Abstract
We present a comprehensive analysis of ADP-glucose pyrophosphorylase (AGP)-repressed pea (Pisum sativum) seeds using transcript and metabolite profiling to monitor the effects that reduced carbon flow into starch has on carbon-nitrogen metabolism and related pathways. Changed patterns of transcripts and metabolites suggest that AGP repression causes sugar accumulation and stimulates carbohydrate oxidation via glycolysis, tricarboxylic acid cycle, and mitochondrial respiration. Enhanced provision of precursors such as acetyl-coenzyme A and organic acids apparently support other pathways and activate amino acid and storage protein biosynthesis as well as pathways fed by cytosolic acetyl-coenzyme A, such as cysteine biosynthesis and fatty acid elongation/metabolism. As a consequence, the resulting higher nitrogen (N) demand depletes transient N storage pools, specifically asparagine and arginine, and leads to N limitation. Moreover, increased sugar accumulation appears to stimulate cytokinin-mediated cell proliferation pathways. In addition, the deregulation of starch biosynthesis resulted in indirect changes, such as increased mitochondrial metabolism and osmotic stress. The combined effect of these changes is an enhanced generation of reactive oxygen species coupled with an up-regulation of energy-dissipating, reactive oxygen species protection, and defense genes. Transcriptional activation of mitogen-activated protein kinase pathways and oxylipin synthesis indicates an additional activation of stress signaling pathways. AGP-repressed embryos contain higher levels of jasmonate derivatives; however, this increase is preferentially in nonactive forms. The results suggest that, although metabolic/osmotic alterations in iAGP pea seeds result in multiple stress responses, pea seeds have effective mechanisms to circumvent stress signaling under conditions in which excessive stress responses and/or cellular damage could prematurely initiate senescence or apoptosis.
The plastidial ADP-Glc pyrophosphorylase (AGP) catalyzes the conversion of Glc-1-P and ATP to inorganic pyrophosphate and ADP-Glc, the substrate for starch synthase, and is a key regulatory enzyme of starch biosynthesis (Preiss et al., 1991; Tetlow et al., 2004). AGP mutants in maize (Zea mays) and pea (Pisum sativum) show dramatic decreases in seed starch content (Dickinson and Preiss, 1969; Smith et al., 1989). However, unlike in leaves, flux control coefficients for AGP in legume seeds are below 0.1 (Denyer et al., 1995; Rolletschek et al., 2002), indicating a low level of control for carbon (C) flux into starch. Seed starch biosynthesis mutants often accumulate soluble sugars instead of starch, leading to specific metabolic adjustments, altered water uptake, wrinkled phenotypes, and increased protein contents (Perez et al., 1993; Casey et al., 1998). This metabolic shift could be of biotechnological importance in legumes, in which protein is the more valuable seed compound. The higher protein content is, at least in part, due to the increased availability of C acceptors in the form of organic acids (Miflin and Lea, 1977), which indicates general C limitation for amino acid/seed protein synthesis (Weigelt et al., 2008). A key enzyme connecting C and nitrogen (N) metabolism is phosphoenolpyruvate carboxylase (Golombek et al., 2001). Its overexpression in Vicia narbonensis seeds channels more C into organic acids, stimulates amino acid biosynthesis, and increases protein content (Rolletschek et al., 2004; Radchuk et al., 2007). Accumulation of Suc, as shown for AGP-repressed Vicia (Weber et al., 2000; Rolletschek et al., 2002) and maize (Cossegal et al., 2008) seeds, causes repartitioning of C into glycolysis, tricarboxylic acid (TCA) cycle, and amino acid biosynthesis.
Apart from its metabolic role, Suc acts as a signal molecule regulating gene expression and seed maturation (Borisjuk et al., 2002; Weber et al., 2005; Osuna et al., 2007). In maturing seeds, Suc is present at high concentrations, promotes cell enlargement and endopolyploidization, and induces starch and protein storage in pea (Wang and Hedley, 1993), Vicia faba (Barratt and Pullen, 1984; Weber et al., 1996), and wheat (Triticum aestivum; Jenner et al., 1991).
Pea seeds overexpressing a Suc transporter show stimulated storage protein biosynthesis at the level of transcripts, proteins, and protein bodies. It has been concluded that Suc functions as both a signal and a fuel to stimulate storage protein accumulation (Rosche et al., 2002, 2005).
In addition, Suc signaling has been demonstrated to interact with that of abscisic acid (ABA; Finkelstein et al., 2002). Possibly, Suc increases ABA sensitivity or levels (Smeekens, 2000) or ABA modulates the response to sugar signals. Several ABA biosynthesis (aba) and ABA-insensitive (abi) mutants are also sugar-sensing mutants, indicating that sugar signaling requires the ABA transduction chain (Rook et al., 2001). ABA action in seeds may involve SnRK1 kinases (Radchuk et al., 2006; Rolland et al., 2006). Decreased ABA levels affect the proper metabolism of Suc in SnRK1-repressed pea (Radchuk et al., 2006; R. Radchuk, R.J.N. Emery, and H. Weber, unpublished data).
While Suc limitation is detrimental to the plant, an excess of sugar can lead to stress responses caused directly by the sugar source itself, by its metabolism (Price et al., 2004), or via indirect effects such as increased water uptake and hyperosmotic stress (Rolletschek et al., 2002). Such stress conditions can initiate complex multiple responses involving hormonal, metabolic, and transcriptional changes (Price et al., 2004; Sanchez et al., 2008; Shulaev et al., 2008).
The aim of this study was to analyze the effects of reduced C flux into starch on seed metabolic pathways by in-depth phenotypic analysis of pea seeds with reduced AGP using a combination of transcript and metabolite profiling. The results indicate that decreasing the C flux through the starch pathway, on the one hand, leads to repartitioning of C into alternative pathways and stimulates storage protein synthesis, but, on the other hand, produces a number of pleiotropic, mainly stress-induced, effects as a consequence of increased sugar accumulation and metabolism. The findings in iAGP-3 seeds highlight the importance of a balanced C-N status for proper seed development and further gives insight into the sugar-mediated regulation of seed maturation. Potential abilities and strategies of legume seeds are discovered to cope with detrimental stress situations. Thus, seed models like the iAGP-3 line with altered pathways contribute substantially to a better understanding of “normal” seed metabolism and toward a more profound knowledge of seed biochemistry.
RESULTS
Generation of AGP-Deficient Pea Lines by RNA Interference
Three homozygous pea lines (iAGP-1, iAGP-2, and iAGP-3) containing single inserts of the AGP-RNA interference (RNAi; small subunit) construct were selected by Southern-blot and segregation analyses. Supplemental Figure S1 and Supplemental Table S1 show that the lines behave identically. Northern-blot analysis performed on embryos at 15, 20, 25, 30, and 35 d after pollination (DAP) revealed strongly decreased AGP mRNA levels and AGP activity from 20 DAP onward. Starch levels between 20 and 35 DAP were only reduced by approximately 50% (Supplemental Fig. S1), indicating a low control coefficient for pea seed AGP on starch synthesis, in accordance with what has already been reported for AGP-reduced Vicia seeds (Weber et al., 2000). In transgenic seeds, mRNA levels of the major storage protein vicilin were increased after 20 DAP, whereas those of legumin decreased to 40% of the wild-type level (Supplemental Fig. S1, D and E). Supplemental Table S1 presents the mature seed composition of all three lines. The wrinkled seed phenotypes are shown in Supplemental Figure S2.
AGP-Deficient Pea Seeds Reveal a Shift in Seed Composition
Analysis of seed composition in mature dry iAGP-3 seeds revealed more total N, C, albumins, globulins, lipids, and Suc per gram dry weight. Seed starch was reduced by 40% to 50% of wild-type levels, and the residual starch was altered in composition, containing considerably less amylose. Seed dry weight was decreased by approximately 20% (Table I).
Table I.
Compositional analysis of mature iAGP-3 seeds
Values represent means ± sd (n = 10). Significant increases compared with the wild type are designated as follows: b, P ≤ 0.01; c, P ≤ 0.001. Significant decreases compared with the wild type are designated as follows: x, P ≤ 0.05; z, P ≤ 0.001.
| Component | iAGP-3 | Wild Type |
|---|---|---|
| Total N (%) | 4.29 ± 0.05 c | 2.87 ± 0.018 |
| Total C (%) | 44.17 ± 0.092 b | 42.77 ± 0.053 |
| Albumins (mg g−1) | 24.1 ± 1.6 c | 19.1 ± 1.02 |
| Globulins (mg g−1) | 64.5 ± 8.2 b | 51.6 ± 2.7 |
| Starch (mg g−1) | 216.8 ± 4.3 z | 393.3 ± 3.8 |
| Suc (mg g−1) | 109 ± 23 c | 71.5 ± 5.4 |
| Lipids (mg g−1) | 57.6 ± 2.8 c | 40.1 ± 0.99 |
| Amylose (mg g−1) | 32.7 ± 0.28 z | 43.3 ± 0.2 |
| Seed weight (mg) | 302 ± 16 x | 345 ± 42 |
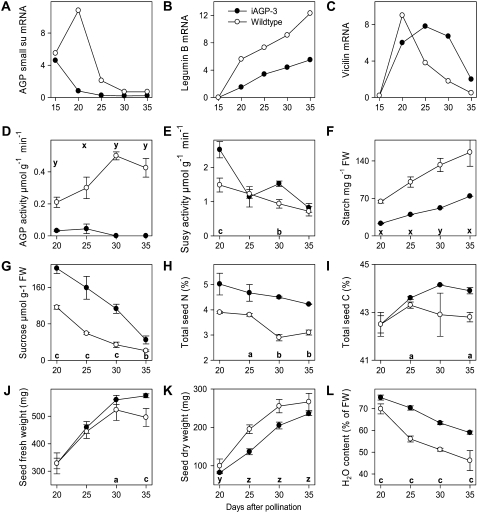
Analysis of seed composition and developmental parameters was performed at 20, 25, 30, and 35 DAP. AGP mRNA was lower at all stages analyzed (Fig. 1A), legumin B mRNA was lower between 20 and 35 DAP (Fig. 1B), vicilin mRNA was higher between 25 and 35 DAP (Fig. 1C), AGP activity was lower at all stages (Fig. 1D), whereas Suc synthase activity was higher at 20 and 30 DAP (Fig. 1E). Starch accumulation was lower, reaching only 40% to 50% of wild-type levels (Fig. 1F); however, iAGP-3 seeds exhibited 50% higher Suc levels at all stages (Fig. 1G). Total seed N and C percentages were higher at 25, 30, and 35 DAP (Fig. 1, H and I). Seed fresh weight was higher at 30 and 35 DAP (Fig. 1J), whereas seed dry weight was decreased at all stages (Fig. 1K). Consistently, iAGP-3 seeds have higher water content at all stages (Fig. 1L), most likely due to the higher sugar content.
