The reverse genetics detection of single base changes in genes of interest, whether induced or endogenous, is an extremely powerful tool for functional genomics. Two major thrusts of research in the grasses are characterizing the function of all the genes in various genomes and then comparing those functions among species. Targeting Induced Local Lesions IN Genomes (TILLING) has proven to be a valuable methodology for finding these polymorphisms in a wide range of plant and animal species and providing mutants carrying them to the researcher. With the advent of NextGen sequencing, targeted evaluation of thousands of genes across mutagenized populations with thousands of individuals will become much more widely available, and for a wide range of grass species. Completed genome sequences from multiple grass species greatly increase the power of reverse genetic approaches to understanding gene function. Perhaps the greatest advantage is the ability to triangulate gene functions among multiple taxa such as rice (Oryza sativa), maize (Zea mays), and sorghum (Sorghum bicolor), and, together with efforts to create transposon and deletion collections in these plants, reverse genetics analysis of functionally important single nucleotide polymorphisms (SNPs) and mutations is a crucial part of the grass functional genomics toolkit.
Transposon and T-DNA approaches can be used in some species, but they are less applicable to most others, particularly grasses, where agroinfection needs to be coerced. Instead, chemical mutagenesis and TILLING have come into wide use (Comai and Henikoff, 2006), particularly for the grasses domesticated as crops. In 2002, there was a single TILLING facility in Seattle, a year later several more, and now there are over a dozen worldwide, both public and private. This marked increase in the number of TILLING projects under way, the mention of these methods and resources in grant proposals, and statements of future directions in various research communities and even the availability of commercial kits are, perhaps, the strongest pieces of evidence that the reverse genetics of point mutations is now a fixture in the genomics landscape.
At the core of each of these efforts are the creation, increase, maintenance, and distribution of large, mutagenized populations. With the exception of maize, where pollen mutagenesis is possible, these populations have been developed through seed treatment, primarily with ethyl methane sulfonate (EMS) but occasionally with the use of other chemicals such as sodium azide and/or methylnitrosourea (MNU) in species where EMS proves less effective. Typically, the approach has been to try a range of treatment severity and select the treatment that gives a desired amount (typically 30%–40%) of M2 families segregating for embryo and seedling lethality (Till et al., 2006). Where TILLING is already established for a species, new mutagenized populations can also be assessed directly by TILLING a sampling of five to 10 genes. These mutant populations have also proven to be valuable resources for forward genetic screening as well, with research communities taking advantage of the growouts for seed increase by various TILLING projects to walk fields looking for novel phenotypes of interest. In maize, mutants with altered recombination frequency (C. Weil, H. Dooner, W. Eggleston, S. Stack, and P. Schnable, unpublished data), inflorescence architecture mutants (R. Schmidt, unpublished data), mutants with altered starch digestion rates (Groth et al., 2008), and defective kernel mutants identified in forward screens of the TILLING populations are just some of those under investigation. The TILLING populations are also being screened as a whole for photosynthetic mutants and cold tolerance mutants (J. Langdale and P. Rivera, unpublished data).
As originally designed (McCallum et al., 2000), the methodology of TILLING screens pooled DNA samples taken from individuals within a mutagenized population; 8-fold pools have proven to be the most generally robust. Using informatics tools such as CODDLe (www.proweb.org/coddle/) that compare the sequence of interest against the known database for similarities, an approximately 1,500-bp region of the target sequence is identified that has the highest likelihood of producing mutations that will have detrimental effects on the gene. These predictions are based on the ability of a mutation to create either a nonsense codon, an alteration of a transcript splicing site, or a nonconservative missense mutation in a highly conserved domain of the predicted protein. The selected region (or any alternative that can be hand designated by a user of the TILLING services) is then PCR amplified from each of the pooled DNAs and screened enzymatically for the presence of mismatched bases that result if one member of a pool carries a mutation that the other members do not. Heteroduplex molecules that form at the last heating and reannealing step of the amplification can be cleaved at any mismatch by S1-family nucleases such as Cel1, found in crude celery (Apium graveolens) juice extracts (Till et al., 2004a). The cleaved products are then resolved, either by HPLC or in sequence analyzers (slab gel or capillary format), and sized. The pooling strategy allows identification of the particular mutant individual within a pool. After reamplifying the target from specific, putative mutants, it is sequenced to identify the specific base that has been altered. Information about the mutation (its position, predicted effect on the protein, and stock number for seed of the line carrying the mutation) is then returned to users of TILLING for further study and characterization.
