Scaffold materials that deliver angiogenic factors to stimulate and increase neovascularization of the graft have also been studied[26,31]. However, in all such products, nutrient mass transfer still occurs primarily by passive diffusion until the product is vascularized in vivo through angiogenesis.
The lack of a functioning vascular network presents a major barrier for the engineering of thick three-dimensional tissues. Until now, there have been only a few attempts in creating microvasculature in vitro. Hepatocytes and endothelial cells were grown on silicon-machined microchannels by Kaihara, et al.[15] for TE liver development. Powers, et al.[29,30] showed approaches for a microfabricated perfused TE liver by micropatterning hepatocytes with flow channels. Ko and Iwata[18] have attempted to reconstruct threedimensional tissue with capillaries using hollow fibers. More recently, Neumann, et al. [27] examined the possibility of creating a smooth muscle cell based microvessel system perfused in vitro for tissue engineering applications. In the above approaches, the design of vascular flow channels was not linked to the mass transfer requirement of the tissue construct.
The last couple of decades have seen numerous efforts garnered towards exploring the use of mathematical modeling approaches based on optimization techniques for the computer simulation of arterial trees and microvascular growth and remodeling. In order to perform the complex energy-intensive task of supplying blood to every tissue at physiologically required levels of pressure and flow, it is supposed that the arterial tree system must have been governed by certain optimization principles during phylogeny[35]. One approach for constructing arterial tree models on the computer is through the Constrained Constructive Optimization (CCO) method[16,34]. Almost all the optimization strategies based on this method deal with minimizing the total intravascular volume of blood, subject to geometric constraints and boundary conditions on pressure drop and flow. Other optimization strategies are based on discrete mathematical modeling[24], truss-type topology optimization[17] and combined geometric and functional vascular growth models[11]. However, none of these methods have paid any attention to the optimization of mass transport characteristics of the vascular networks. Moreover, the results of these models and other angiogenesis-based models[22,23] are not readily transferable to the formulation of tissue constructs with micro flow channels. Diffusion and transport of metabolites and waste products to and from the vasculature depend to a large extent on the net surface area. In order to address our original transport problem of maximizing nutrient transfer in a piece of tissue, it is essential to optimize the net surface area per unit volume of the tissue space.
In this paper, we describe the first step towards the rational design of vascular flow networks with mass transport characteristics that would meet the metabolic demand of tissues, through principles of optimization and mathematical modeling. Here we formulate an overall optimization model in the micro-scale for a two-dimensional bifurcating flow network design. We solve the model using a generalized reduced gradient methodology to obtain optimal network designs with maximal mass transport characteristics. We investigate two types of network geometries, rectangular ducts and square ducts, choosing skin as our tissue model. We explore the effect of network variables such as geometry, porosity and generations on the optimal network designs and their mass and fluid transport characteristics. The network designs are found to exhibit significantly greater microvascular area per volume values than those recorded in the literature. Further, the rectangular duct designs are characterized by ease of microfabrication as well as superior mass transfer efficiencies than the corresponding square ducts.
2. Mathematical Model
Our main objective is to rationally design vascular networks of micron dimensions with optimal transport characteristics to be integrated with TE scaffolds. In this section we develop a mathematical model to answer the following fundamental question: what fluid transport design will result in maximum nutrient transport in a piece of tissue, T(x,y,z)? We start by choosing our basic network design to consist of dichotomous branching of capillaries in a planar geometry, a schematic of which is shown in Figure 1A. The planar geometry as opposed to a three-dimensional geometry was chosen as current techniques do not allow microfabrication of micron precision three-dimensional structures in biopolymeric matrices. In addition, a duct geometry instead of a circular one was chosen for a similar reason that circular geometries are harder to microfabricate. It should be mentioned, however, that three-dimensional networks will have several advantages over two-dimensional networks. For example, fluid distribution is an important issue in several engineering processes such as separations and reactions. A flow network that optimizes fluid distribution will be, in practice, three-dimensional.