Figure 1.
Compositional analysis of maturing iAGP-3 embryos (black circles) compared with the wild type (white circles). A, AGP mRNA. B, Legumin B mRNA. C, Vicilin mRNA. D, AGP activity. E, Suc synthase activity. F, Starch. G, Suc. H, Total seed N. I, Total seed C. J, Seed fresh weight (FW). K, Seed dry weight. L, Seed water content. Data points are means from four replicates ± se. Significantly higher levels according to t test are as follows: a, P < 0.05; b, P < 0.01; c, P < 0.001. Significantly lower levels according to t test are as follows: x, P < 0.05; y, P < 0.01; z, P < 0.001.
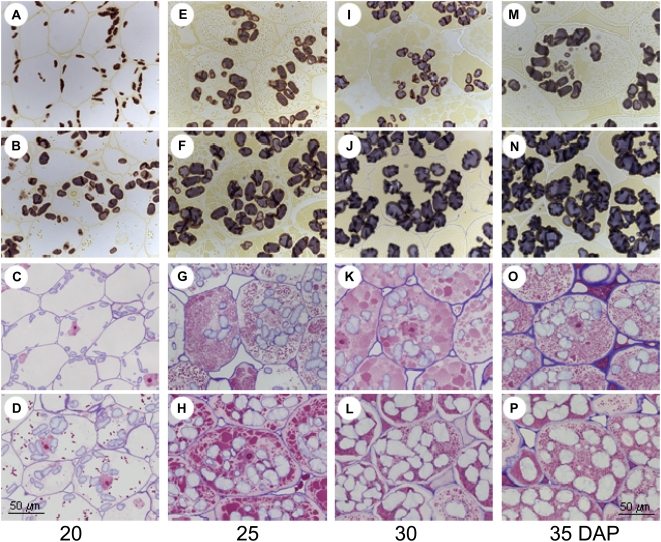
AGP-Deficient Pea Embryos Reveal Altered Cell Morphology
Following staining for starch and proteins, cell morphology was analyzed by light microscopy in iAGP-3 and wild-type embryos at 20, 25, 30, and 35 DAP. At 20 DAP, transgenic cells contain elongated starch grains that are tiny (Fig. 2A) compared with the wild type (Fig. 2B). Almost no storage protein vacuoles are visible in iAGP-3 cells (Fig. 2C) compared with the wild type (Fig. 2D). At 25 DAP, the transgenic cells contain fewer and smaller starch grains (Fig. 2, E and F), whereas storage protein vesicles are larger but less densely filled (Fig. 2, G and H). At 30 DAP, starch grains are again less numerous in iAGP-3 cells and exhibit light brown color (Fig. 2I), whereas wild-type grains are larger and of dark blue color (Fig. 2J). Moreover, in transgenic cells, storage vesicles are larger and appear to be less dense (Fig. 2K) than the wild type (Fig. 2L). A similar pattern with regard to grain size and color between transgenic (Fig. 2M) and wild-type (Fig. 2N) cells occurs at 35 DAP. However, there is no difference in the size of the storage protein vacuoles at this time point, although they appear to be less densely packed in iAGP-3 cells (Fig. 2O) than in the wild type (Fig. 2P).
Figure 2.
Histology of iAGP-3 embryos at 20 DAP (A and C), 25 DAP (E and G), 30 DAP (I and K), and 35 DAP (M and O) in comparison with the wild-type control at 20 DAP (B and D), 25 DAP (F and H), 30 DAP (J and L), and 35 DAP (N and P).
Transcript Profiling of iAGP-3 Embryos
We analyzed differential gene expression between phytochamber-grown embryos at 20, 25, 30, and 35 DAP using microarrays enriched in seed-expressed genes (Weigelt et al., 2008). Transcript levels are given as ratios between wild-type and iAGP-3 embryos (fold up- or down-regulated). Genes were regarded as differentially expressed if they varied by a factor of at least 2.0-fold with statistical differences (P < 0.05). Transcript abundances do not necessarily reflect transcriptional activity, protein content, or enzyme activity; therefore, all of the following statements on gene identity and function have to be considered as “putative.” For reasons of simplicity, higher or lower transcript levels are referred to as down- or up-regulated. A total of 203, 395, 300, and 205 genes were up-regulated (3.9%, 7.5%, 5.7%, and 3.9% from 5,246) at 20, 25, 30, and 35 DAP, respectively. From these, 87, 122, 99, and 71 genes (corresponding to 43%, 31%, 33%, and 35% of up-regulated genes in the respective stages) showed no homology to annotated sequences. A total of 119, 173, 207, and 157 genes were down-regulated (2.3%, 3.3%, 3.7%, and 3.0% of total) at 20, 25, 30, and 35 DAP, respectively. From these, 47, 72, 90, and 60 genes (corresponding to 39%, 42%, 43%, and 60% of down-regulated genes in the respective stages) showed no homology to annotated sequences. The number of differentially expressed genes is highest at 25 and 30 DAP and lowest at 20 and 35 DAP. Two- to 3-fold more genes are up-regulated than down-regulated. Genes with known annotation were assembled into 10 functional groups: metabolism, transport, protein processing, protein turnover, cell proliferation, storage, stress and disease response, signaling, photosynthesis, and transcription and translation (Supplemental Fig. S3; Supplemental Table S2). The largest group of genes up-regulated at 25 DAP (116) and 30 DAP (54) belongs to the transcription and translation group and encodes ribosomal proteins, histones, translation initiation factors, and RNA-processing enzymes. In comparison, only three genes are down-regulated at 25 DAP.
AGP Repression Affects Carbohydrate Metabolism in Different Compartments
Transcriptional profiling revealed 32 differentially expressed genes encoding carbohydrate-metabolizing enzymes targeted to cytosol, plastid, or mitochondrion (Supplemental Table S3). Cytosolic up-regulation involves interconversion of Suc and hexose-P, namely by Suc-P synthase, Suc phosphatase, Suc synthase-1, UDP-Glc pyrophosphorylase, P-glucomutase (PGM), and P-glucoisomerase. In contrast, neutral and vacuolar invertases and Suc synthase-3 were down-regulated. Pea possesses at least three Suc synthase genes differing in expression, kinetic properties, and regulation. Isoform 1 accounts for more than 90% of embryonic activity (Barratt et al., 2001). Glycolytic genes encoding enolase and gyceraldehyde-3-phosphate dehydrogenase (GAPDH) were up-regulated, whereas Fru-P2 aldolase and P-glycerate mutase were down-regulated. Two up-regulated genes encode subunits A and B of ATP-citrate lyase, which generates cytosolic pools of acetyl-CoA, a key precursor for many pathways, including lipid biosynthesis (Fatland et al., 2000). Plastidial GAPDH, PGM, Fru-P2 aldolase, and NADP-Mal-DH were up-regulated, whereas Pyr kinase and 6-P-gluconate-DH in plastids were down-regulated. As expected, AGP small subunit was down-regulated at all stages together with large subunit at 25 DAP. Some up-regulated genes are involved in starch synthesis (granule-bound starch synthase-1, branching and debranching enzymes). A β-amylase gene was down-regulated at 25 and 35 DAP (Supplemental Table S4). Mitochondrial genes encoding Pyr-DH isoforms E1α and E1β, Pyr-DH E2, and 2-oxoglutarate-DH were up-regulated as well as a nucleoside sugar hydratase putatively involved in the cleavage of reactive nucleoside diphosphate sugars (Muñoz et al., 2006).
AGP Repression Stimulates Amino Acid Metabolism and Storage Protein Synthesis
Eighteen genes involved in amino acid metabolism were differentially expressed (Supplemental Table S4), with only two being down-regulated, 3-isopropylmalate dehydratase and cytosolic Gln synthetase-1 (GS-1), which has been shown to be ammonia responsive (Ishiyama et al., 2004). Three up-regulated genes were involved in aromatic amino acid metabolism, 3-dehydroquinate synthase, indole-3-glycerol phosphate synthase, and fumarylacetoacetase, the latter of which catalyzes Phe degradation. In addition, asparaginase, putatively involved in N mobilization (Murray and Kennedy, 1980), was transcriptionally up-regulated at all four stages. Its product Asp can be converted to Glu via Asp transaminase, which was also up-regulated. Two genes encoding Arg biosynthesis enzymes were up-regulated, argininosuccinate synthase and the bifunctional Orn acetyltransferase/N-acetylglutamate synthase, which is involved in Orn production and the recycling of acetyl groups during Arg biosynthesis (Slocum, 2005). Two genes involved in the Asp branch were also up-regulated, ketol acid reductoisomerase and Met synthase. Expression of several genes of Ser/Gly and Cys metabolism was activated. Thr aldolase converts Thr into Ser and acetaldehyde (Joshi et al., 2006) and plastidial phosphoserine phosphatase, catalyzing the terminal step in Ser biosynthesis. Ser acetyltransferases and the plastidial O-acetyl-Ser(thiol)-lyase are both involved in Cys biosynthesis.
