Abstract
Haemochorial placentation is a unique physiological process in which the fetal trophoblast cells remodel the maternal decidual spiral arteries to establish the fetoplacental blood supply. Pregnancy-specific glycoproteins (PSGs) are members of the carcinoembryonic antigen family. PSGs are produced by the placenta of rodents and primates and are secreted into the bloodstream. PSG23 is one of 17 members of the murine PSG family (designated PSG16 to PSG32). Previous studies determined that PSGs have immunoregulatory functions due to their ability to modulate macrophage cytokine secretion. Here we show that recombinant PSG23 induces transforming growth factor (TGF) beta1, TGFB1, and vascular endothelial growth factor A (VEGFA) in primary murine macrophages and the macrophage cell line RAW 264.7 cells. In addition, we identified new cell types that responded to PSG23 treatment. Dendritic cells, endothelial cells, and trophoblasts, which are involved in maternal vasculature remodeling during pregnancy, secreted TGFB1 and VEGFA in response to PSG23. PSG23 showed cross-reactivity with human cells, including human monocytes and the trophoblast cell line, HTR-8/SVneo cells. We analyzed the binding of PSG23 to the tetraspanin CD9, the receptor for PSG17, and found that CD9 is not essential for PSG23 binding and activity in macrophages. Overall these studies show that PSGs can modulate the secretion of important proangiogenic factors, TGFB1 and VEGFA, by different cell types involved in the development of the placenta.
Keywords: angiogenesis, mouse pregnancy-specific glycoproteins
Murine PSG23 induces TGFB1 and VEGFA in macrophages, dendritic cells, endothelial cells, and trophoblasts.
INTRODUCTION
In mammals with haemachorial placentation, pregnancy represents a physiological state in which intimate contact between maternal and fetal tissues must occur. The factors that promote or hamper proper trophoblast invasion of the maternal spiral arteries are still unknown; however, the involvement of the proangiogenic factors transforming growth factor beta (TGFB) [1] and vascular endothelial growth factor (VEGF) [2] have been implicated in the process of proper placental development. Thus, signals responsible for regulating these two molecules during pregnancy warrants further investigation.
Pregnancy-specific glycoproteins (PSGs), also referred to as pregnancy-specific β1-glycoprotein (SP1), are among the most abundant fetal proteins, reaching concentrations of 200–400 μg/ml in the serum of pregnant women at term [3]. PSGs are members of the carcinoembryonic antigen (CEA) family of immunoglobulin (Ig)-like genes [4]. PSGs are highly glycosylated, with N-linked glycosylation composing approximately 30% of their molecular mass [4]. Although the exact function of PSGs is still unknown, previous studies have focused on their role as immunomodulators and have defined monocytes/macrophages as their target cells [5–8].
To date, 17 murine Psg genes (Psg16–32) have been identified. PSG protein and mRNA have been detected in trophoblast giant cells and spongiotrophoblasts of the mouse as early as Embryonic Day 6.5 [9]. Murine PSGs consist of a varying number of Ig variable (IgV)-like N-domains and one Ig constant (IgC)-like A-domain [9]. Murine PSGs have a 59%–72% homology in their N-domains, and they likely arose by duplication from a single ancestral mouse Psg gene [9]. Although individual murine PSGs are expressed at different times and levels during pregnancy [9] and may play unique roles in gestation, it is also likely that they have some overlapping functions. Psg23 is one of the most abundantly expressed murine PSGs during midgestation as determined by RT-PCR [10], but its functions have never been investigated.
Monocytes and macrophages work in numerous processes during pregnancy, including at the sites of fetal trophoblast invasion and migration [11]. Macrophages have been shown to promote angiogenesis in several systems [12, 13]. Wynne et al. [14] recently showed that murine PSG21 is associated predominantly with endothelial cells lining the vascular channels in the decidua. Therefore, we hypothesized that PSGs could promote angiogenesis.
Here we report that murine PSG23 induces TGFB1 and VEGF in monocytes, macrophages, and dendritic cells, immune cells at the implantation site that have been shown to secrete angiogenic factors [11, 15]. In addition, we demonstrate that treatment of endothelial and trophoblast cell lines with PSG23 leads to induction of TGFB1 and VEGF. Our results strongly suggest a new function for PSGs during maternal vasculature remodeling and identify novel targets for PSG activity.
We have previously shown that the tetraspanin CD9 is the receptor for murine PSG17 and PSG19 [16, 17]. Tetraspanins are a family of membrane proteins expressed in most cell types, and many different tetraspanins are coexpressed in the same cell [18]. The data presented here indicates that not all PSGs utilize the same receptor, as expression of CD9 is not required for the PSG23-induced secretion of TGFB1 and VEGF in macrophages. Further studies are required to establish whether human PSGs share these newly described properties and to further dissect the role of the different members of this family in the establishment and maintenance of pregnancy.
MATERIALS AND METHODS
Antibodies
For flow cytometry analysis the following antibodies were used: streptavidin-fluorescein isothiocyanate (FITC), streptavidin-phycoerythrin (PE), and PE Rat IgG2b isotype control (eBioscience, Inc., San Diego, CA); anti-Flag BioM2 (Sigma, St. Louis, MO); and rat anti-mouse IgG1 PE (BD Biosciences, San Jose, CA).
For immunoblotting, the following antibodies were employed: His-probe (H-15, clone sc-803; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-Flag M2, and anti-Flag M2 horseradish peroxidase (HRP; Sigma).
Neutralization of TGFB1 was performed by adding the anti-human TGFB1 neutralizing antibody (R & D Systems, Minneapolis, MN), and normal chicken Ig antibody was used as a control (R & D Systems).
Protein Production and Purification
Recombinant murine PSG23N1A-His-Flag (hereafter referred to as PSG23N1A) was synthesized by the GenScript Corporation (Piscatawy, NJ). The cDNA encoding for the leader peptide, N1, and A domains of Psg23, along with the nucleotide sequence for six histidines (6× His) and a Flag tag (Sigma) were inserted into the SmaI site of pUC57 (EZBiolab, Inc., Westfield, IN). Psg23N1A-His-Flag was subcloned into the HindIII and EcoRI sites of the pEAK10CV vector (Edge Biosystems, Gaithersburg, MD) and transfected into dihydrofolate reductase-negative CHO cells, along with the pDCHIP plasmid (obtained from Dr. Kaplan, FDA, MD), which encodes the Dhfr minigene. Positive transformants were obtained by methotrexate selection, propagated, and seeded in a 5-kDa molecular mass cutoff hollow fiber cartridge (FiberCell Systems, Inc., Frederick, MD). The supernatant from the cartridge was harvested daily and kept frozen until it was processed as described below.
