Abstract
Wheat (Triticum spp.) grains contain large protein polymers constituted by two main classes of polypeptides: the high-molecular-weight glutenin subunits and the low-molecular-weight glutenin subunits (LMW-GS). These polymers are among the largest protein molecules known in nature and are the main determinants of the superior technological properties of wheat flours. However, little is known about the mechanisms controlling the assembly of the different subunits and the way they are arranged in the final polymer. Here, we have addressed these issues by analyzing the formation of interchain disulfide bonds between identical and different LMW-GS and by studying the assembly of mutants lacking individual intrachain disulfides. Our results indicate that individual cysteine residues that remain available for disulfide bond formation in the folded monomer can form interchain disulfide bonds with a variety of different cysteine residues present in a companion subunit. These results imply that the coordinated expression of many different LMW-GS in wheat endosperm cells can potentially lead to the formation of a large set of distinct polymeric structures, in which subunits can be arranged in different configurations. In addition, we show that not all intrachain disulfide bonds are necessary for the generation of an assembly-competent structure and that the retention of a LMW-GS in the early secretory pathway is not dependent on polymer formation.
The unique ability of wheat (Triticum spp.) flour to form a dough that has the rheological properties required for the production of leavened bread and other foods is largely due to the characteristics of the proteins that accumulate in wheat endosperm cells during seed development (Gianibelli et al., 2001). Among these endosperm proteins, a major role is played by prolamines, a large group of structurally different proteins sharing the characteristic of being particularly high in Pro and Gln.
On the basis of their polymerization status, wheat prolamines can be subdivided into two groups, the gliadins and the glutenins. While gliadins are monomeric, glutenins are heterogeneous mixtures of polymers where individual subunits are held together by interchain disulfide bonds (Galili et al., 1996; Tatham and Shewry, 1998). The subunits participating to the formation of these large polymers have been classified into four groups according to their electrophoretic mobility (Gianibelli et al., 2001). The A group is constituted by the so-called high-molecular-weight glutenin subunits (HMW-GS), while polypeptides in groups B, C, and D are collectively termed low-molecular-weight glutenin subunits (LMW-GS). While only three to five HMW-GS are expressed in common wheat endosperm, LMW-GS include a very large number of different polypeptides.
Different models of glutenin assembly have been proposed (see Gianibelli et al., 2001 for a review), but the determination of their precise structure and Mr distribution has been hampered by their large size and complex subunit composition. Crucially, because disulfide bonds appear to be the major factor affecting polymer stability, it would be very useful to know whether the pairing between specific Cys residues, rather than random assembly, controls glutenin polymer formation. Indeed, data obtained with HMW-GS indicate that the formation of certain types of intermolecular disulfide bonds is particularly favored (Tao et al., 1992; Shimoni et al., 1997). In the case of LMW-GS, at least two functionally distinct types of subunits can be distinguished. Subunits of the first type, to which the majority of B-type subunits belong, would act as chain extenders, because they contain two Cys residues that remain available for the formation of interchain disulfide bonds. Subunits of the second type, containing a single Cys residue able to form an interchain disulfide bond, would instead act as chain terminators (Kasarda, 1989). Most of the members of this second group are indeed modified gliadins that participate to polymer formation thanks to the presence of extra Cys residues (D'Ovidio and Masci, 2004). Given the complexity of the situation found in wheat endosperm, where many different subunits are synthesized at the same time and can participate in the formation of complex high-Mr polymers, the study of glutenin polymer formation can take advantage of the use of heterologous expression systems where the behavior of individual subunits can be more easily monitored. For instance, the expression of HMW-GS in transgenic tobacco (Nicotiana tabacum) has provided insights into the rules governing the assembly of some of the subunits belonging to this class (Shani et al., 1994; Shimoni et al., 1997). In this work, we have used heterologous expression of wild-type and modified LMW-GS in tobacco protoplasts to study the assembly of this class of gluten polypeptides. Our results confirm that disulfide bonds are crucial for the assembly of these proteins and indicate that a relaxed specificity in Cys pairing from different subunits can drive the formation of complex glutenin polymers.
RESULTS
Polymer Formation in Tobacco Protoplasts
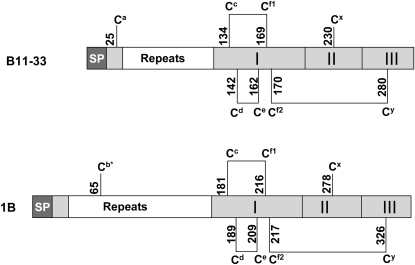
The B11-33 clone encodes a protein that has the typical features of B-type LMW-GS (Okita et al., 1985; Fig. 1). Like other prolamines, LMW-GS are synthesized as precursors containing an N-terminal signal peptide for import into the endoplasmic reticulum (ER). In the B11-33 protein, the signal peptide for insertion into the ER is followed by a short N-terminal region that contains a first Cys residue (Ca) and by a repetitive domain rich in Gln and Pro residues. The C-terminal part of the protein consists of a domain that is stabilized by the presence of three intrachain disulfide bonds and that can be further subdivided into three regions (I, II, and III in Fig. 1): a first region containing 5 Cys residues, Cc, Cd, Ce, Cf1, and Cf2, all involved in the formation of intrachain disulfides; a Gln-rich domain containing a Cys residue (Cx) that remains available for polymer formation; and a C-terminal conserved sequence containing one further Cys residue (Cy) that is again involved in the formation of an intrachain bond. The position of these Cys residues is indicated in Figure 1. In other LMW-GS, such as the one encoded by the pLDNLMW1B clone isolated from durum wheat (Triticum durum), the Cys residue in the N-terminal nonrepetitive region is absent and is replaced by a Cys residue (Cb*) in the repetitive domain (Fig. 1). In other members of the class, all eight Cys are located in the C-terminal part of the molecule (D'Ovidio and Masci, 2004). Whether the different Cys distribution in these polypeptides leads to crucial functional differences is currently unknown.
Figure 1.
B11-33 and 1B LMW-GS structure. Schematic diagram showing the proposed disulphide structure of the B11-33 (Okita et al., 1985) and 1B (D'Ovidio et al., 1997) LMW-GS. Designation of Cys residues is according to Müller et al. (1998). Numbers indicate their position in the primary structure, starting from the first amino acid in the signal peptide. Nonrepetitive regions are shaded. I, II, and III indicate the three regions that constitute the nonrepetitive C-terminal domain. SP, Signal peptide.
