Abstract
The possibility of using microbes to maintain health, and to prevent or treat disease is a topic as old as microbiology. However, one factor impeding the introduction of effective probiotics has been our very limited understanding of the composition of the human microbiome, as well as the biological requirements for these organisms. With advances in understanding the microbiome and its metagenome in humans and other mammals, we now can build a more robust scientific basis to develop probiotic strategies. Increasing knowledge of intramicrobial competition and cooperation, as well as host-microbe cross-signaling, will facilitate design of new probiotics and the modeling of their deployment, leading to eventual clinical trials.
Introduction
The microbial component of humans or animals has been termed the indigenous microbiota. However, since “biome” refers to “ecosystems” in ecology, the term microbiome, introduced by Joshua Lederberg (Lederberg 2000) also applies. Despite interest in the human microbiome, going back more than a century (reviewed by Mackowiak, 1982), it remains largely unexplored (Blaser 2006). Experimental evidence shows that the microbiome is needed for the health of the host and that alterations in the ecological equilibrium of microbes can lead to disease. Therefore, it is logical to expect that the use of microbes that are members of the microbiome might help us restore balance. We use the term “probiotics” to refer to microbes that are administered to produce beneficial effects on health, and the term “prebiotics” to indicate substrates that improve the growth or metabolic activities of particular indigenous organisms. Long ago, physicians and others started the practice of administering intestinal microbes (as probiotics) to maintain health or correct disease, before they clearly understood the mechanisms involved in microbial metabolism and signaling. The knowledge base underlying the use of probiotics is small today, but is an important frontier for scientific inquiry to improve human health.
The Human Microbiome
The first life forms on earth were bacteria, over 4 billion years ago, and we continue to live in the age of bacteria (Gould 1994). All subsequent forms of life evolved in their presence, interacting and integrating with them. Mammals appear to be born free of bacteria, fungi, or protozoa, but in the microbial world in which we reside, our exposed organs -skin, body invaginations, and digestive tract- become niches for adapted microbes. In a sense, these spaces reflect in part the exterior environment crossing our body. The contents of these organs are kept separated from the “interior” of the body by barriers that effectively cordon the luminal microbes. Humans have a developmental program for expression of antimicrobial peptides modulating the microbial ecosystem that begins to form shortly after birth (Menard, Forster et al. 2008). The process of colonization is dynamic, creating the structured populations reached in the climax community, which itself is subject to perturbations (Caufield, Cutter et al. 1993; Schwiertz, Gruhl et al. 2003; Hopkins, Macfarlane et al. 2005). The choreography of such events is modulated by the complex interactions involving microbial signaling with other microbes and with the host.
The best studied microbe-dominated organ is the human intestinal ecosystem, estimated to contain 500–1,000 bacterial species (Mackowiak 1982; Eckburg, Bik et al. 2005). However, amplifying and sampling signature genes for taxonomic purposes provides incomplete definition, due to PCR biases and insufficient sample coverage. Analyses of the microbiomes of the skin (Gao, Tseng et al. 2007), mouth (Kroes, Lepp et al. 1999), esophagus (Pei, Bini et al. 2004), stomach (Bik, Eckburg et al. 2006) and vagina (Hyman, Fukushima et al. 2005), also have provided preliminary views. Table 1 shows current examples of the roles of digestive tract microbes on intestinal function; however, in reality we know little about the roles of our microbial populations and their interactions with other microbes and with the host. Detailed metagenomic analyses will provide the next level of understanding.
Table 1.
Non-immunologic function of the gastrointestinal microbiome
| Physiological benefit | Demonstrated effects | Reference |
|---|---|---|
| Host nutrition | Synthesis of vitamins | (Stevens and Hume 1995), (Savage 1986) |
| Degradation of indigestible plant cell walls | (Savage 1986; Mackie 2002) | |
| Integrity of intestinal barrier | induction of epithelial growth by microbial butyrate | (Gaudier, Forestier et al. 2004) |
| Colonization resistance | Protection against pathogen colonization | (Boullier, Nougayrede et al. 2003) |
| Regulation of angiogenesis | Stimulation of the elaboration of microvasculature | (Stappenbeck, Hooper et al. 2002)] |
| Hormonal regulation | Energy homeostasis: modulation of leptin and ghrelin Estrogen metabolism in the intestine |
(Roper, Francois et al. 2007) (Tamura, Tsushida et al. 2007) |
The structure of the intestinal microbiome in humans has high individual variability. Essentially all humans living in primitive societies harbor protozoa and helminths (Marini, Maldonado et al. 2007), probably resembling the microbiomes of humans in prehistoric times. With modern lifestyles, which involve improved hygiene, small family size, and intensive exposure to antibiotics from early ages, protozoa and helminths are disappearing from the digestive tract of modern populations, and some bacterial species also are trending towards extinction [for example H. pylori (Perez-Perez, Salomaa et al. 2002; Blaser 2005)]. The health implications of these trends focus in part our current interests towards probiotics.