PSG23N1A was dialyzed into 20 mM sodium phosphate buffer containing 50 mM imidazole and purified from the harvested supernantant using a HisTrap column on the ÄKTAprime Plus system (GE Healthcare, Piscataway, NJ). Positive fractions eluted from the HisTrap column were identified by immunoblotting, pooled, buffer-exchanged into PBS, applied to an anti-Flag M2 agarose column (Sigma), and eluted with 3× Flag peptide (Sigma). Fractions, which reacted with the anti-Flag monoclonal antibody, were collected, pooled, and buffer-exchanged with PBS. The purified protein was run on an SDS-PAGE gel, stained with GelCode Blue Stain Reagent (Pierce, Rockford, IL), and quantitated against BSA standards.
Recombinant murine PSG17N-myc-His was generated and purified as previously described [19].
PSGs were determined to be endotoxin-free by the Limulus Amebocyte Lysate assay and its inability to induce tumor necrosis factor α (TNFA) in macrophages by ELISA. Endotoxin has been shown to up-regulate cytokines and growth factors in macrophages [20]; the absence of endotoxin in the protein preparations further ensures that the cell response is specific to the presence of PSG23N1A.
Cell Lines
All cells were maintained in a 37°C humidified incubator with 10% CO2. For protein activity assays we tested RAW 264.7 (American Type Culture Collection [ATCC], Manassas, VA), C166 (ATCC), human invasive trophoblast (HTR-8/SVneo) cells [21] (kind gift from Charles Graham, Queen's University, Kingston, ON, Canada), and a murine trophoblast cell line derived from C57BL/6 × 129 placenta [22] (kind gift from Joan Hunt, University of Kansas Medical Center, Kansas City, KS).
HEK 293T (ATCC) and BHK-21 cells (ATCC) were maintained in Dulbecco modified Eagle medium (DMEM; Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT), 10 mM Hepes (Mediatech, Inc., Manassas, VA), and antibiotics. RAW 264.7 and C166 cells were cultured in DMEM supplemented with 10% fetal clone III serum alternative (Hyclone), 10 mM Hepes (Mediatech, Inc.), and penicillin-streptomycin (PS; Mediatech, Inc.). HTR-8/SVneo cells were maintained in HyQ RPMI 1640-RS (Hyclone) media. Human monocytes were maintained in RPMI 1640 (Gibco) supplemented with 2 mM glutamine.
Primary Cells
All cells were maintained in a 37°C humidified incubator with 10% CO2. Six- to eight-week-old C57BL/6 mice deficient in CD9 were bred from a CD9+/− breeding pair obtained from Dr. Claude Boucheix (Hôpital Paul Brousse, Villejuif, France). Six- to eight-week-old BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Briefly, mice were injected i.p. with 3 ml of sterile 3% thioglycollate broth (BD Biosciences). Three days after i.p. injection, macrophages were obtained by peritoneal lavage. Cells were seeded at 1.5 × 106 per well in a 24-well plate (BD Biosciences). Twenty-four hours after seeding the cells, macrophages attached to wells and non-adherent cells were removed. Plating media (RPMI supplemented with 2% FBS and 0.5× PS) was replaced with treatment media (RPMI supplement with 2% delipidized FBS and 0.5× PS) prior to addition of protein or PBS control. All samples were collected in accordance with Uniformed Services University Institutional Animal Care and Use Committee-approved protocol.
Murine bone marrow-derived dendritic cells (BMDCs) were isolated and differentiated as previously described [23]. Human monocytes were isolated from the peripheral blood of healthy adult donors and purified as previously described in accordance with human subject protocols approved by the Internal Review Board of the National Institutes of Health [7].
Plasmids and Cell Transfection
For cell transfection, HEK 293T or BHK-21 cells were seeded at a density of 4.5 × 106 in a 60-mm poly-L-lysine culture dish (BD Biosciences). After cells attached, culture media was removed and 20 ml of plating media was added to each plate. Plasmids were incubated with FuGENE 6 Transfection Reagent (Roche Diagnostics, Basel, Switzerland) as per manufacturer's instructions and added to the cells. Forty-eight hours posttransfection, cells were removed from the culture dishes using CellStripper (MediaTech, Inc.) and used for binding experiments. The green fluorescent protein (GFP) murine CD9 fusion cDNA was constructed as previously reported [19].
Enzyme-Linked Immunosorbent Assays
For ELISAs cells were plated in triplicate wells for each treatment in 24-well plates and incubated in a 37°C humidified incubator with 10% CO2. RAW 264.7 cells were seeded at a density of 1 × 106 cells per well, C166 cells were seeded at a density of 0.25 × 106 cells per well, HTR-8/SVneo cells were seeded at 0.4 × 106 cells per well, human monocytes were seeded at a density of 1.2 × 106 cells per well, and peritoneal macrophages were seeded at a density of 1.5 × 106 per well. Cells were incubated overnight and treated on the following day in 300 μl of fresh media for treatment times less than 6 h or 1 ml of fresh media for treatment times greater than 6 h. In some experiments, cells were treated with PSG23N1A protein after it had been boiled for 15 min, endotoxin-free BSA (Sigma), or control protein as negative controls.
After treatment, the supernatants were collected and centrifuged at 3000 rpm for 5 min to remove cell debris. For TGFB1 ELISA, supernatant or media alone was activated as per the manufacturer's instructions (R & D Systems). Murine VEGFA and placental growth factor (PlGF) and human VEGFA and VEGFC expression were measured by ELISA. Murine VEGFC ELISA was performed using a rat VEGFC ELISA kit (Bender MedSystems, Burlingame, CA), which cross-reacts with murine VEGFC. TNFA in the supernatants from both human and murine cells was determined using the BD OptEIA Mouse TNF (Mono/Mono) ELISA Set (BD Biosciences). All cells were treated in triplicate wells, and supernatants were analyzed as individual samples in each ELISA. At least three different protein preparations were tested, and experiments were repeated a minimum of three times.
Binding Experiments
For fluorescence-activated cell sorting (FACS) binding experiments, 1 × 106 cells in 100 μl were treated with Multiple Tag, which contains a Flag tag (GenScript Corporation) employed as control protein, or PSG23N1A for 1 h on ice. Cells were incubated with 0.5 μg of biotinylated anti-Flag antibody (Sigma) to detect protein binding, followed by incubation with 0.5 μg FITC- or PE-conjugated streptavidin. Excess protein and antibodies were removed by washing with FACS wash buffer (PBS supplemented with 3% FBS and 0.05% NaN3). In the experiments indicated, a live/dead cell discriminate dye was used (LIVE/DEAD Fixable Dead Cell Stain Kit; Molecular Probes, Carlsbad, CA). Cells were fixed with Cytofix (BD Bioscences) and analyzed using the Coulter Epics XL Flow Cytometer (Beckman Coulter, Fullerton, CA). For flow cytometry analysis of peritoneal macrophages, cells were first blocked with anti-mouse CD16/CD32 (Fcγ III/II receptor; BD Biosciences) antibody prior to incubation with the control or PSG23N1A proteins.