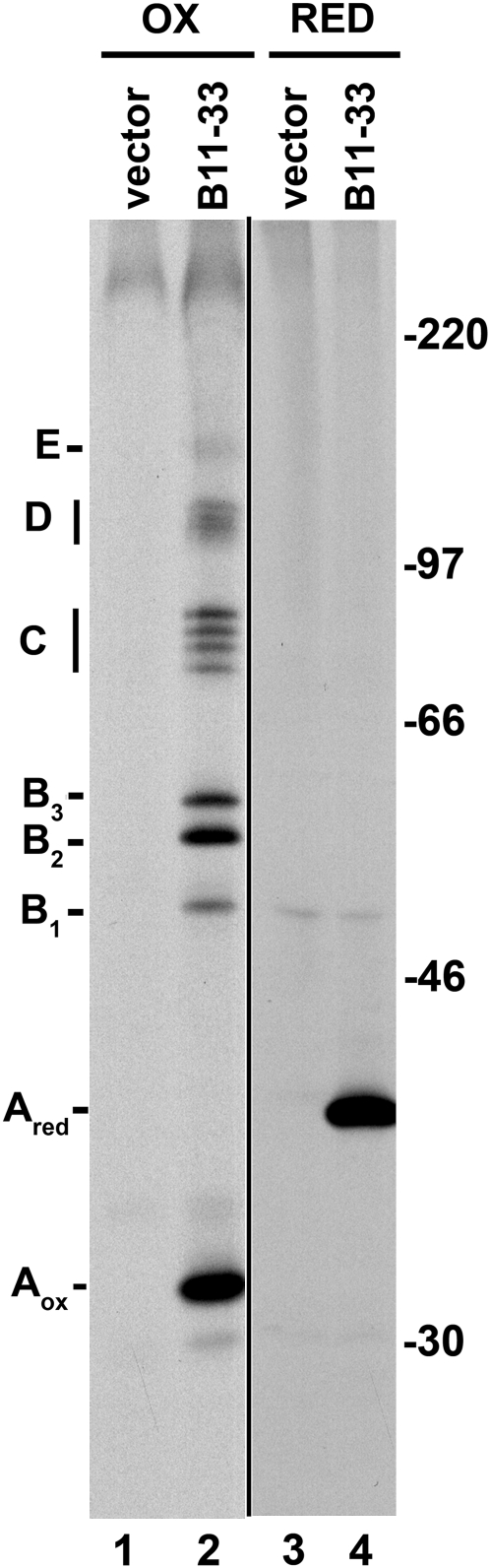
We have previously shown that when B11-33 polypeptides are synthesized in an in vitro translation system based on a wheat germ extract and bean (Phaseolus vulgaris) microsomes, the protein is stabilized by three intrachain disulfide bonds (Orsi et al., 2001). However, no assembly could be observed in this system. To assess whether B11-33 polypeptides could assemble into oligomeric forms in vivo, tobacco cv SR1 protoplasts were transfected with a construct encoding the wild-type B11-33 protein. After pulse-labeling, polypeptides were immunoselected with an anti-glutenin antiserum and analyzed by SDS-PAGE under nonreducing conditions (Fig. 2). Several bands that were absent in the control lane were evident in immunoprecipitates from protoplasts expressing the B11-33 protein (Fig. 2, lane 2). The faster migrating band (32 kD, Aox in Fig. 2) had the expected mobility for monomeric B11-33 polypeptides bearing three intrachain disulfide bonds (Orsi et al., 2001). Four more groups of bands were also evident. The first group consisted of three polypeptides (designated B1, B2, and B3) migrating between the 46- and the 66-kD markers. The second group (C in Fig. 2) consisted of four bands migrating between the 66- and the 97-kD markers. Two slower migrating bands were also evident (D and E in Fig. 2). These smeared bands likely represent groups of larger oligomeric forms. Separation in the molecular mass range of these groups was not sufficient to allow determining the number of bands within each group.
Figure 2.
LMW-GS polymer formation in tobacco protoplasts. Tobacco protoplasts were transfected with a plasmid encoding the B11-33 LMW-GS or with vector as control. Protoplasts were labeled with [35S]Cys and [35S]Met for 1.5 h and then homogenized. Proteins immunoselected with an anti-glutenin antiserum were analyzed by nonreducing (OX, lanes 1 and 2) or reducing (RED, lanes 3 and 4) SDS-PAGE and fluorography. Aox, Oxidized monomer; Ared, reduced monomer. B1, B2, B3, C, D, and E indicate the positions of B11-33 containing complexes. The positions of molecular mass markers (kD) are shown on the right.
All these bands collapsed in a single band (Ared, lane 4) with an apparent molecular mass of 39 kD when the same sample was run under reducing conditions, indicating that the different forms resolved in the nonreduced sample were indeed either disulfide-linked oligomers of the B11-33 protein or covalent adducts with endogenous tobacco proteins.
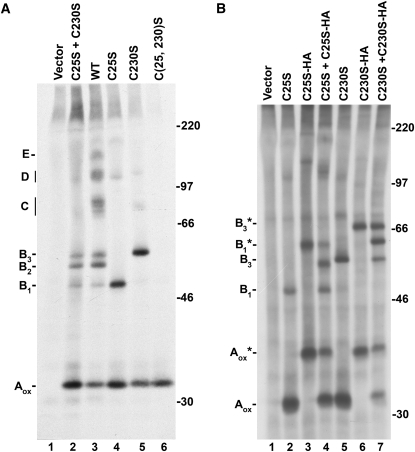
To further characterize the nature of the polypeptides immunoisolated from B11-33 expressing protoplasts, we analyzed a set of mutants in which Cys residues potentially involved in interchain disulfide bond formation were mutated to Ser (Fig. 3A). Mutation of both C25 and C230 led to the accumulation of a single major band with a migration corresponding to fully oxidized monomeric B11-33 (Aox, compare lane 3 and lane 6). Two major immunoreactive bands were instead evident in the sample derived from protoplasts expressing a mutant (C25S) in which C25 was mutated to Ser (lane 4). While the faster of these bands had the same mobility of oxidized monomeric B11-33, the second one comigrated with the B1 form accumulating in protoplasts expressing wild-type B11-33 (compare lane 3 with lane 4). This suggests that this second band corresponds to dimers in which the two monomers are connected by a C230-C230 bond.
Figure 3.
Analysis of B11-33 Cys mutants. A, Tobacco protoplasts were transfected with vector DNA or plasmids encoding the wild-type B11-33 protein (WT) or the C25S, C230S, C(25, 230)S mutants, alone or in combination, as indicated. Protoplasts were labeled with [35S]Cys and [35S]Met for 1.5 h and then homogenized. Proteins immunoselected with an anti-glutenin antiserum were analyzed by SDS-PAGE under nonreducing conditions and fluorography. Aox, Oxidized monomer. B1, B2, B3, C, D, and E indicate the positions of the different B11-33-containing complexes. B, Tobacco protoplasts were transfected with vector DNA or plasmids encoding the C25S, C25S-HA, C230S, C230S-HA mutants, alone or in combination as indicated. Protoplast labeling, immunoselection, and analysis were performed as indicated in A. Aox, Oxidized untagged monomer; B1, B3, untagged dimers; Aox*, oxidized HA-tagged monomer; B1*, B3*, HA-tagged dimers. In both segments, the positions of molecular mass markers (kD) are shown on the right.
Similarly, two major bands were evident in samples derived from protoplasts expressing the C230S mutant (lane 5), the faster migrating one again corresponding to monomeric B11-33 polypeptides. However, in the case of this mutant, the second band comigrated with the B3 form accumulating in protoplasts expressing wild-type B11-33 (compare lane 3 with lane 5). This suggests that this band represents dimers in which the two monomers are connected via a C25-C25 disulfide bond. The difference in mobility between the B1 and B3 complexes likely reflects differences in the hydrodynamic volume of the two disulfide-linked forms during electrophoretic separation. In addition to the major forms described above, small amounts of larger oligomeric forms were evident in the case of both the C25S and C230S mutants (lanes 4 and 5). The nature of these forms was not further investigated, but they could represent oligomers containing misfolded polypeptides or adducts with endogenous tobacco proteins.
These findings raised the possibility that the central band (B2) in the first group of putative oligomers may represent a dimer in which the subunits are linked via a C25-C230 disulfide bond. To test this hypothesis, we coexpressed the C25S and the C230S mutant. Indeed, in addition to the monomeric form and to the two dimeric forms synthesized when the mutants were expressed alone, a third major band was evident, corresponding in migration to the B2 polypeptide (compare lane 2 with lane 3). All together, these data indicate that B11-33 polypeptides assemble into covalent dimers by forming interchain disulfide bonds between C25 (“head” Cys) and C230 (“tail” Cys) in head-to-head, tail-to-tail, or head-to-tail configuration.