Mechanisms of immune modulation by microbes
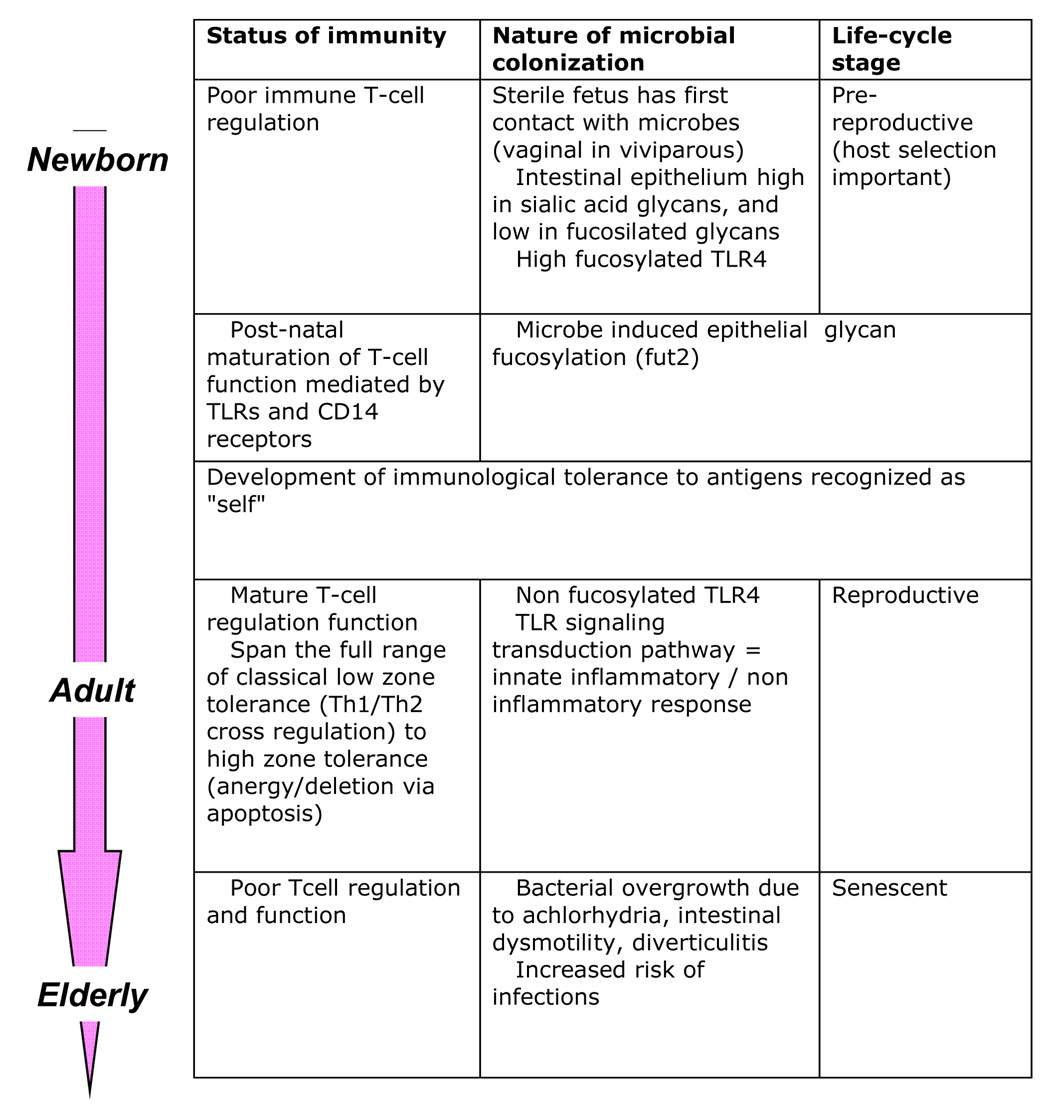
Newborns have poorly developed immune regulation, involving T-cells, and regulatory and antigen-presenting cells (Figure 1). Post-natal development, intrinsically modulated by both genetic and microbial factors, leads to a functionally healthy intestine that maintains a dynamic balance between microbes, host epithelial cells and mucosal immunity. Eukaryotic cell TLR recognition provides a molecular basis for bacterial-epithelial cross-talk, with effects on both innate and acquired immune responses [(Bach 2005; Walker 2008); (Table 2)]. Indigenous microbes also may protect against autoimmune and inflammatory diseases (Ismail and Hooper 2005), using mechanisms including antigenic competition (immune responses against pathogens compete with autoimmune and allergic responses) and immunoregulation [stimulation of regulatory cells (Th2, CD25+, Tr1, and NKT)]. Since the microbial population structure helps maintain mucosal immunologic balance, factors affecting the community may disrupt mucosal tolerance. Decreases in infectious diseases with modernity, related to frequent antibiotic use and possibly excessive hygiene, have been associated with a concomitant increase in the prevalence of autoimmune and chronic inflammatory diseases involving the mucosa [respiratory allergies, rhino-sinusitis, inflammatory bowel disease, and possibly some lymphomas(Bach 2005; Praprotnik, Sodin-Semrl et al. 2008)]. For example, helminths affect host mucosal and systemic immunity, inhibiting dysregulated inflammatory responses and inducing regulatory T-cell activity (Weinstock, Summers et al. 2005).
Fig 1.
Developmental cross-talk between colonizing microbes and the immune response across the human lifespan
Table 2.
