Abstract
The human amygdala preferentially responds to objects of potential value, such as hedonically valenced and novel stimuli. Many studies have documented age-related differences in amygdala responses to valenced stimuli, but relatively little is known about age-related changes in the amygdala’s response to novelty. This study examines whether there are differences in amygdala novelty responses in two different age groups. Healthy young and elderly adults viewed both young and elderly faces that were seen many times (familiar faces) or only once (novel faces) in the context of an fMRI study. We observed that amygdala responses to novel (versus familiar) faces were preserved with aging, suggesting that novelty processing in the amygdala remains stable across the lifespan. In addition, participants demonstrated larger amygdala responses to target faces of the same age group than to age out-group target faces (i.e., an age in-group effect). Differences in anatomic localization and behavioral results suggest that novelty and age in-group effects were differentially processed in the amygdala.
Keywords: functional magnetic resonance imaging, aging, human, face perception, emotion, neuroimaging, amygdala
Introduction
Many neuroimaging studies in young healthy adults have demonstrated responses of the amygdala to valenced stimuli, particularly during emotion perception (for a meta-analytic summary, see Wager et al., in press). A number of studies now confirm that as people age, they develop a preference for positive (relative to negative) information, and this shift is associated with changes in the neural activity of the amygdala and closely related structures. Recent neuroimaging studies in the elderly show that the amygdala responds weakly to negative stimuli (Iidaka et al., 2002; Gunning-Dixon et al., 2003; Mather et al., 2004; Fischer et al., 2005; Tessitore et al., 2005; Williams et al., 2006), and in certain cases shows stronger responses to positive stimuli (Mather et al., 2004; Williams et al., 2006). Yet, the amygdala is engaged by more than just hedonically potent stimuli. The amygdala routinely responds to novel stimuli (e.g., Dubois et al., 1999; Schwartz et al., 2003; Wilson & Rolls, 1990; Wright et al., 2003; Wright et al., 2006, 2007) and quickly habituates to stimuli as they become familiar (Breiter et al., 1996; Wedig et al., 2005; Wright et al., 2003, Wright, Fischer et al., 2001). In fact, recent evidence suggests that caricatures of fear faces reliability engage the human amygdala because they are unfamiliar and rarely seen in real life (Somerville & Whalen, 2006). The amygdala preferentially responds to stimuli of uncertain value (Herry et al., 2007), and the act of reducing the ambiguity in the meaning in a stimulus (e.g., providing a face with an emotional and opposed to a gender label) decreases amgydala response (Lieberman et al., 2007). Furthermore, amygdala lesions disrupt normal responses to novelty in primates (e.g. Prather et al., 2001). One function of the amygdala may be to direct attention and physiological responses (Holland & Gallagher, 1999) allowing mammals to learn more about a stimulus when its current predictive value is unknown (cf. Barrett, Lindquist et al., 2007). Currently, little is known about whether amygdala responses to novel stimuli change with age.
The only existing studies to examine age-related changes in amygdala responses to novelty found that novel emotional stimuli (e.g., faces depicting fear that were never before seen) engaged the amygdala similarly in both young and elderly adults (when compared to familiar neutral expressions) (Wright et al., 2006, 2007). Given that emotion and novelty were combined in the target stimuli of that study, it is not clear whether the amygdala activation observed in those elderly participants was due to novelty or a novelty-emotion interaction. If the amygdala responds similarly to novelty in both young and elderly adults, it would indicate that novel stimuli remain salient across the lifespan, and therefore that novelty processing – a potentially survival-relevant function – is preserved with aging.
Preserved age-related amygdala responses to novelty might suggest that, although the amygdala is involved in affective processing, it also performs the more basic function of maintaining vigilance (Whalen, 2007) by directing an organism to learn more about a stimulus so as to better determine its predictive value for well-being or survival (Barrett et al., 2007; Rolls, 2005; Whalen, 1998). Alternatively, it might be that novelty is itself a basic dimension of affective processing. Appraisal models of emotion, for example, propose that novel stimuli attract attention and set the stage for further affective processing and other meaning analysis in which emotion is grounded (Frijda et al., 1989; Roseman, 1996; Scherer, 1984; Smith & Ellsworth, 1985).
The main goal of the current study was to examine amygdala novelty responses to neutral stimuli in young and elderly adults. During an fMRI paradigm, all participants viewed both young and elderly target faces portraying a neutral expression during a passive viewing task.
A second goal of this study was to examine amygdala responses to in-group and out-group faces as defined by age. People often show enhanced amygdala responses to out-group (vs. in-group) faces when groups status is defined by skin color (i.e., race) (Hart et al., 2000; Phelps et al., 2000), and this response may be partly governed by novelty and familiarity. Age and race based in-group faces are more easily recognized (Ellis and Deregowski, 1981; Wright and Stroud, 2002; Walker and Tanaka, 2003; Anastasi and Rhodes, 2005; Perfect and Moon, 2005) and amygdala responses habituate more rapidly to race in-group versus out-group faces (Hart et al., 2000). A more recent study has shown that both African American and European American participants have larger amygdala responses to African American (vs., European American) target faces (Lieberman et al., 2005), suggesting that the degree of contrast in the face (perhaps around the eye, involving the sclera; Whalen, Kagan, et al., 2004) or some other feature than novelty per se, may driving amygdala differential amygdala response to African American faces. In any case, no studies to date have examined amygdala responses to stimulus group membership as defined by age. Therefore, in the context of studying novelty responses in young and elderly participants, we also investigated amygdala responses to the effects of target face age.
Materials and Methods
Subjects
Sixteen healthy young adults (Table 1; 8 females, 8 males; age M=22.9, SD=2.5, Range=19–30 years) and 16 healthy elderly adults (8 females, 8 males; age M=72.4, SD=6.9, Range=60–84 years) were included in the study. Twenty young adults were initially studied but four were excluded due to excessive motion (total motion vector >3mm) or scanner/image-related difficulties. Nineteen elderly adults were initially studied, but three were excluded because of scanner/image-related difficulties or failing to meeting inclusion criteria (i.e., left-handed). This study was approved and conducted in accordance with guidelines established by the Partners Human Research Committee. Written informed consent was obtained from each subject.
Table 1. Participant Charactersitics and Neuropsychological Variables.
Mean and standard deviations are reported. Superscripts across cell rows indicate statistical significance at p<.05. Note that years of education and BNT data were missing for one participant in the young cohort.
| Young | Elderly | |
|---|---|---|
| Subject Characteristics | ||
| Age | 22.9 (2.5)a |
72.4 (6.9)a |
| Education - yrs | 15.9 (1.9)a |
16.7 (3.1)a |
| American National Reading Test | 123.5 (7.4)a |
123.8 (10.7)a |
| Mini Mental State Exam (MMSE) | 28.8 (1.2)a |
28.2 (1.7)a |
| Boston Naming Test (BNT) | 14.7 (0.6)a |
14.6 (0.7)a |
| Extraversion T-score | 60.2 (9.9)a |
53.9 (10.2)a |
| Neuroticism T-score | 42.3 (7.5)a |
40.0 (8.2)a |
| Openness T-score | 57.6 (8.6)a |
52.9 (11.5)a |
| Agreeableness T-score | 53.6 (11.1) |
57.2 (11.7)a |
| Conscientiousness T-score | 53.9 (11.8)a |
49.0 (10.3)a |
| CERAD | ||
| Delayed Recall | 8.4 (1.8)a |
6.8 (2.3)b |
| Recognition | 19.8 (0.4)a |
19.5 (0.7)a |
All participants underwent the Structured Clinical Interview for DSM-IV (First et al., 1995) to confirm the absence of DSM-IV Axis I diagnoses (American Psychiatric Association, 1994). All were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971) and free of psychoactive medications. In addition, participants completed the American National Reading Test (Nelson, 1982), Mini-Mental State Exam (Folstein et al., 1975), Boston Naming Test (Kaplan et al., 1983), Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Test of Memory (Morris et al., 1989), the 6 front-view faces of the Benton Face Recognition Test (Benton et al., 1983), and the NEO Five Factor personality inventory (NEO-FFI) (McCrae and Costa, 1997).