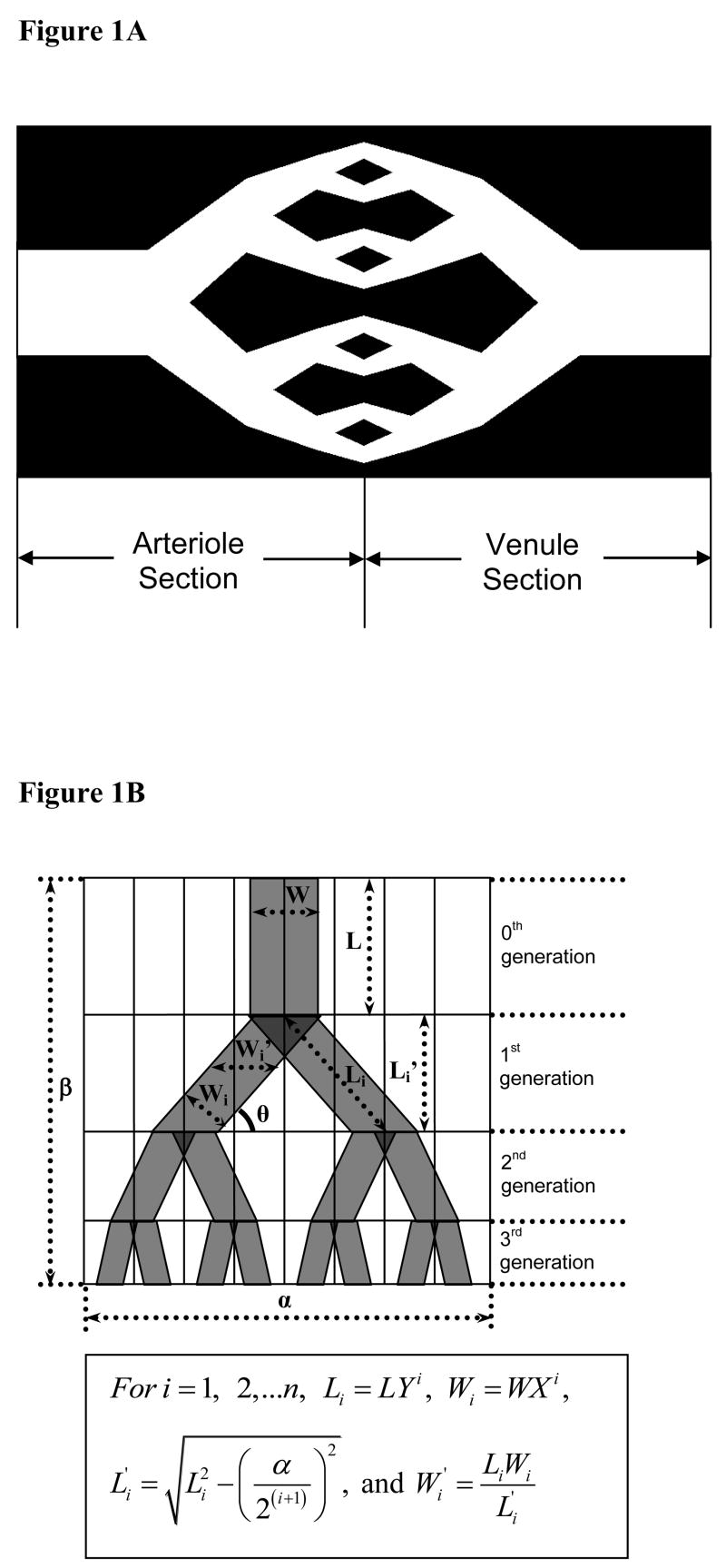
Figure 1.
(A) – Basic network design: Planar dichotomous branching of capillaries showing the arteriole and venule sections (B) – Schematic of the network design in 3 generations showing the geometric dimensions and the relevant expressions
In our designs, the overall design consists of an upstream arteriole side, where the parent blood vessel branches into two daughter vessels and a downstream venule side where two daughter blood vessels merge into one. We assume that the flow rate in each daughter vessel is equal to one half of that in the parent vessel. Further, the arteriole and venule sides are assumed to be mirror images of each other. The optimization model is solved to generate optimal values for the dimensions of the network designs. These planar network structures are characterized by maximum mass transport efficiency for a specified maximum pressure loss across them and a fixed porosity. Skin is chosen as our tissue model for the current study.
The three important constituents of any optimization model are the decision variables, the objective function and the constraints. While this general approach is equally applicable to a network that is three-dimensional, the type of constraints and the number of decision variables will depend on the base design of the three-dimensional network.
2.1 Decision Variables
For a tissue section of given width α and half-length β, the extent of branching in the planar dichotomous network depends on the number of generations, n. For example, Figure 1B illustrates a sample network design in 3 generations. As shown in this figure, the dichotomous branching results in 2i equidistant branches in the ith generation. We assume that the length of the vessel from one generation to another reduces by a factor Y, which is the same for each generation. Likewise, the width of the vessel reduces by a constant factor X. Thus, for a given value of width (α), half-length (β), and number of generations (n), the design of the network requires optimal values for the following decision variables:
L : Length of the vessel at the 0th generation (Initial Length, cm)
W : Width of the vessel at the 0th generation (Initial Width, cm)
Y : Length scaling factor
X : Width scaling factor
For ease of exposition, we define the following additional variables, which, as shown in Figure 1B, can be derived as a function of above four decision variables.
Li: Length of the vessel at the ith generation (cm)
Wi: Width of the vessel at the ith generation (cm)
: Projected length of the vessel at the ith generation (cm)
: Projected width of the vessel at the ith generation (cm)
In this work, we have used duct flow as the principle flow model. The reason for using duct geometry is the ease of micro-fabrication of the resulting flow networks. Further, two types of duct geometry are explored: rectangular ducts and square ducts. Rectangular ducts are defined to have a constant depth throughout the network, equal to one-tenth the initial width (W) of the network. The depth of such ducts is of the order of magnitude of 0.1 cm. Square ducts are defined to have varying depths with the depth at each generation equal to the width (Wi) at that generation. Such ducts have depths of the order of magnitude of 1 cm at the 0th generation.
2.2 The Objective Function
Our design objective is to maximize the mass transfer efficiency of the nutrients in a piece of tissue. The overall mass transfer efficiency will depend on several factors such as local flow characteristics, tissue and nutrient transport characteristics, vascular geometry and capillary distribution [1,9,40]. We chose net vascular surface area, through which nutrients diffuse into the adjoining tissue, per unit volume of the tissue space as a simple indicator of mass transport rates. Surface area/volume is widely used as an important indicator of mass transport effectiveness in various industrial mass transfer processes such as extraction and distillation.
