Abstract
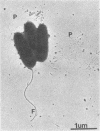
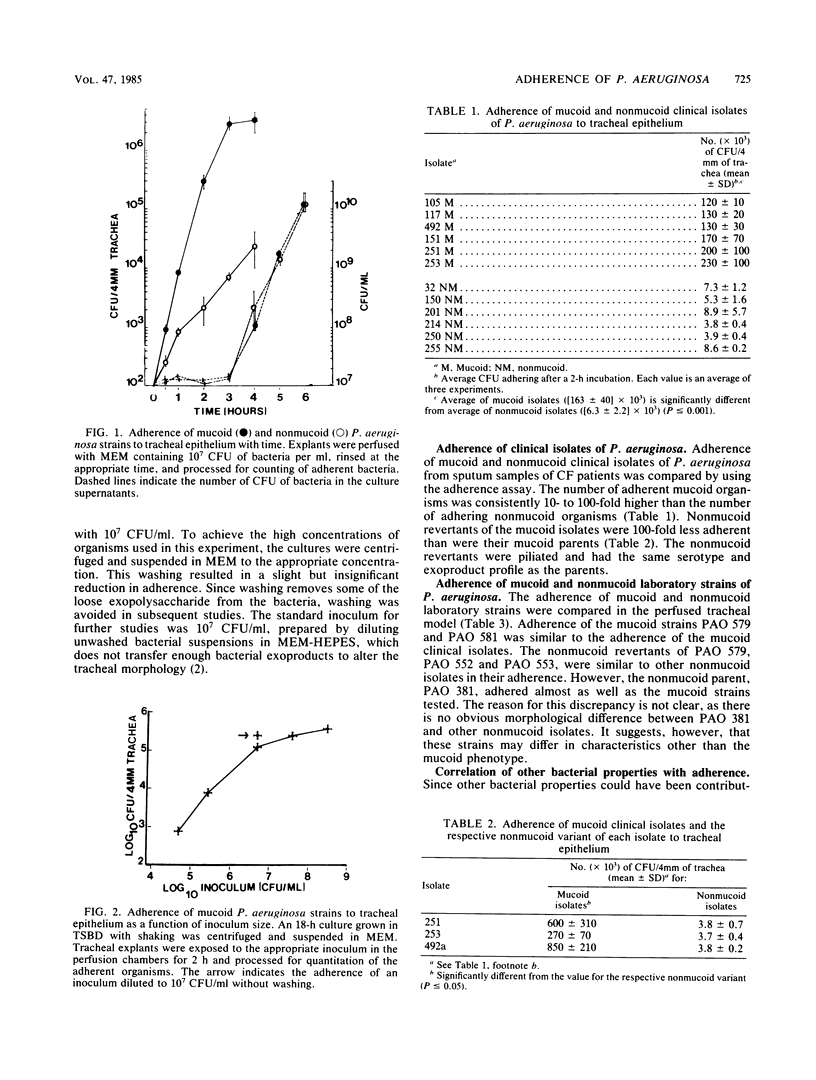
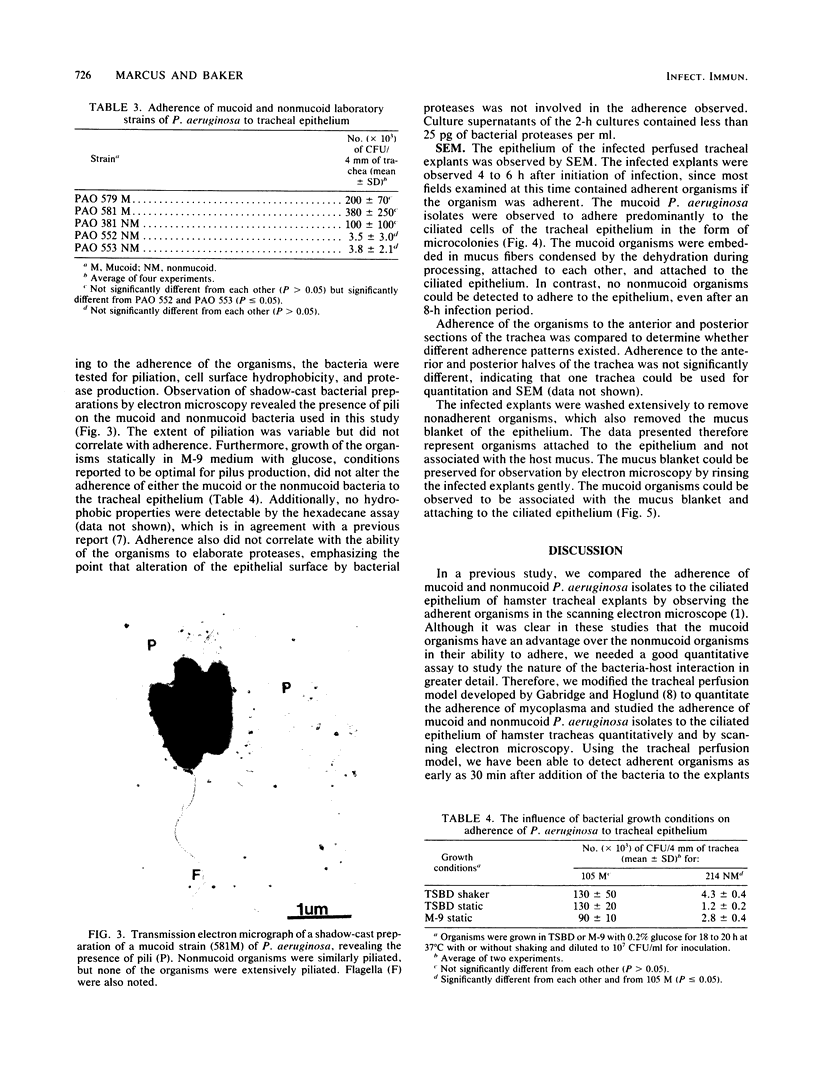
Adherence of mucoid and nonmucoid isolates of Pseudomonas aeruginosa to tracheal epithelium was quantitated by using hamster tracheas mounted in a perfusion chamber. The strains of P. aeruginosa used were clinical isolates from cystic fibrosis patients and a series of laboratory strains. Aseptically excised hamster tracheas were mounted in perfusion chambers and embedded in minimal essential medium containing 1.5% agarose. The tracheas were infected with various numbers of bacteria for various periods, rinsed, homogenized, and plated on Trypticase soy agar. A 4-mm segment from each trachea was prepared for quantitation, and the other segment was prepared for examination by scanning electron microscopy. Adherence increased with time and with increasing concentrations of inoculum. Standard conditions of inoculation were set at an inoculum of 10(7) CFU/ml and a 2-h incubation. Under these conditions, the mucoid organisms adhered to the ciliated epithelium 10- to 100-fold better than did the nonmucoid organisms. Adherence of the mucoid isolates did not appear to be pilus mediated and did not involve hydrophobic interactions. The mucoid P. aeruginosa isolates could be seen adhering to the epithelium in the form of microcolonies embedded in an extracellular matrix which attaches the organisms to the cilia and to each other. The adherence may be involved in the establishment of infection of the lungs of these patients and in the inability to clear the organisms from the lungs. The model will be useful in determining the mechanism of adherence of the bacteria to the ciliated epithelium of the respiratory tract.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. R., Tao Y. A tracheal culture model of respiratory tract infection with Pseudomonas aeruginosa. In Vitro. 1982 Apr;18(4):369–376. doi: 10.1007/BF02796337. [DOI] [PubMed] [Google Scholar]
