Abstract
In Angiosperms, the male gametes are delivered to the female gametes through the maternal reproductive tissue by the pollen tube. Upon arrival, the pollen tube releases the two sperm cells, permitting double fertilization to take place. Although the critical role of the female gametophyte in pollen tube reception has been demonstrated, the underlying mechanisms remain poorly understood. Here, we describe lorelei, an Arabidopsis thaliana mutant impaired in sperm cell release, reminiscent of the feronia/sirène mutant. Pollen tubes reaching lorelei embryo sacs frequently do not rupture but continue to grow in the embryo sac. Furthermore, lorelei embryo sacs continue to attract additional pollen tubes after arrival of the initial pollen tube. The LORELEI gene is expressed in the synergid cells prior to fertilization and encodes a small plant-specific putative glucosylphosphatidylinositol-anchored protein (GAP). These results provide support for the concept of signaling mechanisms at the synergid cell membrane by which the female gametophyte recognizes the arrival of a compatible pollen tube and promotes sperm release. Although GAPs have previously been shown to play critical roles in initiation of fertilization in mammals, flowering plants appear to have independently evolved reproductive mechanisms that use the unique features of these proteins within a similar biological context.
INTRODUCTION
In plants, the gametes are not directly generated as products of meiosis, but result from division and differentiation of the multicellular haploid gametophytes. In angiosperms, the gametophytes are the pollen and the embryo sac, producing the male and female gametes, respectively. Furthermore, the male gametes are nonmobile and are instead carried, after germination of the pollen grain on the female stigma, by the pollen tube. The pollen tube emanates from the pollen grain and finds its way in the female tissues, ultimately delivering two sperm cells to the embryo sac, at the mycropylar end. The canonical mature embryo sac, as in Arabidopsis thaliana, is composed of seven cells: the egg cell and the central cell, which will receive the two sperm cells, two synergids at the micropylar end, and three antipodal cells at the chalazal end. The proper delivery of the male gametes relies on a series of signals from the embryo sac. Through laser ablation experiments in Torenia fournieri, Higashiyama et al. (2001) showed that the synergid is the direct source of attractant for the pollen tube, while recent work in maize (Zea mays) has shown that the egg cell may also play a role in this guidance (Dresselhaus, 2006). Furthermore, work by Chen et al. (2007) has shown an involvement of the central cell in the guidance of the pollen tube in Arabidopsis. Upon arrival at the embryo sac, the pollen tube releases the sperm cells for the double fertilization to proceed. Furthermore, the embryo sac loses its ability to attract pollen tubes after fertilization is initiated.
Recently, the identification in Arabidopsis of the receptor-like kinase FERONIA has shed some light on potential signaling mechanisms responsible for these processes. In feronia and sirène mutants, the pollen tube can reach the embryo sac, but instead of arresting and delivering the two sperm cells, the pollen tube does not arrest and continues to grow and invades the embryo sac (Huck et al., 2003; Rotman et al., 2003). In addition, feronia/sirène embryo sacs can attract supernumerary pollen tubes (Escobar-Restrepo et al., 2007). FERONIA encodes a receptor-like kinase and is expressed in the filliform apparatus, on the surface of the synergid cells (Escobar-Restrepo et al., 2007). FERONIA may thus be part of a signaling cascade from the embryo sac to the pollen tube causing growth arrests and allowing sperm cells release. In this model, the ligand of FERONIA can be produced by the pollen. Alternatively, the ligand could be produced the embryo sac itself and FERONIA would be involved in the maturation of functional synergids able to interact with the pollen tube. The abstinence by mutual consent (amc) Arabidopsis mutant (Boisson-Dernier et al., 2008) displays an absence of release of the sperm cells from the pollen tube similar to what was observed in feronia/sirène. However, this mutant differs from feronia in that the defect appears only when a pollen tube from an amc pollen grain encounters an amc embryo sac, and mutant embryo sacs are fully competent to receive wild-type pollen. The AMC gene encodes a peroxin expressed in a wide range of vegetative tissues and strongly expressed throughout both male and female gametophytes. AMC appears to be necessary for PTS-1–dependent peroxisome import and may be involved in the processing of a ligand or some other signaling steps in the recognition pathway for both the pollen and the embryo sac. In this study, we describe an Arabidopsis mutant, lorelei, whose phenotype is reminiscent of the feronia/sirène mutation. lorelei embryo sacs show an impairment of fertilization caused by an inability of the pollen tube to release the sperm cells upon arrival at the lorelei embryo sac. The pollen tube subsequently experiences a continuous growth, resulting in an invasion of the embryo sac. The LORELEI gene encodes a putative plant-conserved glucosylphosphatidylinositol (GPI)-anchored protein (GAP) and is expressed in the synergid cells of the embryo sac. We thus identify a second female gametophyte-specific component of the signaling pathway or pathways required for fertilization in higher plants.
RESULTS
The lorelei Mutation Causes an Invasive Pollen Tube Phenotype and Failure of Fertilization
To obtain more data on the role of the embryo sac in pollen tube guidance, we performed a genetic screen to isolate mutants deficient in pollen tube reception and pollen tube arrest typical of the sirène class represented by the two alleles srn and fer. Plants from a population of Columbia (Col) wild-type seeds irradiated by gamma rays were used as pollen donors to fertilize wild-type ovules. We did not obtain male gametophytic mutants defective for fertilization among 2000 lines. However, we isolated mutant lines that showed >25% of seeds arrested at early stages after self-fertilization. The line GM474 showed a phenotype similar to srn and fer. Ovules from self-fertilized lre-1/+ plants showed failure of pollen tube arrest with further curling or invasive growth of the pollen tube in the embryo sac (Figure 1B). We tentatively named the mutation carried by GM474 lorelei-1 (lre-1) in reference to the mermaid names used for this class of phenotype. Genetic mapping based on 2108 plants determined that the mutation lre-1 location is within an interval between the BACs F14M19 and F10M23.
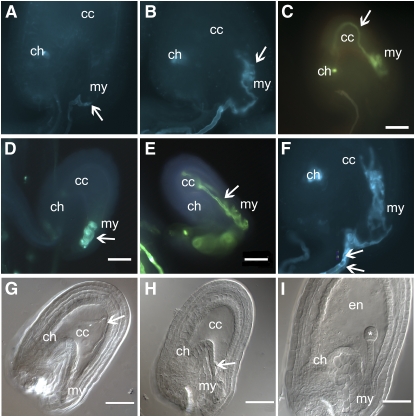
Figure 1.
Phenotype of lorelei Mutants.
(A) to (F) Aniline blue staining of ovules from different lorelei mutants pollinated with wild-type pollen.
(A) Wild-type arrival of the pollen tube (arrow) to the micropyle (my). There is no fluorescence inside the ovule.
(B) Pollen tube curling at the micropyle of an lre-1 ovule.
(C) Pollen tube invading the central cell (cc) and turning back toward the micropyle of an lre-2 ovule.
(D) Pollen tube curling inside the micropylar end of an lre-3 mutant.
(E) Pollen tube entering the central cell of an lre-4 ovule and heading back toward the micropyle.
(F) An example of two pollen tubes reaching the same lre-1 ovule. The two pollen tubes crawl along the funiculus and enter the embryo sac before forming coils at the micropylar end.
(G) to (I) DIC pictures of ovules the same lre-2/+ silique pollinated with wild-type pollen.
(G) An example of pollen tube entering deep inside the central cell and turning back toward the micropyle.
(H) Pollen tube coiling at the micropylar end of the embryo sac.
(I) Wild-type-looking ovule with a developed endosperm (en) and an embryo at the dermatogen stage (asterisk).
Arrow, pollen tube; cc, central cell; my, micropylar end; ch, chalazial end; en, endosperm; *, embryo. Bars = 50 μm.