Mechanisms of immunity modulated by bacteria
| Function | Mechanism | Reference |
|---|---|---|
| Intestinal homeostasis | Stimulation of the gut lymphoid tissue | (Kerneis, Bogdanova et al. 1997; Cherayil and Walker 2003) |
| Mucosal defense | Intraepithelial and lamina propria production of cytokines | (Kohler, McCormick et al. 2003), |
| Maturation of helper T cells into mature TH1/Th2 and Th3/Trl | (Calder, Krauss-Etschmann et al. 2006; Walker 2008). | |
| Secretion of IgA by mesenteric lymph nodes | (Macpherson and Uhr 2004) | |
| Decreased IgE production in response to antigens (oral tolerance; eg. IgE production to ovalbumin ingestion is higher in germfree mice) | Sudo, Sawamura et al. 1997; Spiekermann and Walker 2001; Bashir, Andersen et al. 2002) | |
| Stimulation of antigen-presenting cells | (Cherayil and Walker 2003; Walker, Rhubart-Berg et al. 2005) | |
| Immunological tolerance | Dendritic cell sampling of bacterial antigens through Toll like receptors (TLR) | (Rescigno, Urbano et al. 2001) |
| Interaction of microbes with host CD14 | (Akira, Takeda et al. 2001; Elson, Dunn-Siegrist et al. 2007; Udan, Ajit et al. 2008), |
Can we improve upon the human microbiome? The use of prebiotics and probiotics and sustainability of the microbiome
Current probiotics are naturally occurring indigenous microbes that are aimed to restore lost bacteria or metabolic activities in colonized organs, restore a balanced immune response similar to that induced by the usual indigenous microbiota, or to suppress pathogenic microbes (Kalliomaki and Isolauri 2003). The scope of probiotics being used is narrow: several intestinal species predominate, including Bifidobacterium and Lactobacillus species, Streptococcus thermophilus, Enterococcus and Bacillus species, E. coli and yeasts, including Sacharomyces boulardii.