Procedure
The overall paradigm was similar to that reported in previous studies (Schwartz et al., 2003; Wright et al., 2003; Wright et al., 2006a; Wright et al., 2007b). All participants passively viewed neutral faces from the University of Illinois Urbana Champain (IUIC) face database (Minear and Park, 2004) in three 4 min 30 sec runs [one familiarization run, and two test runs (Fig. 1)]. In the familiarization run, participants viewed young (n=4; 2 female) and elderly (n=4; 2 female) faces in 8 alternating 20 sec face blocks (with repeated presentations of neutral faces), interspersed with 10s fixation blocks. Each face was presented for 500 ms with a 500 ms interstimulus interval during which a fixation cross was shown. In the two test runs, participants viewed alternating blocks of young or elderly familiar faces (those seen in the familiarization blocks) and novel during imaging (not yet seen before) faces of both age groups. Novel faces were presented once throughout the imaging experiment. The timing of the test and familiarization runs was similar. All runs were bracketed by two 20s fixation blocks. All face stimuli were presented in a pseudorandom order, such that no familiar face was presented twice before all others were presented, and no more than two male or two female faces were presented in a row. The order of presentation was counterbalanced across participants.
Figure 1. Novel and Face-Age Paradigm.
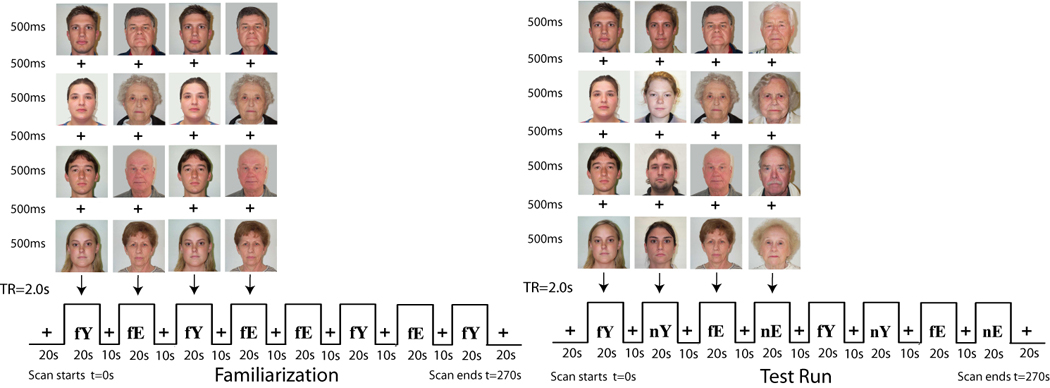
Stimulus presentations using neutral faces were divided into one familiarization run and two test runs. The familiarization run included repeated presentations of 8 unique identities, 4 elderly (fE) and 4 young (fY), viewed over eight 20s blocks with an interspersed 10s fixation. Each test run included eight alternating blocks of the following four stimuli types: familiarized elderly and young faces (fE, fY) and novel elderly and young faces (nE, nY). An example of only one test run and a single run order is shown. Each face stimulus was presented for 500ms with a 500 ms interstimulus interval. Each run was bracketed by two 20s fixation blocks, for a total of 4 minutes 30 seconds scan. The repetition time (TR) for obtaining whole brain images was 2s.
The face stimuli (in PICT format) were displayed using standardized software (MacStim 3.2.) and a Sharp XGNV6 color LCD projector (Osaka, Japan). Subjects were instructed to look at the faces at the eye-level, and remain awake and alert. An instruction reminder was given prior to each successive run.
Immediately after the scanning session, participants performed a recognition task where they judged whether or not they saw a series of faces in the scanner. For recognition task, three types of faces were used: all 8 which had been seen many times (“familiar faces”), 16 faces that had been viewed once during scanning (“novel during scanning faces”), and, 16 faces not seen during scanning (“single presentation faces”). An equal number of male and female faces were presented across face type. In addition, participants were asked to rate each face stimulus according to valence (negative-positive; −3 to +3) and arousal value (low-high; 0–6).
Image Acquisition
An Avanto 1.5 Tesla whole body high-speed imaging device equipped for echo planar imaging (EPI) (Siemens Medical Systems, Iselin NJ) was used with a 12-channel gradient head coil. Head movement was restricted using expandable foam cushions. After an automated scout image was acquired and shimming procedures were performed to optimize field homogeneity, high-resolution 3D MPRAGE sequences (TR/TE/flip angle=2730ms/3.31ms/7°) with an in-plane resolution of 1.3 X 1.0 mm, and 1.3 mm slice thickness were collected for spatial normalization and for positioning the slice prescription of the subsequent sequences. Then a T1-EPI (TR/TE/flip angle=8.0sec/39ms/90°) and a T2-weighted (TR/TE/flip angle= 5.64sec/95ms/150°) sequences were collected to assist in registration of the functional data to the high-resolution anatomical scan. Functional MRI images (blood oxygenation level dependent or BOLD) (Kwong et al., 1992) were acquired using a gradient echo T2*-weighted sequence (TR/TE/flip angle= 2.0sec/40ms/90°). Prior to each scan, four time points were acquired and discarded to allow longitudinal magnetization to reach equilibrium. The T1, T2, and gradient-echo functional images were collected in the same plane (26 coronal slices angled perpendicular to the ac-pc line) with the same slice thickness (5mm; voxel size 3.125 × 3.125 × 5 mm), excitation order (interleaved) and phase encoding (foot-to-head). These parameters were used as earlier work suggested that they helped minimize susceptibility in medial temporal lobe regions (Wright et al., 2001).
Image Pre-preprocessing
Functional and structural MRI data were analyzed using the FreeSurfer analysis stream developed at the Martinos Center for Biomedical Imaging (http://surfer.nmr.mgh.harvard.edu/). fMRI data were motion-corrected and inspected for gross-motion. The average motion vector within each of the three runs was less than 1.3mm and was not significantly different (t(30)=1.78, p=0.09) between the groups (young: M=0.43, SD=0.35; elderly: M=0.63, SD=0.28). Data in each functional run was intensity normalized and spatially smoothed (full-width half-maximum = 7mm) using a 3D Gaussian filter. Preprocessing steps included polynomial drift correction entailing 2 nuisance regressors spanning the space of a 2nd order polynomial to account for low-frequency drift and whitening based on a single autocorrelation function estimated across all brain voxels to remove temporally autocorrelation noise (Burock and Dale, 2000).
After preprocessing, functional images for each participant were averaged according to condition (i.e., fixation, familiar young, familiar elderly, novel young and novel elderly) and registered to an average 3D structural image created from the two high-resolution 3D MPRAGE images. Functional data were modeled using a gamma function and visualized over the averaged 3D image for each individual to ensure that the fMRI signal in the amygdala was not obscured by susceptibility artifact. No participants were excluded on this basis.
fMRI Data Analyses Overview
Anatomically-based functional analyses
For our main analyses, we performed region of interest (ROI) analyses of the functional data using an anatomic-based approach. Compared with whole brain techniques, this method has the benefits of focusing on an a priori ROI with less concern about multiple comparisons. In addition, this method allows a single analysis of both novelty and in- versus out-group effects without the bias of performing an initial contrast of interest to select the anatomic region for analyses. Furthermore, as we compare young and elderly participants, the anatomic-based approach in native space helps protect against group-specific age effects during spatial normalization (Vandenbroucke et al., 2004).
Automated subcortical segmentation methods were applied to the native 3D MPRAGE structural images of each individual subject to create anatomically-defined amygdala ROIs (Fischl et al., 2002). These amygdala ROIs were manually verified and minor edits were performed according to our previously published protocols (Wedig et al., 2005; Wright et al., 2006a; Wright et al., 2006b). Good reliability and comparability to manual labeling has been demonstrated for these methods with intraclass correlation coefficients (ICC), which are consistently >.8 (Rademacher et al., 1993; Caviness et al., 1996; Seidman et al., 1997; Goldstein et al., 1999; Seidman et al., 1999). For the current study, we independently compared the volumes of 12 manual amygdala tracings and 12 automated amygdala ROIs and observed a high level of reliability (ICC=.88). Volume of the amygdala ROIs was also measured and utilized to assess the influence of age-related atrophy on the fMRI results in our control analyses. Amygdala volumes were corrected for estimated intracranial volumes (eTIV). The eTIV measurements and their reliability are detailed in other recent studies (Buckner et al., 2004; Wright et al., 2006b; Wright et al., 2007b; Wright et al., 2007a).
The anatomically-defined amygdala ROIs were registered to the fMRI data from each scanning session and BOLD signal was extracted from each participant’s fMRI data for analysis. Our main index was mean percent (%) signal change compared to baseline in all voxels of the ROI that were positively associated with the task at any level (e.g. all versus fixation).
The fusiform gyrus was utilized as a secondary site of interest because this area is both responsive to faces and novelty, and it is closely connected to the amygdala. An anatomically-defined fusiform ROI was made using an automated surface-based technique for parcellating the cerebral cortex and verified manually for accuracy (Fischl et al., 2004; Wright et al., 2007b). This label was used to calculate fusiform volumes and to extract fMRI signal intensity data in an identical manner as described above for the amygdala ROI.