In parallel, we previously described a Ds element insertion collection in Arabidopsis for gametophytic mutants (Pagnussat et al., 2005). One of these mutants, called une17 (for unfertilized embryo sac 17), displayed a segregation ratio of the kanamycin resistance, carried by the Ds element, of 1.09:1, clearly indicative of a gametophytic defect. Furthermore, it proved impossible to recover a plant homozygous for the Ds element. Reciprocal crosses with the wild type as an ovule or pollen donor showed a mainly female lethality (0.12:1 and 0.84:1, respectively) and reduced genetic transmission from the female but not from the male gametes (Table 1). Differential interference contrast (DIC) observation of cleared heterozygous siliques after pollination showed that 28.6% of the embryo sacs displayed a failure of pollen tube arrest and sperm discharge. Instead, these embryo sacs contained an invasive pollen tube, either curling at the micropylar end (Figure 1H) or entering in the central cell region and then turning back toward the micropyle (Figure 1G). Because of its similarity to the previously identified lorelei mutation, which mapped to the same chromosomal region (see below), this mutant was renamed lorelei-2 (lre-2).
Table 1.
Transmission of lre for the lre-1 and lre-2 Deletions
| Ovule × Pollen | lre/+ | Wild Type | Ratio lre-1/Wild Type |
|---|---|---|---|
| lre-1/+ × Col | 19 | 224 | 0.08:1 |
| Col × lre-1/+ | 142 | 181 | 0.78:1 |
| Kanr | Kans | Ratio Kanr/Kans | |
| lre-2/+ self | 983 | 902 | 1.09:1 |
| lre-2/+ × Ler | 43 | 361 | 0.12:1 |
| Ler × lre-2/+ | 224 | 266 | 0.84:1 |
The genetic transmission of the lre mutation (ovule donor listed first) of the lre-1 and lre-2 deletions was established by observation of lorelei phenotype (invasive pollen tube) occurrence for lre-1 and by assaying the transmission of the linked nptII gene in lre-2.
Crosses between ovules from lre/+ plants and wild-type pollen produced siliques in which approximately one-quarter of the embryo sacs were aborted and had invasive pollen tube growth (Table 2). By contrast, there was very limited ovule abortion in crosses between wild-type ovules and pollen from lre/+ plants. The proportion of aborted ovules with invasive pollen tube growth between lre/+ siliques pollinated with wild-type pollen and self-pollinated lre/+ siliques showed little variation. However, ∼10% of the seeds aborted in self-pollinated lre/+ plants as a result of early embryo and endosperm arrest between the 2- to 8-cell stage in the embryo. Although the Ds element insertion primarily affected the embryo sac function, there was also some reduced transmission of the transposon through the lre-2 pollen, but we did not detect a reduced transmission through lre-1 pollen (Table 1), indicating that the pollen defect might be allele specific. In conclusion, the mutations lre-1 and lre-2 primarily affect the capacity of the embryo sac to be fertilized, and the phenotype exhibits a moderate penetrance.
Table 2.
Phenotypic Analysis of lre-1 and lre-2 by DIC Analysis
| Ovule × Pollen | Col × Col | lre-1/+ × lre-1/+ | lre-1/+ × Col | Col × lre-1/+ | lre-2/+ × lre-2/+ | lre-2/+ × Ler |
|---|---|---|---|---|---|---|
| Normal | 257 (95.5%) | 140 (50.9%) | 149 (58.5%) | 202 (95.7%) | 431 (55.8%) | 138 (65.7%) |
| Invasive pollen tube | 0 (0%) | 82 (29.8%) | 70 (27.4%) | 0 (0%) | 221 (28.6%) | 54 (25.7%) |
| Unfertilized | 2 (0.8%) | 39 (14.2) | 31 (12.1%) | 6 (2.8%) | 95 (12.3%) | 3 (1.4%) |
| Aborted | 10 (3.8%) | 14 (5.1%) | 5 (2.0%) | 3 (1.5%) | 26 (3.4%) | 15 (7.1%) |
The flowers were emasculated and fertilized manually with pollen. The phenotype was scored 48 h after pollination using DIC on cleared ovules.
To confirm the observations made with DIC on cleared ovules, the phenotypes were rescored using samples stained with aniline blue. This method allow for easy visualization of the pollen tube (Figures 1A to 1C, Table 3). In both lre deletions, the proportion of embryo sacs showing an invasion by a pollen tube was similar to the one observed with DIC. We were also able to score the number of pollen tubes reaching a single ovule using aniline blue staining. Table 4 shows that embryo sacs in lre-/+ can attract supernumerary pollen tubes, as >20% of the ovules had two or more pollen tubes reaching them (Figure 1F). Overall, the defects observed in lre/+ siliques are quite similar to those described in the feronia/sirène (Huck et al., 2003; Rotman et al., 2003) and amc mutants (Boisson-Dernier et al., 2008), with an overgrowth of the pollen tube in the embryo sac and attraction of supernumerary pollen tubes. However, the penetrance of the lre-1 and lre-2 mutations is lower than what was observed in feronia/sirène. While ovules from fer/+ siliques showed invasion by the pollen tube almost 50% of the time, the percentage of occurrence in lre-2 is lower at 25%. These observations, in addition to the possible transmission of lre through the female suggest that the defect is not as drastic in lre-2 compared with fer and srn, which are not transmitted through the female gametes (Huck et al., 2003; Rotman et al., 2003). A direct comparison with amc is not possible because while lre-2 and fer are independent of the male gametophyte, the amc phenotype appears only if both the pollen and the embryo sac carry the mutation, and amc embryo sacs are able to receive wild-type pollen normally (Boisson-Dernier et al., 2008).
Table 3.
Phenotypic Analysis of lre-1 and lre-2 by Aniline Blue Staining
| lre-1/+ × Col Pollen | lre-2/+ × Ler Pollen | |
|---|---|---|
| Normal | 305 (78.8%) | 338 (67.6%) |
| Invasive pollen tube | 82 (21.2%) | 136 (27.2%) |
| No fluorescence | 0 (0%) | 26 (5.2%) |
The phenotype scoring was repeated using aniline blue staining 48 h after pollination on lre siliques pollinated with wild-type pollen.
Table 4.
Polyspermy in lre Mutants
| Col × Col | lre-1/+ × Col pollen | lre-2/+ × Ler Pollen | |
|---|---|---|---|
| One pollen tube | 493 | 239 | 193 |
| Two pollen tubes | 8 (1.6%) | 49 (20.5%) | 48 (19.8) |
| Three pollen tubes | 0 (0%) | 2 (0.1%) | 1 (0.4%) |
Supernumerary pollen tubes reaching the embryo sac were counted using aniline blue staining 48 h after pollination.
To examine whether the invasive pollen tube phenotype observed in lre/+ siliques arose from defective embryo sac development, analysis using DIC of the developing embryo sacs was performed. No defects could be observed during embryo sac development, and the mature, unpollinated embryo sacs appeared uniformly normal in lre/+ siliques. Furthermore, ovules from lre-1/+ plants expressed the β-glucuronidase reporters for the genes MEA and FIS2 the same those from the wild type. Similarly, lre-2/+ correctly expressed the β-glucuronidase genes associated with the embryo sac marker lines ET119 and ET2634 (Gross-Hardt et al., 2007) in specific cell types in the embryo sac, the egg cell, and the synergids (data not shown). Similar experiments performed on feronia also failed to show defects during the embryo sac development (Huck et al., 2003).