It should be noted that, although all possible types of dimers were formed in this system, they were not equally represented. Indeed, we consistently found that the B2 band, corresponding to a C25-C230 dimer was the most abundant, while the B1 band, corresponding to a C230-C230 dimer, was the less represented.
The above experiments did not allow us to formally exclude the possibility that the B1 and B3 forms accumulating in protoplasts expressing the C25S and C230S mutants represented complexes with an endogenous tobacco protein, rather than B11-33 homodimers. To investigate this possibility, we coexpressed untagged and hemagglutinin epitope (HA)-tagged forms of the C25S and C230S mutants. The addition of a triple HA tag did not affect the overall assembly pattern of the B11-33 protein (not shown here, but see Fig. 6B). If the B1 and B3 forms corresponded to B11-33 homodimers, coexpression of untagged and tagged forms of the C25S or C230S mutants should lead to the formation of three different types of dimer (untagged + untagged, untagged + tagged, and tagged + tagged). Conversely, if they represented heterodimers with endogenous tobacco proteins, only two forms should accumulate (one containing the untagged and one containing the tagged protein).
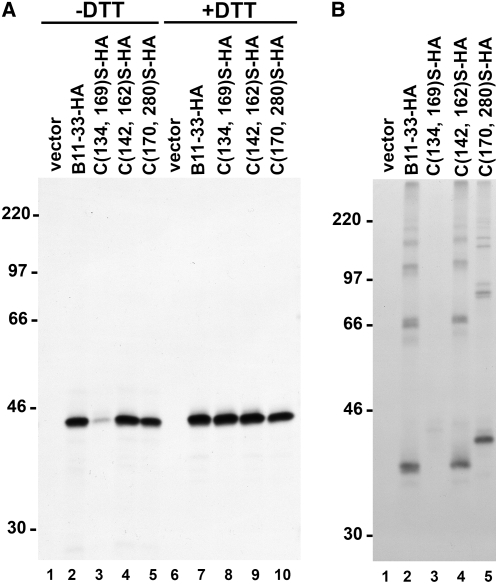
Figure 6.
Role of intrachain disulphide bonds in the assembly of B11-33 LMW-GS. Protoplasts were transfected with vector DNA or with plasmid encoding either the B11-33-HA protein or mutants of this protein in which specific pairs of Cys residues were mutated to Ser (C(134, 169)S-HA; C(142, 162)S-HA; C(170, 280)S-HA). Protoplasts were labeled with [35S]Cys and [35S]Met for 1.5 h. A, Protoplasts were homogenized under denaturing conditions in the absence (−) or in the presence (+) of DTT, as indicated, and proteins were immunoselected from protoplast homogenates using an anti-HA monoclonal antibody. Immunoselected proteins were analyzed by reducing SDS-PAGE. B, Protoplasts were homogenized under denaturing conditions in the absence of DTT, and proteins were immunoselected from protoplast homogenates using an anti-HA monoclonal antibody. Immunoselected proteins were analyzed by nonreducing SDS-PAGE. In both segments, the positions of molecular mass markers (kD) are shown on the left.
Protoplasts were therefore cotransfected with plasmids encoding the C25S or the C230S mutant and the corresponding HA-tagged form. Untagged (Aox, B1, B3) and tagged (Aox*, B1*, B3*) forms can be easily distinguished on SDS-PAGE (Fig. 3B). Coexpression of either the C25S or the C230S mutant with the corresponding HA-tagged form led to the appearance of one extra band of intermediate mobility (Fig. 3B, lanes 4 and 7). These results are therefore consistent with the hypothesis that the B1 and B3 forms represent different homodimers of the B11-33 protein.
Our approach did not allow us to determine the covalent structure of the set of four polypeptides migrating above the dimeric forms (C in Fig. 2). To get some hints about the possible structure of these putative trimeric forms, we calculated the number of different trimers that would be expected if C25 and C230 could form the same three types of disulfide bonds found in dimers. This calculation predicts the formation of four different trimeric forms (see Supplemental Data S1), suggesting that three different types of disulfide bonds (C25-C25, C230-C230, C25-C230) connect individual subunits within trimers and possibly higher order oligomers.
Coexpression of Two Different LMW-GS Leads to Heterodimer Formation
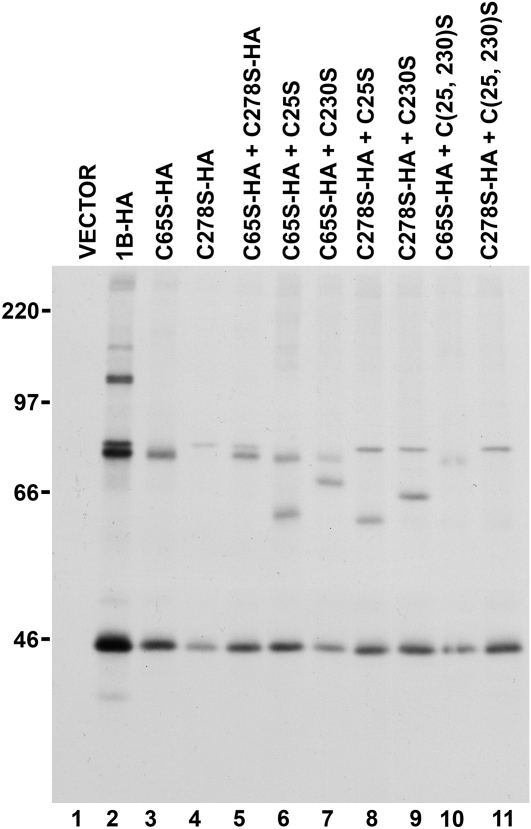
We next wished to investigate whether the B11-33 protein could form mixed dimers with a different glutenin subunit. For this purpose, we chose the LMW-GS encoded by the pLDNLMW1B clone from durum wheat (D'Ovidio et al., 1997). In this protein (hereafter indicated as 1B), one of the two Cys residues (C65 and C278; see Fig. 1) that should remain available to form interchain disulfides is located in the repetitive region. Expression of an HA-tagged version of the 1B protein (1B-HA) in tobacco protoplasts led to the accumulation of several forms that could be specifically selected with an anti-HA monoclonal antibody (Fig. 4, lane 2). Only two major bands, likely representing monomeric and homodimeric forms of the 1B-HA protein, were instead evident in the case of the C65S-HA and the C278S-HA mutants (lanes 3 and 4, respectively). By analogy with the results obtained in the case of the B11-33 protein, this indicates that the 1B-HA LMW-GS can form homodimers in both head-to-head and tail-to-tail configurations. When the two mutants were coexpressed, no additional band was generated (lane 5), indicating either that dimers linked by a C65-C278 disulfide bond do not form or that the resulting dimeric form comigrates with one of two other types of dimer.
Figure 4.
Heterodimer formation in tobacco protoplasts. Tobacco protoplasts were transfected with plasmids encoding the 1B-HA protein or different mutants in which individual Cys residues were mutated to Ser. When indicated, the 1B-HA protein or its Cys mutants (C65S-HA, C278S-HA) were coexpressed with Cys mutants of the untagged B11-33 protein (C25S, C230S, C(25, 230)S). Protoplasts were labeled with [35S]Cys and [35S]Met for 1.5 h. Proteins were immunoselected from protoplast homogenates using an anti-HA monoclonal antibody and analyzed by non-reducing SDS-PAGE. The positions of molecular mass markers (kD) are shown on the left.