Preliminary studies indicate that probiotics can affect innate immunity, as evidenced by oral tolerance, which cannot be achieved in germ-free animals (Sudo, Sawamura et al. 1997), is reduced by antibiotic use (Bashir, Louie et al. 2004), and can be restored by administering probiotics (Braat, van der Brande et al. 2004). Probiotics also protect against pathogens by strengthening the intestinal mucosal (immune) barrier, decreasing pathogen adherence (Mack, Michail et al. 1999), or by production of acid or antibiotics inhibitory to pathogens (Gibson, McCartney et al. 2005). Our scientific understanding of currently used probiotics is limited; their beneficial effects are usually low, with strong placebo effects, and a substantial lack of robustness across experiments.
Modern era lifestyles are impacting our microbiome in ways we are just beginning to elucidate. We might need to have a probiotic in our future, but first, well-designed clinical trials are needed. The future of probiotics might expand in several ways:
1. Use of non-bacterial members of the human microbiota as probiotics
Fungi also are known to be constituents of the intestinal microbiota in mice (Scupham, Presley et al. 2006), fish (Andlid, Blomberg et al. 1999) and humans, and they are being investigated as intestinal probiotics (Martins, Nardi et al. 2005). S. boulardii may bind pathogenic Escherichia coli strain and salmonellae (Buts, Dekeyser et al. 2006)]. Effective probiotics for the skin, vagina, nose, and ear microbiota are yet to be discovered. It also is possible that constituents of other microbiomes from other animals might have specific utilities for humans; these, too, would need to be carefully tested so that the zoonotic costs do not exceed the potential benefits.
2. Administration of bacteriophages
Bacteriophages may be considered as either probiotics or as prebiotics , in the sense that they would inhibit specific bacterial populations, promoting the growth of other species. Bacteriophages also are potential alternatives to antibiotics (Sulakvelidze and Morris 2001). Discovered independently by Frederick W. Twort and Felix d’Herelle (Stone 2002), phages have been extensively used to prevent and treat bacterial infections, mainly in Eastern Europe and the former Soviet Union (Shasha, Sharon et al. 2004). Phages have been used in humans against diarrheal diseases caused by E. coli, Shigella or Vibrio, and to eliminate Campylobacter, Escherichia and Salmonella in farm animals (Lederberg 1996; Sulakvelidze and Morris 2001). Although more specific, the lysogenic (non-lytic) pathway can limit the effectiveness of phage therapy.
3. Development of new prebiotics
Deeper knowledge of human microbial ecology will lead to specific prebiotic approaches to promote growth of favorable microbes, or to provide substrate for favorable metabolic pathways. In that sense, lactulose, a non-absorbable polysaccharide currently used as a prebiotic to treat hepatic encephalopathy, has multiple effects; next generation prebiotics may have much greater specificity.
Conclusions
It is likely that many potentially useful microbes remain unculturable. Better elucidation of the microbiome will lead to improved techniques to culture and evaluate candidate probiotic organisms. An alternative possibility is the banking of each individual’s intestinal contents (obtained as a fecal sample during health) that could be administered at the time of disease. Either the total (undefined) biota could be useful or the specimen cultured to isolate particular host-specific organisms that had become depleted. The former approach is closer to the current state-of-the-art, but it is the latter that is most desirable.
In any event, a fundamental need to advance the use of probiotics and prebiotics is to improve our knowledge of the microbiome and its function, which will facilitate identifying and evaluating microbes that can be given to many hosts. Technical advances involving metagenomic, transcriptomic, and proteomic analyses of our microbial communities promise to increase our understanding of the human microbiome. This improved knowledge will aid the design of new probiotics and prebiotics, leading to the necessary clinical trials to assess efficacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Takeda K, et al. Toll-like receptors: critical problems linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Andlid T, Blomberg L, et al. Characterization of Saccharomyces cerevisiae CBS 7764 isolated from rainbow trout intestine. Systematic and Applied Microbiology. 1999;22(1):145–155. doi: 10.1016/S0723-2020(99)80037-X. [DOI] [PubMed] [Google Scholar]