Molecular Characterization of lre-1 and lre-2
The lre-1 mutation was caused by gamma ray irradiation, and we could not obtain new recombinants after scoring 1200 plants in our collection of 2100 F2 mapping population. We made the hypothesis that a deletion took place at the lre-1 locus. To determine the extent of the potential deletion, we made crosses between wild type Landsberg erecta (Ler) and lre-1/+ in the Col background to produce F1 hybrids carrying the mutation. We tried to detect polymorphism between Col and Ler across the genetic location previously determined by mapping. Polymorphism was not detected in lre-1/+ Col-0/Ler hybrids inside a region delimited by the genes At4g26190 and At4g26570, comprising at least 63 open reading frames (ORFs). The lack of polymorphism indicates the deletion causing the lre phenotype must encompass this region spanning 160 kb on the chromosome 4 (Figure 2A). In the case of lre-2, the Ds element mutagenesis allows for recovery of the sequences flanking the insertion site by thermal asymmetric interlaced (TAIL)-PCR (Liu et al., 2005). Initial experiments placed the 5′ end of the Ds element of une17/lre-2 in the second exon of the gene At4g26330 (Pagnussat et al., 2005). Further TAIL-PCR analysis indicated that the 3′ end of the transposon is inserted in 5th exon of At4g26660, at a distance of 128.2 kb from the 5′ flanking sequence. PCR using gene-specific primers confirmed the results of the TAIL-PCR on lre-2/+ genomic DNA. Therefore, the insertion of the Ds element seems to have caused the deletion of a 128-kb stretch of chromosome IV, affecting 41 annotated genes or pseudogenes (Figure 2A).
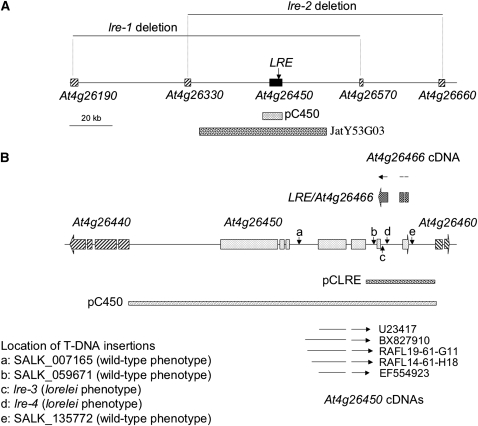
Figure 2.
Structure of the LORELEI Locus and Its Vicinity.
(A) A schematic view of the region of chromosome 4 showing the position of At4g26450 and At4g26466 as well as the extent of the deletions present in lre-1 ad lre-2. The sequences covered by the complementation constructs JatY53G03 and pC450 are displayed as well.
(B) Close-up drawing of the At4g26450/At4g26466 locus. The map shows the structure of At4g26450 (seven exons, light gray boxes on the map) and At4g26466 (three exons, dark-gray boxes above the map) as annotated by the Arabidopsis Genome Initiative consortium as well as the two bordering genes, At4g26440 and At4g26460. The full-length cDNAs for At4g26450 present in the database are shown (as arrows indicating the exons, below the schematic) as well at the cDNA for At4g26466 (arrow above the schematic) produced during this work. Furthermore, the positions of the five T-DNA lines investigated are also marked by the vertical arrows. The two T-DNA lines with an lre phenotype (lre-3 and lre-4) are marked as c and d. The three other lines had a wild-type phenotype. Finally, the length of complementing constructs pC450 and pCLRE is also displayed.
To confirm that the phenotype observed in lre-2/+ siliques was indeed caused by this deletion, we transformed lre-2/+ plants with a binary vector, JatY53G03, containing a section of the chromosome IV, including the genes from At4g26350 to At4g26530 (Figure 2A). The resulting T2 plants were screened for complementation of the Ds transmission frequency using the Kanr/Kans ratio. Several independent transformants displaying an increase in Kanr/Kans were recovered (Table 5). The line lre-2/+;JatY53G03-1/+ was further characterized for pollen tube invasion in the embryo sac as well as the Barr/Bars ratio, indicative of the complementing construct that confers resistance to Basta. As shown in Tables 6 and 7, the invasive pollen tube phenotype was entirely rescued in the progeny of the T2 plants 1.2 of the complementing line, corresponding to the complementing construct in a homozygous state, as indicated by the complete Basta resistance in this family.
Table 5.
Complementation of lre-2
| Kanr | Kans | Ratio Kanr/Kans | |
|---|---|---|---|
| lre-2/+; JatY53G03-1 | 185 (68.5%) | 85 (31.5%) | 2.18:1 |
| lre-2/+; JatY53G03-5 | 231 (74.3%) | 80 (25.7%) | 2.89:1 |
lre-2/+ plants were transformed with the JatY53G03 binary vector, and the segregation of the linked nptII gene conferring kanamycin resistance (Kanr) was scored in the T2 generation. Kans, kanamycin sensitivity.
Table 6.
Rescue of the Invasive Pollen Tube Phenotype by JatY53G03
| lre-2/+; JatY53G03-1.2 | lre-2/+; JatY53G03-1.4 | lre-2/+; JatY53G03-1.6 | |
|---|---|---|---|
| Normal | 164 (92.1%) | 151 (77.3%) | 168 (77.1%) |
| Invasive pollen tube | 0 (0%) | 36 (18.6%) | 32 (14.7%) |
| No pollen tube | 8 (4.5%) | 4 (2.1%) | 10 (4.6%) |
| Aborted | 6 (3.4%) | 3 (1.5%) | 8 (3.7%) |
Plants carrying the lre-2 mutation and the JatY53G03 construct were tested for invasive pollen tubes 48 h after pollination using DIC.
Table 7.
Segregation of the Kanr and Bars Markers in the lre-2/+ Transformed Lines
| Kanr | Kans | Barr | Bars | |
|---|---|---|---|---|
| lre-2/+; JatY53G03-1.2 | 366 (71.9%) | 143 (28.1%) | 100% | / |
| lre-2/+; JatY53G03-1.4 | 157 (66.8%) | 78 (32.2%) | 355 (79.1%) | 89 (20.1%) |
| lre-2/+; JatY53G03-1.6 | 156 (63.9%) | 88 (36.1%) | 426 (75.4%) | 112 (20.8%) |
Segregation ratio for kanamycin and Basta resistance (Kanr and Bars, respectively) in the rescued lines.
This complementation experiment indicated that the locus responsible for the lorelei phenotype was located between At4g26350 and At4g26530. To identify the specific gene, all of the 19 annotated genes present on JatY53G03 were independently cloned in binary vectors and transformed in lre-2/+ plants. The F2 progeny from those transformants were screened for their Kanr/Kans ratio. However, we noted that one of the genes contained in the original deletion, and also on the complementing BAC JatY53G03, was At4g26500, which has been identified as embryo lethal (Xu and Møller, 2006). Therefore, the expected ratio after rescue in the case of a single copy of the complementing construct containing a single gene would be 2:1 at best. Of the 17 constructs tested, only one construct, pC450 (Figure 2A), resulted in a significant increase of Kanr/Kans ratio, as shown in Table 8.
Table 8.
Complementation of lre-2/+ by the pC450 Construct
| Kanr | Kans | Ratio Kanr/Kans | |
|---|---|---|---|
| lre-2/+; pC450.6-2 | 235 (67.0%) | 116 (33.0%) | 2.02:1 |
| lre-2/+; pC450.6-3 | 155 (51.8%) | 144 (48.2%) | 1.08:1 |
| lre-2/+; pC450.7-4 | 249 (66.6%) | 125 (33.4%) | 1.99:1 |
| lre/-2+; pC450.7-7 | 255 (66.4%) | 129 (33.6%) | 1.98:1 |
lre-2/+ plants were transformed with the binary vector pC450 carrying the annotated At4g26450 gene. Complementation was confirmed in several independent transformed lines by checking the segregation of the nptII gene conferring kanamycin resistance (Kanr) linked to the lre-2 mutation. Kans, kanamycin sensitivity.