- Blackwood L. L., Pennington J. E. Influence of mucoid coating on clearance of Pseudomonas aeruginosa from lungs. Infect Immun. 1981 May;32(2):443–448. doi: 10.1128/iai.32.2.443-448.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Hoglund L. E. Mycoplasma pneumoniae infection of intact guinea pig tracheas cultured in a unique matrix-embed/perfusion system. In Vitro. 1981 Oct;17(10):847–858. doi: 10.1007/BF02618279. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A., Baker N. R. Heterogeneity and reduction in pulmonary clearance of mucoid Pseudomonas aeruginosa. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S874–S879. doi: 10.1093/clinids/5.supplement_5.s874. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Phillippe L., Teng Tseng J., Stemke G. W., Campbell J. N. Purification and characterization of exocellular proteases produced by a clinical isolate and a laboratory strain of Pseudomonas aeruginosa. Can J Microbiol. 1980 Jan;26(1):77–86. doi: 10.1139/m80-012. [DOI] [PubMed] [Google Scholar]
- Kulczycki L. L., Murphy T. M., Bellanti J. A. Pseudomonas colonization in cystic fibrosis. A study of 160 patients. JAMA. 1978 Jul 7;240(1):30–34. [PubMed] [Google Scholar]
- Laraya-Cuasay L. R., Cundy K. R., Huang N. N. Pseudomonas carrier rates of patients with cystic fibrosis and of members of their families. J Pediatr. 1976 Jul;89(1):23–26. doi: 10.1016/s0022-3476(76)80920-1. [DOI] [PubMed] [Google Scholar]
- McArthur H. A., Ceri H. Interaction of a rat lung lectin with the exopolysaccharides of Pseudomonas aeruginosa. Infect Immun. 1983 Nov;42(2):574–578. doi: 10.1128/iai.42.2.574-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I. Identification of Pseudomonas aeruginosa in the clinical laboratory. J Med Microbiol. 1969 Feb;2(1):9–16. doi: 10.1099/00222615-2-1-9. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Adherence of mucoid and nonmucoid Pseudomonas aeruginosa to acid-injured tracheal epithelium. Infect Immun. 1983 Jul;41(1):345–351. doi: 10.1128/iai.41.1.345-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Bass J. A., Johanson W. G., Jr, Straus D. C. Role of adherence in the pathogenesis of Pseudomonas aeruginosa lung infection in cystic fibrosis patients. Infect Immun. 1980 Dec;30(3):694–699. doi: 10.1128/iai.30.3.694-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Bass J. A. Role of fibronectin in the prevention of adherence of Pseudomonas aeruginosa to buccal cells. J Infect Dis. 1981 Jun;143(6):784–790. doi: 10.1093/infdis/143.6.784. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]