Although iAGP seeds have higher globulin contents, vicilin and legumin transcripts respond differently. Six and nine sequences encoding vicilins and vicilin-like USP proteins were up-regulated. Six legumin genes were down-regulated. This is also confirmed by RNA-blot analysis (Supplemental Fig. S1). One albumin and one glycinin gene were up-regulated, whereas two albumins were down-regulated (Supplemental Table S2).
AGP Repression Affects Transport-Related Gene Expression
Several genes encoding plastid-localized transport proteins were up-regulated in the iAGP-3 seeds, including Glc-6-P translocator, ADP/ATP translocator, ATP-synthetase-δ, and three inner membrane transport proteins. In contrast, a Glc transporter and several zinc transporter genes were down-regulated as well as two genes encoding isoforms of OEP16, a plastidial outer membrane amino acid-selective channel (Pohlmeyer et al., 1997), four genes encoding inner membrane translocase proteins, and two PIP-type aquaporins reported as turgor responsive (Guerrero et al., 1990; Supplemental Table S2). Up-regulated genes encoding mitochondrial proteins include two subunits of complex 1 of NADH-ubiquinone oxidoreductase, mitochondrial uncoupling protein, and three subunits of ATP synthase. Other up-regulated transport-related genes encode monosaccharide and peptide transporters and two subunits of plasma membrane-located H+-ATPases.
AGP Repression Up-Regulates Cytokinin Levels and Gene Expression Related to Cell Proliferation
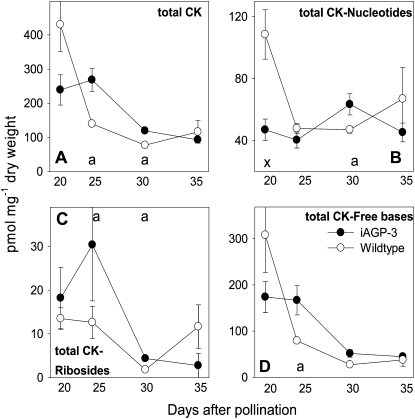
Twenty-three up-regulated genes encode proteins involved in cell proliferation (Supplemental Table S5), six annexins, four tubulins, two tubulin-specific chaperones, two actins and two actin-binding factors, two cell division control proteins, and one prohibitin, which is involved in mitochondrial biogenesis and maintenance (Ahn et al., 2006). Two genes encoding cyclin B and cyclin D3, demonstrated to be Suc responsive (Riou-Khamlichi et al., 2000), were up-regulated. Direct measurement of cytokinin (CK) levels revealed higher total levels of CKs in the iAGP-3 seeds at 25 and 30 DAP (Fig. 3A). However, the total amount of CK nucleotides, which represent precursors or reversible conjugates of the active forms of CK, is lower in transgenic seeds at 20 DAP and elevated at 30 DAP (Fig. 3B). In contrast, the most active isoforms, CK ribosides and CK free bases, are increased at 25 and 30 and at 25 DAP, respectively (Fig. 3, C and D).
Figure 3.
CK content of iAGP-3 embryos. A, Total CKs. B, Total CK nucleotides. C, Total CK ribosides. D, Total CK free bases. Data points are means from three to four replicates ± se. Significantly higher levels according to t test are follows: a, P < 0.05. Significantly lower levels according to t test are as follows: x, P < 0.05.
AGP Repression Alters Gene Expression Related to Hormonal and Stress Signaling
Three up-regulated genes in iAGP-3 seeds are involved in oxylipin/jasmonate metabolism, namely allene oxide cyclase, 12-oxophytodienoic acid (OPDA) reductase, and S-adenosyl-l- Met:jasmonic acid (JA) carboxyl methyltransferase. Two genes associated with GA metabolism were up-regulated, ent-kaurenoic acid oxidase and GA-regulated protein 1. In accordance with this, one member of the GRAS/DELLA/GAI transcription factor family, which is thought to repress transcription of GA-inducible genes, was down-regulated at all four stages.
The A-type response regulator gene ARR5, probably acting in a negative feedback loop of CK signaling (Hwang and Sheen, 2001), was transcriptionally up-regulated. The auxin-responsive protein IAA8, a negative regulator of auxin (Dreher et al., 2006), was also up-regulated, whereas IAA-amido synthetase, involved in IAA homeostasis, was down-regulated. Genes encoding two Myb factors, a WRKY factor and a FUS3-like transcription factor, were up-regulated, whereas Lec-1 was down-regulated. Finally, changes in key gene expression of ABA signaling seemed to be functionally consistent with the negative regulation of ABA. Phosphatase 2A, a negative ABA regulator, was up-regulated, whereas 9-cis-epoxycarotenoid dioxygenase (NCED-9), encoding a key ABA biosynthesis gene, was down-regulated (Supplemental Table S6).
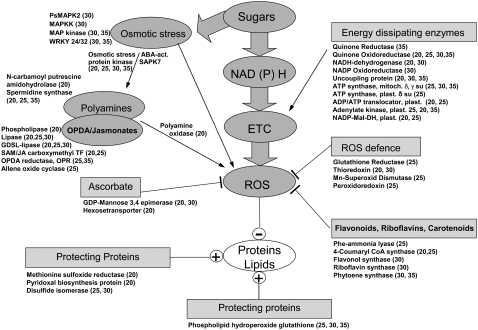
iAGP-3 seeds displayed up-regulation of gene expression related to stress responses, with 24 genes encoding protein kinases/phosphatases differentially expressed. Up-regulated gene expression is related to osmotic stress signaling (SAPK Ser/Thr kinase, mitogen-activated protein [MAP] kinase, MAP kinase kinase, Ca-dependent protein kinase, and WRKY transcription factor), JA/oxylipin metabolism (Supplemental Table S7), energy dissipation within mitochondria, ROS defense, ascorbate synthesis, flavonoid and riboflavin synthesis, protein and lipid protection, peroxidation, proteolysis, and chlorophyll degradation. A set of genes related to abiotic and biotic stress were variously up- and down-regulated (Supplemental Table S2; see Fig. 11 below).
Figure 11.
Summary of multiple stress responses on the basis of changed gene expression in AGP-repressed embryos. For more details, see text.
AGP Repression Shifts the Levels of Certain Amino Acids
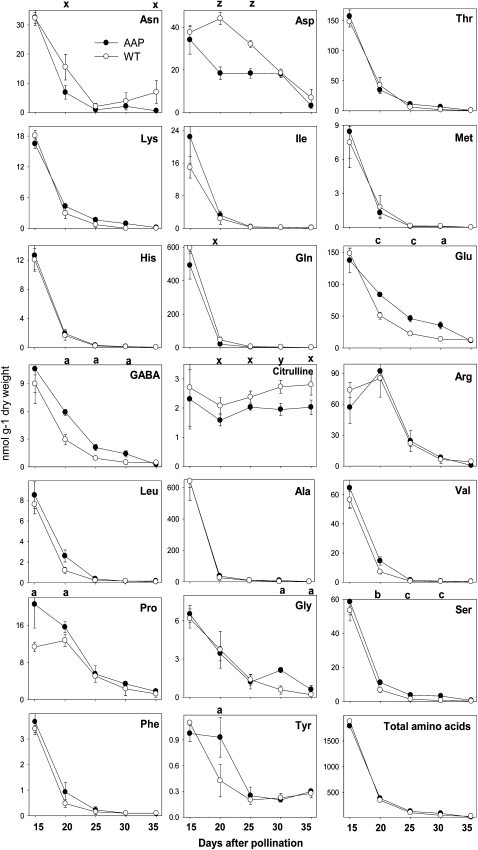
Individual amino acids and the related metabolites GABA and citrulline were analyzed by HPLC from greenhouse-grown iAGP-3 and wild-type embryos. Levels were highest at earlier (15 DAP) and lower at later maturation, with the most abundant amino acids being Ala, Gln, Glu, Thr, Arg, Val, and Ser. In iAGP-3 embryos, some amino acids were present at higher levels, namely Glu (20, 25, and 30 DAP), GABA (20, 25, and 30 DAP), Gly (30 and 35 DAP), Ser (20, 25, and 30 DAP), Pro (15 and 20 DAP), and Tyr (20 DAP). Others were present at lower levels, namely Asn (20 and 35 DAP), Asp (20 and 25 DAP), Gln (20 DAP), and citrulline (20, 25, 30, and 35 DAP). Despite major changes in the pattern, the sum of total amino acids was unaltered (Fig. 4).
Figure 4.
Levels of free amino acids in iAGP-3 and wild-type embryos during maturation. Data points are means from four replicates ± se. Significantly higher levels according to t test are as follows: a, P < 0.05; b, P < 0.01; c, P < 0.001. Significantly lower levels according to t test are as follows: x, P < 0.05; y, P < 0.01; z, P < 0.001.