In contrast to typical forward mutagenesis screens, TILLING populations aim for the maximum number of mutations per plant that still allow the plants to be viable and fertile. Depending on the mutation density within the population being screened (a rate of one mutation per 500 kb or less is regarded as serviceable) and the random distribution of individual mutations throughout the genome of a given mutant population, TILLING can return allelic series for specific genes, with a wide variety of phenotypic effects. Certain types of mutations (for example, those that are gametophytic lethals and thus are not transmitted) simply cannot be recovered, but a major advantage to these populations is that most lethal and sterile alleles are maintained as heterozygotes and can still be assessed. In addition, the range of severity for point mutations means that, oftentimes, sublethal alleles of essential genes and substerile alleles of fertility genes can be analyzed as part of an allelic series. For maize, of 576 mutations delivered to date, informatics (e.g. SIFT [www.proweb.org]) based on nonconservative substitutions in conserved sequence motifs suggest that 14% are damaging alleles; however, 258 (or approximately 45%) of the mutations overall are nonsilent, and it is important to emphasize that any of these have the potential to show a biologically interesting phenotype.
The genetic diversity among grasses has also proven to be an advantage from a reverse genetic standpoint, using a variation on TILLING dubbed EcoTILLING after its introduction looking at natural variation among Arabidopsis (Arabidopsis thaliana) ecotypes (Comai et al., 2004). This method examines a cultivar/inbred line/accession against a reference genome in a one-on-one comparison to a reference genome, usually the genome that has been sequenced (where that is an option). SNPs are detected in the same manner that induced mutations are in EMS TILLING. There are often more than one such SNP within a target in EcoTILLING, and there will therefore often be multiple bands in a lane (Fig. 1). Notice that patterns unique to individual accessions, relatively rare SNPs common among only a small number of accessions, and more common SNPs are all visible on the same gel. Comparison of many different accessions to a reference genome by EcoTILLING can define unique patterns for individual accessions, identify patterns of SNPs shared among accessions that suggest relatedness, and reveal differing levels of selection in different regions of genes (e.g. decreased diversity within exons versus introns) as well as among genes. Valuable, practical information can be gotten from EcoTILLING analysis as well. For example, analysis of simple sequence repeat markers from ESTs reveals very high levels of divergence between accessions of switchgrass (Panicum virgatum), an outcrossing polyploid, as much as 20% divergence between populations of the same accession, and even higher variation between plants of the same population (80%; Narasimhamoorthy et al., 2008). In contrast, the switchgrass accessions shown in Figure 1 show levels of SNP haplotype diversity within exons (of the ago111 gene pictured as well as two others, sdg105 and sdg 125; data not shown) that are very similar to those typical of maize genes that do not show signatures of selection (Whitt et al., 2002). Thus, assessing sequence diversity by multiple methods will probably be an especially useful approach in some of these new crop species. In domesticated crop species, EcoTILLING has also proven to be a useful, relative measure of the sequence diversity remaining in highly inbred, elite germplasm (Table I; R. Johnson and C. Weil, unpublished data). In addition, comparison of various targets across diverse germplasm has revealed that, interestingly, two genes, encoding maize RNA-dependent RNA polymerases, show more haplotype variation than most other genes (Table I), raising interesting questions of what advantages the increased variability in RNA-dependent RNA polymerases might confer.
Figure 1.
EcoTILLING gel image of switchgrass. Using primers designed against the maize Argonaute-like gene ago111, 48 accessions of switchgrass were compared to the upland variety Cave-in-Rock. Two duplicate sets of samples are loaded, and mismatches (SNPs) between the reference genome and the variety tested appear as bands indicated by a matching pair of squares in both duplicates. For simplicity, only the LI-COR image from the 800-nm channel is shown. The black bar at the left indicates that the entire 1,401-bp target is within exon sequence; size standards are in base pairs.
Table I.