When the C65S-HA mutant of the 1B-HA protein was coexpressed with the untagged C25S mutant of the B11-33 protein, one additional band could be immunoselected using anti-HA antibodies (lane 6). This band was not generated when the C65S-HA mutant was coexpressed with the B11-33 C(25, 230)S protein (lane 10), suggesting that it represents a covalent dimer in which C278 in the C65S-HA protein forms an interchain disulfide with C230 in the B11-33 C25S protein. Similar results were obtained when the C65S-HA mutant of the 1B-HA protein was coexpressed with the B11-33 C230S mutant (lane 7), except that the 1B-HA C65S/B11-33 C230S heterodimer migrated more slowly than the 1B-HA C65S/B11-33 C25S heterodimer. Analogous results were obtained in the case of the C278S mutant of the 1B-HA LMW-GS (lanes 8, 9, and 11). When FLAG-tagged forms of the two B11-33 Cys mutants were individually coexpressed with HA-tagged mutants of the 1B protein, a band of the expected size could be invariably immunoselected by both the anti-FLAG and anti-HA antibodies (Supplemental Fig. S1). This confirmed the formation of four different types of covalent heterodimers between the two proteins.
All together, these results indicate that 1B and B11-33 proteins can form mixed disulfides involving any possible combination of the two Cys residues that remain available for interchain disulfide bond formation in the folded monomers.
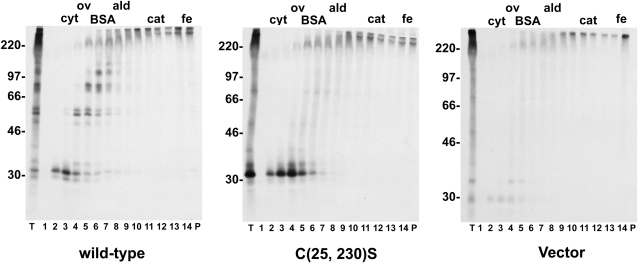
Sedimentation Velocity Analysis of B11-33 Polypeptides
We next wished to investigate whether the different monomeric/oligomeric forms of the B11-33 protein identified on nonreducing SDS-PAGE were noncovalently associated. Protoplasts expressing the B11-33 and the C(25, 230)S mutants were therefore labeled with radioactive amino acids, and protoplasts homogenates were fractionated by sedimentation velocity centrifugation on Suc gradient (Fig. 5). In the case of the wild-type protein, it was evident that monomers and individual disulfide-linked oligomers were recovered in different gradient fractions. Consistently, the C(25, 230)S mutant was recovered as a single peak in the upper part of the gradient. We therefore conclude that monomeric and lower order oligomeric forms of the B11-33 protein do not form stable protein complexes, or, if they do form complexes in vivo, the interaction is too weak to withstand even mild extraction conditions.
Figure 5.
Sedimentation behavior of monomeric and oligomeric forms of the B11-33 protein. Protoplasts were transfected with vector DNA or with plasmids encoding either the wild-type B11-33 protein or the C(25, 230)S mutant. Protoplasts were labeled with [35S]Cys and [35S]Met for 1.5 h. Protoplast homogenates were fractionated by sedimentation velocity centrifugation on Suc gradients. Fractions were collected from the top and proteins were immunoselected with an anti-glutenin antiserum from an aliquot of the total homogenate (T), from the material recovered at the bottom of the tube (P), and from each gradient fraction (1–14). Immunoselected proteins were analyzed by nonreducing SDS-PAGE and fluorography. The approximate position of sedimentation markers is shown at the top. cyt, Cytochrome C; ov, ovalbumin; BSA, bovine serum albumin; ald, aldolase; cat, catalase; fe, ferritin. The positions of molecular mass markers (kD) are shown on the left.
Analysis of Mutants Lacking Intrachain Disulfide Bonds
We have previously shown that the disulfide bond linking C134 and C169 is essential to prevent the aggregation of B11-33 polypeptides expressed in an in vitro translation/translocation system (Orsi et al., 2001). Conversely, mutation of Cys residues involved in the formation of the two other intrachain disulfide bonds (C142-C162 and C170-C280) had no effect on the solubility of in vitro synthesized B11-33 polypeptides. Because those in vitro experiments did not allow us to assess the role of intrachain disulfide bonds in the assembly process, we expressed the same mutants in tobacco protoplasts and investigated the effect of the mutations on the solubility and assembly of B11-33 polypeptides.
Compared to the B11-33-HA protein or to the other Cys pair mutants, immunoselection of the C(134, 169)S-HA mutant was very inefficient when homogenization was performed under nonreducing conditions (Fig. 6A, compare lane 3 with lanes 2, 4, and 5). However, similar amounts of the four proteins were immunoselected when a reducing agent was included in the homogenization buffer (Fig. 6A, lanes 7–10). These results confirm the in vitro data and indicate that, in the absence of the C134-C169 intrachain disulfide bond, B11-33 polypeptides form disulfide-linked aggregates that cannot be efficiently recovered unless a reducing agent is included in the homogenization buffer.
Analysis under nonreducing conditions allowed us to determine the role of the individual intrachain disulfides in the assembly process (Fig. 6B). The assembly pattern of the C(142, 162)S-HA mutant was very similar to the one of the wild-type protein (compare lanes 2 and 4 in Fig. 6B). However, all forms migrated slightly slower, indicating that, in the absence of the C142-C162 intrachain disulfide bond, B11-33 polypeptides assume a less compact conformation during electrophoresis (Orsi et al., 2001). The ratio between the different oligomeric forms was not significantly affected by the elimination of the C142-C162 disulfide bond, clearly indicating that this bond does not play any major role in the formation of covalent polymers.
Elimination of the C170-C280 bridge had a much larger effect on the migration of the monomeric protein and on the assembly pattern (compare lanes 2 and 5 in Fig. 6B). Still, the presence of a set of distinct oligomeric forms indicates that the absence of the C170-C280 bond does not compromise the ability of B11-33 polypeptides to participate to the formation of covalent polymers.
Oligomeric Forms of the B11-33 Protein Are Associated with the ER
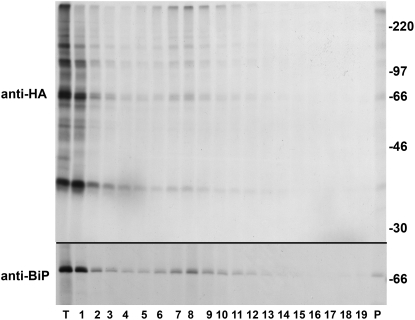
The ER is the subcellular compartment where various enzymes able to catalyze oxidative folding and assembly are located (Sevier and Kaiser, 2002). We therefore wished to determine whether covalent glutenin polymers were assembled in the ER of tobacco cells expressing a LMW-GS. Protoplasts expressing the B11-33-HA protein were pulse-labeled and fractionated by centrifugation on isopycnic Suc gradient. B11-33-HA polypeptides were immunoisolated using an anti-HA monoclonal antibody and analyzed under nonreducing conditions (Fig. 7). The tobacco homolog of the immunoglobulin heavy chain binding protein (BiP) was also immunoprecipitated as a marker for the ER. The distribution of B11-33-HA monomer and oligomers along the gradient could be superimposed to the one of BiP, indicating that both monomeric and oligomeric forms of the B11-33-HA protein are present in the ER.
Figure 7.
Glutenin polymers are associated with the ER. Protoplasts expressing the B11-33-HA protein were labeled with [35S]Cys and [35S]Met for 1 h, and total homogenates were fractionated on a 16% to 55% Suc gradient. Fractions were collected from the top and proteins were immunoselected with an anti-HA monoclonal antibody from total homogenate (T), from each gradient fraction (1–19), and from the material recovered at the bottom of the gradient (P). The supernatant from the first immunoprecipitation was then subjected to a second round of immunoselection using an anti-BiP antiserum. Immunoselected proteins were analyzed by nonreducing (anti-HA) or reducing (anti-BiP) SDS-PAGE. The positions of molecular mass markers (kD) are shown on the right.