Metabolic Profiling of AGP-3 Embryos
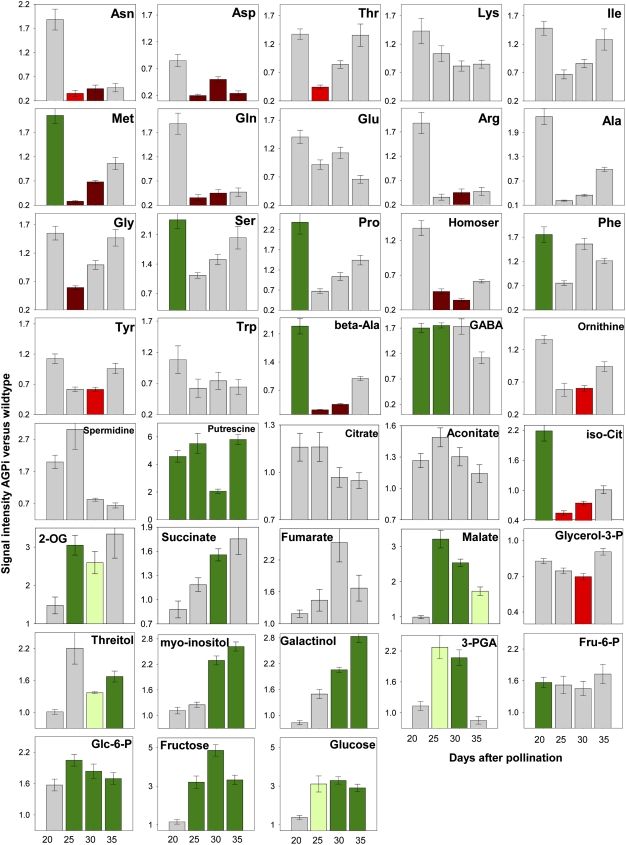
Metabolic profiling was done using gas chromatography-mass spectrometry analysis on growth chamber-grown embryos at 20, 25, 30, and 35 DAP (Fig. 5). Significantly increased amino acids were Met (20 DAP), Ala (20 DAP), Ser (20 DAP), Pro (20 DAP), Phe (20 DAP), and GABA (20 and 25 DAP). Lower levels were found for Asn (25 and 30 DAP), Asp (25, 30, and 35 DAP), Thr (25 DAP), Met (25 and 30 DAP), Gln (25 and 30 DAP), Arg (30 DAP), Gly (25 DAP), Homoser (25 and 30 DAP), Tyr (30 DAP), and Orn (30 DAP). Lys, Ile, Glu, and Trp were unchanged. Putrescine was largely increased at all stages, and spermidine was higher at 20 and 25 DAP, albeit not significantly. The changes were accompanied by up-regulated gene expression related to polyamine synthesis, with transcripts for both N-carbamoylputrescine amidohydrolase and putrescine aminopropyltransferase 2 (spermine synthase). Levels of β-Ala displayed a contrary pattern, increasing at 20 DAP but decreasing at 25 and 30 DAP.
Figure 5.
Relative changes of metabolites in iAGP-3 embryos in comparison with the wild type as determined by gas chromatography-mass spectrometry. Values represent means ± sd (n = 6). Dark green bars, Increased at P < 0.05; light green bars, increased at P < 0.1; dark red bars, decreased at P < 0.05; light red bars, decreased at P < 0.1.
Levels of sugars and sugar alcohols were increased, namely Glc (25, 30, and 35 DAP), Fru (25, 30, and 35 DAP), Glc-6-P (25, 30, and 35 DAP), Fru-6-P (20 DAP), myoinositol (30 and 35 DAP), galactinol (30 and 35 DAP), and threitol (30 and 35 DAP). Organic acids were increased, namely malate (25, 30, and 35 DAP), 2-oxoglutarate (25 and 30 DAP), succinate (30 DAP), and isocitrate (20 DAP), with decreases of isocitrate at 25 and 30 DAP.
AGP Repression Leads to Changes in JA Metabolites and Hydrogen Peroxide Content
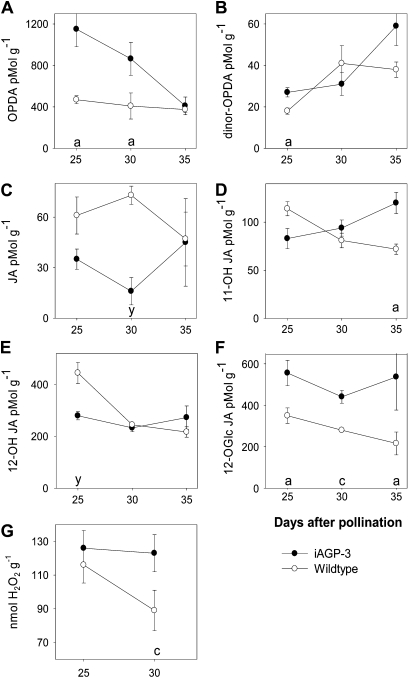
To analyze whether a possible stress situation within the transgenic seeds results in the synthesis of specific signaling substances, we measured OPDA, JA, some of its metabolites, and hydrogen peroxide (H2O2) at 25, 30, and 35 DAP in iAGP-3 and wild-type embryos. OPDA and dinor-OPDA were increased at 25, 30, and 35 DAP (Fig. 6, A and B). JA was significantly lower in iAGP-3 seeds at 30 DAP (Fig. 6C), whereas the hydroxylation product 11-OH-JA was increased at 35 DAP and 12-OH-JA was decreased 25 DAP (Fig. 6, D and E). Remarkably, levels of 12-O-Glc-JA were higher at all stages (Fig. 6F). Hydroxylation and O-glycosylation of JAs may represent an off switch of JA signaling (Miersch et al., 2008). Measurements of H2O2 revealed higher levels in iAGP-3 embryos at 30 DAP (Fig. 6G).
Figure 6.
Levels of OPDA, jasmonate derivatives, and H2O2 in iAGP-3 seeds. A, OPDA. B, Dinor-OPDA. C, JA. D, 11-OH-JA. E, 12-OH-JA. F, 12-O-Glc-JA. G, H2O2. Data points are means from four replicates ± se. Significantly higher levels according to t test are as follows: a, P < 0.05; c, P < 0.001. Significantly lower levels according to t test are as follows: y, P < 0.01.
Levels of ABA were measured in three independent batches of seeds, but always with high variations and no agreement between repetitions (data not shown).
DISCUSSION
In order to analyze seed storage metabolism and pathway interactions, we adopted a combinatorial transcriptome and metabolite profiling study of AGP-repressed pea seeds. Moderately decreasing starch levels led to increased carbohydrate oxidation as well as osmotic effects, including higher seed fresh weight and water content, increased vacuolization, delayed cellular differentiation, and wrinkled seed shape. The severe phenotype reveals that the metabolic change results in strong primary and pleiotropic effects that ultimately influencing seed metabolism and morphology as well as potentially affecting seed persistence. We identified and, at least partially, dissected strategies by which seeds maximize their potential to cope with adverse conditions.
AGP-Repressed Pea Seeds Have Higher Protein Content but Lower Seed Weight
Increasing seed proteins at the expense of starch might enhance levels of more valuable feed and food materials and is interesting from a biotechnological perspective. Thus, a metabolic shift from starch to protein could be biotechnologically important in legume seeds, in which protein is the more valuable compound. However, our results show that iAGP seeds display a potential yield penalty in combination with possible harmful stress situations. Therefore, a strategy to simply repress starch synthesis in order to increase the levels of pea seed protein has to cope with multiple and unexpected negative effects.
AGP-repressed pea seeds exhibit metabolic shifts in composition from starch toward the accumulation of sugars, lipids, and proteins, a phenotype already reported for AGP-repressed Vicia seeds (Weber et al., 2000; Rolletschek et al., 2002) and rb/rr pea mutants (Denyer et al., 1995). However, in iAGP-3 seeds, higher N-C ratios and N contents were partially compensated by reduced individual seed weight. In contrast to AGP-deficient Vicia seeds (Weber et al., 2000), seed fill duration was apparently unchanged in the transgenic plants described here, and, as a consequence of lower dry weight accumulation, final seed weight was decreased whereas the seed number per plant was unaltered (data not shown).
AGP Repression Stimulates Carbohydrate Metabolism
Transcriptional and metabolic changes indicate that sugar accumulation due to AGP repression stimulates carbohydrate degradation via glycolysis, the TCA cycle, and the mitochondrial respiration chain as well as providing precursors such as acetyl-CoA and organic acids for other processes.
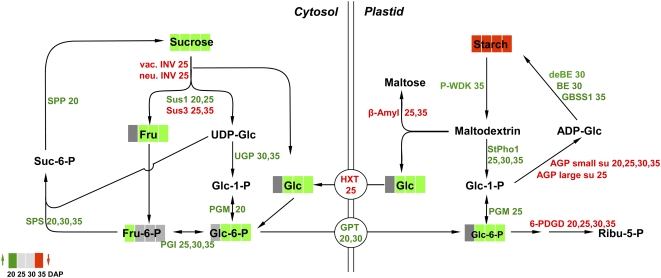
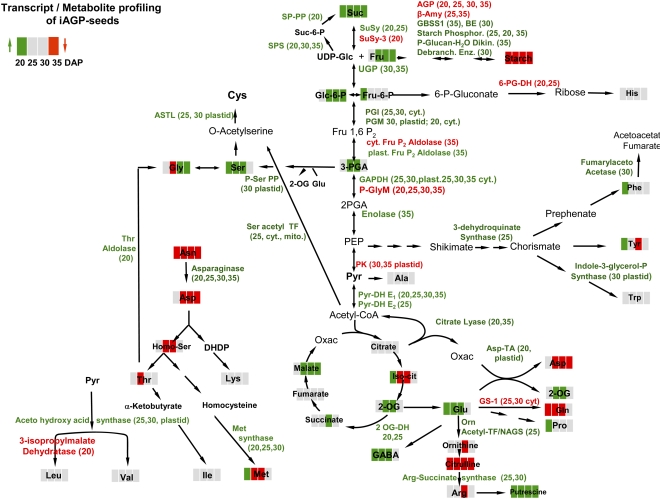
Higher levels of sugars and their phosphorylated intermediates indicate specific modulations in carbohydrate metabolism within cytosol, plastids, and mitochondria. In the cytoplasm, up-regulated expression of Suc synthase-1, SPS, SPP, UGP, PGM, and PGI suggest activated cycling of hexose-P and Suc. Such metabolic phenomena have been commonly observed in plant cells, where the consumption and/or production of ATP (Alonso et al., 2005) can be offset against the change of expression and/or activity of common respiratory enzymes. Vacuolar and neutral invertases were down-regulated, potentially to keep free hexoses low, which avoids hexose-mediated sugar sensing (Koch, 2004). AGP repression lowers ADP-Glc levels (Rolletschek et al., 2002), which may initiate compensatory pathways toward Glc-1-P production, such as P-glucan-water-dikinase and starch phosphorylase. Gene expression of β-amylase and a plastidial hexose transporter was down-regulated, suggesting that such compensation only occurs in the phosphorolytic pathway. Decreased starch levels may activate the expression of biosynthetic enzymes in order to compensate for this, such as plastidial Glc-6-P translocator, plastidial PGM, branching and debranching enzymes, and GBSS1. The reaction scheme in Figure 7 integrates transcript and metabolite changes associated with cytosolic Suc/hexose-P interconversion and plastidial starch metabolism.