Identification of Additional lorelei Alleles
The vector pC450 contained ∼10 kb of Arabidopsis genomic DNA surrounding At4g26450. Several T-DNA insertion lines were available in this region, and five of them were investigated further (Figure 2B). Three of those lines did not display any invasive pollen tube phenotype (SALK_007165, SALK_059671, and SALK_135772). However, the lines SALK_040289 and SAIL_8_C08 presented the same invasion of the embryo sac by the pollen tube as the lre mutants (Figures 1D and 1E) and were renamed lre-3 and lre-4, respectively. Observations made on siliques from lre-3/+ and lre-4/+ showed a similar penetrance of the invasive pollen tube phenotype in those lines (Table 9). A homozygous line for lre-3 was recovered and analyzed. The occurrence of the lorelei phenotype in the homozygote was double what was observed in the lre-3 heterozygote, as expected from twice the number of mutant embryo sacs in the homozygote (Table 9). Importantly, the homozygous knockout plants were healthy and had no other visible phenotypes, suggesting that the LRE gene functions specifically in fertilization. While the lre-3 homozygous line displayed a strong increase in invasive pollen tube frequency, half of the mutant embryo sacs are able to receive the pollen tube normally. It could therefore be hypothesized that the function of LRE in the signaling pathway is partially redundant and/or is, to some extent, dispensable (see below).
Table 9.
Phenotypic Analysis of lre-3 and lre-4 by Aniline Blue Staining
| lre-3/lre-3 × Col pollen | lre-4/+ × Col pollen | |
|---|---|---|
| Normal | 195 (54.0%) | 280 (74.3%) |
| Invasive pollen tube | 166 (46.0%) | 97 (25.7%) |
Phenotypic analysis of lre-3/lre-3 and lre-4/+.
Isolation of the LORELEI Gene
When the work to identify the LORELEI gene was performed, the gene At4g26466 was not annotated. In silico analysis of the region lead to the discovery of another gene at the At4g26450 locus, which was identified as LORELEI and appears under the number At4g26466 in the AGI V8 annotation (see Supplemental Results online). To confirm that At4g26466 was indeed LORELEI, a construct covering the sequences between the exon 5 of At4g26450 and the stop codon of At4g26460 was transformed in lre-3 homozygotes and able to rescue the phenotype (Table 10). Furthermore, a cDNA matching the sequence of the predicted At4g26466 was recovered through RT-PCR (see Supplemental Results online).
Table 10.
Complementation of lre-3 by pC450 and pCLRE
| lre-3/lre-3; pC450 | lre-3/lre-3; pCLRE-1 | lre-3/lre-3; pCLRE-3 | |
|---|---|---|---|
| Normal embryo sacs | 246 (89.13%) | 161 (85.63%) | 206 (81.74%) |
| Invasive pollen tube | 29 (2.42%) | 11 (5.85%) | 31 (12.30%) |
| No pollen tube | 4 (1.45%) | 31 (12.30%) | 15 (5.95%) |
lre-3 homozygous plants were transformed by pC450 carrying the annotated At4g26450 gene or pCLRE, a binary vector carrying the lorelei genomic sequence. Complementation was assayed by scoring the lre phenotype 48 h after pollination using aniline blue staining.
LORELEI Is Expressed in the Synergid Cells of the Embryo Sac
Preliminary studies showed that LORELEI was expressed at a low level throughout the plants, including in floral organs (see Supplemental Results online). To determine the pattern of localization of LORELEI mRNA, we performed in situ hybridization in longitudinal sections of wild-type ovules, using a digoxygenin-labeled probe of 181 bp corresponding to a small portion of the 3rd exon in At4g26466. We find that LORELEI expression occurs in differentiated ovules that contain cellularized female gametophytes prior to pollination. Specifically, LORELEI mRNA was localized in the egg apparatus, predominantly in both synergids prior to degeneration (Figure 3A). Sporadically, a weak signal was detected in the cytoplasm of the egg cell (Figure 3A); however, no mRNA was localized in the central cell and the antipodals either before or after pollination. After pollination, abundant mRNA was found localized in synergids at the micropylar pole of the female gametophyte (Figures 3B and 3C). Although this signal could reflect a higher concentration of mRNA confined to a restricted portion of cytoplasm in degenerating synergids, it could also be the result of an increase in LORELEI expression following pollination. Developing ovules undergoing megasporogenesis or megagametogenesis did not show LORELEI mRNA localization at any developmental stage, indicating that LORELEI is active only in the fully differentiated cells of the egg apparatus.
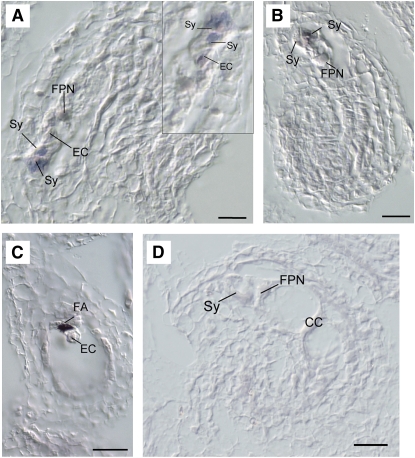
Figure 3.
Localization of LORELEI mRNA in Wild-Type Ovules of Arabidopsis.
(A) Longitudinal section of a fully differentiated ovule prior to pollination, showing predominant LORELEI expression (purple color) in both synergids. Inset: a higher magnification of the egg apparatus in a consecutive section of the same ovule shows additional weak expression in the egg cell cytoplasm.
(B) Longitudinal section of a fully differentiated ovule following pollination. LORELEI mRNA is localized in both degenerating synergids but not in the central cell.
(C) Longitudinal section of a fully differentiated ovule following pollination. Abundant LORELEI mRNA is localized in the remnants of a degenerating synergid.
(D) Longitudinal section of a fully differentiated ovule hybridized with a LORELEI sense probe.
Sy, synergid; EC, egg cell; FPN, fused polar nuclei; FA, filiform apparatus; CC, central cell. Bars = 20 μm.
LORELEI Encodes a Conserved, Plant-Specific, GPI Anchor Protein
Sequence comparison of the predicted protein showed strong similarity with a small family of GAPs with three known members in Arabidopsis and more in other plants, including rice (Oryza sativa), maize, and Physcomitrella patens. Using BLAST (Altschul et al., 1990) with the LORELEI sequence against the Medicago truncatula and Populus trichocarpa genome allowed the identification of partial sequences of three putative orthologs in Medicago and one in Populus. Performing the same type of analysis as used for LORELEI (see above and Supplemental Results online), it was possible to reconstruct four full ORFs sharing similar gene structures between themselves and with LORELEI. The newly identified ORFs have been named Mt LORELEI-related 1, 2, 3, and Pt LORELEI-related1 for the Medicago and poplar sequences, respectively.
No significant similarities were found with animal or yeast proteins. Further in silico analysis of the LORELEI sequence using SignalP (Nielsen et al., 1997; Bendtsen et al., 2004) and TargetP (Emanuelsson et al., 2000) predicted a signal peptide for the secretory pathway containing the first 20 amino acids. BigPI (Eisenhaber et al., 2003), a program designed to detect GPI modification site in plant proteins, found a putative GPI anchor domain in the C terminus, along with a GPI anchor attachment site, Ser-139 (Figure 4A). The presence of an N-terminal signal peptide and a C-terminal anchor signal leaves a central portion of LRE as the potential active domain.
Figure 4.
Analysis of the LORELEI Sequence.
(A) Sequence of the LORELEI protein, with the position of the predicted signal peptide (box), the GPI anchor domain (underline), and the Ser binding to the GPI moiety (in bold).