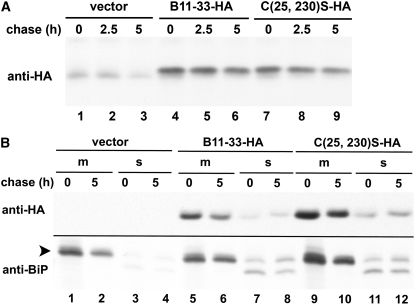
In wheat endosperm cells, storage proteins are transported to the vacuole where they accumulate in the form of protein bodies (Galili et al., 1996). We therefore investigated whether the B11-33 LMW-GS was retained in the ER or transported out of this compartment when expressed in tobacco protoplasts. Pulse-chase analysis showed that neither the B11-33-HA protein nor the C(25, 230)S-HA mutant was secreted in the incubation medium (not shown). Rather, the proteins stably accumulated within the cells, only a small proportion of the signal present at the end of a pulse not being recovered at the end of a 5-h chase (Fig. 8A). Upon subcellular fractionation into a microsomal and a soluble fraction (the latter containing, in addition to cytosolic proteins, soluble proteins released from broken central vacuoles) the vast majority of the B11-33-HA protein and of the C(25, 230)S-HA mutant was recovered in the microsomal fraction indicating that little, if any, accumulation of these proteins in the vacuole occurs during the first 5 h following synthesis (Fig. 8B). These results also indicate that polymer formation does not play any major role in determining the short-term fate of the LMW-GS in tobacco leaf protoplasts.
Figure 8.
Stability and intracellular transport of LMW-GS in tobacco protoplasts. A, Protoplasts were transfected with vector alone (vector) or constructs encoding either the B11-33-HA or the C(25, 230)S-HA protein, labeled for 1 h with [35S]Cys and [35S]Met and then chased for the times indicated. Proteins were immunoselected from cell homogenates with anti-HA antibodies and analyzed by reducing SDS-PAGE and fluorography. B, Protoplasts were transfected and pulse-labeled as in A and then chased for the times indicated. After fractionation into a membrane and a soluble fraction as in “Materials and Methods,” proteins were immunoselected with anti-HA or anti-BiP antibodies and analyzed by reducing SDS-PAGE and fluorography. The arrowhead indicates the position of the BiP polypeptide.
The localization of the B11-33-HA and C(25, 230)S-HA proteins was also analyzed by immunofluorescence (Fig. 9). Forty-eight hours after transfection, the B11-33-HA and C(25, 230)S-HA proteins were found to be mainly confined to regions of the cell that were also stained by the anti-BiP antibody. Still, rather than being evenly distributed within the ER, the B11-33-HA and C(25, 230)S-HA proteins were concentrated in discrete spots, a phenotype that was particularly evident in the case of the C(25, 230)S-HA mutant. The presence of some of the spots in areas where the BiP signal is rather weak or even absent might be due to exclusion of the marker from regions of the ER in which the LMW-GS are highly concentrated. Consistent with the accumulation of the two proteins in the ER, the nuclear membrane was decorated by the anti-HA antibody. Conversely, no signal was evident in the central vacuole.
Figure 9.
Subcellular localization of glutenin subunits. Tobacco leaf protoplasts were transiently transformed with a plasmid encoding either the B11-33-HA (A–D) or the C(25, 230)S-HA (E–H) proteins and fixed with 4% paraformaldehyde 48 h after trasfection. The subcellular distribution of the two proteins and of the ER marker BiP was examined by confocal laser scanning microscopy using a combination of an anti-HA monoclonal antibody and a rabbit anti-BiP antiserum. A and E, Distribution pattern of the heterologous proteins revealed by the anti-HA and Alexa Fluor 488 goat anti-mouse antibodies. B and F, Reticular fluorescence of endogenous BiP visualized by the anti-BiP and Alexa Fluor 568 goat anti-rabbit secondary antibody. C and G, Chlorophyll autofluorescence. D, Overlaid images: A (green) + B (magenta). H, Overlaid images: E (green) + F (magenta). n, Nucleus; v, central vacuole. Scale bar = 5 μm.
All together, these results indicate that glutenin polymers can be found within the ER and that the bulk of the B11-33-HA and C(25, 230)S-HA proteins is retained in this compartment for several hours after synthesis. They also indicate that polymer formation is not required to limit the trafficking of this LMW-GS along the secretory pathway.
DISCUSSION
While a large amount of information is available about the primary structure of LMW-GS and HMW-GS, relatively little is known about the way these proteins assemble to form glutenin polymers. Direct information about the assembly pattern derives either from the analysis of cystine peptides isolated from protease digests of gluten proteins (Tao et al., 1992; Köhler et al., 1993; Keck et al., 1995), from partial reduction of glutenin polymers (Werner et al., 1992), or from the expression in heterologous systems (Shani et al., 1994; Shimoni et al., 1997). Different models of polymer formation have been proposed, but our overall understanding of the rules governing glutenin subunit assembly remain rather limited. Much of the difficulties in analyzing glutenin polymer structure derive from the complexity of the system, where tens of subunits are synthesized in the same endosperm cell, and from the size and physical properties of the resulting polymers.
Here, we have used transient expression in tobacco protoplasts to determine some essential features of the disulfide-mediated polymerization process. We found that expression of individual LMW-GS in this system resulted in the accumulation of both monomeric and oligomeric forms, clearly indicating that these proteins can form homopolymers. A considerable fraction of the protein remained monomeric, suggesting that redox conditions or the concentration of LMW-GS in the tobacco ER do not support full conversion of the subunits into glutenin polymers. This is in contrast with the situation found in wheat, where LMW-GS are efficiently incorporated into polymeric structures. However, it should be noted that the polymerization assay presented in this work only allows us to monitor the short-term fate of these proteins. Long-term maturation processes occurring in wheat endosperm cells (Gupta et al., 1996) may be essential for the quantitative incorporation of LMW-GS into high-Mr polymers.
Our results obtained by expressing Cys mutants provide an overall view of the possible disulfide bonds that can link LMW-GS in glutenin oligomers. Analysis of cystine peptides derived from wheat gluten has previously revealed the presence of disulfide bonds connecting Cb* with Cx and Cb* with Cb* (Köhler et al., 1993; Keck et al., 1995), while it has not revealed the presence of Cx-Cx disulfides and of any disulfide involving Ca. The results obtained with the B11-33 proteins indicate that Ca (C25) can indeed be involved in homodimer assembly by forming an interchain bond either with a second Ca residue or with a Cx (C230) residue. In addition, B11-33 Ca was found to mediate the assembly of B11-33/1B heterodimers by forming an interchain disulfide with either a Cb* (C65) or a Cx (C278) residue in the 1B protein. Thus, the Ca residue appears to be able to form disulfide bonds with any free Cys residue present in the B11-33 and 1B proteins. Analogous conclusions can be drawn concerning the Cx Cys in the B11-33 sequence.
The results obtained from the expression of the 1B mutants were similar to those obtained with the B11-33 mutants, except that we could not identify a form corresponding to a dimer in which C65 (Cb*) forms an interchain disulfide with C278 (Cx). It is, however, possible that such a dimer was indeed present but could not be resolved from the two other dimeric forms of the 1B protein.
It should also be noted that the different dimeric forms of the B11-33 protein were not equally represented; while the B2 head-to-tail form was the most abundant, the B1 tail-to-tail dimer was the less represented. This suggests that in glutenin polymers, certain arrangements of disulfide bonds may be more represented than others.