Figure 7.
Summary of transcript and metabolite changes associated with cytosolic Suc/hexose-P interconversion and plastidial starch metabolism in iAGP-3 embryos. Red, Down-regulated in iAGP-3 embryos with respect to the wild type; green, up-regulated in iAGP-3 embryos with respect to the wild type. Numbers 20, 25, 30, and 35 refer to DAP.
Major changes from the combined transcriptome and metabolic profiling of iAGP-3 compared with wild-type seeds are summarized in Figure 8. Transcriptional up-regulation of cytosolic (enolase, GAPDH) and mitochondrial (Pyr-DH E1 and E2, 2-OG-DH) enzymes indicate activated sugar metabolization via glycolysis and the TCA cycle, an assumption supported by increased levels of 3-PGA, malate, 2-OG, and succinate. Citrate is not increased, possibly due to stimulated export from mitochondria and further metabolism via citrate lyase (up-regulated at 20 and 30 DAP), which yields cytosolic acetyl-CoA (Fatland et al., 2005) and oxalacetate, the substrate for Asp-transaminase (up-regulated at 20 DAP). Acetyl-CoA is a substrate for fatty acid elongation. In this respect, it is indicative that long-chain fatty acid-CoA ligase is up-regulated at 25 DAP, together with increased levels of total fatty acids (Table I). Cytosolic acetyl-CoA is also required for Cys biosynthesis by cytosolic and mitochondrial Ser-acetyl-transferase (both up-regulated at 25 DAP) and plastidial O-acetlyserine-thio-lyase (up-regulated at 25 and 30 DAP).
Figure 8.
Summary of changed transcript and metabolite levels in iAGP-3 embryos. Data are derived from Supplemental Figure S1, Supplemental Tables S2 to S6 (transcripts), and Figure 7 (metabolites). See Figure 7 legend for additional details.
Stimulation of carbohydrate oxidation in mitochondria is indicated by up-regulation of two subunits of NADH ubiquinone oxidoreductase, three subunits of F0F1-ATP synthase, and mitochondrial uncoupling protein involved in energy dissipation (Pastore et al., 2007).
Increased Availability of C Activates Amino Acid and Storage Protein Synthesis
Analysis of iAGP-3 seeds along with other seed models (for review, see Weigelt et al., 2008; Weber et al., 2009) reveals that legume seeds are potentially C and N limited with respect to storage protein synthesis. However, the comprehensive analysis of metabolic alterations reveals specific mechanisms that can be activated to adjust the C-N ratio, indicating that C-N balances, rather than levels of N or C per se, are important for efficient seed maturation.
Seventeen genes involved in amino acid synthesis are up-regulated. Several use metabolites from glycolysis and the TCA cycle, namely acetohydroxy acid synthase (Pyr), P-Ser phosphatase (3-PGA), cytosolic and mitochondrial isoforms of Ser acetyl transferase (acetyl-CoA), and Asp-transaminase (oxalacetate). Such stimulation is not reflected in the free amino acid level, which is unchanged, but rather by an altered pattern. Pro and GABA are increased (15 and 20 DAP), possibly in response to osmotic stress (Verbruggen and Hermans, 2008). Ser/Gly, Phe, Tyr, and Glu are slightly increased, whereas Gln, Arg, Orn, citrulline, Asn, and its follow-up products Asp/Homoser/Thr/Met are decreased. Asn and Gln are mainly delivered from the phloem (Macnicol, 1977). Its lower levels indicate either stimulated metabolism in sink organs or decreased rates of import. Down-regulation of GS-1, known to be ammonia responsive (Ishiyama et al., 2004), supports this view. In pea seeds, Asn serves as transient N storage and accumulates under surplus N conditions and/or carbohydrate limitation due to Asn synthetase 1 induction, as shown in pea seeds overexpressing an amino acid permease (Weigelt et al., 2008). The iAGP seeds have lower Asn levels and display Asn mobilization, as evidenced by up-regulated asparaginase at all stages. Arg represents another transient N storage, which normally accumulates during maturation (Micallef and Shelp, 1989; Slocum, 2005). Thus, lower levels of the “N storage amino acids” Asn and Arg indicate increased demand for N, possibly caused by stimulated storage protein synthesis. Because free amino acids are unchanged, the increased total N content is probably due to higher levels of globulins and albumins, which is supported by the observed up-regulation of gene expression related to storage protein synthesis, such as transcription/translation, endomembrane transport, and protein processing, especially at 25 and 30 DAP. Gene expression is stimulated for vicilins rather than legumins. Accordingly, AGP-repressed Vicia seeds have lower legumin-vicilin ratios (Rolletschek et al., 2002). In wrinkled pea mutants, legumin mRNA stability is more sensitive toward high Suc levels (Turner et al., 1990). In contrast, carboxylase-overexpressing seeds with lower sugar levels (Rolletschek et al., 2004), due to the additional C sink, show mainly up-regulated legumin rather than vicilin transcription, suggesting fundamental differences in their (metabolic) regulation.
Apparently, blocking seed starch synthesis increases the C state and hence activates amino acid and storage protein synthesis, which leads to higher N demand. Similarly, in carboxylase-overexpressing seeds with increased anaplerotic C fluxes, an improved organic acid supply can also stimulate amino acid biosynthesis (Rolletschek et al., 2004; Radchuk et al., 2007). Metabolic changes in iAGP seeds are somewhat contrary to those observed by overexpressing an amino acid transporter (Weigelt et al., 2008). In this study, increased amino acids similarly stimulate storage protein synthesis but surplus N is channeled into the transient storage pools Asp and Arg. Moreover, increased C demand, due to the elevated N-activated C limitation response pathways such as amino acid catabolism in mitochondria, demonstrates the importance of properly maintaining N-C ratios during seed maturation.
iAGP Seeds Display a Signaling Network of Sugars and Hormonal Signals
Metabolic alterations in iAPG-3 seeds cause sugar accumulation. Such signals are apparently counteracted by pathways involving ABA, SnRK1, and Tre-6-P. In addition, sugar availability, besides its nutrient role, also has a signaling function to stimulate cell proliferation pathways mediated by CKs (Hartig and Beck, 2006).
Activation of amino acid and storage protein synthesis is metabolically regulated and stimulated by carbohydrate feeding and/or supply of C precursors (Osuna et al., 2007; Radchuk et al., 2007; this work). Suc in seeds can also signal storage-associated processes (Weber et al., 2005), a function interacting with ABA signaling. The hormone is necessary to metabolize Suc or modulate the response to sugar signals (Smeekens, 2000; Finkelstein et al., 2002). Pea embryos with decreased ABA levels and/or sensitivity have lower sink strength with impaired conversion of Suc into storage products (Radchuk et al., 2006; R. Radchuk, U. Conrad, I. Saalbach, and H. Weber, unpublished data). In iAGP-3 seeds, ABA functions seem to be down-regulated on transcriptional levels. PPC2A, a negative regulator of ABA, is up-regulated, and NCED-9, the major ABA biosynthesis enzyme, is down-regulated. However, no consistency was observed for ABA levels in three independent experiments. Because metabolic signaling can interfere with osmotic stress, it is difficult to predict the significance of ABA levels/functions. Down-regulated ABA functions at the transcript level may modulate the effects from elevated Suc. This is supported by down-regulated Tre-6-P synthase (30 DAP), which may respond to an increased sugar state. In Arabidopsis (Arabidopsis thaliana), TPS mRNA is rapidly decreased upon Suc feeding (Usadel et al., 2008). A SnRK1 subunit, possibly involved in regulating Suc metabolism, is also down-regulated at 20 DAP.
Increased sugar availability in iAGP-3 seeds may stimulate cell proliferation, as indicated by the up-regulation of 23 related genes, alongside the observation that most active CKs were increased. d-Cyclins (up-regulated at 30 DAP) are inducible by sugar and/or CKs and integrate hormonal and nutritional signals (Gaudin et al., 2000; Riou-Khamlichi et al., 2000; Dewitte et al., 2007). In yeast, activities of mcm proteins (up-regulated at 35 DAP), initiating DNA replication, can be modulated by altering glycolysis (Chen and Tye, 1995). On the other hand, nutrient starvation diminishes mitotic activity in pea root tips (Van't Hof, 1966), producing cell cycle arrest (Van't Hof et al., 1973).
AGP-Repressed Seeds Show Multiple Stress Responses
In iAGP seeds, metabolic/osmotic alterations potentially lead to multiple stress responses, such as ROS production and polyamine and oxylipin synthesis. However, pea seeds have specific and effective mechanism to switch off stress signaling, such as the JA signal, in order to prevent cell damage or excessive up-regulation of stress-related gene expression, which could finally initiate senescence and apoptotic pathways (Puppo et al., 2005; Miao and Zentgraf, 2007).