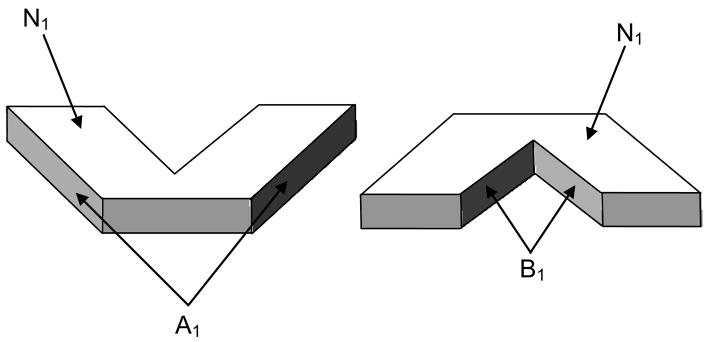
As shown for the 1st generation in Figure 2, at the ith generation of a bifurcating network, the total surface area is contributed by three parts. The first part consists of the top and the bottom vessel areas, each designated as Ni. The second part is the sum over all bifurcations, of the left side of the left branch and the right side of the right branch of each bifurcation. This is designated as Ai. Finally the third part is made up of the right side of the left branch and the left side of the right branch of each bifurcation. This is again summed over all the bifurcations in the generation and is designated as Bi.
Figure 2.
Bifurcation at the 1st generation to illustrate the contributions to the surface area; Net surface area contributed by the 1st generation = 2N1+A1+B1
We are then able to obtain the following expressions, for rectangular ducts, for the three parts of the surface area in terms of the lengths and widths of the network at each generation:
The net surface area per unit volume, which is also the objective function to be maximized, is then given as,
For square ducts, the expressions for A and B are slightly different from those above and can be obtained as,
The corresponding surface area per unit volume for the square ducts can be written as:
2.3 Constraints
In this work, we imposed the following constraints:
Pressure drop constraint, which fixes the maximum value of pressure drop across the network
Porosity constraint, which fixes the porosity of the network to a specified value, γ
Geometric constraints, which impose some logical limitations on the net length and net width at any point of the network and on the length and width scaling factors
2.3.1 Pressure Drop
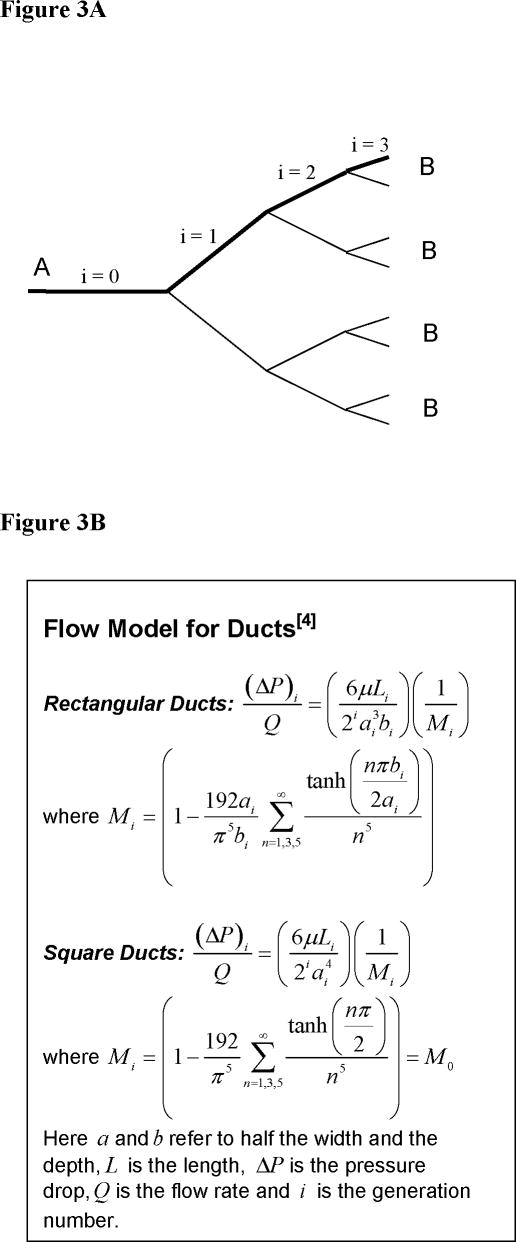
For a symmetric dichotomous branching network, the net pressure drop (pA − pB) is equal to the pressure drop in the bold segment of the network shown in Figure 3A. This is based on the premise that at each generation pressure drop is the same in all the branches, and that resistances can be added when they are in series[39]. Here, the pressure losses due to branching itself are assumed to be negligible. Physiologically, the average functional pressure drop across arterioles and venules in the systemic circulation is of the order of 17 mmHg [13]. In this work, we have constrained this value to be less than or equal to 1 mmHg.
Figure 3.