Statistical Analysis
SPSS (SPSS, Inc., Chicago, IL) and Microsoft EXCEL were used for data statistical analysis. The two-tailed Student t-test was used to determine statistical significance in experiments comparing protein treated versus untreated cells, with a P value of <0.05 as a cut-off. One-way ANOVA was used to determine statistical significance of dose-response assays, with a P value of <0.001 as a cut-off. Quantitative RT-PCR results were analyzed using the 7500 System Sequence Detection Software version 1.2.3 (Applied Biosystems). FACS data were analyzed using WinList 6.0 (Verity Software House, Topsham, ME). All data is representative of at least three independent experiments.
RESULTS
Generation of Recombinant PSG23N1A
Previous studies indicate that the N1 domain of murine PSGs is sufficient for binding and activity in macrophages [5, 16, 17]. The CFG face in the murine PSG N1 domain includes the putative integrin-interacting RGD-like motif on a solvent-exposed loop, which has been postulated to have functional significance in rodent and human PSGs [24]. Supporting the importance of the N-domain for PSG function, McLenachan et al. [25] demonstrated that the vast majority of human PSG splice variants retain the N-domain and that there has been nonneutral evolution of the N-domain. We generated recombinant PSG23N1A protein that consists of the leader peptide, the N1 domain, and the constant C2-like Ig A domain, followed by 6X His and a C-terminal Flag tag. The 36-kDa recombinant protein was harvested from the supernatant of stably transfected CHO cells generated as described in Materials and Methods and was sequentially purified on a HisTrap and anti-Flag affinity column. These two purification steps rendered a glycoprotein that was approximately 90% pure based on Coomassie Blue staining (Fig. 1).
FIG. 1.
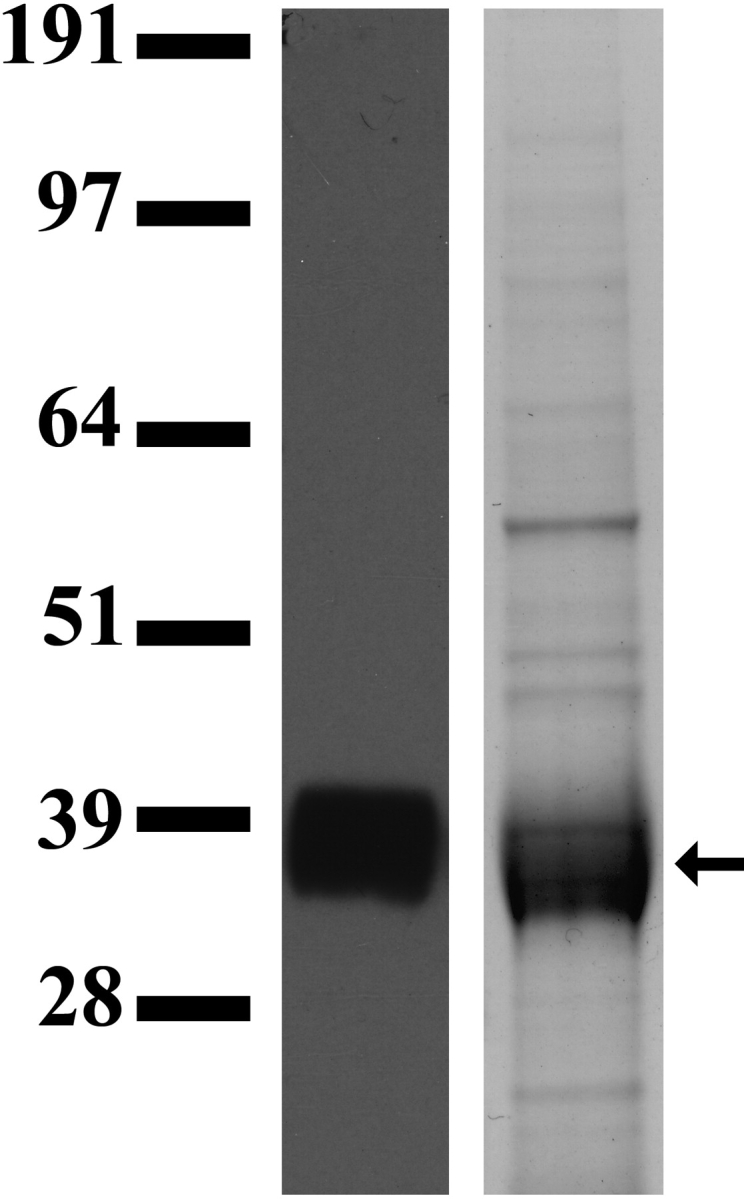
Recombinant PSG23N1A protein produced in CHO cells. Lane 1: PSG23N1A secreted by CHO cells was analyzed by Western blot using an anti-Flag antibody followed by an HRP-conjugated secondary antibody; lane 2: the same protein after two rounds of purification in a Coomassie stained gel. Arrow indicates the size of the purified protein, approximately 36 kDa.
Media harvested from non-PSG23 expressing CHO cells grown in a hollow fiber cartridge was passed through a HisTrap column, and the eluted material was used as an additional control after the protein concentration was determined by the BCA protein assay.
Recombinant PSG23N1A Binds to Macrophages and Dendritic Cells
Previously, it was demonstrated that murine PSGs elicit an anti-inflammatory cytokine response in monocytes and macrophages [5–8, 17] and that the N1 domain of three different murine PSGs is sufficient for binding to target cells [7, 17]. We first tested the response of the murine macrophage cell line, RAW 264.7, to different concentrations of PSG23N1A, and RAW cells secreted TGFB1 in response to PSG23N1A starting at 5 μg/ml (Fig. 2A). This response was observed as early as 4 h after treatment (data not shown). Although we could observe up-regulation of TGFB1 by ELISA in response to PSG23N1A treatment, there was no increase of Tgfb1 mRNA at 0.5, 1, 2, and 4 h (data not shown). Therefore, PSG23N1A, as previously observed for other murine PSGs [7], did not up-regulate TGFB1 at the transcriptional level.
FIG. 2.
