Abstract
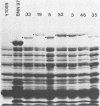
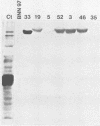
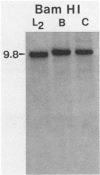
DNA obtained from Chlamydia trachomatis (serovar L2) was partially digested with DNase I and inserted into the beta-galactosidase gene of bacteriophage lambda gt11. Seven recombinants were selected that produced immunoreactive fusion proteins which were detected with anti-C. trachomatis rabbit serum. One recombinant, designated lambda gt11/L2/33, reacted with various monoclonal antibodies that recognize species-, subspecies-, and type-specific determinants on the chlamydial major outer membrane protein (MOMP). Immunoblot analysis of a lambda gt11/L2/33 lysogen revealed a fusion protein that expressed a approximately 15,000-dalton carboxyl-terminal peptide of the chlamydial MOMP. This moiety of the MOMP possesses epitopes responsible for each of the unique reactivities demonstrated by anti-MOMP monoclonal antibodies. The lambda gt11/L2/33 recombinant contained a 1.1-kilobase DNA insert which hybridized to DNA isolated from each of the 15 C. trachomatis serovars.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell H. D., Judd R. C. Structural analysis of chlamydial major outer membrane proteins. Infect Immun. 1982 Dec;38(3):960–968. doi: 10.1128/iai.38.3.960-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Vance D. W., Jr, Al-Hossainy E. Identification of a major envelope protein in Chlamydia spp. J Bacteriol. 1981 Apr;146(1):426–429. doi: 10.1128/jb.146.1.426-429.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Narita T., Manire G. P. Protein-carbohydrate-lipid complex isolated from the cell envelopes of Chlamydia psittaci in alkaline buffer and ethylenediaminetetraacetate. J Bacteriol. 1976 Jan;125(1):308–316. doi: 10.1128/jb.125.1.308-316.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Sippel A. E., Müller-Hill B. Exon cloning: immunoenzymatic identification of exons of the chicken lysozyme gene. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6852–6855. doi: 10.1073/pnas.79.22.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970 Sep;70(3):367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]
- Weiss E., Schramek S., Wilson N. N., Newman L. W. Deoxyribonucleic Acid Heterogeneity Between Human and Murine Strains of Chlamydia trachomatis. Infect Immun. 1970 Jul;2(1):24–28. doi: 10.1128/iai.2.1.24-28.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]