(A) – Net pressure drop between two points in a symmetric dichotomous branching network (B) – Flow models for rectangular and square ducts
The pressure drop across the network is a function of the network geometry and the fluid characteristics for the type of flow model employed. As mentioned earlier, we have used duct flow in rectangular and square duct geometries as the flow model. Figure 3B summarizes the pressure drop equations for both types of duct flow. Here, Poiseuille’s flow was assumed in individual segments. If a non-Newtonian fluid is used, a corresponding expression for pressure drop can easily be developed. The inlet flow rate, Q, was obtained by assuming constant flow rate per tissue weight ratio, which is in the range of 0.125–0.13 ml min−1 g−1 for skin tissue[10]. For rectangular ducts, the total volume of the tissue sample can be given as V = (α cm × 2β cm × W/10 cm) = αβW/5(cm3). Correspondingly, the flow rate through the skin tissue sample assuming that the density of the sample tissue equals 1 (g cm−3) can be calculated as Q= (0.125) αβW/5 ml/min.. In all the computational work so far, we have considered α = β = 1 cm, so the arteriole and venule sections are each 1 cm × 1 cm in dimension, and Q = 0.025W (ml min−1) Similarly, we can obtain the flow rate for square ducts as Q = 0.025W (ml min−1)
2.3.2 Porosity
We restrict the porosity to a specified value γ. The value of γ is varied from 0.4 to 0.7 [8,25,33,36]. Porosity of the network is indicative of the percent of blood in microvasculature and, in general, can vary depending on the permeability of vasculature. Here it is defined 12 as the ratio of vascular volume to the tissue volume. For rectangular ducts, it is straightforward to note that the porosity is simply (N/αβ), where N is derived in Section 2.2. For square ducts, the expression is not as trivial and can be written as,
2.3.3 Geometric Constraints
These constraints include the limitations on the net length and net width at any generation due to the network geometry as well as the fact that both length and width scaling factors cannot exceed 1[38,40]. The associated constraint equations are listed here with a brief explanation for each.
-
The sum of the projected lengths must be equal to the net length of the arteriole/venule section. That is,
-
Due to fabrication limitations, we have constrained the minimal vessel width in the network to 50 microns. In addition, the maximum width of the vessel at any generation comes from the number of branches that need to be accommodated within the overall width at that generation. These give rise to the following set of constraints:
-
The length constraint requires the length at each generation to have a lower bound, below which the projected length becomes imaginary.
Or
Depending on the value that Y takes, either will assume the maximum value amongst the set of n values, . Correspondingly, the above set of n constraints can be reduced to one constraint as follows: -
The length and width scaling factors must be less than or equal to one.
3. Solution Methodology
Clearly, the optimization model is highly non-linear in nature and can be classified as a constrained non-linear optimization problem. We solved the model using the generalized reduced gradient algorithm (GRG, Lasdon, et al.[20]) for solving nonlinear programming problems (NLPs). Although other algorithms like Sequential Quadratic Programming (SQP) and Interior Point or Barrier Method can also be used for solving NLPs, the GRG algorithm happens to be one of the most robust and reliable approaches to solve difficult NLP problems. While the GRG algorithm is available through the Solver function within MS Excel (Microsoft Corporation, Redmond, WA), in order to customize the GRG algorithm for our problem and expand it to accommodate additional functions, we implemented it by a custom-code written on the MATLAB platform (Mathworks, Natick, MA). The main motivation for generating a custom programming code was that it would provide us with more flexibility and a better understanding of the problem at hand. MATLAB platform was chosen for its powerful built-in matrix manipulation functions. In addition, the problem could be further expanded in many ways to perform various functions with a tightly integrated and inter-connected approach. The model solution was checked for consistency by verifying convergence for various initial values of the decision variables. Moreover, for simpler cases the results were verified by solving the nonlinear optimization problem through the Solver function within MS Excel. The computational times varied depending on the generations and the initial values and were approximately 15 – 45 seconds on a Windows XP Pro (Microsoft Corp, Redmond, WA) machine with Intel® Pentium 4-M, 1.2 GHz processor and 512 MB RAM.
Simulations of the model were carried out to obtain bifurcating vascular flow network designs with optimal transport characteristics. Briefly, for every simulation, the initial length, initial width and the length and width scaling factors were changed by the program to maximize the surface area per unit volume subject to the constraint functions. In all the simulations, the net length, 2α and net width, β of the network structures for both rectangular and square duct geometries were set to 2 cm and 1 cm respectively. Thus the arteriole and the venule sections each were 1cm × 1cm in area. Further, the depth of the rectangular ducts was set to be constant throughout and equal to a tenth of the initial width, i.e. one-tenth the width of the vessel at the 0th generation. On the other hand, the square ducts featured a varying depth, such that the depth at each generation was equal to the width at that generation. The number of generations was varied discreetly from 0 to 6 in steps of 1. For each generation, the model was solved for porosities ranging from 0.4 to 0.7 in intervals of 0.05.
For every combination of the porosity and the number of generations that we solved, our model generated a set of optimal values for the decision variables. It was required to convert the optimal numbers into flow network designs for visualization and fabrication, using a rapid and effective technique. We accomplished this by a custom-written macro in Visual Basic (Microsoft Corporation, Redmond, WA), which took the optimization results and mapped them onto AutoCAD (Autodesk, San Rafael, CA) to obtain optimal network designs.