(B) ClustalW alignment of LRE-related proteins from Arabidopsis (At), O. sativa (Os), Z. mays (Zm), M. truncatula (Mt), P. trichocarpa (Pt), and P. patens (Pp). Similar residues are highlighted in gray and identical residues in black.
A ClustalW alignment (Larkin et al., 2007) is shown on Figure 4B, displaying a central conserved domain. The domain does not show any similarity with previously described protein domains and appears specific to the LORELEI protein family. Within the domain, several amino acids are conserved in all sequences, such as a series of Cys residues (C62, C72, C73, C81, C117, and C126) as well as several hydrophobic residues (M98, F99, F113, and L124). However, the significance of those conserved residues is unclear. Outside of the central domain, little conservation is observed, particularly in the N-terminal signal peptide and the C-terminal GPI anchor signal. Out of the three identified LRE paralogs, At5g56170 is the closest, with 58% identity and 76% similarity. The two remaining (At4g28280 and At2g20700) show more differences, with 55 and 46% identity and 71 and 65% similarity, respectively. However, At4g28280 and At2g20700 are highly similar to each other, showing 83% identity. Both At2g20700 and At4g28280 have been found to be expressed strongly in the mature pollen tube in an earlier study, while At5g56170 was not detected in the same experiment (Lalanne et al., 2004).
To address the intracellular localization of the LRE protein, a green fluorescent protein (GFP) fusion was engineered between the signal peptide and the rest of LRE. First, a modified GFP protein was created with the N-terminal signal peptide of LRE. This modified GFP was fused to the central domain and the GPI anchor domain of LRE to generate an N-signal peptide-GFP-LRE-C fusion (Figure 5A). Unfortunately, efforts to obtain GFP fluorescence in a transient expression experiment by particle bombardment in intact onion cells were not successful with this fusion, although strong expression was obtained with a 35S-GFP control plasmid (data not shown). This suggested the possibility that the fusion protein might be unstable or that it was quenching the GFP fluorescence. Another possible reason for the failure to detect GFP fluorescence in intact tissues might be that localization of the fusion protein on the outer leaflet of the plasma membrane, predicted for a GPI anchor protein, results in exposure of GFP to unfavorable conditions in the extracellular environment, such as low pH, as noted by others (Nadeau and Sack, 2002; Zheng et al., 2003). Therefore, an alternate system for expression was attempted, which relied upon transfection into isolated Arabidopsis mesophyll protoplasts (Yoo et al., 2007). This system permits use of a buffer-controlled environment for the outer surface of the plasma membrane and also has the advantage that it avoids having to employ a heterologous plant system. Using this expression system, with the 35S promoter driving transcription of the LRE-GFP fusion, GFP fluorescence was observed in 10% of the transfected cells, and was in all cases localized to the surface of the protoplasts (Figures 5D and 5G). No GFP signal was detected in the untransfected samples (Figures 5B and 5E), while a positive control using 35S-GFP resulted in very strong cytoplasmic signal that did not localize to the surface of the protoplasts (Figures 5C and 5F). These results are consistent with the predicted localization of LRE to the plasma membrane, at least in Arabidopsis protoplasts.
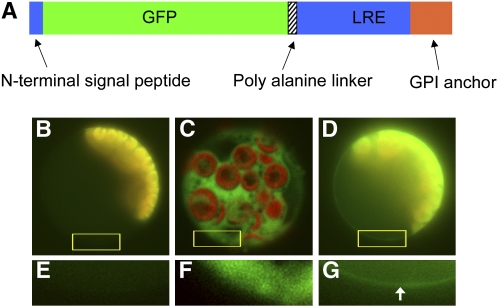
Figure 5.
Subcellular Localization of LORELEI.
(A) Schematic representation of the GFP-LRE construct. The GFP is sandwiched between the N-terminal signal peptide and the rest of the LORELEI protein (central domain and GPI anchor domain). The predicted GPI anchor domain is shown in orange. An 8x Ala linker was added at the junction between the GFP and the major part of LORELEI.
(B) to (G) Epifluorescence detection of GFP-LRE in transfected protoplasts. The GFP fusion protein (green) appeared at the cell periphery as a line in transfected mesophyll protoplasts in which chloroplast autofluorescence (red) was also detected ([D]; detail from boxed region is shown in [G], with the arrow showing the thin fluorescent band around at the periphery of the cell). In protoplasts transfected with GFP only, the GFP protein (green) was seen throughout the cell, with no preference for the cell periphery. The autofluorescence from the chloroplasts appears in red ([C]; detail from boxed region is shown in [F]). In untransfected protoplasts, only chloroplast autofluorescence was detected (red) ([B]; detail from boxed region is shown in [E]). Exposure times for the pictures were as follows: 1 s for the green and red channels of the untransfected control ([B] and [E]), 0.6 s for the green and red channels of GFP ([C] and [F]), and 0.8 s for the green channel and 1 s for the red channel of GFP-LRE ([D] and [G]).
DISCUSSION
GAPs are eukaryotic proteins known to function in cell–cell signaling, and are anchored at their C termini to the extracellular surface of the plasma membrane of the cell though the GPI moiety (Muñiz and Riezman, 2002). The GPI anchor is synthesized in the endoplasmic reticulum membrane in a multistep process, and the processed protein is ultimately trafficked to the cell surface, where it is believed to be tethered to lipid rafts (Orlean and Menon, 2007). GAPs can be released from their anchor into the extracellular medium; this release can be catalyzed by phospholipase C or by ACE (reviewed in Sharom and Lehto, 2002; Mayor, 2005). Only a few genes encoding GAPs have been characterized in terms of function in Arabidopsis. The gene COBRA is required for polarized cell expansion in the root (Schindelman et al., 2001), and the gene NDR-1 is involved in signal transduction during disease resistance (Coppinger et al., 2004). A classical arabinogalactan protein containing a GPI anchor has been shown to be involved in the initiation of female gametogenesis in Arabidopsis and has been proposed to be involved in intercellular signaling between sporophytic and gametophytic cells (Acosta-Garcia and Vielle-Calzada, 2004). In addition, three Arabidopsis mutants affecting biosynthesis of the GPI anchor have also been described. Mutations in two Arabidopsis genes, SETH1 and SETH2, encoding Arabidopsis homologs of two subunits of the enzymatic complex responsible for the first step of the GPI synthesis, have been shown to be male sterile, preventing pollen germination and pollen tube growth. The peanut1 mutant, which is defective for another enzyme of the GPI synthesis pathway, is seedling lethal and causes severe morphological defects in the embryo (Gillmor et al., 2005). However, the lack of GPI synthesis did not affect the fertility of the embryo sac in the above mutants. It is plausible that maternally produced enzymes are carried over during the development of the female gametophyte and can synthesize sufficient GPI for LRE function, as illustrated by mutations in many essential biosynthetic genes that result in embryo arrest rather than in gametophytic defects (Tzafrir et al., 2004).
The function of LRE appears specific to the process of fertilization because lre mutant homozygous plants are otherwise normal with no observable growth or developmental defects, consistent with the observed expression pattern of LRE with the highest expression in the synergids within the ovule. In mammals, GAPs appear to be critical for fertilization, as mouse eggs lacking GAPs on their surface, generated by conditional knockout of GPI synthesis, are unable to fuse with the sperm (Alfieri et al., 2003; Primakoff and Myles, 2007). GAPs expressed on the surface of mammalian sperm cells are also important for fertilization because cleavage of these proteins by ACE and release into the extracellular medium are required for sperm cell capacitation in oocyte binding (Kondoh et al., 2005). In mammals, species specificity in fertilization occurs through the interactions between sperm cell and egg cell, with cell surface proteins acting as key determinants (reviewed in Wassarman, 1999; Vieira and Miller, 2006). In flowering plants, barriers are provided by the sporophytic tissues, such as the stigma and transmitting tract, and play a major role in preventing interspecies fertilization for distantly related species. In the case of more closely related plant species, it has been demonstrated that species specificity occurs at the stage of the release of sperm from the pollen tube after penetration of the synergid cell (Escobar-Restrepo et al., 2007). Our study shows that although flowering plants and mammals have evolved very divergent mechanisms for fertilization and species recognition in reproduction, in both cases, GPI anchor proteins are used to regulate key steps in fertilization.