Using the amygdala and fusiform gyrus anatomic ROIs as described above, percent signal change for each type of stimulus face (familiar young, novel young, familiar elderly, novel elderly) versus baseline (fixation) was calculated and entered into a repeated measures ANOVA with participant age (young and elderly) as the between-subject factor and both novelty (familiar, novel) and age of target face (young, elderly) as within-subjects factors. Separate ANOVAs were performed for the amygdala and fusiform gyrus of each hemisphere. Additional repeated measures ANOVAs were utilized to directly assess effects of hemisphere and gender on the group findings of interest (i.e. novelty and target face-age). The significance threshold was p < 0.05. Appropriate post-hoc tests were used to determine the sources of significant findings. Multiple regression models were utilized to control for the influence of variables with significant between-group differences on novelty and target face age or group membership effects. The regional brain activation measures were utilized as the dependent variable, age was the independent variable, and the variance attributable to these potentially confounding variables was removed from the model.
Functionally-based ROI analyses
Because anatomic based-ROI approaches may dilute focal effects within a region or miss other brain areas with significant but unexpected effects, we followed our anatomical-ROI analyses with functional ROIs defined by the novel versus familiar contrast, and extracted % signal change from those voxels based on all versus fixation contrast maps. A functional ROI approach allowed confirmation of anatomic-ROI based analyses for novelty as well as an examination of whether in- versus out-group face effects were related to novelty responsive regions. Based on the number of resolution elements in each of these regions, the significance threshold for amygdala activation was p ≤.001 and for fusiform cortex it was p ≤.0001. These thresholds represent an approximate small-volume Bonferroni correction for multiple comparisons.
To begin, we performed a whole-brain analysis. Data were spatially normalized into Talairach space (Talairach and Tournoux, 1988) and a cortical surface-based spherical coordinate system (Dale et al., 1999; Fischl et al., 1999). For Talairach spatial normalization, procedures developed and distributed by Montreal Neurological Institute were used to compute a transformation matrix from the high-resolution MPRAGE volumes (Collins et al., 1994). For spherical spatial normalization (software and documentation is available at http://www.nmr.mgh.harvard.edu/freesurfer), the averaged high-resolution 3D MPRAGE volume was used to create a segmentation of the gray/white matter boundary and outer cortical surface for each subject using a semi-automated procedure. This surface was then smoothed using a topology-preserving deformable surface algorithm, allowing specification of which voxels in the original volume correspond to the cortical surface.
Each participant’s data were selectively averaged for each condition and re-sampled into Talairach space or spherical space for within and between-group random-effects analyses. The young and elderly groups were also examined separately for direct visual comparison of the activation patterns when relevant.
To avoid bias from sampling different sites in each group, the functionally-defined ROI analyses were performed on statistical maps for the relevant contrasts collapsed across all subjects. In a similar manner as for the anatomically-defined ROI based analyses described above, fMRI data at the sites of significant activation (i.e. amygdala, fusiform) were extracted, % signal change was calculated and entered into a repeated measures ANOVA.
Whole brain analyses
Finally, we also examined the whole-brain analysis for other areas that displayed a differential BOLD response to our contrasts of interest (novel versus familiar and the in- versus out-group effect). For these remaining gray matter areas outside our two a priori ROIs, a significance threshold of p ≤ .00001 was used.
Cognitive, Behavioral and Volumetric Statistical Analyses
Young and elderly participant performance on neuropsychological variables, face recognition accuracy rates, as well as their valence and arousal ratings of the young and elderly faces were compared using multivariate analysis of variance (MANOVA) in addition to independent or paired t-tests. The volumes of the amygdala and fusiform in the young and elderly were also compared with independent t-tests.
Results
Neuropsychological, Behavioral and Volumetric Measures
Young and elderly participants performed equally well on all measures of cognitive functioning except for delayed recall performance on the CERAD memory test (see Table 1, second to last row). Compared to young participants, elderly participants had worse recall on average, but their performance was within the age-adjusted norms (Welsh et al., 1994).
Valence and arousal ratings of the face stimuli were subjected to a 2 (participant age: young/elderly) by 2 (novelty: novel/familiar) by 2 (age of target face: elderly/young) MANOVA. Means and standard errors are presented in Table 2. The only significant effect to emerge was a participant age by target face interaction for valence ratings, F(1,28) = 9.29, p < .005. Young participants rated elderly faces as significantly more negative (M = −.76) than young target faces (M = .30), t(14) = 3.10, p < .008. Elderly participants did not rate the young and old target faces differently, t(14) = 1.27, p < .22, but they rated elderly target faces as more positive (M = .37) than did the young participants (M = −.76), t(29) = 2.05, p < .05.
Table 2. Mean Valence and Arousal Ratings.
Mean and standard errors are reported. Superscripts within cells for the same variable indicate statistical significance at p<.05.
| Face Ratings | Participant Group | |
|---|---|---|
| Young | Elderly | |
| Arousal | ||
| Elderly | 2.5 (0.34)a |
2.1 (0.44)a |
| Young | 2.5 (0.24)a |
2.0 (0.38)a |
| Valence | ||
| Elderly | −0.76 (0.30)a |
0.37 (0.39)b |
| Young | 0.30 (0.13)b |
−0.1 (0.37)b |
The accuracy for familiar faces, faces seen once during scanning, and faces not seen during scanning (single presentation faces) was assessed by computing the hit rate for each type of face stimulus [hits /( hits + misses)*100]. Hit rates were subjected to a 2 (participant age: young/elderly) by 2 (age of face : young/elderly) by 3 (number of times face was seen during scanning: familiar, seen once during scanning, not seen during scanning) ANOVA. Means and standard errors for accuracy rates are presented in Table 3. Overall, young participants were more accurate in correctly classifying faces than were elderly participants, M = .63 vs. M = .51, F(1,26) = 20.49, p < .001. All participants were significantly more accurate in identifying familiar faces than faces seen once during scanning (M =.84 versus M = .63), and they were least accurate in identifying faces never seen during scanning (M = .24), F (2,52) = 57.81, p < .001. The age of participant × age of face × face repetition during scanning interaction was significant, F (2,52) = 4.39, p < .02. The specific pattern of findings are reported in Table 3.
Table 3. Recognition.
Mean percent correct and standard errors are reported. Superscripts across cell rows indicate statistical significance at p<.05. Face recognition data were missing for three participants (1 in the young cohort and 2 in the elderly cohort).
| Face Type | Participant Group | |||
|---|---|---|---|---|
| Young | Elderly | |||
| Face Age | Face Age | |||
| Young | Elderly | Young | Elderly | |
| Familiar | 88.3 (5.9)a |
88.3 (5.9)a |
70.5 (6.3)b |
90.9 (6.4)a |
| Novel (Faces Seen Once During Scanning) | 78.2 (6.6)a |
66.7 (6.9)ab |
61.0 (7.1)b |
47.4 (7.4)c |
| Single Presentation | 27.5 (4.9)a |
29.7 (4.8)a |
26.9 (5.3)a |
10.9 (5.1)b |
We also computed the rates of recognition mistakes. Specifically, we examined the percentage of familiar faces mistakenly judged as “seen once during scanning” and the percentage of faces seen once during scanning that were mistakenly judged as “familiar.” Faces judged as completely new (not seen at all during scanning) were not included in this analysis. Mean error rates are presented in Table 4. A 2 (participant age: young/elderly) by 2 (age of face : young/elderly) by 2 (number of times face was seen during scanning: familiar, seen once during scanning) indicated that faces seen once during scanning (i.e., faces that were novel during scanning) were more likely to be mistakenly judged as familiar than vice versa, F(1,26) = 29.65, p <.001. This mistake (judging faces as familiar when they had in fact only been seen once during scanning) was stronger for elderly faces than for young faces, F(1,26) = 7.28, p <.012. Marginal means analysis (see Table 4) indicated that elderly participants were likely to make this recognition error for elderly faces when compared to young faces, whereas in young participants the mistake rates were in the same direction but were not significantly different. These findings suggest that during scanning, elderly participants may have experienced some of the novel faces as familiar; if this mistake had any effect at all, it would be to reduce the BOLD signal changes to novelty in the amygdala and fusiform gyrus. Nonetheless, in all analyses, we used these recognition error rates as covariate in the analyses of BOLD signal changes in amygdala and fusiform gyrus. The opposite mistake rate (judging familiar faces as novel or “seen once during scanning”) was low for all participants.
Table 4. Mistakes.