Sugar accumulation and increased metabolism within the cytosol and by plastidial glycolysis and mitochondria may cause overreduced NAD(P)H and ubiquinone pools, with potential harmful consequences such as ROS production (Moller, 2001; Pastore et al., 2007). These observations probably explain the increased H2O2 levels at 30 DAP.
Transcript profiling of iAGP-3 seeds revealed many up-regulated genes associated with protective functions in plastids: increased ATP synthesis (F0F1-ATP synthase δ, up-regulated at 25 DAP), ATP export (ADP/ATP translocator, up-regulated at 20 and 25 DAP), elevated export of reductants via the NADP-Mal-DH-mediated malate valve (up-regulated at 20 and 25 DAP), an indirect export system to balance ATP-NADPH ratios in plastids (Scheibe et al., 2005), control of substrate flux and NADPH production within the oxidative pentose-P cycle by down-regulation of 6-P-gluconate-DH (20 and 25 DAP), and adenine nucleotide equilibration by up-regulation of adenylate kinase (25, 30, and 35 DAP), a key enzyme of energy metabolism (Pradet and Raymond, 1983; Regierer et al., 2002).
Plant mitochondria are well suited to oxidize cytosolic pyruvate and NAD(P)H and integrate plastidial and cytosolic metabolism (Sweetlove et al., 2007). Up-regulated subunits of complex 1 NADH-ubiquinone oxidoreductase and F0F1-ATP synthase subunits β, γ, and δ (20, 25, 30, and 35 DAP) indicate increased mitochondrial electron transport chain (ETC) activity. NADP dehydrogenase (30 DAP) adjusts NAD(P)/NAD(P)H homeostasis and uncoupling protein (20, 30, and 35 DAP) depolarizes membrane potential, suggesting activated mitochondrial energy dissipation in response to overreduced ETC.
Increased oxidative activity, as an unavoidable consequence, produces ROS (Moller, 2001). Hyperosmotic stress, apparently occurring in iAGP seeds, represents another signal, potentially transmitted by specific kinases such as SAPK7, an osmotic stress/ABA-induced Ser/Thr kinase (Kelner et al., 2004), and three MAP kinases, which together with 10 other kinases/phosphatases are up-regulated. Their potential involvement in abiotic stress signaling is well known (Zhu, 2002; Chinnusamy et al., 2004).
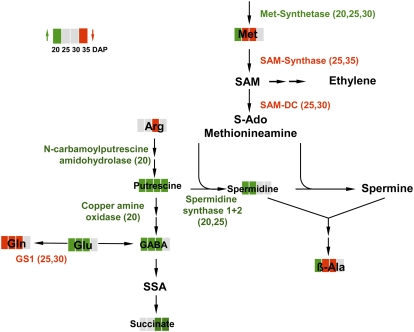
Putrescine and spermidine levels are increased, whereas Arg is decreased. This suggests putrescine synthesis from Arg via N-carbamoylputrescine amidohydrolase (up-regulated at 20 DAP) and spermidine via spermidine synthase 1 and 2 (up-regulated at 20 and 25 DAP). Putrescine might be degraded by oxidation to GABA (increased at 20, 25, and 30 DAP) via copper amine oxidase (up-regulated at 20 DAP). This reaction releases ROS (Cona et al., 2006), indicating that osmotic stress in iAGP seeds generates ROS via putrescine metabolism. Similarly, osmotic stress increases polyamine contents in Lupinus seedlings (Legocka and Kluk, 2005). The reaction scheme in Figure 9 summarizes transcript and metabolite levels associated with polyamine metabolism.
Figure 9.
Summary of changed transcript and metabolite levels associated with polyamine metabolism that appears to be initiated in iAGP-3 embryos. See Figure 7 legend for additional details.
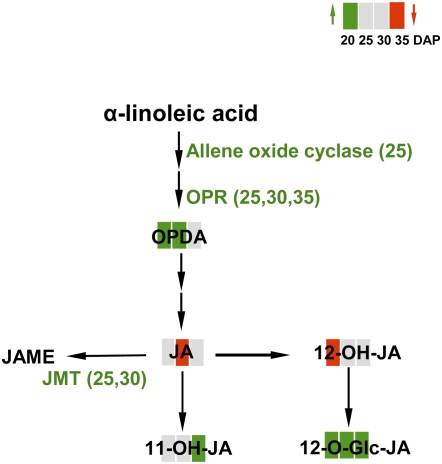
Plants respond to osmotic stress and wounding by the generation of lipid-derived signals such as JAs, its derivatives, and its precursor OPDA (Wasternack, 2007; Glauser et al., 2008; Miersch et al., 2008). Figure 10 summarizes changes of JA and its metabolites and related transcripts. OPDA accumulates and is obviously synthesized from α-linolenic acid via allene oxide cyclase and OPDA reductase (up-regulated at 25 and 25 DAP and at 30 and 35 DAP, respectively). However, JA itself is decreased; instead, the hydroxylated products 11-OH-JA and 12-O-Glc-JA were elevated. Many biotic and abiotic stress genes are only inducible by JA/JA methyl ester but not by 12-OH-JA or 12-O-Glc-JA. Thus, hydroxylation and metabolism of 12-OH-JA leads to a fine-tuning of JA-dependent gene expression and can down-regulate JA-specific responses (Miersch et al., 2008).
Figure 10.
Summary of changed levels of JA and its metabolites and of transcripts involved in JA biosynthesis and metabolism as changed in iAGP-3 embryos. See Figure 7 legend for additional details.
The generation of excess ROS in iAGP embryos can have detrimental effects and may damage proteins and other macromolecules (Bailly, 2004). Protective actions prevent the overreduction of mitochondrial ETC by energy dissipation strategies or the synthesis of compounds to oxidize ROS, such as ascorbate. GDP-Man 3,4-epimerase (Wheeler et al., 1998), which is also inducible by jasmonates (Wolucka et al., 2005), is transcriptionally up-regulated (20 and 30 DAP). Other up-regulated genes are involved in ROS defense, such as glutathione reductase (25 DAP), thioredoxin (20 and 30 DAP), manganese-superoxide dismutase (25 DAP), and peroxidoredoxin (25 DAP), or in the production of flavonoids, riboflavins, and carotenoids such as Phe ammonia lyase (25 DAP), coumaryl-CoA synthase (20 and 25 DAP), flavonol synthase (30 DAP), riboflavin synthase (30 DAP), and phytoene synthase (30 and 35 DAP). Figure 11 depicts the multiple stress responses on the basis of changed gene expression that appears to operate in AGP-repressed seeds.
MATERIALS AND METHODS
Plant Material and Transformation
Pea plants (Pisum sativum ‘Eiffel’) were grown in 2-L pots in growth chambers under a light/dark regime of 16 h (20°C)/8 h (18°C) or alternatively in greenhouses during the spring/summer season of 2007 without additional light or temperature regulation (Weigelt et al., 2008). A 550-bp fragment of pea AGP small subunit (accession no. X96765.1; Burgess et al., 1997) was amplified by reverse transcription-PCR from pea cotyledon RNA (Heim et al., 1993; primers 5′-TGTTGAAGTTCTTGCTGCT-3′ and 5′-CAATGTCTTCCCAGTAGCC-3′) to design a hairpin construct according to Chen et al. (2003). The construct was fused to the LeB4 promoter (Bäumlein et al., 1992), inserted into PZP 200, and introduced into pea by Argobacterium tumefaciens-mediated gene transfer (Rolletschek et al., 2005). For metabolite measurements and enzyme assays of embryos, pods were tagged according to DAP, collected in the middle of the light phase, and snap frozen in liquid N.
RNA Isolation and Hybridization Techniques
Nucleic acids were isolated and northern hybridization was performed as described by Heim et al. (1993). The Psagps1 cDNA fragment was used as a probe after labeling with [32P]dCTP as described by Miranda et al. (2001).
Determination of Suc, Starch, Globulins/Albumins, Total C and N, and Total Lipids, and Enzyme Assay
Extraction and determination of Suc, starch, and globulins/albumins was as described by Rolletschek et al. (2002). Total lipids were measured by a gravimetric method according to Rolletschek et al. (2002). Relative contents of total C and N in dried, powdered samples were measured using an elemental analyzer (Vario EL; Elementaranalysensysteme). Suc synthase activity was performed as described by Heim et al. (1993). Statistical analysis was done using Student's t test using SigmaStat software (SPSS; http://www.systat.de).
Ps6kOLI1 Microarrays, Microarray Hybridizations, and Data Evaluation
Microarray hybridization and data analysis were performed as described by Weigelt et al. (2008) and Hohnjec et al. (2005) with three independent replicates. Image processing data were evaluated using EMMA 2.0 software (Dondrup et al., 2003). Lowess normalization was performed for each microarray hybridization using a floor value of 20 and Student's t test to identify differentially expressed genes (P < 0.05). Transcriptome profile data sets can be viewed at http://www.ebi.ac.uk/arrayexpress/ under ArrayExpress accession number E-MEXP-1749 and experiment name ihAGPase_pea seed.
Metabolite, Hormone, and H2O2 Analysis
Amino acid analysis by HPLC and metabolite analysis by gas chromatography-mass spectrometry were carried out as described previously (Lisec et al., 2006; Weigelt et al., 2008). CKs were measured using the protocol of Götz et al. (2007). OPDA and jasmonate derivatives were measured as described by Miersch et al. (2008). H2O2 was quantified using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (A22188; Molecular Probes).