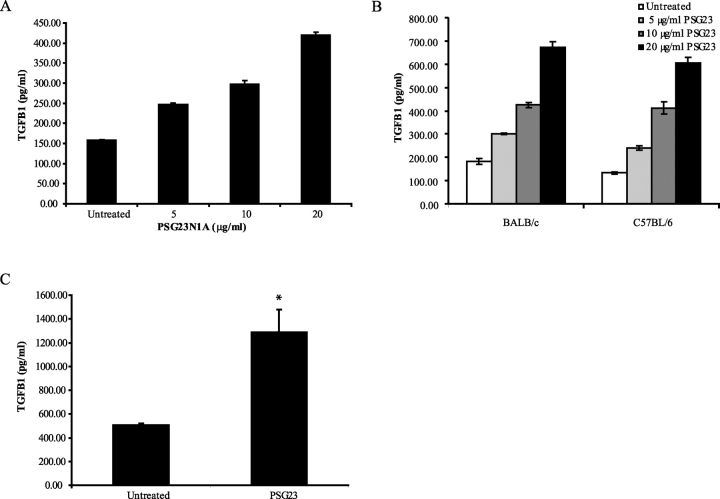
PSG23N1A induces TGFB1 in macrophages and BMDCs. A) RAW 264.7 cells were treated with 5–20 μg/ml of PSG23N1A for 24 h. B) Peritoneal macrophages isolated from BALB/c or C57BL/6 mice were treated with PSG23N1A (PSG23) at 5–20 μg/ml of protein for 4 h. C) Following differentiation, BMDCs isolated from C57BL/6 mice were treated with 30 μg/ml of PSG23N1A for 24 h. TGFB1 in the supernatants was analyzed by ELISA. All cells were treated in triplicate, and data is representative of at least three independent experiments. For A and B, statistical significance between doses was determined by one-way ANOVA, and the P value between doses was <0.001. For C, statistical significance between the protein-treated and untreated samples was determined by two-tailed Student t-test, and * indicates P < 0.05. Error bars represent mean ± SEM.
We also tested primary macrophages, obtained by peritoneal lavage of C57BL/6 and BALB/c mice and found that PSG23N1A induced TGFB1 in these cells at concentrations ranging from 5–20 μg/ml (Fig. 2B), with a peak expression at 8 h after treatment.
Dendritic cells (DCs) have also been implicated in maintaining maternal immune tolerance to the developing fetus [26] because of their ability to influence the differentiation of T cells to a T helper type 2 (Th2) subset [27] and their cross-talk with the most abundant leukocyte population at the maternofetal interface, uterine natural killer (uNK) cells [28]. Therefore, we investigated whether DCs could respond to PSG treatment. We harvested BMDCs of C57BL/6 mice as previously described [23], and after 7 days in culture we treated the cells with PSG23N1A at 15–30 μg/ml for 24 h. We found that PSG23N1A induces TGFB1 in BMDCs (Fig. 2C).
In addition, we measured the production of pro-inflammatory cytokines in RAW cells in response to PSG23N1A treatment. PSG23N1A did not up-regulate TNFA, IL12A, IL23A, or IL6 mRNA in RAW cells as determined by quantitative real-time PCR (data not shown). Analysis of the supernatants using specific ELISAs for TNFA, IL12, and IL6 at 24 h post-PSG23N1A treatment demonstrated a lack of up-regulation of these cytokines at the protein level.
PSG23N1A Induces VEGFA in Macrophages and Dendritic Cells
TGFB is a proangiogenic factor that plays an important role in the development of the fetoplacental capillary system during implantation [1]. TGFB secretion has been correlated to up-regulation of VEGF family members [1, 29], and both angiogenic factors are essential in pregnancy. Upon the observation that PSG23N1A treatment of macrophages and BMDCs induced TGFB1, we investigated the possibility that PSG23N1A might regulate VEGFA, which is expressed at the site of trophoblast invasion [2].
RAW cells secreted VEGFA in response to PSG23N1A treatment in doses ranging from 10–30 μg/ml (Fig. 3A) after 24 h of treatment. Primary macrophages from BALB/c and C57BL/6 mice also secreted VEGFA after 4 h of PSG23N1A treatment and showed peak expression of VEGFA after 8 h of treatment (Fig. 3B). As observed in macrophages, PSG23N1A treatment resulted in the up-regulation of VEGFA secretion in BMDCs isolated from C57BL/6 mice measured at 24 h (Fig. 3C).
FIG. 3.
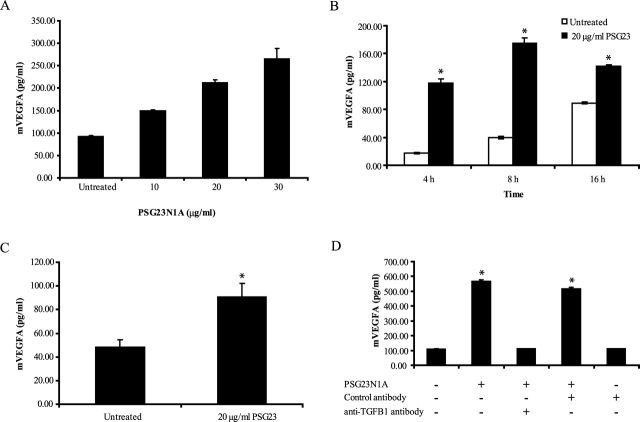
PSG23N1A induces VEGFA in macrophages and BMDCs. A) RAW 264.7 cells were treated with 10–30 μg/ml PSG23N1A protein for 24 h. Statistical significance between doses was determined by one-way ANOVA, and the P value between doses was <0.001. B) Peritoneal macrophages isolated from BALB/c mice were treated with 20 μg/ml of PSG23N1A in a time-course study. C) Following differentiation, BMDCs isolated from C57BL/6 mice were treated with 20 μg/ml of PSG23N1A for 24 h. D) RAW 264.7 cells were pretreated with 20 μg/ml control antibody or neutralizing anti-TGFB1 antibody. After 30 min of incubation with antibodies, cells were treated with 20 μg/ml PSG23N1A in the indicated wells and incubated for 24 h. VEGFA in the supernatants was analyzed by ELISA. All cells were treated in triplicate, and data is representative of at least three independent experiments. For B and C, statistical significance between the protein-treated and untreated samples was determined by two-tailed Student t-test, and * indicates P < 0.05. For D statistical significance between protein-treated or antibody-treated samples versus the untreated samples was determined by two-tailed Student t-test, and * indicates P < 0.05. Error bars represent mean ± SEM.
To test whether PSG23N1A induction of VEGFA in macrophages was TGFB dependent, we pretreated RAW cells with 20 μg/ml of neutralizing anti-TGFB or control antibody for 30 min prior to the addition of PSG23N1A. The anti-TGFB antibody, but not the antibody control, effectively inhibited PSG23N1A-induced VEGFA secretion (Fig. 3D).
VEGFC is another VEGF family member important in pregnancy and is expressed by trophoblasts, endothelial cells, and uNK cells during pregnancy [30]. The factors that promote VEGFC secretion remain to be elucidated. We investigated whether PSG23N1A induced VEGFC in macrophages both at the mRNA and protein levels. We did not see Vegfc mRNA, measured by quantitative RT-PCR, or VEGFC protein, measured by ELISA, in RAW cells or peritoneal macrophages (data not shown).