4. Results and Discussion
4.1 Variations in the Scaling Factors
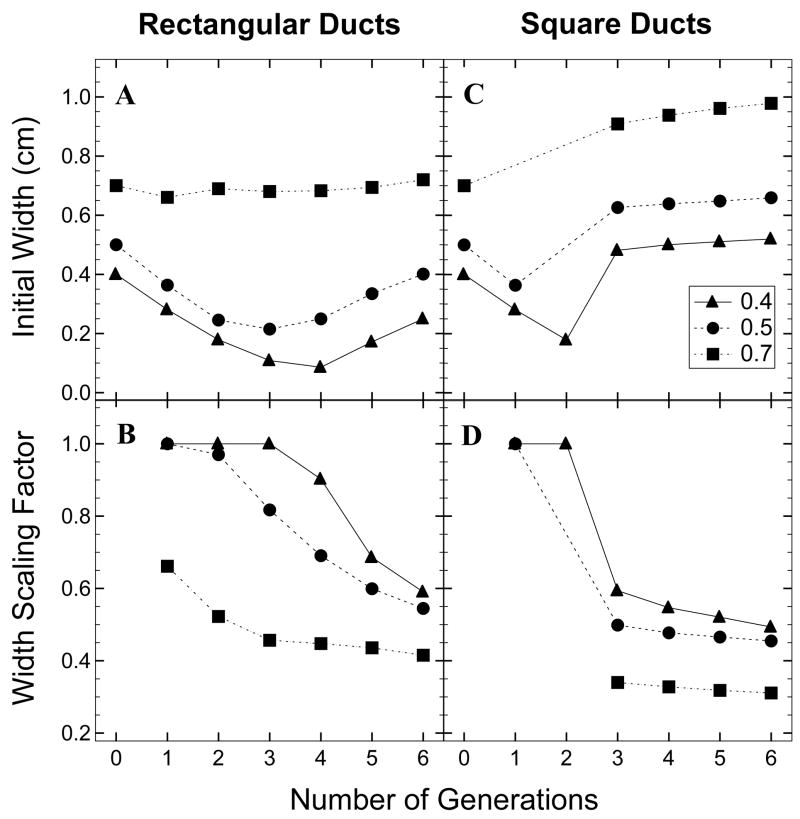
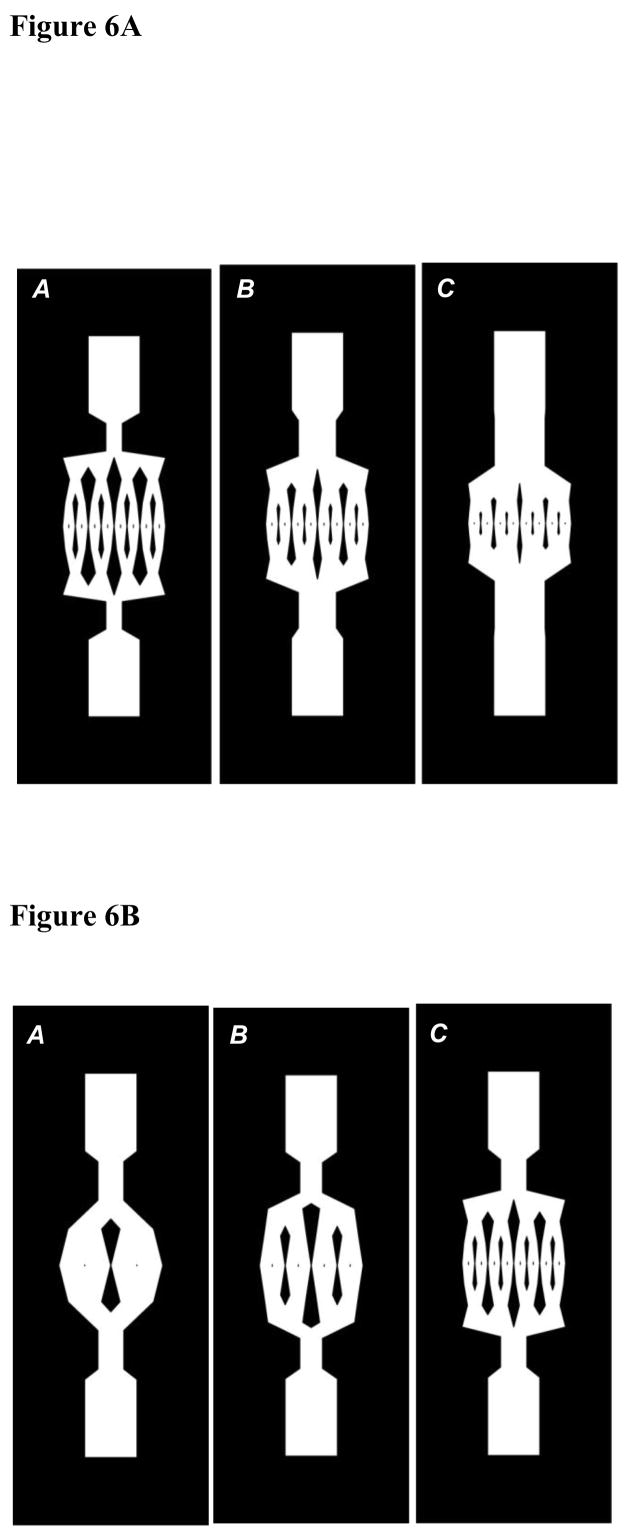
The variations of the four decision variables, L, Y, W and X are summarized in Figures 4 and 5 for select porosities and number of generations for optimized rectangular and square ducts. It may be noted that an increase in the porosity is brought about by an increase in the initial length and the initial width of the flow networks (Figures 4A, C and 5A, C). This is also depicted in Figure 6A, which shows rectangular duct network designs of increasing porosity for number of generations equal to 4. For a particular number of generations, corresponding to the increase in the initial length and width, there is a decrease in the scaling factors to satisfy the length and width constraints (Figures 4A, B and 5C, D). With number of generations, the initial length and width of rectangular and square ducts decrease and reach a minimum value before further increasing (Figure 6B). This minimum value corresponds to the optimal number of generations, where the transport efficiency attains a maximum. As will be discussed in sections 4.2 and 4.3, the optimum number of generations is 3 or 4 for rectangular ducts and 1 or 2 for square ducts.
Figure 4.
Plots of initial length (A, C) and length scaling factor (B, D) of rectangular and square ducts as a function of number of generations for different porosities
Figure 5.
Plots of initial width (A, C) and width scaling factor (B, D) of rectangular and square ducts as a function of number of generations for different porosities
Figure 6.