Histological Analysis
Seeds were fixed in 2% glutaraldehyde, 2% formaldehyde, and 50 mm phosphate buffer, pH 7.0, for 16 h. After three 15-min washes, samples were dehydrated in a graded ethanol series, embedded in Spurr's low-viscosity resin, sectioned (700 nm) on a Reichert-Jung Ultracut S (Leica), and stained with basic fuchsin followed by brief counterstaining with crystal violet or stained with iodine to visualize starch. Digital recordings were made with a Zeiss Axiovert microscope equipped with an Axiocam (Carl Zeiss).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Characterization of iAGP pea lines.
Supplemental Figure S2. Phenotypes of mature dry seeds of iAGP-1, -2, and -3 and wild-type plants.
Supplemental Figure S3. Numbers of genes up- or down-regulated (numbers at top and bottom, respectively) in iAGP-3 embryos at 20, 25, 30, and 35 DAP.
Supplemental Table S1. Seed compositional analysis of mature iAGP seeds ± sd grown in the phytochamber: significantly increased compared with wild type, a ≤ 0.05, b ≤ 0.01, c ≤ 0.001; significantly decreased compared with wild type, x ≤ 0.05, y ≤ 0.01, z ≤ 0.001.
Supplemental Table S2. Genes up- and down-regulated in iAGP-3 embryos at 20, 25, 30, and 35 DAP.
Supplemental Table S3. Carbohydrate metabolism for up-regulated genes (boldface) and down-regulated genes (italics) in iAGP-3 seeds in comparison with the wild type (ratios are significant at P < 0.05).
Supplemental Table S4. Amino acid metabolism for up-regulated genes (boldface) and down-regulated genes (italics) in iAGP-3 seeds in comparison with the wild type (ratios are significant at P < 0.05).
Supplemental Table S5. Cell proliferation for up-regulated genes (boldface) and down-regulated genes (italics) in iAGP-3 seeds in comparison with the wild type (ratios are significant at P < 0.05).
Supplemental Table S6. Hormonal and signaling functions for up-regulated genes (boldface) and down-regulated genes (italics) in iAGP-3 seeds in comparison with the wild type (ratios are significant at P < 0.05).
Supplemental Table S7. Protein kinases/phosphatases for up-regulated genes (boldface) and down-regulated genes (italics) in iAGP-3 seeds in comparison with the wild type (ratios are significant at P < 0.05).
Supplementary Material
Acknowledgments
We are grateful to Petra Hoffmeister, Katrin Blaschek, and Susanne Knüpffer for excellent technical assistance, to Ursula Tiemann and Karin Lipfert for figure artwork, and to Manuela Meyer for performing Ps6kOLI1 microarray hybridizations. We thank Winfriede Weschke for discussions, continuous support, and critically reading the manuscript.
This work was supported by the European Union (GRAIN LEGUMES Integrated Project), the Deutsche Forschungsgemeinschaft (grant no. WE 1641/9–1), and the Sachsen-Anhalt (Innoplanta).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hans Weber (weber@ipk-gatersleben.de).
The online version of this article contains Web-only data.
References
- Ahn CS, Lee JH, Reum Hwang A, Kim WT, Pai HS (2006) Prohibitin is involved in mitochondrial biogenesis in plants. Plant J 46 658–667 [DOI] [PubMed] [Google Scholar]
- Alonso AP, Vigeolas H, Raymond P, Rolin D, Dieuaide-Noubhani M (2005) A new substrate cycle in plants: evidence for a high glucose-phosphate-to-glucose turnover from in vivo steady-state and pulse-labeling experiments with [13C]glucose and [14C]glucose. Plant Physiol 138 2220–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14 93–107 [Google Scholar]
- Barratt DH, Barber L, Kruger NJ, Smith AM, Wang TL, Martin C (2001) Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol 127 655–664 [PMC free article] [PubMed] [Google Scholar]
- Barratt DHP, Pullen A (1984) Control of seed protein accumulation in field bean. Ann Bot (Lond) 54 31–38 [Google Scholar]
- Bäumlein H, Nagy I, Villarroel R, Inzé D, Wobus U (1992) Cis-analysis of a seed protein gene promoter: The conservative RY repeat CATGCATG within the legumin box is essential for tissue-specific expression of a legumin gene. Plant J 2 233–239 [PubMed] [Google Scholar]
- Borisjuk L, Walenta S, Rolletschek H, Mueller-Klieser W, Wobus U, Weber H (2002) Spatial analysis of plant development: sucrose imaging within Vicia faba cotyledons reveals specific developmental patterns. Plant J 29 521–530 [DOI] [PubMed] [Google Scholar]
- Burgess D, Penton A, Dunsmuir P, Dooner H (1997) Molecular cloning and characterization of ADP-glucose pyrophosphorylase cDNA clones isolated from pea cotyledons. Plant Mol Biol 33 431–444 [DOI] [PubMed] [Google Scholar]
- Casey R, Domoney C, Forster C, Hedley C, Hitchin E, Wang T (1998) The effect of modifying carbohydrate metabolism on seed protein gene expression in peas. J Plant Physiol 152 636–640 [Google Scholar]
- Chen S, Hofius D, Sonnewald U, Börnke F (2003) Temporal and spatial control of gene silencing in transgenic plants by inducible expression of double-stranded RNA. Plant J 36 731–740 [DOI] [PubMed] [Google Scholar]
- Chen Y, Tye BK (1995) The yeast Mcm1 protein is regulated posttranscriptionally by the flux of glycolysis. Mol Cell Biol 15 4631–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Schumaker K, Zhu JK (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot 55 225–236 [DOI] [PubMed] [Google Scholar]
- Cona A, Rea G, Angelini R, Federico R, Tavladoraki P (2006) Functions of amine oxidases in plant development and defence. Trends Plant Sci 11 80–88 [DOI] [PubMed] [Google Scholar]
- Cossegal M, Chambrier P, Mbelo S, Balzergue S, Martin-Magniette ML, Moing A, Deborde C, Guyon V, Perez P, Rogowsky P (2008) Transcriptional and metabolic adjustments in ADP-glucose pyrophosphorylase-deficient bt2 maize kernels. Plant Physiol 146 1553–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer K, Foster J, Smith AM (1995) The contributions of ADP-glucose pyrophosphorylase and starch-branching enzyme to the control of starch synthesis in developing pea embryos. Planta 197 57–62 [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, Maughan SC, Menges M, Braun N, Collins C, Nieuwland J, Prinsen E, Sundaresan V, et al (2007) Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci USA 104 14537–14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DB, Preiss J (1969) Presence of ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize endosperm. Plant Physiol 44 1058–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondrup M, Goesmann A, Bartels D, Kalinowski J, Krause L, Linke B, Rupp O, Sczyrba A, Pühler A, Meyer F (2003) EMMA: a platform for consistent storage and efficient analysis of microarray data. J Biotechnol 106 135–146 [DOI] [PubMed] [Google Scholar]
- Dreher KA, Brown J, Saw RE, Callis J (2006) The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell 18 699–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatland BL, Ke J, Anderson MD, Mentzen WI, Cui LW, Allred CC, Johnston JL, Nikolau BJ, Wurtele ES (2000) Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-CoA in Arabidopsis. Plant Physiol 130 740–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatland BL, Nikolau BJ, Wurtele ES (2005) Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis. Plant Cell 17 182–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin V, Lunness PA, Fobert PR, Towers M, Riou-Khamlichi C, Murray JA, Coen E, Doonan JH (2000) The expression of D-cyclin genes defines distinct developmental zones in snapdragon apical meristems and is locally regulated by the Cycloidea gene. Plant Physiol 122 1137–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Grata E, Dubugnon L, Rudaz S, Farmer EE, Wolfender JL (2008) Spatial and temporal dynamics of jasmonate synthesis and accumulation in Arabidopsis in response to wounding. J Biol Chem 283 16400–16407 [DOI] [PubMed] [Google Scholar]
- Golombek S, Rolletschek H, Wobus U, Weber H (2001) Control of storage protein accumulation during legume seed development. J Plant Physiol 158 457–464 [Google Scholar]
- Götz KP, Staroske N, Radchuk R, Emery RJN, Wutzke KD, Herzog H, Weber H (2007) Uptake and allocation of carbon and nitrogen in Vicia narbonensis plants with increased seed sink strength achieved by seed-specific expression of an amino acid permease. J Exp Bot 58 3183–3195 [DOI] [PubMed] [Google Scholar]
- Guerrero FD, Jennifer T, Jones J, Mullet JE (1990) Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted: sequence and expression of three inducible genes. Plant Mol Biol 15 11–26 [DOI] [PubMed] [Google Scholar]
- Hartig K, Beck E (2006) Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biol 8 389–396 [DOI] [PubMed] [Google Scholar]
- Heim U, Weber H, Bäumlein H, Wobus U (1993) A sucrose-synthase gene of Vicia faba L.: expression pattern in developing seeds in relation to starch synthesis and metabolic regulation. Planta 191 394–401 [DOI] [PubMed] [Google Scholar]
- Hohnjec N, Vieweg MF, Pühler A, Becker A, Küster H (2005) Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different Glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza. Plant Physiol 137 1283–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sheen J (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413 383–389 [DOI] [PubMed] [Google Scholar]
- Ishiyama K, Inoue E, Watanabe-Takahashi A, Obara M, Yamaya T, Takahashi H (2004) Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in Arabidopsis. J Biol Chem 279 16598–16605 [DOI] [PubMed] [Google Scholar]
- Jenner CF, Ugalde TD, Aspinall D (1991) The physiology of starch and protein deposition in the endosperm of wheat. Aust J Plant Physiol 18 211–226 [Google Scholar]
- Joshi V, Laubengayer KM, Schauer N, Fernie AR, Jander G (2006) Two Arabidopsis threonine aldolases are nonredundant and compete with threonine deaminase for a common substrate pool. Plant Cell 18 3564–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner A, Pekala I, Kaczanowski S, Muszynska G, Hardie DG, Dobrowolska G (2004) Biochemical characterization of the tobacco 42-kD protein kinase activated by osmotic stress. Plant Physiol 136 3255–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7 235–246 [DOI] [PubMed] [Google Scholar]