Discovery of induced point mutations in maize genes by TILLING
Maize haplotype variation as revealed by EcoTILLING.
| MaizeGDB Identifier | Best BLASTx Match and Score | No. Exon Haplotypes in 10 PVPa Inbreds | No. Exon Haplotypes in 86 MDLb Inbreds |
|---|---|---|---|
| mtp0176 | Phosphatidylinositol-4-P 5-kinase; 7e-136 | 1 | 2 |
| mtp0162 | FASS1; 1e-47 | 1 | 1 |
| mtp0174 | Transcription activator DEMETER 6e-58 | 2 | 4 |
| mtp0156 | Inositol monophosphatase 4e-19 | 3 | 4 |
| mtp0140 | Inositol polyphosphate 2-kinase 3e-103 | 3 | 4 |
| mtp0150 | Lys ketoglutarate reductase/saccharopine dehydrogenase 7e-46 | 3 | 3 |
| mtp0160 | Myb-like protein 1e-94 | 1 | 1 |
| mtp0192 | Pectinesterase 6e-52 | 2 | 4 |
| mtp0016 | DNA methyltransferase 1e-86 | 2 | 2 |
| mtp0158 | Dihydropicolinate synthase 3e-164 | 1 | 2 |
| mtp0177 | RNA-dependent RNA polymerase 2 2e-144 | 4 | 8 |
| mtp0179 | RNA-dependent RNA polymerase 6 8e-167 | 3 | 12 |
| mtp0175 | SET domain-containing protein SET118 3e-54 | 1 | 2 |
| mtp0045 | MADS box containing protein 2e-18 | 2 | 2 |
| mtp0182 | R-interacting factor (regulator of anthocyanin) 2e-123 | 2 | 2 |
| mtp0173 | Autophagocytosis transporter 10-like protein 1e-17 | 2 | 2 |
| mtp0166 | Autophagocytosis transporter 1-like protein 4e-42 | 1 | 5 |
| mtp0164 | Zn-finger CONSTANS-like protein 3e-88 | 1 | 2 |
| mtp0126 | Phytochrome C1 apoprotein 0.0 | 3 | 6 |
| mtp0184 | Rolled leaf 1 homeobox-Leu zipper protein 1e-60 | 3 | 4 |
PVP, Plant variety protection; these lines are the recently off-patent inbreds LH1, LH123Ht, LH82, PH207, PHG35, PHG39, PHG47, PHG84, PHJ40, and PHZ51.
MDL, Maize diversity lines (Liu et al., 2003).
TILLING projects among major grass crop species (maize, barley [Hordeum vulgare], rice, wheat [Triticum aestivum]) have been in operation for several years (Table II), using a variety of mutagens, and the reader is referred to these project Web pages for detailed information about each project. In addition, projects using TILLING, EcoTILLING, or a combination of the two are initiating or are being planned for several other grass species, including sorghum (M. Tuinstra, G. Ejeta, Z. Xin, and C. Weil, personal communication), oats (Avena sativa; O. Olsson, personal communication), the model cool season grass Brachypodium (Garvin et al., 2008), tef (Eragrostis tef; J. Bennetzen, personal communication), and switchgrass (M. Casler, D. Costich, and C. Weil, personal communication). One factor that has contributed to the rapid growth in applying this technique, particularly in food crops, is the idea that chemically mutagenized material does not fall under the heading of genetically modified organism. The single base changes that are created are no different than spontaneous single base changes that occur naturally, and no exogenous DNA is introduced into the plant. Indeed, EcoTILLING uses existing, natural variation as its source. In addition, the relatedness among grasses, the completion of genome sequences for rice, maize, and sorghum, and the projects beginning to sequence several other grass genomes now permit comparison of sequence information among these species in extremely powerful ways. Conservation of protein domains is quite high because the species are relatively close to one another evolutionarily. Furthermore, in regions where homologous proteins show less sequence identity, highly conserved individual amino acids begin to stand out as likely to be important in the structure and function of the protein even though they are not part of longer motifs. TILLING and EcoTILLING reveal point mutations and naturally occurring SNPs that impact amino acid sequence that can provide reverse genetic approaches to functional genomics. Where function is not yet known for a gene in a grass species, the ability to draw on the databases of many close relatives makes testing hypotheses about function of new homologs very straightforward.
Table II.