Overall, these results suggest a model for glutenin polymer assembly where a large number of different polymers can be formed starting from even a limited number of subunits. Conceivably, the different structural characteristics of individual subunits would determine which kind of bonds prevail in the glutenin polymers, and this may have an impact on the chemico-physical properties of the resulting molecule. Formation of interchain disulphide bonds between LMW-GS and HMW-GS (Keck et al., 1995) would further add to the complexity of the polymers and may have a profound impact on their structural and functional characteristics.
Our data also indicate that formation of interchain disulfide bonds is essential for the assembly of stable polymers. After sedimentation velocity centrifugation, monomeric and oligomeric forms of the B11-33 protein were recovered at distinct positions in the gradient, indicating that they were not associated to form stable protein complexes. Consistently, the vast majority of the C(25, 230)S mutant was recovered as a single peak corresponding to the monomeric protein. Although noncovalent interactions must contribute to the assembly process, these do not appear to be sufficient for the formation of complexes able to withstand mild extraction and separation conditions.
Using the tobacco expression system, we could also investigate the role of intrachain disulfide bonds in assembly. Previous in vitro studies have shown that the C134-C169 intrachain disulfide bond plays an essential role in B11-33 structural maturation by preventing the aggregation of the newly synthesized protein (Orsi et al., 2001). Here, we have confirmed that this intrachain disulfide, which is highly conserved throughout the 2S and prolamin families of seed storage proteins (Shewry et al., 1995), plays a crucial role also in an in vivo situation. Indeed the C(134, 169)S mutant of the B11-33 protein could be efficiently immunoselected only after being reduced and denatured, but not under nonreducing conditions. This strongly suggests that, as it occurs in vitro, this mutant rapidly aggregates, forming aberrant interchain disulfide bonds and that subunits present in these aggregates are not available for immunoselection.
The disulfide bond connecting Cd and Ce (C142 and C162 in the B11-33 protein) was instead found to play a minor role in folding and assembly; the protein was soluble, could be efficiently immunoselected, and the assembly pattern was similar to the one of the wild-type protein. The electrophoretic migration of monomeric and oligomeric forms of this mutant was slightly reduced, likely due to a less compact conformation of the subunits in the absence of the C142-C162 bond (Orsi et al., 2001). This intrachain disulfide bond is present in LMW-GS and γ-gliadins but is absent in α-gliadins. This incomplete conservation is consistent with the nonessential role of this bridge in the structural maturation of wheat sulfur-rich prolamins.
The third intrachain disulfide bond (C170-C280) was also found not to be essential to maintain the B11-33 protein in a soluble and assembly-competent configuration. Although we did not establish whether the mechanism of assembly was altered upon the elimination of this bridge, the presence of distinct oligomeric forms suggests that C25 and C230 remain available for interchain disulfide bond formation even in the absence of the C170-C280 bond.
Overall, our results indicate that, rather than being governed by highly specific protein-protein interactions, the assembly of LMW-GS seems to rely on the ability of each Cys residue to form disulfide bonds with a variety of companion Cys present on identical or different subunits. It would be now interesting to exploit this system to examine the interaction between LMW-GS and HMW-GS. In addition, because certain LMW-GS have been associated with good technological characteristics of wheat flours (D'Ovidio and Masci, 2004), it should be possible to determine whether these subunits present different polymerization patterns and/or whether they significantly differ from other LMW-GS in the propensity to form disulfide-linked oligomers. While our experimental approach only allows us to study the early events in glutenin assembly, these initial steps are bound to define the structure of the building blocks that will become the substrates of further polymerization processes during endosperm development (Gupta et al., 1996). These early events might therefore play a crucial role in determining the final technological characteristics of glutenin polymers.
Subcellular fractionation of tobacco protoplasts expressing the B11-33-HA protein revealed the presence of monomers and oligomers in ER-derived vesicles, indicating that the covalent assembly process begins in this compartment. While the donor(s) of oxidizing equivalents for interchain disulfide bond formation remain to be identified, ER-located oxidoreductases (Houston et al., 2005) and plant homologs of the yeast and mammalian Ero proteins (Dixon et al., 2003) are possible players in the pathway leading to the covalent assembly of glutenin subunits.
Whether covalent polymer formation plays any specific role in the deposition of wheat storage proteins is still an open question. Analysis of the fate of the B11-33-HA protein and of the assembly-defective C(25, 230)S-HA mutants did not reveal any major difference in stability and intracellular transport. Both proteins were not secreted and were retained in a microsomal fraction during the first 5 h after synthesis. These findings argue against rapid Golgi-mediated intracellular transport and indicate that retention within the cell is not mediated by the formation of interchain disulfide bonds. Because interchain disulphide bond formation has been found to be essential for the retention of a phaseolin/maize γ-zein fusion (Pompa and Vitale, 2006), our findings suggest that different mechanisms may be involved in the retention of different cereal storage proteins within the ER.
Previous studies performed in Xenopus laevis oocytes revealed that while a γ-gliadin was partially secreted by these cells, the B11-33 protein was fully retained (Altschuler and Galili, 1994). Our data corroborate and extend these findings, suggesting that structural features of the monomer, rather than the ability to form covalent polymers, determine the short-term fate of LMW-GS in the plant secretory pathway. Further studies performed in transgenic tobacco plants will be necessary to establish whether long-term accumulation of a single LMW-GS would result in the transport of this protein to the vacuole via the autophagic route that has been shown to operate in wheat endosperm cells (Levanony et al., 1992).
MATERIALS AND METHODS
Materials
The anti-glutenin antiserum was raised in rabbits using standard procedures using a partially purified HMW-GS fraction from the wheat (Triticum aestivum) cv Yecora Rojo. Immunoglobulins were purified using a HiTrapProtein G column (GE Healthcare). Before use, the purified immunoglobulins were preincubated with a tobacco (Nicotiana tabacum) cv SR1 leaf extract.
Recombinant DNA
To construct plasmids for the transient expression of the wild-type B11-33 protein and the C25S, C230S, C(25, 230)S mutants in tobacco protoplasts, the sequences encoding these proteins (Orsi et al., 2001) were amplified by PCR with oligonucleotides GGATATCTAGAAATGAAGACCTTCCTCGTC and CCGATATCCTGCAGTCAGTAGGCACCAACTCCGGTGCC. The amplification products were digested with XbaI and PstI and cloned in XbaI-PstI-cut pDHA (Tabe et al., 1995).
To construct the pD3 vector, a DNA fragment encoding the triple HA tag was amplified using plasmid pRT104C-3HA as template (a kind gift from Markus Fauth) and oligos GGTTATCTAGAGCTAGCGCAGTCGACCTTCCCGGGTACCCATACGATGTTCCTGAC and CCGATATCCTGCAGTTAGTAATCTGGAACGTCATATG. The amplification product was digested with XbaI and PstI and cloned into XbaI-PstI-cut pDHA.
To place a triple HA tag at the C terminus of the B11-33 and C(25, 230)S proteins, sequences encoding these proteins were amplified using oligonucleotides GGATATCTAGAAATGAAGACCTTCCTCGTC and GGGGTAGGCACCAACTCCGGTGC. The amplification products were cut with XbaI and cloned into XbaI-SmaI-cut pD3. HA-tagged versions of the C25S and C230S mutants were then generated by site-directed mutagenesis using the QuickChange in vitro mutagenesis system (Stratagene) and oligonucleotides GCACAGATGGAGACTAGCTCGATCTCTGGTTTGG and CCAAACCAGAGATCGAGCTAGTCTCCATCTGTGC or GCAGCAGCAGCTCGGACAGAGCTCTTTCCAACAACC and GGTTGTTGGAAAGAGCTCTGTCCGAGCTGCTGCTGC, respectively.