- Legocka J, Kluk A (2005) Effect of salt and osmotic stress on changes in polyamine content and arginine decarboxylase activity in Lupinus luteus seedlings. J Plant Physiol 162 662–668 [DOI] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry based metabolite profiling in plants. Nat Protocols 1 387–396 [DOI] [PubMed] [Google Scholar]
- Macnicol PK (1977) Synthesis and interconversion of amino acids in developing cotyledons of pea (Pisum sativum L.). Plant Physiol 60 344–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Zentgraf U (2007) The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 19 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef BJ, Shelp BJ (1989) Arginine metabolism in developing soybean cotyledons. III. Utilization. Plant Physiol 91 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C (2008) Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol 177 114–127 [DOI] [PubMed] [Google Scholar]
- Miflin BJ, Lea PJ (1977) Amino acid metabolism. Annu Rev Plant Physiol 28 299–329 [Google Scholar]
- Miranda M, Borisjuk L, Tewes A, Heim U, Sauer N, Wobus U, Weber H (2001) Amino acid permeases in developing seeds of Vicia faba L.: expression precedes storage protein synthesis and is regulated by amino acid supply. Plant J 28 61–72 [DOI] [PubMed] [Google Scholar]
- Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52 561–591 [DOI] [PubMed] [Google Scholar]
- Muñoz FJ, Baroja-Fernández E, Morán-Zorzano MT, Alonso-Casajús N, Pozueta-Romero J (2006) Cloning, expression and characterization of a nudix hydrolase that catalyzes the hydrolytic breakdown of ADP-glucose linked to starch biosynthesis in Arabidopsis thaliana. Plant Cell Physiol 47 926–934 [DOI] [PubMed] [Google Scholar]
- Murray DR, Kennedy IR (1980) Changes in activities of enzymes of nitrogen metabolism in seedcoats and cotyledons during embryo development in pea seeds. Plant Physiol 66 782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, Gibon Y, Bläsing OE, Höhne M, Günter M, Kamlage B, Trethewey R, Scheible WR, et al (2007) Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J 49 463–491 [DOI] [PubMed] [Google Scholar]
- Pastore D, Trono D, Laus MN, Di Fonzo N, Flagella Z (2007) Possible plant mitochondria involvement in cell adaptation to drought stress: a case study. Durum wheat mitochondria. J Exp Bot 58 195–210 [DOI] [PubMed] [Google Scholar]
- Perez MD, Chambers SJ, Bacon JR, Lambert N, Hedley CL, Wang T (1993) Seed protein content and composition of near-isogenic and induced mutant pea lines. Seed Sci Res 3 187–194 [Google Scholar]
- Pohlmeyer K, Soll J, Steinkamp T, Hinnah S, Wagner R (1997) Isolation and characterization of an amino acid-selective channel protein present in the chloroplastic outer envelope membrane. Proc Natl Acad Sci USA 94 9504–9509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradet A, Raymond P (1983) Adenine-nucleotide ratios and adenylate energy charge in energy metabolism. Annu Rev Plant Physiol Plant Mol Biol 34 199–224 [Google Scholar]
- Preiss J, Ball K, Smith-White B, Iglesias A, Kakefuda G, Li L (1991) Starch biosynthesis and its regulation. Biochem Soc Trans 19 539–547 [DOI] [PubMed] [Google Scholar]
- Price J, Laxmi A, St Martin SK, Jang JC (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16 2128–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Lucas MM, de Felipe MR, Harrison J, Vanacker H, Foyer CH (2005) Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165 683–701 [DOI] [PubMed] [Google Scholar]
- Radchuk R, Radchuk V, Götz K-P, Weichert H, Richter A, Emery RJN, Weschke W, Weber H (2007) Ectopic expression of PEP carboxylase in Vicia narbonensis seeds: effects of improved nutrient status on seed maturation and transcriptional regulatory networks. Plant J 51 819–839 [DOI] [PubMed] [Google Scholar]
- Radchuk R, Radchuk V, Weschke W, Borisjuk L, Weber H (2006) Repressing the expression of the sucrose nonfermenting-1-related protein kinase gene in pea embryo causes pleiotropic defects of maturation similar to an abscisic acid-insensitive phenotype. Plant Physiol 140 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regierer B, Fernie AR, Springer F, Perez-Melis A, Leisse A, Koehl K, Willmitzer L, Geigenberger P, Kossmann J (2002) Starch content and yield increase as a result of altering adenylate pools in transgenic plants. Nat Biotechnol 20 1256–1260 [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Menges M, Healy JMS, Murray JAH (2000) Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-Type cyclin gene expression. Mol Cell Biol 20 4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57 675–709 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Borisjuk L, Radchuk R, Miranda M, Heim U, Wobus U, Weber H (2004) Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in Vicia narbonensis increases protein content and improves carbon economy. Plant Biotechnol J 2 211–219 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Hajirezaei M, Wobus U, Weber H (2002) Antisense-inhibition of ADP-glucose pyrophosphorylase in Vicia narbonensis seeds increases soluble sugars, causes higher uptake of water and amino acids which leads to higher protein content. Planta 214 954–964 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Hosein F, Miranda M, Heim U, Götz KP, Schlereth A, Borisjuk L, Saalbach I, Wobus U, Weber H (2005) Ectopic expression of an amino acid transporter (VfAAP1) in seeds of V. narbonensis and P. sativum increases storage proteins. Plant Physiol 137 1236–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW (2001) Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J 26 421–433 [DOI] [PubMed] [Google Scholar]
- Rosche E, Blackmore D, Offler CE, Patrick JW (2005) Increased capacity for sucrose uptake leads to earlier onset of protein accumulation in developing pea seeds. Funct Plant Biol 32 997–1007 [DOI] [PubMed] [Google Scholar]
- Rosche E, Blackmore D, Tegeder M, Richardson T, Schroeder H, Higgins TJ, Frommer WB, Offler CE, Patrick JW (2002) Seed-specific overexpression of a potato sucrose transporter increases sucrose uptake and growth rates of developing pea cotyledons. Plant J 30 165–175 [DOI] [PubMed] [Google Scholar]
- Sanchez DH, Siahpoosh MR, Roessner U, Udvardi M, Kopka J (2008) Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol Plant 132 209–219 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56 1481–1489 [DOI] [PubMed] [Google Scholar]
- Shulaev V, Cortes D, Miller G, Mittler R (2008) Metabolomics for plant stress response. Physiol Plant 132 199–208 [DOI] [PubMed] [Google Scholar]
- Slocum RD (2005) Genes, enzymes and regulation of arginine biosynthesis in plants. Plant Physiol Biochem 43 729–745 [DOI] [PubMed] [Google Scholar]
- Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51 49–81 [DOI] [PubMed] [Google Scholar]
- Smith AM, Bettey M, Bedford ID (1989) Evidence that the rb locus alters the starch content of developing pea embryos through an effect on ADP glucose pyrophosphorylase. Plant Physiol 89 1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Fait A, Nunes-Nesi A, Williams T, Fernie AR (2007) The mitochondrion: an integration point of cellular metabolism and signalling. Crit Rev Plant Sci 26 17–43 [Google Scholar]
- Tetlow IJ, Morell MK, Emes MJ (2004) Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot 55 2131–2145 [DOI] [PubMed] [Google Scholar]
- Turner SR, Barratt DH, Casey R (1990) The effect of different alleles at the r locus on the synthesis of seed storage proteins in Pisum sativum. Plant Mol Biol 14 793–803 [DOI] [PubMed] [Google Scholar]
- Van't Hof J (1966) Experimental control of DNA synthesizing and dividing cells in excised root tips of Pisum. Am J Bot 53 970–976 [Google Scholar]
- Van't Hof J, Hoppin DP, Yagi S (1973) Cell arrest in G1 and G2 of the mitotic cycle of Vicia faba root meristems. Am J Bot 60 889–895 [Google Scholar]
- Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35 753–759 [DOI] [PubMed] [Google Scholar]
- Wang TL, Hedley CL (1993) Genetic and developmental analysis of the seed. In R Casey, DR Davies, eds, Peas: Genetics, Molecular Biology and Biochemistry. CAB International, Cambridge, UK, pp 83–120
- Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U (1996) Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Comparative analysis of a small and a large-seeded genotype. Plant J 10 823–824 [Google Scholar]
- Weber H, Borisjuk L, Wobus U (2005) Molecular physiology of legume seed development. Annu Rev Plant Biol 56 253–279 [DOI] [PubMed] [Google Scholar]
- Weber H, Radchuk R, Weigelt K, Saalbach I (2009) Changing metabolic pathways to manipulate legume seed maturation and composition. In Modification of Seed Composition to Promote Health and Nutrition. Society of Agronomy and Crop Science Society of America, Madison, WI (in press)
- Weber H, Rolletschek H, Heim U, Golombek S, Gubatz S, Wobus U (2000) Antisense inhibition of ADP-glucose pyrophosphorylase in developing seeds of Vicia narbonensis moderately decreases starch but increases protein content and affects seed maturation. Plant J 24 33–43 [DOI] [PubMed] [Google Scholar]
- Weigelt K, Küster H, Radchuk R, Müller M, Weichert H, Fait A, Fernie AR, Saalbach I, Weber H (2008) Increasing amino acid supply in pea embryos reveals specific interactions of N and C metabolism and highlights the importance of mitochondrial metabolism. Plant J 55 909–926 [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393 365–369 [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Goossens A, Inzé D (2005) Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J Exp Bot 56 2527–2538 [DOI] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M (2008) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.