(A) – Rectangular duct designs for number of generations equal to 4 with porosities: A=0.45, B=0.55, C=0.6 (B) – Rectangular duct designs for porosity equal to 0.5 with number of generations: A=2, B=3, C=4
4.2 Characteristics of Rectangular Ducts
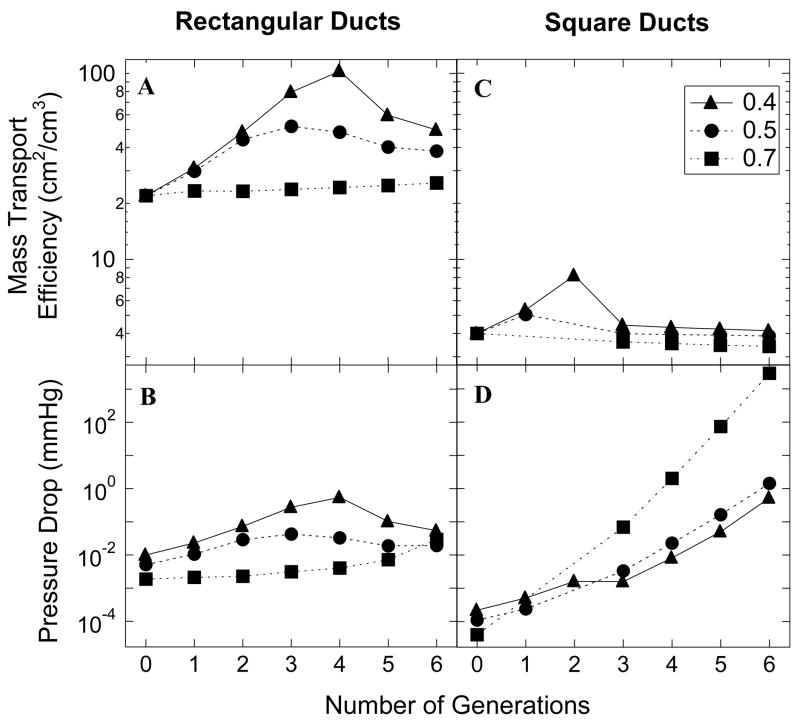
Figure 7 (A and B) shows plots of the mass transport efficiency (surface area/volume) and the pressure drop of optimized rectangular ducts as a function of number of generations for three different porosities. It is evident from these plots that both the mass transport efficiency and the pressure drop decrease with an increase in the porosity of the networks. As mentioned earlier, the initial width of the network increases with porosity. Correspondingly, the depth of the networks increases leading to an increase in the total volume of the tissue space. As a consequence the surface area/volume ratio decreases. The pressure drop is directly proportional to the length and inversely proportional to the cube of the width at each generation. As a result, an increase in the width with porosity would mean a decrease in the pressure loss. We also observed that there seems to be an optimal number of generations, either 3 or 4, for any particular porosity, where the transport efficiency attains a maximum. However the cost is also maximum at this generation number.
Figure 7.
Plot of mass transport efficiency (A) and semi-log plot of pressure drop (B) of rectangular ducts as a function of number of generations for different porosities. Plot of mass transport efficiency (C) and semi-log plot of pressure drop (D) of square ducts as a function of number of generations for different porosities
4.3 Characteristics of Square Ducts
We made a similar analysis for the square ducts. A corresponding plot is shown in Figure 7 (C and D). The mass transport efficiency again decreases with porosity, just like the rectangular ducts. However the pressure drop follows a different behavior. The optimal number of generations for maximum surface area/volume seems to be at 1 or 2 or 3 for square ducts. But the pressure drop doesn’t show a corresponding optimum; rather it increases with number of generations. Although the pressure losses for both the rectangular and square ducts are not the lowest corresponding to maximum mass transport efficiencies, the values that we have obtained are much less than physiologically observed values of pressure drops, indicating we are operating on a safe side.
4.4 Comparison between Rectangular and Square Ducts
In this section we make use of the plots in Figure 7, to compare between the two duct geometries, rectangular and square. Rectangular ducts clearly are more transport efficient, their surface area/volume values are almost an order of magnitude higher than the square ducts. With respect to the pressure drop, the rectangular ducts fare better for higher number of generations and higher porosity values. The square ducts show a much wider range in the pressure drop values and fare better for low number of generations, mainly because of their trend of increasing pressure drop with number of generations. Correspondingly, they have to pay a higher price, as the pressure losses are higher than those for the square ducts.
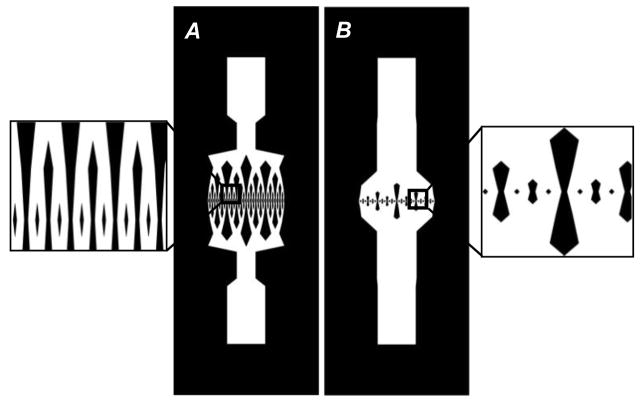
To visualize the effect of duct geometry on the network designs, Figure 8 shows two designs, rectangular and square, for the same porosity and number of generations. The square ducts have a much higher initial length and width, giving rise to much higher tissue volumes, which explains why they have inferior mass transport characteristics as compared to the rectangular ducts. An important point to note here is that both types of ducts are characterized by much higher values of surface area per volume than typical values for microvasculature in the skin tissue[32] which are in the order of 1 cm2/cm3.