Mean and standard errors are reported. Superscripts within target face age indicate statistical significance at p<.05. Mistake rates were computed for familiar faces and faces seen only once during scanning; estimates were missing for 3 participants (1 in the young cohort and 2 in the elderly cohort).
| Face Type | Participant Group | |
|---|---|---|
| Young | Elderly | |
| Faces Seen Once During Scanning Mistakenly Judged as Familiar | ||
| Elderly | 43.8b (9.6) |
65.9a (10.3) |
| Young | 29.6b (8.9) |
42.0b (9.6) |
| Familiar Faces Mistakenly Judged as Seen Once During Scanning | ||
| Elderly | 10.6cd (5.2) |
1.9d (5.6) |
| Young | 10.6cd (5.2) |
15.4c (5.6) |
The elderly had significantly smaller left and right amygdala volumes (corrected for eTIV) than the young (see Table 5). The fusiform volumes were also smaller in the elderly compared to the young, but this difference was not significant.
Table 5. Estimated Total Intracranial Volume.
Mean and standard deviations are reported. ETIV = percentage (%) of estimated total intracranial volume.
| Volume (%of eTIV) | Participant Group | |
|---|---|---|
| Young | Elderly | |
| Right Amygdala | 0.09 (0.01)a |
0.08 (0.01)b |
| Left Amygdala | 0.08 (0.01)a |
0.07 (0.01)b |
| Right Fusiform | 0.29 (0.03)a |
0.28 (0.04)a |
| Left Fusiform | 0.27 (0.04)a |
0.29 (0.04)a |
Effects of Novelty in the Amygdala and Fusiform Gyrus
All participants showed significant increases in left and right amygdala response to novel (versus familiar) faces in anatomically-defined ROI analyses (left: F(1,30)=6.1, p < .02; right: F(1,30)=36.5, p < .0001; Fig. 2). Participants also showed increased activation in fusiform gyrus (left: F(1,30)=13.1, p < .001; right: F(1,30)=29.4, p < .0001; Fig. 3). Similar results were obtained for functionally-defined ROI analyses. Because elderly participants made a greater number of recognition errors for faces seen once during imaging (judging them as familiar after imaging), we performed ANCOVAs by re-running the ANOVAs but controlling for these error rates. The pattern of results was maintained, and all effects remained significant except for activity in both the structural and functional left amygdala ROIs (which continued to show the same pattern but activation levels failed to reach conventional levels of significance).
Figure 2. Amygdala Responses to Novelty in the Young and Elderly.
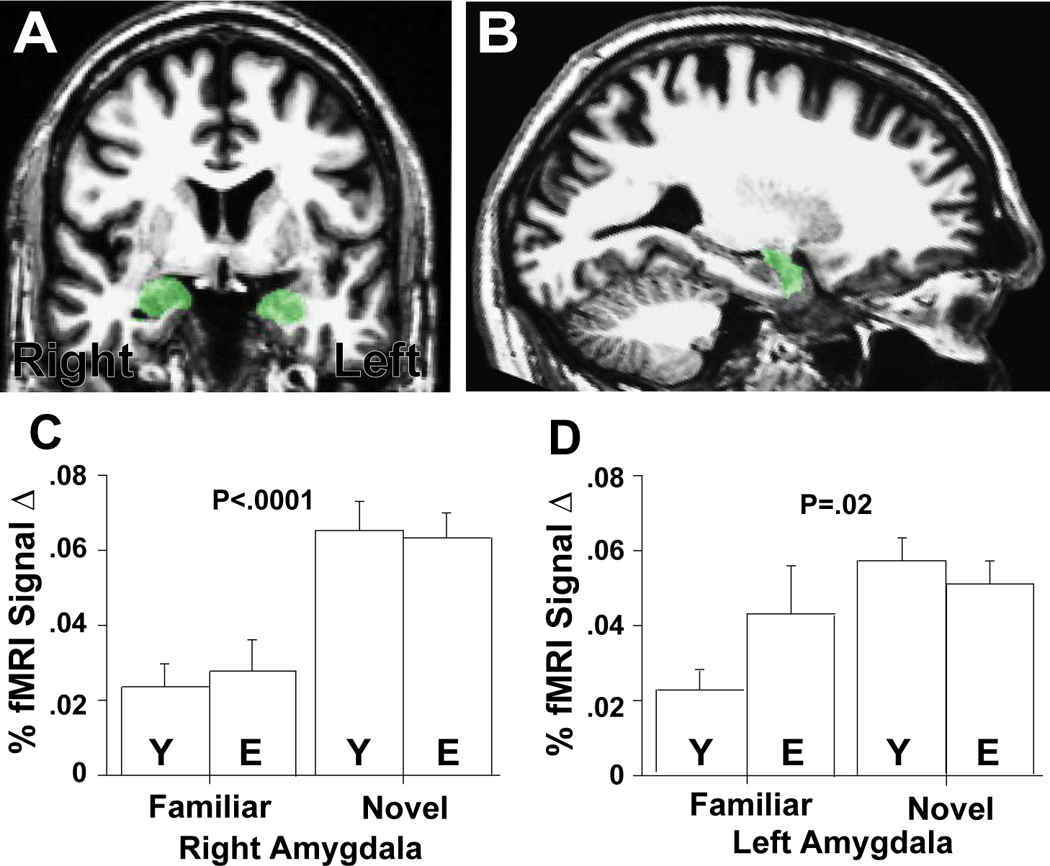
High resolution (A) coronal and (B) sagittal T1 weighted image demonstrating right and left amygdala anatomic ROIs in a 65 year-old male. The anatomically defined right amygdala ROIs for each subject in each group was used to extract fMRI data for the familiar and novel conditions vs. fixation (collapsed across face age). Bar graphs show mean percent (%) fMRI signal change (vs. fixation) for young (Y) and elderly (E) adults in the right (C) and left (D) amygdala. One standard error of the mean is shown. P-values indicate a significant main effect of novelty in the amygdala. There were no group × condition interactions. Left amygdala novelty effects were weaker due to greater responses to familiar faces in the elderly vs. the young, but the difference between the groups was not statistically significant.
Figure 3. Fusiform Gyrus Responses to Novelty in the Young and Elderly.