Existing and proposed TILLING projects in grass species
| Species | Project | Mutagen | Web Site or Contact | References |
|---|---|---|---|---|
| Barley (cv Optic) | DIStilling (SCRI) | EMS | germinate.scri.sari.ac.uk/barley/mutants/ | Caldwell et al. (2004) |
| Barley (cv Barke) | GABI-TILL | EMS | www.gabi-till.de/project/ipk/barley.html | |
| Barley (cv Morex) | TILLMore | EMS | www.distagenomics.unibo.it/TILLMore/ | Talame et al. (2008) |
| Barley (cv Lux) | Risø National Labs, KVL Denmark | EMS | pgrc.ipk-gatersleben.de/barleynet/organisation_kvl.php | |
| Brachypodium | Risø National Labs, KVL, Denmark | Sodium azide | www.risoe.dk/rispubl/BIO/biopdf/ris-r-1510.pdf. | |
| Maize (B73 and W22) | Maize TILLING Project (Purdue) | EMS | genome.purdue.edu/maizetilling/ | Till et al. (2004b); Weil and Monde, (2007) |
| Oat | CropTailor AB | EMS | www.croptailor.com/Engelsk/engindex.htm | |
| Rice (japonica) | RiceTILL (UC Davis) | EMS, MNU + azide | tilling.ucdavis.edu/index.php/Rice_Tilling | Till et al. (2007) |
| Rice (japonica) | Mishima | MNU | Suzuki et al. (2008) | |
| Sorghum (BTx623) | U.S. Department of Agriculture-Agricultural Research Service, Lubbock, TX | EMS | Xin et al. (2008) | |
| Switchgrass (Cave-in-Rock) | Purdue TILLING Project | EMS | Clifford Weil/Michael Casler | |
| Wheat (hexaploid) | Arcadia Biosciences | EMS | Slade et al. (2005) | |
| UC Davis | EMS | Luca Comai/Jorge Dubcovsky | ||
| Rothamstead Research (RRes) | EMS | www.rothamsted.ac.uk | ||
| Wheat (Triticum monococcum) | Rothamstead Research (RRes) | EMS | www.rothamsted.ac.uk | |
| Wheat (durum) | OPTIWHEAT | EMS | www.rothamsted.ac.uk/cpi/optiwheat/indexcontent.html |
PROSPECTS FOR THE FUTURE
TILLING was designed, initially, as a cost-effective means of screening populations for changes in individual genes. At the time, sequencing costs and throughput were such that it was important to have a preliminary screening tool to identify the individuals that carried a mutation before sequencing efforts on that gene were carried out. This generation of sequencing technologies, which produce gigabases of data at extremely low cost, has changed that landscape considerably. Now, simply resequencing the gene of interest in thousands of lines is both feasible and inexpensive, particularly for mutagenized inbred lines where the unmutagenized inbred sequence can serve as a scaffold for sequence assembly. Resequencing entire genomes across a mutagenized population that numbers in the thousands is still not cost effective, and is debatable even once the goal of a $1,000 genome sequence is achieved. However, targeted resequencing of hundreds, or even thousands, of interesting genes within these populations is now within our grasp, cost effective, and can be done for as many as 30 to 50 genes per instrument run, potentially exceeding 2,000 genes analyzed per year per instrument. Various TILLING and SNP discovery projects are moving toward these sequencing approaches, using all three of the major platforms currently available (Roche 454, Illumina/Solexa, and ABI SOLiD; Comai and Henikoff, 2006; L. Comai and E. Buckler, personal communication). The number of genes that can be processed can be adjusted in two dimensions, either screening larger numbers of genes across smaller numbers of mutagenized plants or smaller numbers of genes across larger numbers of plants, as needs and mutation densities dictate.
Even where a genome sequence can serve as a scaffold for assembly, care needs to be taken that the sequences being analyzed in any given instrument run do not share long sequence identities, which could complicate processing the data. Figure 2 shows a simple comparison of a concatenated genomic sequence of random maize genes (taken from the Maize Genome Sequencing project and totaling approximately 100,000 bp) to itself, using different potential read lengths and allowing two mismatches in the sequence, a level that could confuse correct identification of induced, single base changes. Ideally, the entire sequence would match only on the diagonal such that all sequences would have only one position into which they could fit in the assembly. For 17 of the 18 genes, the read lengths of 25 bp or more currently available on Illumina and ABI sequencing instruments would already provide straightforward assembly of the sequences, as do the much longer read lengths on Roche 454 instruments. The one gene in this test set that has repeated sequence within it requires significantly longer reads (>135 bp) to assemble easily; however, such exceptions can still be analyzed individually using Cel1 TILLING.
Figure 2.