- Bach JF. Infections and autoimmune diseases. J Autoimmun. 2005;25 Suppl:74–80. doi: 10.1016/j.jaut.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Bashir MEH, Andersen P, et al. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169:3284–3292. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- Bashir MEH, Louie S, et al. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- Bik EM, Eckburg PB, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A. 2006;103(3):732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ. An endangered species in the stomach. Sci Am. 2005;292(2):38–45. doi: 10.1038/scientificamerican0205-38. [DOI] [PubMed] [Google Scholar]
- Blaser MJ. Who are we? Indigenous microbes and the ecology of human diseases. EMBO Rep. 2006;7(10):956–960. doi: 10.1038/sj.embor.7400812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boullier S, Nougayrede JP, et al. Genetically engineered enteropathogenic Escherichia coli strain elicits a specific immune response and protects against a virulent challenge. Microbes Infect. 2003;5(10):857–867. doi: 10.1016/s1286-4579(03)00175-8. [DOI] [PubMed] [Google Scholar]
- Braat H, van der Brande J, et al. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dentritic cell function. Am J Clin Nutr. 2004;80:1618–1625. doi: 10.1093/ajcn/80.6.1618. [DOI] [PubMed] [Google Scholar]
- Buts JP, Dekeyser N, et al. Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatric Research. 2006;60(1):24–29. doi: 10.1203/01.pdr.0000220322.31940.29. [DOI] [PubMed] [Google Scholar]
- Calder PC, Krauss-Etschmann S, et al. Early nutrition and immunity - progress and perspectives. Br J Nutr. 2006;96(4):774–790. [PubMed] [Google Scholar]
- Caufield PW, Cutter GR, et al. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J. Dent Res. 1993;72(1):37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- Cherayil BJ, Walker WA. Microbial pathogenesis and the intestinal epithelial cell. G. Hecht. Washington DC: ASM Press; 2003. Ontogeny of the host to enteric microbial infection; pp. 333–349. [Google Scholar]
- Eckburg PB, Bik EM, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson G, Dunn-Siegrist I, et al. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 2007;109(4):1574–1583. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, et al. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A. 2007;104(8):2927–2932. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudier E, Forestier L, et al. Butyrate regulation of glycosylation-related gene expression: evidence for galectin-1 upregulation in human intestinal epithelial goblet cells. Biochem Biophys Res Commun. 2004;325(3):1044–1051. doi: 10.1016/j.bbrc.2004.10.141. [DOI] [PubMed] [Google Scholar]
- Gibson GR, McCartney AL, et al. Prebiotics and resistance to gastrointestinal infections. Br J Nutr. 2005;93 Suppl 1:S31–S34. doi: 10.1079/bjn20041343. [DOI] [PubMed] [Google Scholar]
- Gould SJ. The Evolution of Life on Earth. Scientific American. 1994 October;:63–69. doi: 10.1038/scientificamerican1094-84. [DOI] [PubMed] [Google Scholar]
- Hopkins MJ, Macfarlane GT, et al. Characterisation of intestinal bacteria in infant stools using real-time PCR and northern hybridisation analyses. FEMS Microbiology Ecology. 2005;54(1):77–85. doi: 10.1016/j.femsec.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Hyman RW, Fukushima M, et al. Microbes on the human vaginal epithelium. Proc Natl Acad Sci U S A. 2005;102(22):7952–7957. doi: 10.1073/pnas.0503236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AS, Hooper LV. Epithelial Cells and Their Neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;289:G779. doi: 10.1152/ajpgi.00203.2005. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol. 2003;3(1):15–20. doi: 10.1097/00130832-200302000-00003. [DOI] [PubMed] [Google Scholar]
- Kerneis S, Bogdanova A, et al. Conversion by Peyer's Patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:910–911. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Kohler H, McCormick BA, et al. Bacterial-enterocyte crosstalk: cellular mechanisms in cell and disease. J Pediatric Gastroenterol Nutr. 2003;36:175–185. doi: 10.1097/00005176-200302000-00005. [DOI] [PubMed] [Google Scholar]