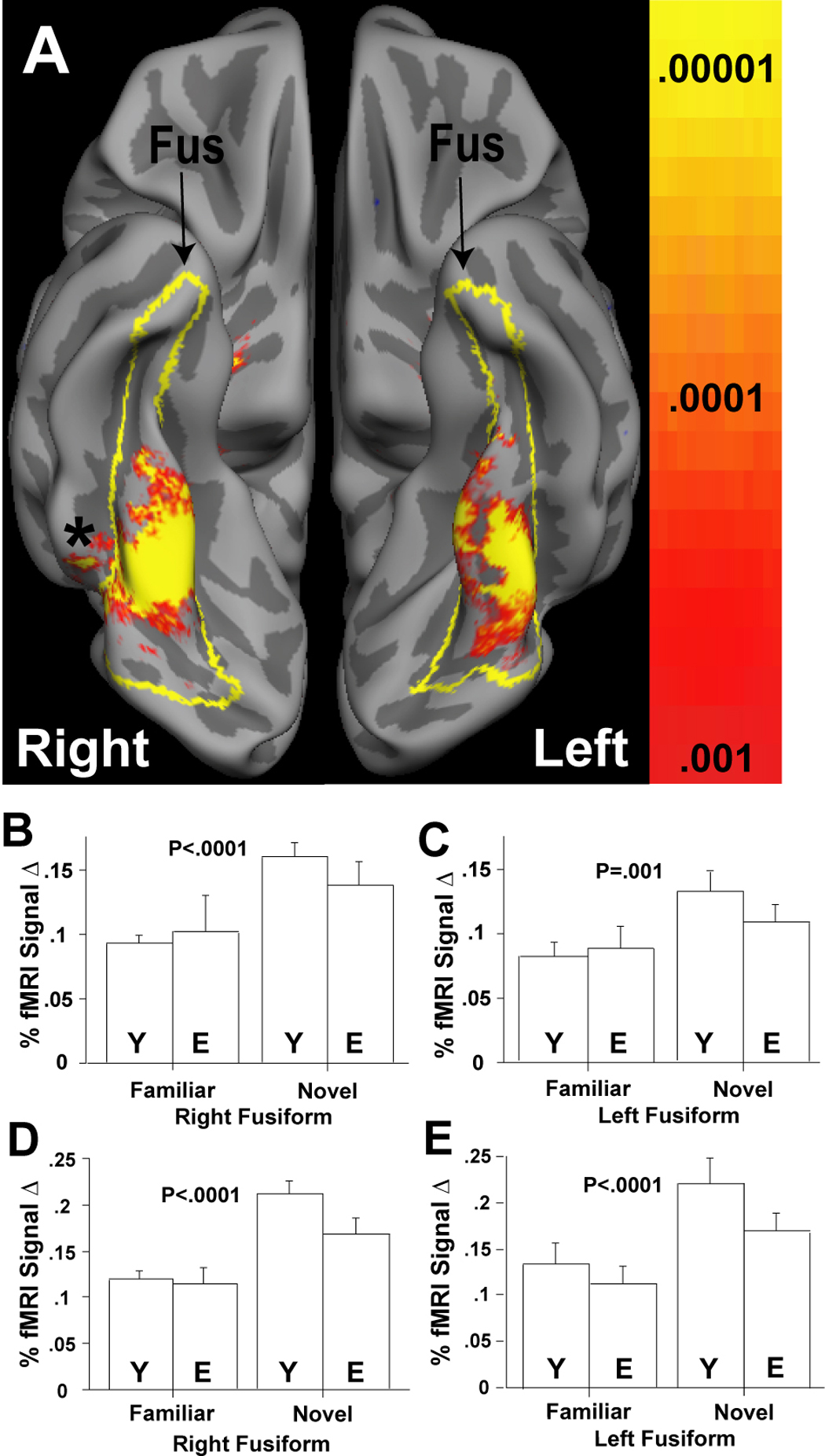
Partially inflated cortical surface group average demonstrating the right and left fusiform gyrus (Fus; yellow outline), and the group statistical maps for the novel vs. familiar contrast (collapsed across all subjects) (A). The inferior surface of each hemisphere is shown. Dark gray regions are sulci. Light gray regions are gyri. The asterix indicates where the activation extends into the right inferior temporal gyrus; on the left the activated area region is buried by the overlying fusiform gyrus. The outlines (yellow) demarcate the fusiform region utilized for the anatomic-ROI analyses (panels B, C). The group statistical maps in (A) were employed to define the sites used for functionally based-ROI analyses (panels D, E). Bar graphs show mean % fMRI signal change for the young (Y) and elderly (E) adults for the familiar and novel conditions vs. fixation, resulting from anatomic (panels B,C) and functionally-based (panels D, E) ROI analyses. One standard error of the mean is shown. P-values indicate significant main effects of novelty. Group × condition interactions were only observed with functionally-based ROI analyses (panels D, E) in which the young had significantly greater fusiform cortex responses to novel vs. familiar faces.
Young and elderly participants did not differ in their amygdala responses in anatomically-defined ROI analyses (left: F(1,30)=2.3, p =.14; right: F(1,30)=0.2, p =.70) (Figs. 2). These results were maintained when we controlled for recognition error rates (judging as familiar the faces that were seen once during scanning). Neither did young and elderly participants differ in their fusiform gyrus responses (right: F(1,30)=4.4, p =.44; left: F(1,30)=2.5, p =.12) (Fig. 3B,C). The only novelty by age of participant interactions reaching significance were in the fusiform gyrus using functionally-defined ROIs analyses (right: F(1,30)=8.1, p <.008; left: F(1,30)=5.4, p <.03) (Fig. 3D,E). These differences remained marginally significant when we controlled for recognition error rates (i.e., the likelihood of experiencing a novel face as familiar), but the pattern of findings remained the same: elderly participants showed increased fusiform activity to novel vs. familiar stimuli; this differential activity was even greater for young participants.
Whole brain within group analyses (i.e. examination of each group separately) confirmed the results of the anatomically and functionally-defined ROI analyses of the amygdala and fusiform gyrus (Fig. 4). Whole brain between-group analyses also confirmed the absence of the novelty × participant age interactions in the amygdala, and suggested their presence in the fusiform gyrus (not shown).
Figure 4. Whole brain analyses of the Novelty Responses in the Young and Elderly.
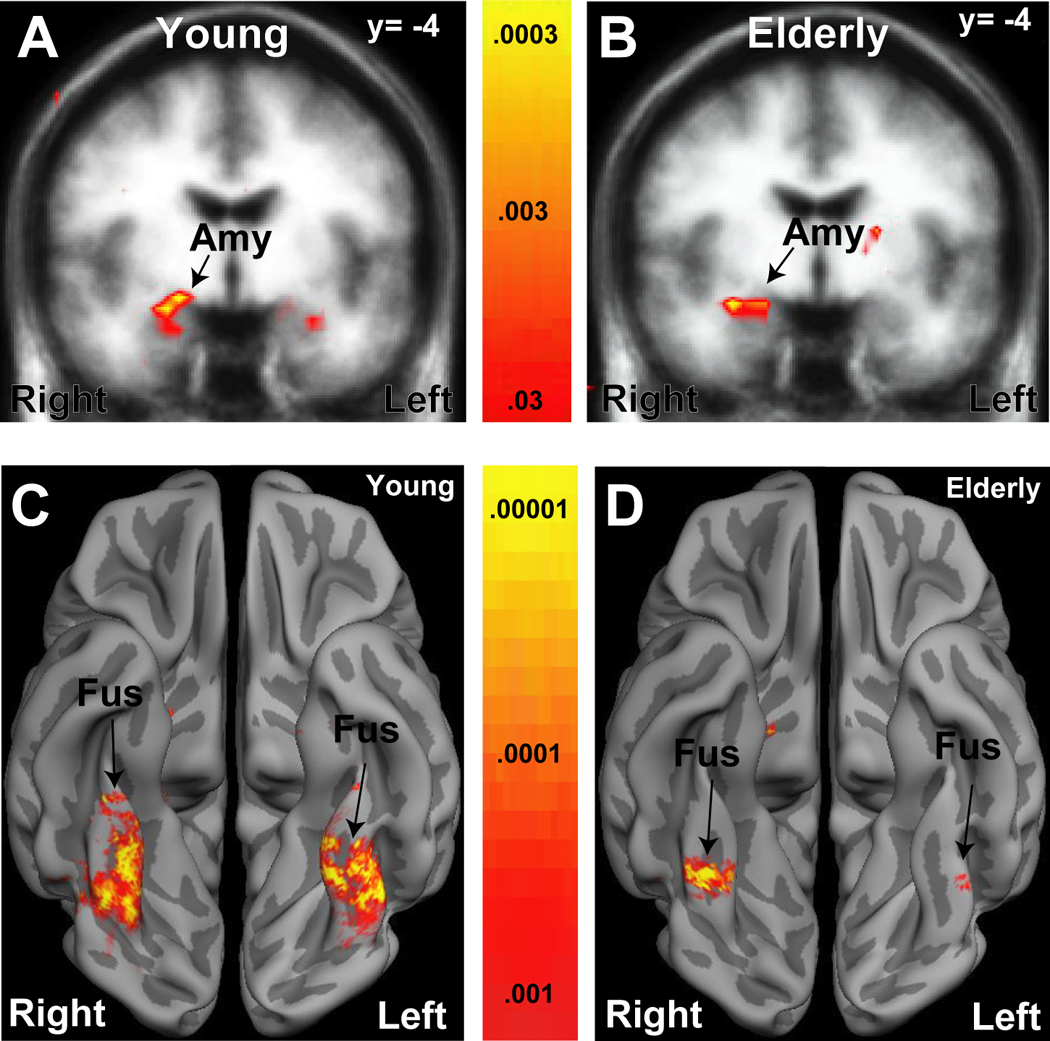
Statistical maps superimposed upon a T1 weighted coronal Talairach group average structural image demonstrating significant amygdala (Amy) activation for the novel vs. familiar contrast in young (A) and elderly (B) adults. The significance and extent of amygdala activation was similar (young: Talairach coordinates x=25, y=−2, z=−18, peak P <10−4; elderly: P <10−4, x=30, y=−7, z=−17). Consistent with the anatomic ROI analyses in Fig. 2 effects are weaker on the left, particularly in the elderly. Statistical maps superimposed on a partially inflated cortical surface group average demonstrating fusiform gryus (Fus) activations to the novel vs. familiar contrasts in young (D) and elderly (E) adults. Dark gray regions are sulci, light gray are gyri. The extent of bilateral fusiform activation was greater in the young than the elderly. The significance of the fusiform activations were similar on the right (young: x=38, y=−32, z=−17, P <10−6; elderly: x=37, y=−53, z=−11; P <10−6) but greater on the left (young: x=−31, y=−56, z=−10, P <10−6; elderly: x=37, y=−53, z=−11; P <10−4) in the young than the elderly.