While the specific role of LRE in the communication between the pollen tube and the embryo sac remains to be elucidated, the known properties of GAPs suggest some models for future studies. LRE might act in the signaling pathway informing the embryo sac of the arrival of a pollen tube, acting in concert with the FER receptor kinase, for example, by modifying the lipid microenvironment (van Meer, 2002) to regulate FER localization or function, or downstream in the intracellular response, as exemplified by the GAP protein NDR1 in pathogen defense (Day et al., 2006). The result would be to trigger a signaling cascade in the synergid cell, eventually sending back a signal to the pollen tube, which causes the release of the sperm cells and also shutting down the attraction signal for other pollen tubes. An alternate hypothesis is that LRE itself could be part of the arrival signal for the pollen tube, either through its presence on the outer surface of the plasma membrane or through release from the membrane by cleavage of the anchor, perhaps in response to activation of the FER kinase. In the former case, the presence of LRE protein would cause an initial response in the pollen tube rather than in the embryo sac. These possible roles of LRE could be explored in the future by studies of LRE protein localization in the embryo sac during fertilization and in wild-type and feronia mutants and of protein interactions with FER kinase. Because the fusion of LRE to a GFP tag did not exhibit fluorescence in intact plant tissues, protein localization studies await the availability of antibodies to the LRE protein. Another question to be addressed is the potential function of the three LRE paralogs. The incomplete penetrance of LRE alone could be explained by redundancy with one or more of those genes that are involved in the same pathway. Examination of knockout mutants for At5g56170, At2g20700, and At4g28240 have not revealed any mutant phenotypes (G. Pagnussat, unpublished results); therefore, construction of double or triple mutants are needed to shed light on this issue. Finally, studies of LRE-related genes in other plant species could yield useful information on the set of the species-specific factors involved in sperm release in flowering plants.
METHODS
Genetic Screen and Mapping
The line GM 474 (Col-0 accession background) was obtained from the genetic screen of γ-ray mutagenized Arabidopsis thaliana seeds previously described (Guitton et al., 2004) and was crossed with wild-type Ler to generate F1 plants heterozygous for polymorphic markers between Col and Ler. The F1 mutant plants were allowed to self to generate F2 mapping populations. A mapping population of 2108 F2 plants from the GM474 line was analyzed. Markers for mapping were designed using the polymorphisms of the Cereon (www.arabidopsis.org/Cereon/index/html) online database.
Plant Transformation and Selection
Three-week-old Arabidopsis plants were transformed by in planta transformation (Bechtold and Pelletier, 1998) with Agrobacterium tumefaciens strain GV3101.
Seeds were surface-sterilized and plated onto medium (half-strength Murashige and Skoog salts, 1% sucrose, and 0.9% agar, pH 5.7) supplemented with 50 mg/L kanamycin (for lre-2/+), 50 mg/L kanamycin and 6 mg/L gluphosinate (for lre-2/+ transformed with JatY53G03), 50 mg/L kanamycin and 15 mg/L hygromycin (for lre-2/+ transformed with pC450), or 15 mg/L hygromycin (for lre-3/- and lre-4/+ transformed with pC450). After 2 d at 4°C, the plates were transferred to a Percival growth chamber (Percival Scientific) with a 16-h-light/8-h-dark cycle at 22°C. Antibiotic resistance was scored after 2 weeks. Plants were then transferred on soil and grown under the condition described above with 60% humidity.
For crosses, flowers of the ovule donor were emasculated and manually pollinated 48 h later.
Cleared Whole-Mount Ovule
For lorelei phenotyping analysis, flowers were emasculated and pollinated 48 h later with either wild-type pollen or self pollen. After 2 d, the fertilized pistils were collected and the replum cut open under a dissecting scope. The pistils were then fixed for 1.5 h in an acetic acid/ethanol solution (1:9 [v/v]) and cleared for 24 h in Hoyers solution (Liu and Meinke, 1998). The pistils were then dissected and the ovules observed under a Zeiss Axioplan imaging 2 microscope under DIC optics. Images were taken with an Axiocam HRC CCD camera (Zeiss) using the Axiovision 3.1 program.
Aniline Blue Staining of Pollen Tubes
Flowers were emasculated and pollinated 48 h later. After 2 more days, the pistils were harvested, and the replum was cut open under a dissecting scope and fixed for 1.5 h in an acetic acid/ethanol solution (1:9 [v/v]). The pistils were then softened overnight in a 5 M NaOH solution. Pistils were then stained for 10 min in a 0.1% aniline blue, 0.1 M K2PO4 buffer, pH 8.3, solution. After this, the pistils were cut open under a dissecting scope and observed with a Zeiss Axioplan imaging 2 microscope fitted with an excitation filter (BP 365/12), a chromatic beam splitter (FT 365), and a barrier filter (LP 397) (lre-2, lre-3, and lre-4) or with a ×40 objective (Nikon) with 340- to 380-nm excitation, 400-nm beamsplitter, and 420 long-pass filter set (Nikon) (lre-1).
In Situ Hybridization
Flowers or isolated gynoecia of wild-type plants were fixed in 4% paraformaldehyde and embedded in Paraplast (Fisher Scientific). Sections of 12-μm thickness were cut using a Leica microtome and mounted on ProbeOnPlus slides (Fischer Biotech). A fragment of 181 bp was amplified using sense (5′-GGTTGGAAACAGAATGTGA-3′) and antisense (5′-CTGTGCACAATCACTATTC-3′) primers, and hybridizations were conducted as described (Vielle-Calzada et al., 1999).
GFP-LORELEI Localization
The GFP fusion construct was introduced into Arabidopsis mesophyll protoplasts by polyethylene glycol–mediated transfection according to Yoo et al. (2007). Twelve to sixteen hours after transfection, the GFP signal was observed under an Eclipse E600 microscope equipped with epifluorescence optics (Nikon) and captured with an Orca CCD camera (Hamamatsu Photonics) using the MetaMorph software package (Molecular Devices). Filter sets for GFP (green) and Texas Red (red) (Semrock) were used. Images were then assembled with the Adobe Photoshop software package.
Arabidopsis Genomic DNA Extraction
One hundred milligrams of tissue was collected and ground in liquid nitrogen. Five hundred microliters of CTAB extraction buffer (2% CTAB, 1.4 M NaCl, 0.2 M EDTA, and 0.1 M Tris-HCl) was added, followed by 30 min of incubation at 65°C. Five hundred microliters of chloroform was added, and the sample was vortexed and spun at 12,000g for 10 min. The aqueous phase was collected and the DNA precipitated by adding 350 μL of isopropanol, followed by spinning at 12,000g for 20 min. The DNA was washed with 500 μL of ethanol 70% and dried. The pellet was resuspended in TE buffer (10 mM Tris-HCl and 1 mM EDTA).
T-DNA Lines
The T-DNA lines SALK_040289, SALK_007165, SALK_059671, and SALK_135772 were described by Alonso et al. (2003). The line SAIL_8_C08 was described by Sessions et al. (2002). The lines SALK_040289 and SAIL_8_C08 are lre-3 and lre-4, respectively.