Figure 8.
Network designs for porosity equal to 0.4 and number of generations equal to 6: A=Rectangular, B=Square
5. Conclusions
In this paper, we have described a first approach towards the rational design of vascular flow networks of micron dimensions, with optimal transport characteristics to be integrated with tissue-engineered scaffolds. We started out with a basic network design of planar dichotomous branching of capillaries, and applied optimization principles and transport criteria to obtain networks with optimal transport characteristics. Here we chose skin as our tissue model, and for two types of duct geometries, rectangular and square, maximized the nutrient transport in a sample tissue section by maximizing the surface area per unit volume of the tissue space. Under the conditions of this study, the surface area/volume values obtained are much higher than the published values of microvascular area per volume for the skin tissue. Further, the pressure drop values we considered are much lower than the physiologically observed range of pressure drops in vascular beds in the skin tissue. We found that the rectangular ducts show much superior mass transport characteristics as compared to the square ducts, and they are also easier to microfabricate for future experimental work. With regards to the results from optimization, two important results need to be summarized: (1) the mass transport efficiency decreases with the increase in porosity of the flow networks and (2) the mass transport efficiency attains a maximum at an optimal number of generations, 2–4, independent of porosity.
7. Nomenclature
L : Initial length of the network (cm)
W : Initial width of the network (cm)
α: Net width of the network (cm)
β: Net length of the arteriole/venule section of the network (cm)
γ: Porosity of the network
Li: Length of the network at the ith generation (cm)
Wi : Width of the network at the ith generation (cm)
: Projected length of the network at the ith generation (cm)
: Projected width of the network at the ith generation (cm)
Y : Length scaling factor
X : Width scaling factor
V : Total volume of the tissue space (cm3)
θi : Angle made by the length axis with the horizontal at the ith generation (°)
: Surface area per unit volume (1/cm)
N : Two dimensional surface area of the arteriole/venule section of the network (cm2)
A : Surface area component (cm2)
B : Surface area component (cm2)
M : Total mass of the tissue space (g)
ρ : Density of the tissue space (g/cm3)
Q : Initial flow rate through the network (ml min−1)
(ΔP)i : Pressure drop across the network at the ith generation (mmHg)
μ: Fluid viscosity (0.01 g/cm/s)
ai: Half of the duct width at the ith generation (cm)
bi: Half of the duct depth at the ith generation (cm)
Acknowledgments
The research was funded in part by grants from the Whitaker Foundation and the National Institutes of Health (EB006203)
References
- 1.Baba K, Kawamura T, Shibata M, Sohirad M, Kamiya A. Capillary-tissue arrangement in the skeletal muscle optimized for oxygen transport in all mammals. Microvasc Res. 1995;49(2):163–179. doi: 10.1006/mvre.1995.1013. [DOI] [PubMed] [Google Scholar]
- 2.Bentley G, Greer RB., 3rd Homotransplantation of isolated epiphyseal and articular cartilage chondrocytes into joint surfaces of rabbits. Nature. 1971;230(5293):385–388. doi: 10.1038/230385a0. [DOI] [PubMed] [Google Scholar]
- 3.Chu TM, Orton DG, Hollister SJ, Feinberg SE, Halloran JW. Mechanical and in vivo performance of hydroxyapatite implants with controlled architectures. Biomaterials. 2002;23(5):1283–1293. doi: 10.1016/s0142-9612(01)00243-5. [DOI] [PubMed] [Google Scholar]
- 4.Constantinescu VN. Laminar Viscous Flow. New York: Springer; 1995. [Google Scholar]
- 5.Dantzer E, Braye FM. Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts. Br J Plast Surg. 2001;54(8):659–664. doi: 10.1054/bjps.2001.3684. [DOI] [PubMed] [Google Scholar]
- 6.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. Faseb J. 1989;3(2):174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 7.Fitton AR, Drew P, Dickson WA. The use of a bilaminate artificial skin substitute (Integra) in acute resurfacing of burns: an early experience. Br J Plast Surg. 2001;54(3):208–212. doi: 10.1054/bjps.2000.3525. [DOI] [PubMed] [Google Scholar]
- 8.Freitas RA. Nanomedicine. Austin, TX: Landes Bioscience; 1999. [Google Scholar]
- 9.Gafiychuk VV, I, Lubashevsky A. On the principles of the vascular network branching. J Theor Biol. 2001;212(1):1–9. doi: 10.1006/jtbi.2001.2277. [DOI] [PubMed] [Google Scholar]
- 10.Ganong WF. Review of Medical Physiology. Norwalk: Appleton and Lange; 1991. [Google Scholar]
- 11.Godde R, Kurz H. Structural and biophysical simulation of angiogenesis and vascular remodeling. Dev Dyn. 2001;220(4):387–401. doi: 10.1002/dvdy.1118. [DOI] [PubMed] [Google Scholar]
- 12.Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295(5557):1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 13.Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia: W.B.Saunders Company; 2000. [Google Scholar]