The VEGF family member PlGF is expressed during and regulates differentiation of vascular endothelia. It is expressed by uNK cells of the mouse pregnant uterus and promotes uNK cell proliferation and differentiation [30]. PlGF has also been shown to be a chemotactic and survival factor for macrophages involved in tumor angiogenesis [31]; therefore, we investigated whether PSG23N1A induced PlGF in macrophages. In our studies, however, PlGF was not induced in macrophages after PSG23N1A treatment (data not shown). The angiopoetins, ANG1 and ANG2, have been shown to synergize with VEGF family members during angiogenesis, and ANG2 production by macrophages was previously reported [32]. We measured Ang1 and Ang2 in peritoneal macrophages and RAW cells by quantitative RT-PCR at different times post PSG23N1A treatment and found that ANG1 was not expressed in these cells and ANG2, while expressed, was not induced by PSG23N1A treatment (data not shown). These results could not be verified at the protein level due to the lack of commercially available reagents to detect murine ANG1 and ANG2 proteins.
Identification of Endothelial Cells and Trophoblasts as Targets for PSG23N1A
Trophoblasts, endothelial cells, and monocytes/macrophages are all involved in the establishment and maintenance of the fetal blood supply during placentation. As PSG23N1A induced TGFB1 and VEGFA in macrophages, we hypothesized that PSGs could affect the functions of other cell types involved in the establishment of the fetoplacental blood supply. Therefore, we included in these studies an endothelial and a trophoblast cell line to determine if these cells responded to PSG23N1A treatment.
The murine endothelial cell line (C166) was derived from a mouse yolk sac and has a normal endothelial cell phenotype [33]. The C166 cells secreted TGFB1 in response to PSG23N1A in a dose-dependent manner (Fig. 4A). Induction of TGFB1 in these cells was seen as early as 1 h after treatment (data not shown). VEGFA was also induced in C166 cells after 24 h of treatment in a dose-dependent manner (Fig. 4B). As observed in macrophages, the secretion of VEGFA in C166 cells was dependent on TGFB1 induction (Fig. 4C).
FIG. 4.
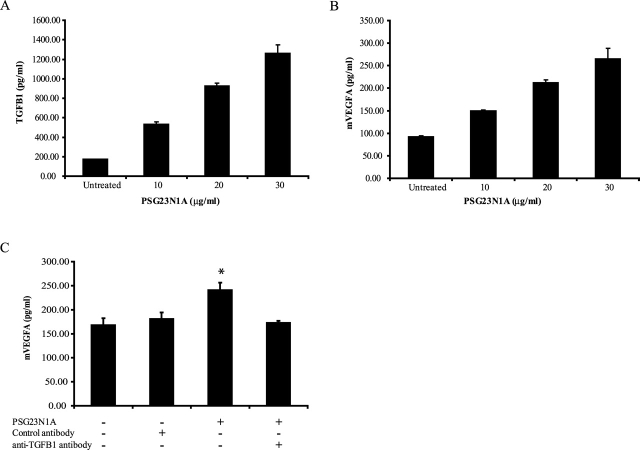
PSG23N1A induces TGFB1 and VEGFA in murine endothelial cells. A, B) C166 cells were treated with PSG23N1A at concentrations of 10–30 μg/ml for 24 h. Statistical significance between doses was determined by one-way ANOVA; the P value between doses was <0.001. C) C166 cells were pretreated with 20 μg/ml of control antibody or neutralizing anti-TGFB1 antibody. Following 30-min incubation with the antibodies, 20 μg/ml of PSG23N1A was added to the indicated wells for 24 h. For C statistical significance between protein-treated or antibody-treated samples versus the untreated samples was determined by two-tailed Student t-test, and * indicates P < 0.05. TGFB1 and VEGFA in the supernatants were analyzed by ELISA. All cells were treated in triplicate, and data is representative of at least three independent experiments. Error bars represent mean ± SEM.
A murine trophoblast cell line responded to PSG23N1A treatment as shown by induction of TGFB1 and VEGFA (data not shown). These trophoblast cells were isolated from mouse placentae and shown to exhibit markers similar to those of labyrinthine trophoblast cells, such as cytokeratin intermediate filaments, transferring receptors, and alkaline phosphatase, but were not differentiated into spongiotrophoblasts [22].
Similar to our results using murine macrophages, PSG23N1A did not induce secretion of mRNA for Vegfc, Ang1, or Ang2 or the cytokines for VEGFC and PlGF, both measured by ELISA, in C166 cells or the murine trophoblast cells (data not shown).
PSG23N1A Shows Cross-Reactivity with Human Cells
Murine and human PSGs share a 60% homology in their N-domains [24], and previous studies showed that murine PSGs induce anti-inflammatory cytokine secretion in human monocytes [7]. We therefore tested the effects of PSG23N1A on human monocytes. Upon PSG23N1A treatment, human monocytes secrete TGFB1 and VEGFA (Fig. 5, A and B). PSG23N1A did not induce VEGFC in human monocytes (data not shown).
FIG. 5.
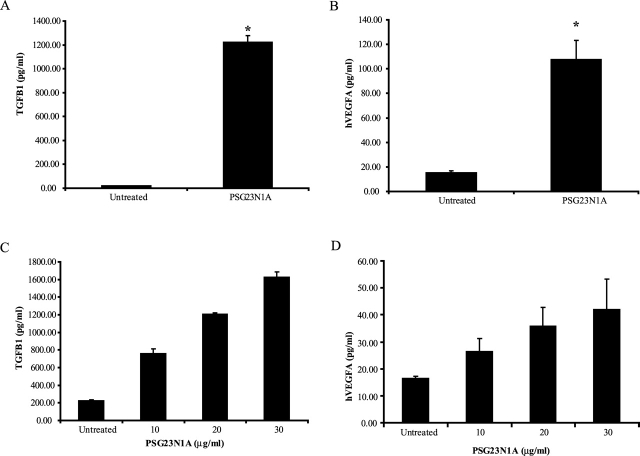
PSG23N1A induces TGFB1 and VEGFA in human monocytes and trophoblast cells. A, B) Human monocytes were treated with 30 μg/ml PSG23N1A for 24 h. Statistical significance between protein-treated and untreated samples was determined by two-tailed Student t-test, and * indicates P < 0.05. C, D) HTR-8/SVneo cells were treated with PSG23N1A in concentrations of 10–30 μg/ml for 24 h. Statistical significance between doses was determined by one-way ANOVA, and the P value between doses was <0.001. TGFB1 and VEGFA in the supernatants were analyzed by ELISA. All cells were treated in triplicate, and data is representative of at least three independent experiments. Error bars represent mean ± SEM.
The invasive trophoblast cell line, HTR-8/SVneo, has proved an important tool in understanding some of the mechanisms involved in trophoblast invasion and migration during pregnancy [21]. We tested the effect of PSG23N1A treatment on secretion of proangiogenic factors by HTR-8/SVneo cells. Cells were treated for 24 h with 10–30 μg/ml of PSG23N1A, and TGFB1, VEGFA, and VEGFC secretion in the supernatants was determined. We observed that PSG23N1A induced TGFB1 and VEGFA in HTR-8/SVneo cells (Fig. 5, C and D) in a dose-dependent manner, but that VEGFC was not up-regulated in these cells (data not shown).