Effects of Target Face Age in the Amygdala and Fusiform Gyrus
Anatomically-defined ROI analyses revealed a significant participant age × target face age interaction in the right amygdala (F(1,30)=6.0, p <.02; Fig. 5A,B). Elderly participants had a significantly larger amygdala response to elderly target faces when compared to young target faces (i.e., greater amygdala response to age-defined in-group versus out-group faces), (t(15)=2.0, p <.05), and these effects continued to be statistically significant when we controlled for recognition error rates. Functionally-defined ROI analyses yielded similar results in the right amygdala (F(1,30)=7.165, p =.01). A similar but non-significant in- versus out-group face effect was observed in the young adults (t(15)= 1.5, p <.16). There were no significant participant age × target face age interactions in the left amygdala (for both anatomically- and functionally-defined ROI analyses, F(1,30)=0, p <1). To confirm the hemispheric specificity of this finding, we performed a direct comparison of the right and left amygdala in an additional ANOVA and found a significant hemisphere × face target age × participant age interaction (F(1,30)=6.36, p <.017). Whole brain voxel-based analyses of participant in- versus out-group effects confirmed these effects in the right amygdala (Fig. 5B). Both anatomically- and functionally-defined ROI analyses showed no significant participant age × target face age interactions in either the left or right fusiform gyrus.
Figure 5. Face-age and age in-group effects in the right amygdala.
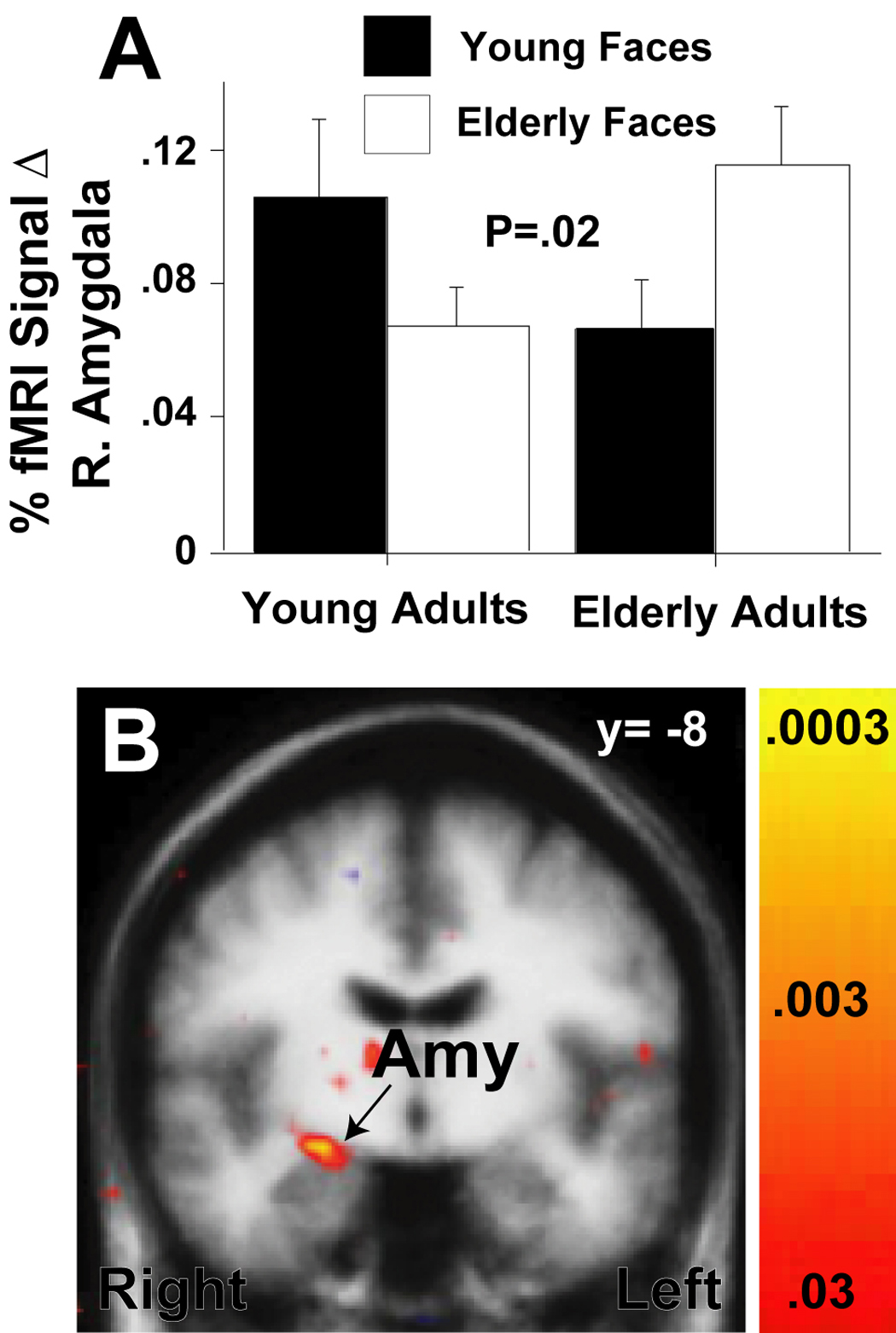
The bar graph in (A) shows right amygdala mean percent (%) fMRI signal change during viewing of young and elderly faces (vs. fixation) for the of young (Y) and elderly (E) study subjects. One standard error of the mean is shown. P-value indicates significant a face-age × group interaction due to greater amygdala responses to age in-group vs. out-group faces. The statistical map superimposed upon a T1-weighted coronal group average structural image in (B) confirms and localizes the face age in- vs. out-group effect in the superior right amygdala across all subjects (coordinates: x=24, y=−8, z=−10; P-value = .0003).
Comparing Novelty and Age-Defined In-Group Effects in the Amygdala
We hypothesized that novelty may be responsible for the in- (versus out-) group effects within the amygdala. To assess this, we performed a formal comparison of the novelty and face-group effects in the amygdala. The novelty effect was observed as diffuse, bilateral amygdala activation. The extent of activation spread from the anterior to posterior limit in both amygdalae, with the peak activation localized to the right amygdala (Talairach coordinates : x=24, y=−3, z=−13, p < .000001). The in-group (versus out-group) effect was partially overlapping with the novelty effect, but encompassed a more restricted region of the right amygdale only, with a peak activation (Talairach coordinates: x=24, y=−8, z=−10; p < .0003) that was slightly posterior and inferior to that found for the novelty effect.
We also created a functionally defined ROI on the peak voxels generated from the in-group (versus out-group) contrast to assess whether voxels preferentially responsive to group status were also responsive to novelty. A 2 (participant age: young/elderly) by 2 (novelty of face : novel/familiar) by 2 (face age: young/elderly) revealed a main effect of novelty (F(1,30)=31.3, p=<.05), indicating that both groups of participants activated to novel (versus familiar) stimuli in those voxels that were also preferentially active to the in-group (versus out-group) contrast.
Other Effects and Interactions in the Amygdala and Fusiform Gyrus
Anatomically-defined ROI analyses demonstrated no significant main effects of participant age for overall face responses in the amygdala (left: F(1,30)=1.3, p <.28; right: F(1,30)=0.08, p <.78) or fusiform gyrus (left: F(1,30)=1.3, p <.27; right: F(1,30)=0.01, p <.92), indicating that activation averaged across stimulus type was similar in young and elderly participants. There were no significant novelty × target face age or novelty × target face age × participant age interactions (all p >.1). Separate ANOVAs examining the effects of gender demonstrated no significant main effects or interactions in the amygdala (all p >.1) or fusiform (all p>.2). Functionally-defined ROIs analyses gave similar results.
Effects of Neuropsychological Variable Group Differences on Novelty and In-Group Face Effects
Variables with significant groups differences (Table 1 and Table 5) were utilized as covariates in multiple regression analyses to assess their influences on our main results. The amygdala novelty and target face age effects remained the same after statistically controlling for the variance contributed by CERAD delayed memory scores, valence ratings and amygdala volume. Likewise, the effect of aging on fusiform gyrus novelty responses remained significant after accounting for the group differences in these variables.
Post-hoc Whole Brain Analyses for Novelty and Target Face Age Effects
The only additional regions that met the whole brain statistical threshold for the novel versus familiar faces contrast were the left (approx. Brodmann Area 37; Talairach coordinates: x=−41, y=−56, z=−17; peak P-value <10−7) and right (approx. Brodmann Area 37; x=50, y=−53, z=−13; P<10−9) inferior temporal gyrus. These regions were contiguous with the activations in the fusiform gyrus (see Fig. 3A). No regions exhibited significant novelty × participant age interactions at the whole brain level. There were also no additional significant target face age effects, participant age × target face age interactions, or age-related in-group vs. out-group effects across all subjects in the whole brain analyses.