- 14.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 15.Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa ER, Ravens M, Pien H, Cunningham B, Vacanti JP. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng. 2000;6(2):105–117. doi: 10.1089/107632700320739. [DOI] [PubMed] [Google Scholar]
- 16.Karch R, Neumann F, Neumann M, Schreiner W. Staged growth of optimized arterial model trees. Ann Biomed Eng. 2000;28(5):495–511. doi: 10.1114/1.290. [DOI] [PubMed] [Google Scholar]
- 17.Klarbring A, Petersson J, Torstenfelt B, Karlsson M. Topology optimization of flow networks. Computer Methods in Applied Mechanics and Engineering. 2003;192(35–36):3909–3932. [Google Scholar]
- 18.Ko IK, Iwata H. An approach to constructing three-dimensional tissue. Ann N Y Acad Sci. 2001;944:443–455. doi: 10.1111/j.1749-6632.2001.tb03854.x. [DOI] [PubMed] [Google Scholar]
- 19.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 20.Lasdon LS, Waren AD, Jain A, Ratner M. Design and testing of a generalized reduced gradient code for nonlinear programming. ACM Trans Math Software. 1978;4(1):34–50. [Google Scholar]
- 21.Ma PX, Zhang R. Microtubular architecture of biodegradable polymer scaffolds. J Biomed Mater Res. 2001;56(4):469–477. doi: 10.1002/1097-4636(20010915)56:4<469::aid-jbm1118>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Mantzaris NV, Webb S, Othmer HG. Mathematical modeling of tumor-induced angiogenesis. J Math Biol. 2004;49(2):111–187. doi: 10.1007/s00285-003-0262-2. [DOI] [PubMed] [Google Scholar]
- 23.McDougall SR, Anderson AR, Chaplain MA. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. J Theor Biol. 2006;241(3):564–589. doi: 10.1016/j.jtbi.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 24.McDougall SR, Anderson AR, Chaplain MA, Sherratt JA. Mathematical modelling of flow through vascular networks: implications for tumour-induced angiogenesis and chemotherapy strategies. Bull Math Biol. 2002;64(4):673–702. doi: 10.1006/bulm.2002.0293. [DOI] [PubMed] [Google Scholar]
- 25.Merchant FA, Diller KR, Aggarwal SJ, Bovik AC. Angiogenesis in cultured and cryopreserved pancreatic islet grafts. Transplantation. 1997;63(11):1652– 1660. doi: 10.1097/00007890-199706150-00020. [DOI] [PubMed] [Google Scholar]
- 26.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21(24):2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 27.Neumann T, Nicholson BS, Sanders JE. Tissue engineering of perfused microvessels. Microvasc Res. 2003;66(1):59–67. doi: 10.1016/s0026-2862(03)00040-2. [DOI] [PubMed] [Google Scholar]
- 28.Porter BD, Oldham JB, He SL, Zobitz ME, Payne RG, An KN, Currier BL, Mikos AG, Yaszemski MJ. Mechanical properties of a biodegradable bone regeneration scaffold. J Biomech Eng. 2000;122(3):286–288. doi: 10.1115/1.429659. [DOI] [PubMed] [Google Scholar]
- 29.Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, Kurzawski P, Wack KE, Stolz DB, Kamm R, Griffith LG. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng. 2002;78(3):257–269. doi: 10.1002/bit.10143. [DOI] [PubMed] [Google Scholar]
- 30.Powers MJ, Janigian DM, Wack KE, Baker CS, Beer Stolz D, Griffith LG. Functional behavior of primary rat liver cells in a three-dimensional perfused microarray bioreactor. Tissue Eng. 2002;8(3):499–513. doi: 10.1089/107632702760184745. [DOI] [PubMed] [Google Scholar]
- 31.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 32.Scheuplein RJ, I, Blank H. Permeability of the skin. Physiol Rev. 1971;51(4):702– 747. doi: 10.1152/physrev.1971.51.4.702. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt RF, Thews G. Human Physiology. 2. New York: Springer- Verlag; 1989. [Google Scholar]
- 34.Schreiner W. Computer generation of complex arterial tree models. J Biomed Eng. 1993;15(2):148–150. doi: 10.1016/0141-5425(93)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Schreiner W, Karch R, Neumann M, Neumann F, Roedler SM, Heinze G. Heterogeneous perfusion is a consequence of uniform shear stress in optimized arterial tree models. J Theor Biol. 2003;220(3):285–301. doi: 10.1006/jtbi.2003.3136. [DOI] [PubMed] [Google Scholar]
- 36.Toyota E, Fujimoto K, Ogasawara Y, Kajita T, Shigeto F, Matsumoto T, Goto M, Kajiya F. Dynamic changes in three-dimensional architecture and vascular volume of transmural coronary microvasculature between diastolic- and systolic-arrested rat hearts. Circulation. 2002;105(5):621–626. doi: 10.1161/hc0502.102964. [DOI] [PubMed] [Google Scholar]
- 37.Vacanti CA, Upton J. Tissue-engineered morphogenesis of cartilage and bone by means of cell transplantation using synthetic biodegradable polymer matrices. Clin Plast Surg. 1994;21(3):445–462. [PubMed] [Google Scholar]
- 38.VanBavel E, Spaan JA. Branching patterns in the porcine coronary arterial tree. Estimation of flow heterogeneity. Circ Res. 1992;71(5):1200–1212. doi: 10.1161/01.res.71.5.1200. [DOI] [PubMed] [Google Scholar]
- 39.Wechsatol W, Lorente S, Bejan A. Optimal tree-shaped networks for fluid flow in a disc-shaped body. International Journal of Heat and Mass Transfer. 2002;45(25):4911–4924. [Google Scholar]
- 40.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276(5309):122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]