PSG23 Activity Does Not Depend on CD9
Murine PSGs share a 59%–70% homology [9]. Previously, murine PSG17 was shown to induce TGFB1, IL-10, and IL-6 secretion in both murine macrophages and human monocytes [5, 7]. We also determined that PSG17 elicits an anti-inflammatory cytokine response in these cells through binding to its receptor CD9 [5], a member of the tetraspanin superfamily. The binding of PSG17 to murine CD9 was determined by panning, ELISA, and FACS and in functional assays with CD9-deficient mice [17]. Because PSG23 also targets monocytes/macrophages, we sought to determine whether PSG23 also utilizes CD9 as its receptor.
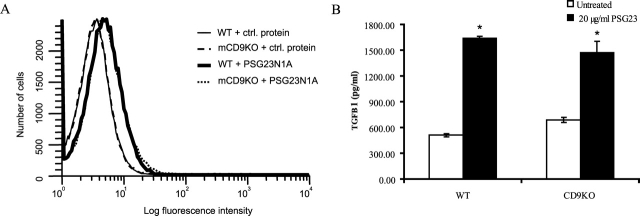
We transfected BHK-21 cells with a plasmid encoding the murine CD9 cDNA or control plasmid. By FACS, we did not observe any differences in PSG23N1A binding to the CD9-expressing cells over the control-transfected cells (data not shown). In an ELISA binding assay, we incubated HEK 293T cells transfected with a murine CD9-GFP fusion or a GFP only-expressing plasmid with PSG23N1A or PSG17. We did not observe binding of PSG23N1A to the CD9-transfected cells over the control-transfected cells, whereas PSG17 bound preferentially to the CD9-transfected cells as previously reported [17] (data not shown). After panning murine CD9-transfected HEK 293T cells on PSG23N1A-coated or uncoated plates, we also did not see a difference in binding to the PSG23N1A-coated plates by the cells overexpressing CD9 (data not shown). In addition, PSG23N1A bound to the same degree to peritoneal macrophages isolated from wild-type and CD9 deficient mice, as determined by FACS (Fig. 6A).
FIG. 6.
PSG23N1A binding and activity is not CD9 dependent. A) Peritoneal macrophages from wild-type (WT) and CD9-null (CD9KO) mice were incubated with 5 μg of PSG23N1A or Multiple Tag control protein, followed by subsequent incubation with biotinylated anti-Flag antibody, and stained with FITC-conjugated streptavidin and a live/dead cell discriminator. Cells were fixed and samples processed with the BD LSRII cell analyzer. Data was analyzed using WinList 3.0 software. B) PSG23N1A induced TGFB1 secretion in wild-type and CD9-null peritoneal macrophages after 24 h of treatment as measured by ELISA. Cells were treated in triplicate, and data is representative of three independent experiments. Statistical significance between protein-treated and untreated samples was determined by two-tailed Student t-test, and * indicates P < 0.05. Error bars represent mean ± SEM. ctrl., Control.
In functional assays, peritoneal macrophages isolated from wild-type and CD9-null mice were treated with PSG23N1A. Cells from wild-type and CD9-deficient mice responded to PSG23N1A by secreting TGFB1 after 24 h of treatment (Fig. 6B). The primary macrophages from both mice also secreted VEGFA in response to PSG23N1A treatment after 4 h of treatment (data not shown). In conclusion, both binding and functional assays showed that PSG23 does not depend on the presence of CD9 to bind to macrophages or to elicit a response.
DISCUSSION
PSGs are among the most abundant proteins in the maternal serum, yet their function during pregnancy is still being explored [3]. Previously, the role of PSGs centered on their functions as immunoregulators during pregnancy [6]. Treatment of monocytes/macrophages with recombinant human and murine PSGs leads to induction of anti-inflammatory cytokines, including IL10 and TGFB1, while there is no induction of pro-inflammatory cytokines [7]. In addition, human PSG1 was shown to induce alternative activation of monocytes and to enhance Th2-type immune responses [6]. Other CEA family members that, unlike PSGs, are membrane-bound have been shown to be involved in angiogenesis and immunoregulation [34]. For example, CEACAM1 has angiogenic properties [35, 36] and inhibits activated decidual lymphocytes [37], and CEACAM6, expressed on placental trophoblast, has been shown to activate a distinct subset of regulatory CD8+ T cells [34].
Recent studies have identified PSG expression at a unique site in nonpregnant mice. Kawano et al. [38] reported expression of murine PSG18 in the follicle-associated epithelium of the Peyer's patches and suggested a role for this murine PSG in the interplay between epithelial cells and immune cells in mucosa-associated lymphoid tissue.
The important players in placentation include macrophages [11], DCs [26], uNK cells [30], endothelial cells, and trophoblasts [39]. Previous work with murine and human PSGs identified monocytes/macrophages as the target cells for protein binding and activity [5, 17]. As previously observed for other human and murine PSGs [5, 7, 8], we found that macrophages up-regulate TGFB1 secretion in response to PSG23 in a dose-dependent manner. In addition, treatment of BMDCs with PSG23 resulted in up-regulation of TGFB1. TGFB1 has multiple roles during pregnancy, including regulation of extravillous trophoblast migration and proliferation [40] and regulation of NK cell function [41, 42]. TGFB1 and VEGFA have additive effects on vasodilation [1] and have been implicated in immunosuppression [43, 44]. Based on the observation that TGFB1 activated macrophages to express VEGF [29], we explored the possible induction of angiogenic factors in response to PSG23 treatment. VEGFA, but not VEGFC, PlGF, Ang-1, or Ang-2, was strongly induced in macrophages and BMDCs upon PSG23 treatment. The induction of VEGFA was found to be TGFB1 dependent.
Our results indicated that PSG23 does not induce TGFB1 at the transcriptional level, which is in agreement with our previous observations [7]. At the posttranscriptional level TGFB is synthesized as an inactive precursor complex comprised of the active TGFB protein bound to a propeptide latency-associated protein [45]. It is possible that PSGs activate effector molecules, such as enzymes, which cleave the inactive precursor and release active TGFB in target cell types [46]. The mechanisms involved in PSG up-regulation of TGFB1 warrant further investigation.
To extend our hypothesis that PSG23 is involved in angiogenesis, we investigated the effect of PSG23 on additional cell types involved in this process. PSG23 treatment of a murine endothelial cell line led to induction of both TGFB1 and VEGFA, and the induction of VEGFA was TGFB-dependent. In a murine trophoblast cell line, we saw that PSG23 treatment induced both TGFB1 and VEGFA. Similar to our results in murine macrophages, treatment with PSG23 did not result in up-regulation of other proangiogenic factors commonly associated with pregnancy. Further studies could elucidate whether PSGs have an effect on uNK cells, which are the most abundant leukocytes in pregnancy [30] and are involved in angiogenesis and trophoblast chemoattraction [47].