Molecular Analysis of the une17 Line and T-DNA Lines
The 5′ flanking sequence isolation of une17 was described by Pagnussat et al. (2005). The 3′ flanking sequence of the Ds element was isolated using TAIL-PCR (Liu et al., 1995). The positions of both the 5′ and 3′ ends of the Ds element were checked using a gene-specific primer and a Ds-specific primer (5′-AGACGATGCGGAACAATCCATGTTG-3′ and 5′-TCCGTTCCGTTTTCGTTTTTTAC-3′ for the 5′ flanking sequence and 5′-GGCTGCAACAATGCAGATCCTGGTA-3′ and 5′-CCGGTATATCCCGTTTTCG-3′ for the 3′ flanking sequence). The PCR products were then purified and sequenced.
The SALK lines flanking sequences were checked using the Lba1 primer (5′-TGGTTCACGTAGTGGGCCATCG-3′) with the following gene-specific primers: 5′-CCTGAAAACGTCTCAGGGAGCTA-3′ for SALK_007165, 5′-AATTGTTGCCGGAGCCAGAT-3′ for SALK_059671, 5′-TCATTATTGCACTATCGACCTTAGGC-3′ for SALK_135772, and 5′-TGGTTGGAAACAGAATGTGAAGTGA-3′ for SALK_040289. For the line SAIL_8_C08, the following primers were used to amplify the left border flanking sequence: 5′-TTCATAACCAATCTCGATACAC-3′ and 5′-TGCTGCAACCGCTGTTTTTG-3′. The right border sequence of SALK_040289 was amplified using 5′-AACGGCTTGTCCCGCGTCAT-3′ and 5′-TGGCTCTTTCAATTTCCTTTGATTG-3′.
Both the left border and right border flanking sequences of SALK_040289 were purified and sequenced.
Plasmids
The plasmid JatY53G03 was obtained from the JatY collection of the John Innes Centre (http://jicgenomelab.co.uk/libraries.html). The pC450 was constructed from a pCAMBIA 1300 vector (Cambia). The annotated At4g26450 gene was amplified with the following primers 5′-ATATATGTCGACTGGGCTCAAGCTGAAACGTG-3′ and 5′-ATATATGTCGACCCGTGTGCTCTGTCTGCATT-3′, producing a 10-kb fragment. The pCAMBIA 1300 and the PCR fragment were digested with SalI and ligated together to form pC450.
A 2.6-kb fragment containing the sequence of LORELEI along with 1.4 kb of promoter and 0.45 kb of terminator sequences was amplified with the following primers 5′-ATATATGTCGACAATTGTTGCCGGAGCCAGAT-3′ and 5′-ATATATGTCGACCCGTGTGCTCTGTCTGCATT-3′. This fragment was cloned in pCAMBIA 1300 with SalI to create pCLRE.
To generate the GFP-LORELEI fusion, the LORELEI sequence, minus the first 69 bp corresponding to the N-terminal signal peptide was amplified by PCR. A HindIII and an 8x Ala linker was added to the 5′ with the primer, and an EcoRI site was added after the STOP codon at the 3′ with the primers. The primers used are 5′-ATATATAAGCTTGCGGCTGCCGCGGCAGCGGCAGCCTCGGATGGTGTGTTTGAAT-3′ and 5′-ATATATGAATTCTCAAGTCAACACTAACAAAG-3′. EGFP was modified through PCR. The sequence corresponding to the N-terminal signal peptide was added in three successive rounds of PCR as well as an Asp-718 restriction site at the 5′ end. A HindIII site was added at the 3′ end. The primers used for the 5′ end elongation were 5′-ATATATTCTCTCTCCTCTTCAAGTTCCATAATGAGTAAAGGAGAAGAACT-3′, 5′-ATATATCTTCTTTCTGATGGCACTGTTGGTATCTCTCTCCTCTTCAAGTTC-3′, and 5′-ATATATGGTACCATGGAGCTGATATTATTATTCTTCTTTCTGATGGCACTGT-3′, successively. The 3′ primer was 5′- ATATATAAGCTTTTTGTATAGTTCATCCATGC-3′. The EGFP fragment and the LORELEI fragment were combined in a vector containing a 35S promoter and a NOS terminator described by Griffith et al. (2007). The resulting plasmid was named p35s:GFP-LRE.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: Lorelei, EU727071; At5g265170, BT001157; At2g20700, BT012124; At4g28280, BT011635; Mt LORELEI-related 1, CU207236; Mt LORELEI-related 2, AC174340; Mt LORELEI-related 3, AC174340; Os 02g0721700, NM_001054497; Os 09g02978, NM_001069341; Pt LORELEI-related1, POP055-H02; Vr Arg8, AB013853; Zm 20282, DQ245780; Zm DQ244578, DQ244578; and Pp 171056, XM_001780105.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. RT-PCR on lre-3 Homozygous and Heterozygous Plants.
Supplemental Table 1. Real-Time PCR for LORELEI.
Supplemental Methods. RT-PCR Using Arabidopsis Total RNA, Real-Time PCR, and RT-PCR on lre-3/+ and lre-3/lre-3 Plants.
Supplemental Results. Identification of a New ORF in the At4g26450 Locus, LORELEI Expression in Arabidopsis Organs, and lre-3 as a Null Mutation.
Supplementary Material
Acknowledgments
We thank Ueli Grossniklaus for generously providing cell-specific marker lines used in this study, Quy Ngo for many helpful suggestions, and Thomas Berleth for discussions. This work was supported by National Science Foundation Grants 2010 0313501 and IOS-0745167 to V.S. and USDA/CSREES/NRICGP Grant 2005-35304-16031 to B.L. Research in the J.-P.V.-C. lab is supported by CONCyTEG, UC-MEXUS, and HHMI. A.C. was supported by an EMBO Long-Term Fellowship.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Venkatesan Sundaresan (sundar@ucdavis.edu).
Online version contains Web-only data.