Discussion
This study contributes two important new findings. First, both young and elderly participants responded similarly to novel neutral faces, indicating that amygdala responses to novelty are preserved with aging. This was also generally true for fusiform gyrus responses, but to a lesser extent. Second, when participants and face stimuli were grouped by age, elderly participants showed a right amygdala in-group response, suggesting a greater sensitivity to the information carried in the faces of others their own age. No statistically significant in-group effect was observed in the young participants.
Amygdala Novelty Responses and Aging
Our findings build on prior studies indicating that the amygdala responds to stimuli of potential value, whether valenced or novel (Wilson and Rolls, 1993; Dubois et al., 1999; Schwartz et al., 2003; Wright et al., 2003; Wright et al., 2006a). The novelty response we observed in this study is consistent with the idea that the amygdala tags a sensory representation when its predictive value is uncertain, and creates a behavioral stance that prioritizes additional processing, to allow the organism to better learn whether this sensory pattern (i.e., the stimulus) predicts a threat or a reward (Barrett et al., 2007). This more basic function may support a host of other psychological phenomena, including establishing the affective meaning of the stimulus, or it may itself be considered as a property of affective perception. In either case, amygdala novelty responses may represent one aspect of the orienting response to new stimuli in the environment. That is, the amygdala is likely important for recognizing new stimuli of as yet undetermined worth, so that the appropriate attentional resources can be marshaled to conclude whether or not the novel stimulus is of harm or benefit to the organism. We found that amygdala novelty responses are preserved with age, suggesting that the amygdala’s role in learning about the predictive value of a stimulus is maintained across the lifespan.
Aging Effects on Fusiform Gyrus Novelty Responses
Although there were significant novelty responses in the fusiform gyrus that did not differ between groups when an anatomic based ROI approach was used, significant differences between groups were present when the most novelty active regions (using functional activation based ROIs) were analyzed, such that elderly participants showed a stronger fusiform response to novel (vs. familiar) faces, but this difference was enhanced for young participants. Other studies using a variety of stimulus types and experimental paradigms have also demonstrated decreased or less selective patterns of activation in the fusiform and other visual association cortices with aging, suggesting that this decrement in fusiform response is a general age-related phenomenon (Iidaka et al., 2001; Iidaka et al., 2002; Gunning-Dixon et al., 2003; Park et al., 2004; Tessitore et al., 2005; Wright et al., 2006a).
Of note, a previous study, in which novelty and emotion were assessed together (Wright et al., 2006a), demonstrated greater fusiform gyrus activation to the novel emotion vs. familiar neutral contrast in young as compared to elderly participants, as well as greater responses to all face conditions (i.e., a group main effect for all faces). This suggests that the presence of emotion in that paradigm may have influenced fusiform responses leading to stronger effects across all stimuli. However, future experiments in which novelty and emotion can be fully dissociated in the same experiment are necessary to accurately understand the interactions between these stimulus features.
Possible Sources of the Amygdala In-group Face Age Response
Our study clearly demonstrates that it is important to consider the age-group membership of target stimuli when comparing neural responses across different age groups. Initial neuroimaging findings of group membership effects based on race suggested greater amygdala activation to race out- vs. in-group faces (Hart et al., 2000; Phelps et al., 2000). In those studies, European-American subjects had greater amygdala responses during viewing of African-American versus European-American target faces. One explanation for that amygdala activation to race out-group faces is that such faces are more novel than race in-group faces because people tend to have more exposure and experience with in-group compared to out-group members. Consistent with this view, European-American perceivers have larger cardiovascular threat responses to race-defined out-group members when they have little experience with a target person (Blascovich, Mendes, Hunter, Lickel, & Kowai-Bell, 2001), or when they are uncertain as to what to expect from the target (Mendes, Blascovich, Hunter, Lickle, & Jost, 2007).
The current results suggest that novelty may not be the driving force in the age-defined in-group effect that we observed in elderly participants, however. The novelty and in-group face effects were largely overlapping (with novelty effects involving much larger areas of the bilateral amygdala, but the in-group effects in only a restricted part of the right amygdala) which might, at first glance, suggest that the two effects are related in some way. Yet, the peak voxels for each effect were somewhat different, suggesting that there are two partially overlapping sets of neurons in the right amygdala that differentially respond to these two stimulus features. The limited anatomic resolution of fMRI data makes deciding between these alternative interpretations difficult, however. Furthermore, this pattern of findings may just as likely indicate that a sub-population of neurons in novelty responsive areas are also responsive to in-group face stimuli; or that there are two partially overlapping sets of neurons in the right amygdala that differentially respond to these two stimulus features. In addition, there are other considerations that line up against a novelty interpretation of the in-group face effect in elderly participants. The in-group effect held in elderly participants even when we statistically controlled for their higher recognition errors in judging novel faces as familiar, and their rates of judging familiar faces as novel (seen once during imaging) were low. Taken together, the findings indicate that a simple tendency to judge familiar elderly faces as novel was not a strong contributing factor to their stronger amygdala responses to elderly faces.
Further research is needed to clarify the mechanisms driving the age-based in-group effect that we observed. Nonetheless, we can rule out several additional interpretations of our amygdala age in-group effects. First, it was not the case that participants found members of their own group either more or less pleasant. Although the young participants rated in-group target faces more positively than out-group target faces, elderly participants did not. In addition, when the relevant valence ratings were entered into control regression analyses, the target face age effects remained significant. A second possibility is that in-group faces were prioritized for sensory processing because they were more important or self-relevant to them (in a valence-independent manner). Appraisal models of emotion propose that self-relevant (also called motivational relevant) stimuli attract attention and set the stage for hedonic processing and other meaning analysis in which emotion is grounded (Ellsworth & Scherer, 2003). Elderly people may have a social network that is populated more by other elderly individuals, such that they may be impacted more by the behaviors of their age-related in-group. This would make elderly targets of more interest and more relevant to the concerns of daily life. We favor this hypothesis, but recognize that more in-depth brain-behavior analyses are necessary to understand the neuropsychological underpinnings of the amygdala-age group membership effects of this initial study.
Strengths and Potential Weaknesses
This study has several strengths. The young and elderly participants were well characterized according to cognitive and personality measures, and were psychiatrically healthy according to a DSM-IV SCID. The presence of similar results using both structural and functional ROI analysis supports the integrity of the between-group comparisons in setting of potentially confounding effects of age-related atrophy or brain shape changes. Likewise, the patterns and regions of activation are highly similar to our earlier studies, indicating the reproducibility of these findings in aging. In addition, this work confirms that the amygdala of the elderly robustly responds to human faces in specific experimental settings (e.g., novelty and age in-group), but not others (Iidaka et al., 2002; Gunning-Dixon et al., 2003; Fischer et al., 2005; Tessitore et al., 2005; Williams et al., 2006).
Potential study limitations include that the study design as cross sectional and involving only two age groups. Therefore, as with many imaging studies on aging, the results could be due to cohort or generational effects as opposed to age per se. It also remains uncertain how novelty and target face age group membership processing may be different in middle age. Additional studies using a longitudinal design and including a wider range of ages will be necessary to address these issues. Furthermore, there were several differences between the young and elderly with regard to memory performance and subjective face ratings, but the significance of these differences is unclear. It is possible that altered memory function, brain atrophy, or different subjective experiences could have led to some of the results, as opposed to effects of age. However, in control regression analyses there were no apparent moderating influences of these factors on our significant main effects or interactions of interest. Nevertheless, caution is still warranted as our sample size is relatively small for the application of regression techniques. In addition, there may be certain weaker age-related effects that did not reach statistical significance due to the modest number of subjects in this study. For example, novelty responses in the left amygdala of the elderly adults appear weaker than in the young adults, but this result was not significant despite being a reproducible pattern from two earlier studies (Wright et al., 2006b; Wright et al., 2007b). Finally, as in previous experiments, this paradigm used passive viewing and did not provide behavioral data during scanning. However, subjects were monitored between each run for wakefulness and the variance in the face recognition measures gathered did not accounted for the effects in control analyses.