Analysis of sequences for repetitive tracts prior to use in a SequeTILLING experiment. Dot matrix analysis of 18 concatenated genomic sequences totaling 101,174 bp compared against itself. Signals off the diagonal line represent identities that pose potential complicating factors for rapid assembly.
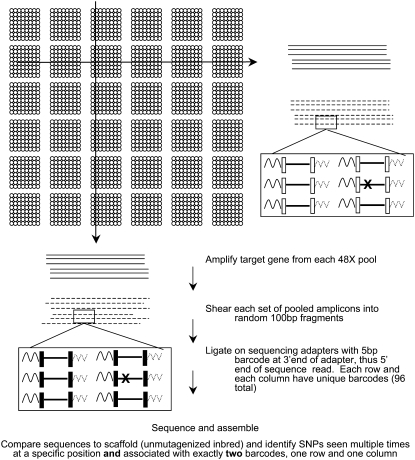
A major advantage to large-scale SequeTILLING is that DNA samples can be analyzed in much deeper pools than with the Cel1 approach. Preliminary data have suggested that for some instruments, pooling can be as high as 40- to 50-fold, compared to the 8-fold pooling typically used for robust Cel1 TILLING (E. Cuppen, unpublished data). The workflow (Fig. 3) generally consists of amplification of a gene from individual pools, followed by random shearing of the amplicons from that pool into pieces approximately 100 to 200 bp in length. Barcode sequences can be attached to the fragments as part of primers that are used to amplify the sequences in preparation for loading on the sequencing instrument. These barcodes are the first bases of the sequence read. Using a two-dimensional pooling strategy, individual mutants are identified by a unique combination of two barcodes (row and column). For example, a 48-fold pooled grid allows 2,304 individuals to be screened in 96 total reactions (48 rows and 48 columns), using 96 unique barcode sequences. It is generally advisable that each barcode sequence varies from all the other barcodes by at least two nucleotides to minimize any error; a five base barcode system can provide all the variability needed without giving up too much of the sequence read, crucial on instruments where read lengths are short. Gene fragments amplified from an individual pool all carry the same barcode at the beginning of their sequence and each individual mutant plant is identifiable by a specific combination of two barcodes (one row and one column in the 48 × 48 grid of 2,304). The unique, gene-specific nature of the rest of each read (within the sequences for that instrument run) allows them to be assembled into gene sequences relatively easily and assessed for single nucleotide changes in comparison to the reference DNA sequence for each gene. Any mutations can be assigned quickly to a specific individual. Error rates of individual sequencing instruments then become the major factor in determining how deeply sequences must be covered to get reliable mutation calling. If the material being analyzed is a mutagenized, elite inbred, then coverage of 3 to 5 times should reliably distinguish real mutations from sequencing artifacts; the same base change repeatedly associated with the same two barcodes would indicate a mutant individual.
Figure 3.
Workflow of SequeTILLING. A 48 × 48 grid of individual DNAs is collapsed into 48-row and 48-column pools for gene amplification. Sequencing adapters can be whatever the manufacturer recommends, as long as the 5-bp barcode sequence for each pool is the first part of the sequence read.
Sequencing approaches can also apply readily to EcoTILLING, assigning individual barcodes to specific inbred lines or accessions rather than pools of mutants, and then resequencing target genes in these collected lines. Sequencing several independent samples of each accession will robustly distinguish real SNPs from sequencing errors although, because of their inherent variability, these sequences will need to be covered at greater depth to make assignment of SNPs to accessions reliable.
One of the important developments to grow out of this quantum leap in throughput is that, even though the research community is still requesting TILLING of a variety of species, the capacity to generate the data is now substantially greater than the user requests for EMS TILLING of specific genes. Consider that a small number of gigabase sequencer runs would quickly equal all the TILLING that has been done to date. The future for these resources may well be that, as mutant populations are being developed and expanded for various grass species, research communities focused on those species need to begin assembling lists of genes for which mutations are desired and develop prioritized approaches for screening them. As massively parallel sequencing methods become more widespread and accessible, the limiting feature of TILLING efforts will be the creation, mutation density testing, curation, and distribution of the mutant populations. Fortunately, once established and especially once shared publicly among researchers, these resources will pay benefits for many years to come as both forward and reverse screening tools.
Acknowledgments
I would like to thank Rita Monde, Dacia Daniel, Courtney Chambers, Leonie Leduc, Heather Sahm, and Tara Breen for technical assistance, and Philip SanMiguel, Paul Parker, Luca Comai, Dick McCombie, and Dick Johnson for valuable discussions.