Plasmids for the expression of HA-tagged mutated forms of the B11-33 protein lacking individual intrachain disulfide bonds were obtained by PCR amplification of the sequences encoding the C134-169S, C142-162S, and C170-280S mutants (Orsi et al., 2001) using oligonucleotides GGATATCTAGAAATGAAGACCTTCCTCGTC and GGGGTAGGCACCAACTCCGGTGC. The amplification products were digested with XbaI and cloned into XbaI-SmaI-cut pD3 vector. The encoded proteins contain a triple-HA tag at the C terminus.
To append the FLAG octapeptide (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys) at the C terminus of the B11-33 C25S and C230S proteins, sequences encoding these proteins were sequentially amplified using the same forward oligonucleotide (GGATATCTAGAAATGAAGACCTTCCTCGTC) and the reverse oligonucleotides TCATCGTCATCCTTGTAATCGTAGGCACCAACTCCGGTGCC and CCGATATCCTGCAGTCACTTGTCATCGTCATCCTTGTAATC. The final amplification product was then digested with XbaI and PstI and cloned into XbaI-PstI-cut pDHA.
To construct an expression plasmid encoding the 1B protein bearing a triple HA tag at the C terminus (1B-HA), the pLDNLMW1B sequence (D'Ovidio et al., 1997) was amplified using oligos GGATATCTAGAAATGAAGACCTTCCTCATC and GGGGTAGGCACCAACTCCGGTGC. The amplification product was cut with XbaI and cloned into XbaI-SmaI-cut pD3.
Plasmids encoding the C65S and C278S mutants of the 1B-HA protein (C65S-HA and C278S-HA) were obtained by site directed mutagenesis using the QuickChange in vitro mutagenesis system (Stratagene) and oligonucleotides CCATTTCCACAACAGCCACCGAGCTCACAGCAACAACAACC and GGTTGTTGTTGCTGTGAGCTCGGTGGCTGTTGTGGAAATGG, or CAGAAGCAGCTCGGACAGAGCTCTTTCCAACAACC and GGTTGTTGGAAAGAGCTCTGTCCGAGCTGCTTCTG, respectively.
Protoplast Isolation, Transfection, Labeling, and Immunoprecipitation of Radiolabeled Proteins
Isolation of tobacco protoplasts from axenic SR1 plants, transfection, and labeling with Pro-mix l-[35S] in vitro cell labeling mix (GE Healthcare) were performed essentially as previously described (Ceriotti et al., 2003). Homogenization and immunoprecipitation under nondenaturing conditions was performed by resuspending the protoplast pellet in protoplast homogenization buffer (150 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1.5 mm EDTA, 1.5% Triton X-100) supplemented with 1× Complete protease inhibitor mixture (Roche Diagnostics), 1.5 mm phenylmethanesulfonyl fluoride (PMSF), and 70 mm iodoacetamide. The samples were clarified by centrifugation at 13,000g for 5 min, diluted with NET-Gel buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 0.1% Igepal CA-630, 0.25% gelatin, 0.02% NaN3), and used for immunoselection with anti-glutenin or anti-HA 12CA5 antibodies. For selection of FLAG-tagged proteins, the cleared homogenates were diluted with 3 volumes of 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100 before immunoselection with ANTI-FLAG M2-Agarose Affinity Gel (Sigma).
Homogenization under denaturing conditions was performed by resuspending the protoplast pellet in 50 mm Tris-HCl, pH 7.4, 1% SDS, 5 mm EDTA, 1× Complete, 1 mm PMSF, 15 units/mL DNase I, and either 10 mm iodoacetamide or 10 mm dithiothreitol (DTT). The samples were heated for 5 min in boiling water, diluted with 9 volumes of 50 mm Tris-HCl, pH 7.4, 1% Triton X-100, 300 mm NaCl, 5 mm EDTA, 10 mm iodoacetamide, 0.02% NaN3 and clarified by centrifugation. Bovine serum albumin was added at 0.1% final concentration before immunoprecipitation with the anti-HA 12CA5 monoclonal antibody and Protein A Sepharose CL-4B (GE Healthcare). Immunoprecipitated proteins were analyzed by SDS-PAGE under nonreducing or reducing conditions (Orsi et al., 2001).
Sedimentation Velocity Centrifugation on Suc Gradients
Protoplast pellets were homogenized in protoplast homogenization buffer supplemented with 1× Complete protease inhibitor mixture, 1.5 mm PMSF, and 70 mm iodoacetamide. The homogenate was clarified by centrifugation (5 min at 13,000g), and one aliquot of the supernatant was used directly for immunoselection of B11-33 polypeptides using anti-glutenin antibodies. The rest was loaded on a 4.3-mL linear Suc gradient (5%–25%, w/v) in 20 mm Tris-HCl, pH 7.5, 100 mm NaCl, 0.1% Triton-X100. Gradients were centrifuged for 16 h at 45,000 rpm in a SW 55 Ti rotor (Beckman) at 4°C and fractionated from the top using an Auto-Densi-Flow apparatus (Labconco). Gradient fractions were diluted with NET-gel, and B11-33 polypeptides were immunoselected with anti-glutenin antibodies. Material sedimented at the bottom of the tube was solubilized by heating for 10 min at 80°C in 20 mm Tris-HCl, pH 7.4, 5 mm EDTA, and 1% SDS. The solubilized material was diluted with 9 volumes of 50 mm Tris-HCl, pH 7.4, 1% Triton X-100, 300 mm NaCl, 5 mm EDTA, 10 mm iodoacetamide, and 0.02% NaN3 and clarified by centrifugation. Bovine serum albumin was added at 0.1% final concentration, and proteins were immunoselected with anti-glutenin antibodies.
Protoplast Fractionation on Suc Step Gradient
Protoplast pellets were resuspended in 550 μL 12% (w/w) Suc in step gradient buffer (100 mm Tris-HCl, pH 7.6, 10 mm KCl, 1 mm EDTA, 70 mm iodoacetamide) supplemented with Complete protease inhibitor mixture and homogenized by pipetting 40 times with a Gilson-type micropipette through a 200-μL tip and then by passing the sample four times through a 25G needle. Intact cells and debris were removed by centrifugation for 5 min at 500g. The supernatant (400 μL) was loaded on a 17% (w/w) Suc pad in step gradient buffer and centrifuged at 35,000 rpm for 30 min at 4°C in a SW 55Ti rotor. Pellets (microsomes) and supernatants (soluble proteins) were diluted in protoplast homogenization buffer and used for immunoprecipitation.
Isopycnic Suc Gradient Centrifugation
Protoplast pellets were homogenized in ice-cold gradient buffer (100 mm Tris-HCl, pH 7.8, 10 mm KCl, 2 mm MgCl2) containing 70 mm iodoacetamide, 1× Complete, and 12% Suc (w/w). The extract was clarified by centrifugation at 1,000g for 5 min. An aliquot of the supernatant was directly used for immunoselection of B11-33-HA polypeptides using the 12CA5 anti-HA monoclonal antibody, while the rest was loaded on a 11-mL Suc (16%–55%, w/w) gradient in gradient buffer made on top of a 1-mL cushion of 55% Suc in the same buffer. After centrifugation at 35,000 rpm for 2 h at 4°C in a Beckman SW40 rotor, fractions were collected from the top using an Auto-Densi-Flow apparatus (Labconco), made 2 mm in EDTA, and then diluted with 0.33 volumes of 10 mm Tris-HCl, pH 7.5, 600 mm NaCl, 8 mm EDTA, 4% Triton X-100, 200 mm iodoacetamide, 1× Complete, and 4 mm PMSF. The samples were then made 0.25% in gelatin, centrifuged 2 min at 10,000g, and used for immunoprecipitation. Material sedimented at the bottom of the tube was recovered in protoplast homogenization buffer. The sample was diluted with NET-gel buffer, centrifuged for 2 min at 10,000g, and used for immunoprecipitation.