References
- Acosta-Garcia, G., and Vielle-Calzada, J.P. (2004). A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. Plant Cell 16 2614–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri, J.A., Martin, A.D., Takeda, J., Kondoh, G., Myles, D.G., and Primakoff, P. (2003). Infertility in female mice with an oocyte specific knockout of GPI-anchored proteins. J. Cell Sci. 111 2149–2155. [DOI] [PubMed] [Google Scholar]
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215 403–410. [DOI] [PubMed] [Google Scholar]
- Bechtold, N., and Pelletier, G. (1998). In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82 259–266. [DOI] [PubMed] [Google Scholar]
- Bendtsen, J.D., Nielsen, H., von Heijne, G., and Brunak, S. (2004). Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340 783–795. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier, A., Frietsch, S., Kim, T.H., Dizon, M.B., and Schroeder, J.I. (2008). The peroxin loss-of-function mutation abstinence by mutual consent disrupts male-female gametophyte recognition. Curr. Biol. 18 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.H., Li, H.J., Shi, D.Q., Yuan, L., Liu, J., Sreenivasan, R., Baskar, R., Grossniklaus, U., and Yang, W.C. (2007). The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell 19 3563–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppinger, P., Repetti, P.P., Day, B., Dahlbeck, D., Mehlert, A., and Staskawicz, B.J. (2004). Overexpression of the plasma membrane-localized NDR1 protein results in enhanced bacterial disease resistance in Arabidopsis thaliana. Plant J. 40 225–237. [DOI] [PubMed] [Google Scholar]
- Day, B., Dahlbeck, D., and Staskawicz, B.J. (2006). NDR1 interaction with RIN4 mediates the differential activation of multiple disease resistance pathways in Arabidopsis. Plant Cell 18 2782–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus, T. (2006). Cell-cell communication during double fertilization. Curr. Opin. Plant Biol. 9 41–47. [DOI] [PubMed] [Google Scholar]
- Eisenhaber, B., Wildpaner, M., Schultz, C.J., Borner, G.H., Dupree, P., and Eisenhaber, F. (2003). Glycosylphosphatidylinositol lipid anchoring of plant proteins. Sensitive prediction from sequence- and genome-wide studies for Arabidopsis and rice. Plant Physiol. 133 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson, O., Nielsen, H., Brunak, S., and von Heijne, G. (2000). Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 300 1005–1016. [DOI] [PubMed] [Google Scholar]
- Escobar-Restrepo, J.M., Huck, N., Kessler, S., Gagliardini, V., Gheyselinck, J., Yang, W.C., and Grossniklaus, U. (2007). The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317 656–660. [DOI] [PubMed] [Google Scholar]
- Gillmor, C.S., Lukowitz, W., Brinistool, G., Sedbrook, J.C., Hamann, T., Pointdexter, P., and Somerville, C. (2005). Glycosylphosphatidylinositol-anchored proteins are required for cell wall synthesis and morphogenesis in Arabidopsis. Plant Cell 17 1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith, M.E., Mayer, U., Capron, A., Ngo, Q.A., Surendrarao, A., McClinton, R., Jürgens, G., and Sundaresan, V. (2007). The TORMOZ gene encodes a nucleolar protein required for regulated division planes and embryo development in Arabidopsis. Plant Cell 19 2246–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Hardt, R., Kägi, C., Baumann, N., Moore, J.M., Baskar, R., Gagliano, W.B., Jürgens, G., and Grossniklaus, U. (2007). LACHESIS restricts gametic cell fate in the female gametophyte of Arabidopsis. PLoS Biol. 5 e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton, A.E., Page, D.R., Chambrier, P., Lionnet, C., Faure, J.E., Grossniklaus, U., and Berger, F. (2004). Identification of new members of Fertilisation Independent Seed Polycomb Group pathway involved in the control of seed development in Arabidopsis thaliana. Development 131 2971–2981. [DOI] [PubMed] [Google Scholar]
- Higashiyama, T., Yabe, S., Sasaki, N., Nishimura, Y., Miyagishima, S., Kuroiwa, H., and Kuroiwa, T. (2001). Pollen tube attraction by the synergid cell. Science 293 1480–1483. [DOI] [PubMed] [Google Scholar]
- Huck, N., Moore, J.M., Federer, M., and Grossniklaus, U. (2003). The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130 2149–2159. [DOI] [PubMed] [Google Scholar]
- Kondoh, G., Tojo, H., Nakatani, Y., Komazawa, N., Murata, C., Yamagata, K., Maeda, Y., Kinoshita, T., Okabe, M., Taguchi, R., and Takeda, J. (2005). Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat. Med. 11 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne, E., Honys, D., Johnson, A., Borner, G.H.H., Lilley, K.S., Dupree, P., Grossniklaus, U., and Twell, D. (2004). SETH1 and SETH2, two components of the glycosylphophatidylinositol anchor biosynthetic pathway, are required for pollen germination and tube growth in Arabidopsis. Plant Cell 16 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, M.A., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23 2947–2948. [DOI] [PubMed] [Google Scholar]
- Liu, C.M., and Meinke, D.W. (1998). The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. Plant J. 16 21–31. [DOI] [PubMed] [Google Scholar]
- Liu, Y.G., Chen, Y., and Zhang, Q. (1995). Amplification of genomic sequences flanking T-DNA insertions by thermal asymmetric interlaced polymerase chain reaction. Methods Mol. Biol. 286 341–348. [DOI] [PubMed] [Google Scholar]
- Liu, Y.G., Mitsukawa, N., Oosumi, T., and Whittier, R.F. (2005). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8 457–463. [DOI] [PubMed] [Google Scholar]
- Mayor, S. (2005). ACEing GPI release. Nat. Struct. Mol. Biol. 12 107–108. [DOI] [PubMed] [Google Scholar]
- Muñiz, M., and Riezman, H. (2002). Intracellular transport of GPI-anchored proteins. EMBO J. 19 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau, J.A. and Sack, F.D. (2002). Control of stomatal distribution on the Arabidopsis leaf surface. Science 296 1697–1700. [DOI] [PubMed] [Google Scholar]
- Nielsen, H., Engelbrecht, J., Brunak, S., and von Heijne, G. (1997). Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10 1–6. [DOI] [PubMed] [Google Scholar]
- Orlean, P., and Menon, A.K. (2007). Thematic review series: Lipid posttranslational modifications. GPI anchoring of protein in yeast and mammalian cells, or: how we learned to stop worrying and love glycophospholipids. J. Lipid Res. 48 993–1011. [DOI] [PubMed] [Google Scholar]
- Pagnussat, G.C., Yu, H.J., Ngo, Q.A., Rajani, S., Mayalagu, S., Johnson, C.S., Capron, A., Xie, L.F., Ye, D., and Sundaresan, V. (2005). Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132 603–614. [DOI] [PubMed] [Google Scholar]
- Primakoff, P., and Myles, D.G. (2007). Cell-cell membrane fusion during mammalian fertilization. FEBS Lett. 581 2174–2180. [DOI] [PubMed] [Google Scholar]
- Rotman, N., Rozier, F., Boavida, L., Dumas, C., Berger, F., and Faure, J.E. (2003). Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr. Biol. 13 432–436. [DOI] [PubMed] [Google Scholar]
- Schindelman, G., Morikami, A., Jung, J., Baskin, T.I., Carpita, N.C., Derbyshire, P., McCann, M.C., and Benfey, P.N. (2001). COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 15 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions, A., et al. (2002). A high-throughput Arabidopsis reverse genetics system. Plant Cell 14 2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharom, F.J., and Lehto, M.T. (2002). Glycosylphosphatidylinositol-anchored proteins: Structure, function, and cleavage by phosphatidylinositol-specific phospholipase C. Biochem. Cell. Biol. 80 535–549. [DOI] [PubMed] [Google Scholar]
- Tzafrir, I., Pena-Muralla, R., Dickerman, A., Berg, M., Rogers, R., Hutchens, S., Sweeney, T.C., McElver, J., Aux, G., Patton, D., and Meinke, D. (2004). Identification of genes required for embryo development in Arabidopsis. Plant Physiol. 135 1206–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer, G. (2002). Cell biology. The different hues of lipid rafts. Science 296 855–857. [DOI] [PubMed] [Google Scholar]
- Vieira, A., and Miller, D.J. (2006). Gamete interaction: is it species-specific? Mol. Reprod. Dev. 73 1422–1429. [DOI] [PubMed] [Google Scholar]
- Vielle-Calzada, J.P., Thomas, J., Spillane, C., Coluccio, A., Hoeppner, M., and Grossniklaus, U. (1999). Maintenance of genomic imprinting in the Arabidopsis medea locus requires zygotic DDM1 activity. Genes Dev. 13 2971–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman, P.M. (1999). Mammalian fertilization: molecular aspects of gamete adhesion, exocytosis, and fusion. Cell 96 175–183. [DOI] [PubMed] [Google Scholar]
- Xu, X.M., and Møller, S.G. (2006). AtSufE is an essential activator of plastidic and mitochondrial desulfurases in Arabidopsis. EMBO J. 25 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, S.D., Cho, Y.H., and Sheen, J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protocols 2 1565–1572. [DOI] [PubMed] [Google Scholar]
- Zheng, H., Kunst, L., Hawes, C., and Moore, I. (2003). A GFP-based assay reveals a role for RHD3 in transport between the endoplasmic reticulum and Golgi apparatus. Plant J. 37 398–414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.