We previously reported cross-reactivity between human cells and murine PSGs [5]; hence, we treated human monocytes and a human trophoblast cell line with PSG23. In the two cell types, PSG23 induced TGFB1 and VEGFA. Induction of proangiogenic factors in human cells with the mouse protein suggests that human PSGs may also play a role in placentation, although this has yet to be explored. Interestingly, a significant reduction in PSGs (SP1) was reported in women with subsequent preeclampsia or growth-retarded fetuses in the early second trimester of pregnancy [48].
Previously, we demonstrated that PSG17, another member of the murine PSG family, induces TGFB1 in RAW 264.7 cells [5]. Upon our observation of PSG23 induction of VEGFA in murine macrophages, we treated RAW cells with PSG17 and found that PSG17 also up-regulates VEGFA secretion (data not shown). Future experiments will be necessary to determine if PSG17 has an effect on endothelial cells and trophoblasts.
VEGFA is the most abundantly expressed VEGF family member during pregnancy and one of the key growth factors in angiogenesis [49]. VEGFA binds to VEGF receptor-1 (VEGFR-1) and VEGFR-2 to induce the growth of new vessels [49]. Macrophages, endothelial cells, and trophoblasts secrete and express receptors for VEGFA, thereby allowing for autocrine and paracrine activity of VEGFA during angiogenesis [2, 29, 50]. Future studies will be necessary to determine whether PSG23 affects the expression of VEGF receptors.
We examined whether PSG23 binds to CD9, the PSG17 receptor. We could not demonstrate binding of PSG23 to CD9 by ELISA and FACS analysis. While we cannot conclusively rule out the possibility that PSG23 binds to CD9, this interaction, if it happens, may be of low affinity. Importantly, expression of CD9 in peritoneal macrophages was not required for induction of TGFB1 and VEGF. Therefore, we conclude that CD9 is not essential for PSG23 function. Tetraspanins are often organized together in a tetraspanin web on the cell surface, and many tetraspanin family members are expressed in the cell types shown to respond to PSG23 in this report [18]. Therefore, the possibility that PSG23 may use another tetraspanin family member as its receptor should be explored.
Our findings indicate that PSGs, besides their immunoregulatory role, may be important in the processes of vasculogenesis and angiogenesis required for the establishment and maintenance of the fetoplacental blood supply. We are currently investigating whether other murine PSGs or human PSGs share the ability with PSG23 to induce proangiogenic factors.
Acknowledgments
We would like to thank Anne Marie Dizon and James Warren for technical assistance and Dr. Jesus Colino for his guidance in the preparation of the bone marrow-derived dendritic cells. In addition, we are grateful to Dr. Larry Wahl (NIH/NIDCR, Bethesda, MD), Dr. Charles H. Graham (Queen's University at Kingston, Ontario, Canada), and Dr. Joan Hunt (University of Kansas Medical Center, Kansas City, KS) for providing us the human monocytes, the HTR-8/SVneo human trophoblast cell line, and the mouse trophoblast cell line, respectively.
Footnotes
1Supported by grant R01HD035832 from the National Institute of Health.
REFERENCES
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006; 12: 642–649.. [DOI] [PubMed] [Google Scholar]
- Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ.Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol 2002; 160: 1405–1423.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TM, Halbert SP, Spellacy WN.Measurement of pregnancy-associated plasma proteins during human gestation. J Clin Invest 1974; 54: 576–582.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Zimmermann W.The carcinoembryonic antigen gene family: structure, expression and evolution. Tumour Biol 1988; 9: 63–83.. [DOI] [PubMed] [Google Scholar]
- Ha CT, Waterhouse R, Wessells J, Wu JA, Dveksler GS.Binding of pregnancy-specific glycoprotein 17 to CD9 on macrophages induces secretion of IL-10, IL-6, PGE2, and TGF-beta1. J Leukoc Biol 2005; 77: 948–957.. [DOI] [PubMed] [Google Scholar]
- Motran CC, Diaz FL, Montes CL, Bocco JL, Gruppi A.In vivo expression of recombinant pregnancy-specific glycoprotein 1a induces alternative activation of monocytes and enhances Th2-type immune response. Eur J Immunol 2003; 33: 3007–3016.. [DOI] [PubMed] [Google Scholar]
- Snyder SK, Wessner DH, Wessells JL, Waterhouse RM, Wahl LM, Zimmermann W, Dveksler GS.Pregnancy-specific glycoproteins function as immunomodulators by inducing secretion of IL-10, IL-6 and TGF-beta1 by human monocytes. Am J Reprod Immunol 2001; 45: 205–216.. [DOI] [PubMed] [Google Scholar]
- Wessells J, Wessner D, Parsells R, White K, Finkenzeller D, Zimmermann W, Dveksler G.Pregnancy specific glycoprotein 18 induces IL-10 expression in murine macrophages. Eur J Immunol 2000; 30: 1830–1840.. [DOI] [PubMed] [Google Scholar]
- McLellan AS, Fischer B, Dveksler G, Hori T, Wynne F, Ball M, Okumura K, Moore T, Zimmermann W.Structure and evolution of the mouse pregnancy-specific glycoprotein (Psg) gene locus. BMC Genomics 2005; 6: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball M, McLellan A, Collins B, Coadwell J, Stewart F, Moore T.An abundant placental transcript containing an IAP-LTR is allelic to mouse pregnancy-specific glycoprotein 23 (Psg23): cloning and genetic analysis. Gene 2004; 325: 103–113.. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schroder W, Kaufmann P, Rath W.The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta 1999; 20: 229–233.. [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Leibovich SJ.Induction of neovascularization by activated human monocytes. J Leukoc Biol 1986; 39: 233–238.. [DOI] [PubMed] [Google Scholar]
- Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ.Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science 1983; 221: 1283–1285.. [DOI] [PubMed] [Google Scholar]
- Wynne F, Ball M, McLellan AS, Dockery P, Zimmermann W, Moore T.Mouse pregnancy-specific glycoproteins: tissue-specific expression and evidence of association with maternal vasculature. Reproduction 2006; 131: 721–732.. [DOI] [PubMed] [Google Scholar]
- Riboldi E, Musso T, Moroni E, Urbinati C, Bernasconi S, Rusnati M, Adorini L, Presta M, Sozzani S.Cutting edge: proangiogenic properties of alternatively activated dendritic cells. J Immunol 2005; 175: 2788–2792.. [DOI] [PubMed] [Google Scholar]
- Ha CT, Waterhouse R, Warren J, Zimmermann W, Dveksler GS.N-glycosylation is required for binding of murine pregnancy-specific glycoproteins 17 and 19 to the receptor CD9. Am J Reprod Immunol 2008; 59: 251–258.. [DOI] [PubMed] [Google Scholar]