Immunofluorescence and Confocal Analysis
Fluorescence immunolabeling of fixed protoplasts was performed as described in Ceriotti et al. (2003). After double staining with the mAb 12CA5 anti-HA in combination with a polyclonal anti-BiP antiserum (Pedrazzini et al., 1997), samples were reacted with Alexa Fluor 488 goat anti-mouse and Alexa Fluor 568 goat anti-rabbit highly cross-adsorbed secondary antibodies (Invitrogen-Molecular Probes). The last wash was performed in the presence of 4′,6-diamidino-2-phenylindole (Merck) at the concentration of 1 μg/mL. Cells were analyzed with a Zeiss LSM 510 microscope at 488 nm excitation for Alexa Fluor 488 and 543 nm excitation for Alexa Fluor 568. Emissions were detected using bandpass filters 505–550 nm or 550–614 nm, respectively.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers M11077 (LMW-GS clone pB11-33) and Y14104 (LMW-GS clone pLDNLMW1B).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Data S1. Computation of the expected number of different polymers made of k subunits.
Supplemental Figure S1. Cys mutants of the B11-33 and 1B LMW-GS form covalently linked heterodimers.
Supplementary Material
Acknowledgments
We thank Nica Borgese, Sara Colombo, and Maura Francolini for assistance in confocal microscopy and Maria Serena Fabbrini and Donald Kasarda for critical reading of the manuscript.
This work was supported by MIUR-FIRB project RBNE01TYZF and by the MIUR-FAR project AGRO-GEN.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Aldo Ceriotti (ceriotti@ibba.cnr.it).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Altschuler Y, Galili G (1994) Role of conserved cysteines of a wheat gliadin in its transport and assembly into protein bodies in Xenopus oocytes. J Biol Chem 269 6677–82 [PubMed] [Google Scholar]
- Ceriotti A, Vitale A, Paris N, Frigerio L, Neuhaus J-M, Hillmer S, Robinson DG (2003) Plant cell biology. In J Davey, JM Lord, eds, Essential Cell Biology, Vol 1. Oxford Univesity Press, Oxford, pp 133–161
- Dixon DP, Van Lith M, Edwards R, Benham A (2003) Cloning and initial characterization of the Arabidopsis thaliana endoplasmic reticulum oxidoreductins. Antioxid Redox Signal 5 389–96 [DOI] [PubMed] [Google Scholar]
- D'Ovidio R, Masci S (2004) The low-molecular-weight glutenin subunits of wheat gluten. J Cereal Sci 39 321–339 [Google Scholar]
- D'Ovidio R, Simeone M, Masci S, Porceddu E (1997) Molecular characterization of a LMW-GS gene located on chromosome 1B and the development of primers specific for the Glu-B3 complex locus in durum wheat. Theor Appl Genet 95 1119–1126 [Google Scholar]
- Galili G, Shimoni Y, Giorini-Silfen S, Levanony H, Altschuler Y, Shani N (1996) Wheat storage proteins: assembly, transport and deposition in protein bodies. Plant Physiol Biochem 34 245–252 [Google Scholar]
- Gianibelli MC, Larroque OR, MacRitchie F, Wrigley CW (2001) Biochemical, genetic, and molecular characterization of wheat glutenin and its component subunits. Cereal Chem 78 635–646 [Google Scholar]
- Gupta RB, Masci S, Lafiandra D, Bariana HS, MacRitchie F (1996) Accumulation of protein subunits and their polymers in developing grains of hexaploid wheats. J Exp Bot 47 1377–1385 [Google Scholar]
- Houston NL, Fan C, Xiang JQ, Schulze JM, Jung R, Boston RS (2005) Phylogenetic analyses identify 10 classes of the protein disulfide isomerase family in plants, including single-domain protein disulfide isomerase-related proteins. Plant Physiol 137 762–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanony H, Rubin R, Altschuler Y, Galili G (1992) Evidence for a novel route of wheat storage proteins to vacuoles. J Cell Biol 119 1117–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasarda DD (1989) Gluten structure in relation to wheat quality. In Y Pomeranz, ed, Wheat Is Unique. American Association of Cereal Chemistry, St. Paul, pp 277–302
- Keck B, Köhler P, Wieser H (1995) Disulphide bonds in wheat gluten: cystine peptides derived from gluten proteins following peptic and thermolytic digestion. Z Lebensm Unters Forsch 200 432–439 [DOI] [PubMed] [Google Scholar]
- Köhler P, Belitz HD, Wieser H (1993) Disulphide bonds in wheat gluten: further cystine peptides from high molecular weight (HMW) and low molecular weight (LMW) subunits of glutenin and from γ-gliadins. Z Lebensm Unters Forsch 196 239–47 [DOI] [PubMed] [Google Scholar]
- Müller S, Vensel WH, Kasarda DD, Köhler P, Wieser H (1998) Disulphide bonds of adjacent cysteine residues in low molecular weight subunits of wheat glutenin. J Cereal Sci 27 109–116 [Google Scholar]
- Okita TW, Cheesbrough V, Reeves CD (1985) Evolution and heterogeneity of the α-/β -type and γ-type gliadin DNA sequences. J Biol Chem 260 8203–8213 [PubMed] [Google Scholar]
- Orsi A, Sparvoli F, Ceriotti A (2001) Role of individual disulfide bonds in the structural maturation of a low molecular weight glutenin subunit. J Biol Chem 276 32322–32329 [DOI] [PubMed] [Google Scholar]
- Pedrazzini E, Giovinazzo G, Bielli A, de Virgilio M, Frigerio L, Pesca M, Faoro F, Bollini R, Ceriotti A, Vitale A (1997) Protein quality control along the route to the plant vacuole. Plant Cell 9 1869–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompa A, Vitale A (2006) Retention of a bean phaseolin/maize γ-Zein fusion in the endoplasmic reticulum depends on disulfide bond formation. Plant Cell 18 2608–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevier CS, Kaiser CA (2002) Formation and transfer of disulphide bonds in living cells. Nat Rev Mol Cell Biol 3 836–847 [DOI] [PubMed] [Google Scholar]
- Shani N, Rosenberg N, Kasarda DD, Galili G (1994) Mechanisms of assembly of wheat high molecular weight glutenins inferred from expression of wild-type and mutant subunits in transgenic tobacco. J Biol Chem 269 8924–8930 [PubMed] [Google Scholar]
- Shewry PR, Napier JA, Tatham AS (1995) Seed storage proteins: structures and biosynthesis. Plant Cell 7 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Blechl AE, Anderson OD, Galili G (1997) A recombinant protein of two high molecular weight glutenins alters gluten polymer formation in transgenic wheat. J Biol Chem 272 15488–15495 [DOI] [PubMed] [Google Scholar]
- Tabe LM, Wardley-Richardson T, Ceriotti A, Aryan A, McNabb W, Moore A, Higgins TJ (1995) A biotechnological approach to improving the nutritive value of alfalfa. J Anim Sci 73 2752–2759 [DOI] [PubMed] [Google Scholar]
- Tao HP, Adalsteins AE, Kasarda DD (1992) Intermolecular disulphide bonds link specific high-molecular weight glutenin subunits in wheat endosperm. Biochim Biophys Acta 1159 13–21 [DOI] [PubMed] [Google Scholar]
- Tatham AS, Shewry PR (1998) Disulphide bonds in wheat gluten proteins. J Cereal Sci 25 207–227 [Google Scholar]
- Werner WE, Adalsteins AE, Kasarda DD (1992) Composition of high-molecular-weight glutenin subunit dimers formed by partial reduction of residue glutenin. Cereal Chem 69 535–541 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.