This work was supported by the National Science Foundation Plant Genome Award (grant no. DBI0604765) and the Purdue Agricultural Research Station.
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Clifford F. Weil (cweil@purdue.edu).
References
- Caldwell DG, McCallum N, Shaw P, Muehlbauer GJ, Marshall DF, Waugh R (2004) A structured mutant population for forward and reverse genetics in Barley (Hordeum vulgare L.). Plant J 40 143–150 [DOI] [PubMed] [Google Scholar]
- Comai L, Henikoff S (2006) TILLING: practical single-nucleotide mutation discovery. Plant J 45 684–694 [DOI] [PubMed] [Google Scholar]
- Comai L, Young K, Till BJ, Reynolds SH, Greene EA, Codomo CA, Enns LC, Johnson JE, Burtner C, Odden AR, et al (2004) Efficient discovery of DNA polymorphisms in natural populations by EcoTILLING. Plant J 37 778–786 [DOI] [PubMed] [Google Scholar]
- Garvin D, Gu YQ, Hasterok R, Hazen S, Jenkins G, Mockler TC, Mur LA, Vogel JP (2008) Development of genetic and genomic research resources for Brachypodium distachyon, a new model system for grass crop research. Crop Sci 48 S69–S84 [Google Scholar]
- Groth D, Santini J, Hamaker BR, Weil CF (2008) High-throughput screening of EMS mutagenized maize for altered starch digestibility. BioEnergy Res 1 118–135 [Google Scholar]
- Liu K, Goodman M, Muse S, Smith JS, Buckler E, Doebley J (2003) Genetic structure and diversity among maize inbred lines as inferred from DNA microsatellites. Genetics 165 2117–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum CM, Comai L, Greene EA, Henikoff S (2000) Targeting induced local lesions IN genomes (TILLING) for plant functional genomics. Plant Physiol 123 439–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhamoorthy B, Saha MCTS, Bouton JH (2008) Genetic diversity in switchgrass collections assessed by EST-SSR markers. BioEnergy Res 1 136–146 [Google Scholar]
- Slade AJ, Fuerstenberg SI, Loeffler D, Steine MN, Facciotti D (2005) A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat Biotechnol 23 75–81 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Eiguchi M, Kumamaru T, Satoh H, Matsusaka H, Moriguchi K, Nagato Y, Kurata N (2008) MNU-induced mutant pools and high performance TILLING enable finding of any gene mutation in rice. Mol Genet Genomics 279 213–223 [DOI] [PubMed] [Google Scholar]
- Talame V, Bovina R, Sanguineti MC, Tuberosa R, Lundqvist U, Salvi S (2008) TILLMore, a resource for the discovery of chemically induced mutants in barley. Plant Biotechnol J 6 477–485 [DOI] [PubMed] [Google Scholar]
- Till BJ, Burtner C, Comai L, Henikoff S (2004. a) Mismatch cleavage by single-strand specific nucleases. Nucleic Acids Res 32 2632–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BJ, Cooper J, Tai TH, Colowit P, Greene EA, Henikoff S, Comai L (2007) Discovery of chemically induced mutations in rice by TILLING. BMC Plant Biol 7 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BJ, Reynolds SH, Weil C, Springer N, Burtner C, Young K, Bowers E, Codomo CA, Enns LC, Odden AR, et al (2004. b) Discovery of induced point mutations in maize genes by TILLING. BMC Plant Biol 4 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BJ, Zerr T, Comai L, Henikoff S (2006) A protocol for TILLING and EcoTILLING in plants and animals. Nat Protocols 1 2465–2477 [DOI] [PubMed] [Google Scholar]
- Weil CF, Monde RA (2007) Getting the point: mutations in maize. Crop Sci 47 S60–S67 [Google Scholar]
- Whitt SR, Wilson LM, Tenaillon MI, Gaut BS, Buckler ES IV (2002) Genetic diversity and selection in the maize starch pathway. Proc Natl Acad Sci USA 99 12959–12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Wang ML, Barkley NA, Burow G, Franks C, Pederson G, Burke J (2008) Applying genotyping (TILLING) and phenotyping analyses to elucidate gene function in a chemically induced sorghum mutant population. BMC Plant Biol 8 103. [DOI] [PMC free article] [PubMed] [Google Scholar]