- Waterhouse R, Ha C, Dveksler GS.Murine CD9 is the receptor for pregnancy-specific glycoprotein 17. J Exp Med 2002; 195: 277–282.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrin S, Manie S, Billard M, Ashman L, Gerlier D, Boucheix C, Rubinstein E.Multiple levels of interactions within the tetraspanin web. Biochem Biophys Res Commun 2003; 304: 107–112.. [DOI] [PubMed] [Google Scholar]
- Ellerman DA, Ha C, Primakoff P, Myles DG, Dveksler GS.Direct binding of the ligand PSG17 to CD9 requires a CD9 site essential for sperm-egg fusion. Mol Biol Cell 2003; 14: 5098–5103.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands JW, Jr, Peavy DL, Gormus BJ, McGraw J.In vitro and in vivo effects of endotoxin on mouse peritoneal cells. Infect Immun 1974; 9: 106–112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK.Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993; 206: 204–211.. [DOI] [PubMed] [Google Scholar]
- Rasmussen CA, Pace JL, Banerjee S, Phillips TA, Hunt JS.Trophoblastic cell lines generated from tumour necrosis factor receptor-deficient mice reveal specific functions for the two tumour necrosis factor receptors. Placenta 1999; 20: 213–222.. [DOI] [PubMed] [Google Scholar]
- Colino J, Shen Y, Snapper CM.Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J Exp Med 2002; 195: 1–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AS, Zimmermann W, Moore T.Conservation of pregnancy-specific glycoprotein (PSG) N domains following independent expansions of the gene families in rodents and primates. BMC Evol Biol 2005; 5: 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLenachan PA, Lockhart PJ, Faber HR, Mansfield BC.Evolutionary analysis of the multigene pregnancy-specific beta 1-glycoprotein family: separation of historical and nonhistorical signals. J Mol Evol 1996; 42: 273–280.. [DOI] [PubMed] [Google Scholar]
- Shojaeian J, Moazzeni SM, Nikoo S, Bozorgmehr M, Nikougoftar M, Zarnani AH.Immunosuppressive effect of pregnant mouse serum on allostimulatory activity of dendritic cells. J Reprod Immunol 2007; 75: 23–31.. [DOI] [PubMed] [Google Scholar]
- Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ.Reciprocal control of T helper cell and dendritic cell differentiation. Science 1999; 283: 1183–1186.. [DOI] [PubMed] [Google Scholar]
- Piccioli D, Sbrana S, Melandri E, Valiante NM.Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med 2002; 195: 335–341.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW, Chun GT, Kim NS, Yie SW, Byeon WH, Eom SH, Ha KS, Kim YM, Kim PH.Mechanisms underlying TGF-beta1-induced expression of VEGF and Flk-1 in mouse macrophages and their implications for angiogenesis. J Leukoc Biol 2007; 81: 557–566.. [DOI] [PubMed] [Google Scholar]
- Tayade C, Hilchie D, He H, Fang Y, Moons L, Carmeliet P, Foster RA, Croy BA.Genetic deletion of placenta growth factor in mice alters uterine NK cells. J Immunol 2007; 178: 4267–4275.. [DOI] [PubMed] [Google Scholar]
- Adini A, Kornaga T, Firoozbakht F, Benjamin LE.Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res 2002; 62: 2749–2752.. [PubMed] [Google Scholar]
- Hubbard NE, Lim D, Mukutmoni M, Cai A, Erickson KL.Expression and regulation of murine macrophage angiopoietin-2. Cell Immunol 2005; 234: 102–109.. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Greer P, Auerbach R.Isolation and propagation of yolk-sac-derived endothelial cells from a hypervascular transgenic mouse expressing a gain-of-function fps/fes proto-oncogene. In Vitro Cell Dev Biol Anim 1996; 32: 292–299.. [DOI] [PubMed] [Google Scholar]
- Shao L, Allez M, Park MS, Mayer L.Immunomodulatory roles of the carcinoembryonic antigen family of glycoproteins. Ann N Y Acad Sci 2006; 1072: 194–209.. [DOI] [PubMed] [Google Scholar]
- Horst AK, Ito WD, Dabelstein J, Schumacher U, Sander H, Turbide C, Brummer J, Meinertz T, Beauchemin N, Wagener C.Carcinoembryonic antigen-related cell adhesion molecule 1 modulates vascular remodeling in vitro and in vivo. J Clin Invest 2006; 116: 1596–1605.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic N, Oliveira-Ferrer L, Wurmbach JH, Loges S, Chalajour F, Neshat-Vahid S, Weil J, Fernando M, Ergun S.Pro-angiogenic signaling by the endothelial presence of CEACAM1. J Biol Chem 2005; 280: 2361–2369.. [DOI] [PubMed] [Google Scholar]
- Markel G, Wolf D, Hanna J, Gazit R, Goldman-Wohl D, Lavy Y, Yagel S, Mandelboim O.Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J Clin Invest 2002; 110: 943–953.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano K, Ebisawa M, Hase K, Fukuda S, Hijikata A, Kawano S, Date Y, Tsuneda S, Itoh K, Ohno H.Psg18 is specifically expressed in follicle-associated epithelium. Cell Struct Funct 2007; 32: 115–126.. [DOI] [PubMed] [Google Scholar]
- Burrows TD, King A, Loke YW.Expression of adhesion molecules by endovascular trophoblast and decidual endothelial cells: implications for vascular invasion during implantation. Placenta 1994; 15: 21–33.. [DOI] [PubMed] [Google Scholar]
- Graham CH, Lysiak JJ, McCrae KR, Lala PK.Localization of transforming growth factor-beta at the human fetal-maternal interface: role in trophoblast growth and differentiation. Biol Reprod 1992; 46: 561–572.. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Meadows SK, Wira CR, Sentman CL.Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-beta. J Leukoc Biol 2004; 76: 667–675.. [DOI] [PubMed] [Google Scholar]
- Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL.TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci U S A 2007; 104: 3378–3383.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP.Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 1996; 2: 1096–1103.. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang P, Zhou H, Meng Q, Huang X.Involvement of Foxp3-expressing CD4+ CD25+ regulatory T cells in the development of tolerance induced by transforming growth factor-beta2-treated antigen-presenting cells. Immunology 2008; 124: 304–314.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Chen YG.Controlling TGF-beta signaling. Genes Dev 2000; 14: 627–644.. [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I.Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 2000; 14: 163–176.. [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12: 1065–1074.. [DOI] [PubMed] [Google Scholar]
- Bersinger NA, Odegard RA.Second- and third-trimester serum levels of placental proteins in preeclampsia and small-for-gestational-age pregnancies. Acta Obstet Gynecol Scand 2004; 83: 37–45.. [PubMed] [Google Scholar]
- Demir R, Kayisli UA, Cayli S, Huppertz B.Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta 2006; 27: 535–539.. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Shibuya M, Wennstrom S.Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res 2004; 299: 476–485.. [DOI] [PubMed] [Google Scholar]