Acknowledgments
The authors wish to thank Mary Foley and Larry White for technical assistance. This work was supported in part by NIMH grant K23MH64806 (Dr. Wright), a Research Incentive Award from Boston College and grants from the National Institutes of Health Director’s Pioneer Award (DP1OD003312), a National Institute of Mental Health’s Independent Scientist Research Award (K02 MH001981), a National Institute of Aging grant (AG030311) and National Science Foundation grants (BCS 0721260; BCS 0527440) (Dr. Barrett), as well as resource grants to the Martinos Center for Biomedical Imaging from the NCRR (P41-RR14075) and the Mental Illness and Neuroscience Discovery (MIND) Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4 Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Anastasi JS, Rhodes MG. An own-age bias in face recognition for children and older adults. Psychon Bull Rev. 2005;12:1043–1047. doi: 10.3758/bf03206441. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K, Varney N, Spreen O. Contributions to neuropsychological assessment: A clinical manual. New York: Oxford University Press; 1983. [Google Scholar]
- Barrett LF, Lindquist K, Bliss-Moreau E, Duncan S, Grendon M, Mize J, Brennan L. Of mice and men: Natural kinds of emotion in the mammalian brain? Perspectives on Psychological Science. 2007;2:297–312. doi: 10.1111/j.1745-6916.2007.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blascovich J, Mendes WB, Hunter SB, Lickel B, Kowai-Bell N. Perceiver threat in social interactions with stigmatized others. Journal of Personality and Social Psychology. 2001;80:253–267. doi: 10.1037/0022-3514.80.2.253. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expressions. Neuron. 1996;17:875–877. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Burock M, Dale A. Estimation and detection of event-related fMRI signals with temporally correlated noise: A statistically efficient approach. Human Brain Mapping. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition. Aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously. A theory of socioemotional selectivity. Am Psychol. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Caviness V, Meyer L, Makris N, Kennedy D. MRI-based topographic parcellation of the human neocortex: An anatomically specified method with estimates of reliability. J Cogn Neurosci. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dubois S, Rossion B, Schiltz C, Bodart JM, Michel C, Bruyer R, Crommelinck M. Effect of familiarity on the processing of human faces. Neuroimage. 1999;9:278–289. doi: 10.1006/nimg.1998.0409. [DOI] [PubMed] [Google Scholar]
- Ellis HD, Deregowski JB. Within-race and between-race recognition of transformed and untransformed faces. Am J Psychol. 1981;94:27–35. [PubMed] [Google Scholar]
- Ellsworth PC, Scherer KR. Appraisal process in emotion. In: Davidson R, Scherer K, Goldsmith H, editors. Handbook of affective sciences. New York: Oxford University Press; 2003. pp. 572–595. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorders - patient edition (SCIP-I/P, version 2.0) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L. Agedifferential patterns of brain activation during perception of angry faces. Neurosci Lett. 2005;386:99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state." a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frijda NH, Kuipers P, ter Schure E. Relations among emotion, appraisal, and emotional action readiness. Personality and Social Psychology. 1989;57:212–228. [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS, Jr, Faraone SV, Tsuang MT. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Archives of General Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE. Age-related differences in brain activation during emotional face processing. Neurobiol Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Lüthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Sadato N, Yamada H, Murata T, Omori M, Yonekura Y. An fMRI study of the functional neuroanatomy of picture encoding in younger and older adults. Brain Res Cogn Brain Res. 2001;11:1–11. doi: 10.1016/s0926-6410(00)00058-6. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, et al. Agerelated differences in the medial temporal lobe responses to emotional faces as revealed by fmri. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activation in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fmri investigation of race-related amygdala activity in african-american and caucasianamerican individuals. Nat Neurosci. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JD, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol Sci. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr Personality trait structure as a human universal. Am Psychol. 1997;52:509–516. doi: 10.1037//0003-066x.52.5.509. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Blascovich J, Hunter SB. Threatened by the unexpected: Physiological responses during social interactions with expectancy-violating partners. Soc Psychology. 2007;92:698–716. doi: 10.1037/0022-3514.92.4.698. [DOI] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behav Res Methods Instrum Comput. 2004;36:630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nelson H. National adult reading test (nart) test manual. Windsor: NFER-Nelson Publishing Co.; 1982. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect TJ, Moon H. The own-age effect of face recognition. In: Duncan J, Phillips L, Mcleod P, editors. Measuring the mind: Speed, control, and age. Oxford: Oxford University Press; 2005. pp. 317–340. [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, Banaji MR. Performance on indirect measures of race evaluation predicts amygdala activation. J Cogn Neurosci. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects with neonatal a mygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Caviness VSJ, Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: Implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex. 1993;3:313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- Roseman IJ, Antoniou AA, Jose PE. Appraisal determinant of emotions: Constructing a more accurate and comprehensive theory. Cognition & Emotion. 1996;10:241–277. [Google Scholar]
- Rolls ET. Neurophysiology and functions of the primate amygdala. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 143–165. [Google Scholar]
- Rolls ET. Emotion explained. Oxford: Oxford University Press; 2005. [Google Scholar]
- Scherer KR. On the nature and function of emotion: A component process approach. In: Scherer KR, Ekman P, editors. Approaches to emotion. Hillsdale, NJ: Erlbaum; 1984. pp. 293–317. [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL. Differential amygdalar response to novel versus newly familiar neutral faces: A functional MRI probe developed for studying inhibited temperament. Biol Psychiatry. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Matsuda G, Hoge EA, Kennedy D, Makris N, Caviness VS, Tsuang MT. Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: A pilot magnetic resonance imaging study. Am J Med Genet. 1997;74:507–514. doi: 10.1002/(sici)1096-8628(19970919)74:5<507::aid-ajmg11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, Tourville J, Kennedy D, Makris N, Caviness VS, Tsuang MT. Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: An MRI-based morphometric analysis. Biol Psychiatry. 1999;46:941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ. Prior experience as a stimulus category confound: An example using facial expressions of emotion. Soc Cogn Affect Neurosci. 2006;1:271–274. doi: 10.1093/scan/nsl040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Ellsworth PC. Patterns of cognitive appraisal in emotion. Journal of Personality and Social Psychology. 1985;48:813–838. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-D proportional system: An approach to cerebral imaging. New York: Thieme Publishers; 1988. [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke MW, Goekoop R, Duschek EJ, Netelenbos JC, Kuijer JP, Barkhof F, Scheltens P, Rombouts SA. Interindividual differences of medial temporal lobe activation during encoding in an elderly population studied by fmri. Neuroimage. 2004;21:173–180. doi: 10.1016/j.neuroimage.2003.09.043. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, Josepsh J, Davidson M, Mize J. The neuroimaging of emotion. Chapter to appear. In: Lewis M, Haviland-Jones JM, editors. The handbook of emotion. 3rd Edition. New York: Guilford; in press. [Google Scholar]
- Walker PM, Tanaka JW. An encoding advantage for own-race versus other-race faces. Perception. 2003;32:1117–1125. doi: 10.1068/p5098. [DOI] [PubMed] [Google Scholar]
- Wedig MM, Rauch SL, Albert MS, Wright CI. Differential amygdala habituation to neutral faces in young and elderly adults. Neurosci Lett. 2005;385:114–119. doi: 10.1016/j.neulet.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The consortium to establish a registry for Alzheimer's disease (CERAD). Part v. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–188. [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG. Human Amygdala Responsivity to Masked Fearful Eye Whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E. The mellow years? Neural basis of improving emotional stability over age. J Neurosci. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Rolls ET. Neuronal responses related to the novelty and familarity of visual stimuli in the substantia innominata, diagonal band of broca and periventricular region of the primate basal forebrain. Exp Brain Res. 1990;80:104–120. doi: 10.1007/BF00228852. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Rolls ET. The effects of stimulus novelty and familiarity on neuronal activity in the amygdala of monkeys performing recognition memory tasks. Exp Brain Res. 1993;93:367–382. doi: 10.1007/BF00229353. [DOI] [PubMed] [Google Scholar]
- Wright CI, Feczko E, Dickerson B, Williams D. Neuroanatomical correlates of personality in the elderly. Neuroimage. 2007a doi: 10.1016/j.neuroimage.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiol Aging. 2006a;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Wright CI, Dickerson BC, Feczko E, Negreira A, Williams D. An fMRI study of amygdala responses to viewing faces: Effects of aging and Alzheimer’s disease. Biological Psychiatry. 2007b doi: 10.1016/j.biopsych.2006.11.013. in press. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer HH, McMullin K, Rauch SL. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18:660–669. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, Wedig MM. Neuroanatomical correlates of extraversion and neuroticism. Cereb Cortex. 2006b;16:1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]
- Wright DB, Stroud JN. Age differences in lineup identification accuracy: People are better with their own age. Law Hum Behav. 2002;26:641–654. doi: 10.1023/a:1020981501383. [DOI] [PubMed] [Google Scholar]