- Kroes I, Lepp PW, et al. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci U S A. 1999;96(25):14547–14552. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. Smaller fleas … ad infinitum: therapeutic bacteriophage redux. Proc Natl Acad Sci U S A. 1996;93(8):3167–3168. doi: 10.1073/pnas.93.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. Infectious history. Science. 2000;288(5464):287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- Mack DR, Michail S, et al. Probiotics inhibit entheropathogenic E. coli adherence in vitro by inducing intestinal mucin gene exoression. Am J Physiol. 1999;276:G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- Mackie RI. Mutualistic Fermentative Digestion in the Gastrointestinal Tract: Diversity and Evolution. Integrative and Comparative Biology. 2002;42(2):319–326. doi: 10.1093/icb/42.2.319. [DOI] [PubMed] [Google Scholar]
- Mackowiak PA. The normal microbial flora. New England Journal of Medicine. 1982;307:83–93. doi: 10.1056/NEJM198207083070203. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Marini E, Maldonado AL, et al. Helicobacter pylori and intestinal parasites are not detrimental to the nutritional status of Amerindians. Am. J. Trop. Med. Hyg. 2007;76(3):534–540. [PubMed] [Google Scholar]
- Martins FS, Nardi RM, et al. Screening of yeasts as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J Gen Appl Microbiol. 2005;51(2):83–92. doi: 10.2323/jgam.51.83. [DOI] [PubMed] [Google Scholar]
- Menard S, Forster V, et al. Developmental switch of intestinal antimicrobial peptide expression. J Exp Med. 2008 doi: 10.1084/jem.20071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z, Bini E, et al. Bacterial biota in the human distal esophagus. PNAS. 2004;101(12):4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez GI, Salomaa A, et al. Evidence that cagA(+) Helicobacter pylori strains are disappearing more rapidly than cagA(−) strains. Gut. 2002;50(3):295–298. doi: 10.1136/gut.50.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praprotnik S, Sodin-Semrl S, et al. The curiously suspicious: Infectious disease may ameliorate an ongoing autoimmune destruction in systemic lupus erythematosus patients. J Autoimmun. 2008;30(1–2):37–41. doi: 10.1016/j.jaut.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Roper J, Francois F, et al. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. Journal of Clinical Endocrinology & Metabolism. 2007;10(1210):2007–2057. doi: 10.1210/jc.2007-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DC. Gastrointestinal microflora in mammalian nutrition. Annu Rev Nutr. 1986;6:155–178. doi: 10.1146/annurev.nu.06.070186.001103. [DOI] [PubMed] [Google Scholar]
- Schwiertz A, Gruhl B, et al. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res. 2003;54(3):393–399. doi: 10.1203/01.PDR.0000078274.74607.7A. [DOI] [PubMed] [Google Scholar]
- Scupham AJ, Presley LL, et al. Abundant and diverse fungal microbiota in the murine intestine. Applied and Environmental Microbiology. 2006;72(1):793–801. doi: 10.1128/AEM.72.1.793-801.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasha SM, Sharon N, et al. Bacteriophages as antibacterial agents. Harefuah. 2004;143(2):121–125. [PubMed] [Google Scholar]
- Spiekermann G, Walker WA. Oral tolerance and its role in clinical disease. J Pediatric Gastroenterol Nutr. 2001;32:237–255. doi: 10.1097/00005176-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Hooper LV, et al. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99(24):15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CE, Hume I. Comparative physiology of the vertebrate digestive system. Cambridge University Press; 1995. [Google Scholar]
- Stone R. Bacteriophage therapy: Stalin's Forgotten Cure. Science. 2002;298(5594):728–731. doi: 10.1126/science.298.5594.728. [DOI] [PubMed] [Google Scholar]
- Sudo N, Sawamura S, et al. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–1745. [PubMed] [Google Scholar]
- Sulakvelidze A, Morris JJ. Bacteriophages as therapeutic agents. Ann Med. 2001;33(8):507–509. doi: 10.3109/07853890108995959. [DOI] [PubMed] [Google Scholar]
- Tamura M, Tsushida T, et al. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe. 2007;13:32–35. doi: 10.1016/j.anaerobe.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Udan ML, Ajit D, et al. Toll-like receptors 2 and 4 mediate Abeta(1-42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104(2):524–533. doi: 10.1111/j.1471-4159.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- Walker P, Rhubart-Berg P, et al. Public health implications of meat production and consumption. Public Health Nutrition. 2005;8(4):348–356. doi: 10.1079/phn2005727. [DOI] [PubMed] [Google Scholar]
- Walker WA. Mechanisms of action of probiotics. Clinical Infectious Diseases. 2008;46:S87–S91. doi: 10.1086/523335. [DOI] [PubMed] [Google Scholar]
- Weinstock JV, Summers RW, et al. Role of helminths in regulating mucosal inflammation. Springer Semin Immunopathol. 2005 doi: 10.1007/s00281-005-0209-3. [DOI] [PubMed] [Google Scholar]