A major part of the daily caloric intake of human societies around the world is derived from a diverse range of foods prepared from members of the grass family, including wheat (Triticum aestivum), rice (Oryza sativa), sorghum (Sorghum bicolor), the millets (Panicum miliaceum and Pennisetum americanum), barley (Hordeum vulgare), and sugar cane (Saccharum officinarum). Grasses cover perhaps 20% or more of the earth's land surface (Gaut, 2002), and many of these are used as forage and fodder for the production of sheep, cattle, and other domesticated livestock. Maize (Zea mays) is also used widely in animal feed diets, while sorghum, switchgrass (Panicum virgatum), and several other perennial grasses are attracting considerable attention as future biomass energy crops (McLaren, 2005).
The grasses are noteworthy for the unusual composition of their cell walls, because walls of grasses have less pectin and xyloglucan, but more heteroxylan, than walls from other higher plants. Most significantly, walls of the grasses contain as major constituents the (1,3;1,4)-β-d-glucans, which are not widely distributed outside the Poaceae. The compositions of walls from selected barley organs are shown in Table I. In many cases, constituents of cell walls in the grasses are closely linked with their widespread adoption, utility, and future potential in agricultural practice and energy production. The noncellulosic polysaccharides of walls in grasses are important components of dietary fiber, which is highly beneficial for lowering the risk of serious human health conditions, including colorectal cancer, high serum cholesterol and cardiovascular disease, obesity, and non-insulin-dependent diabetes (Braaten et al., 1994; Brennan and Cleary, 2005). Conversely, the noncellulosic wall polysaccharides from walls of cereals and grasses have antinutritive effects in monogastric animals such as pigs and poultry (Brennan and Cleary, 2005) and are often considered to be undesirable components of raw materials in the malting and brewing industries (Kuntz and Bamforth, 2007).
Table I.
Comparison of cell wall polysaccharide composition in various barley tissues
Note the relatively low levels of xyloglucans and pectin in these walls, particularly those from grain. ND, Not detected.
| Tissue | Cellulose | (1,3;1,4)-β-Glucan | Heteroxylan | Xyloglucan | Pectin |
|---|---|---|---|---|---|
| Starchy endosperm | 3% | 70% | 20% | Trace | ND |
| Aleurone | 2% | 26% | 71% | ND | ND |
| Coleoptile (4 d) | 35% | 10% | 32% | 10% | 12% |
| Young leaf | 63% | 16% | 11% | Trace | 5% |
| Stem | 65% | 5% | 28% | ND | ND |
Although there have been exciting new discoveries in the synthesis of cellulose, pectic polysaccharides, mannans, and xyloglucans in recent years, these discoveries have been made predominantly in dicotyledonous plants (Ye et al., 2006; Mohnen, 2008; Zabotina et al., 2008) and will not be covered here. This update, therefore, will be restricted to recent advances in our understanding of the biosynthesis of the characteristic and major wall polysaccharides of the grasses, namely the heteroxylans and (1,3;1,4)-β-d-glucans.
So, why might we argue that we have come upon revolutionary times in our understanding of cell wall biosynthesis in the grasses? What new information has come to light in recent years? Progress in defining the genes and biological mechanisms underlying the synthesis of the major polysaccharides of walls in the grasses had remained painfully slow throughout the biochemical and molecular biological eras, mainly because the enzymes that catalyze the biosynthetic reactions are membrane proteins that usually lose activity quickly after cell disruption, before purification of the enzymes can be effected. Without even partially purified enzyme preparations, we were unable to obtain amino acid sequence information and hence could not identify the corresponding genes. However, emerging technologies of forward and reverse genetics and functional genomics have provided new tools to tackle these difficult problems and have yielded spectacular results. Thus, comparative genomics and forward genetics have been used to identify candidate genes that encode polysaccharide synthases involved in (1,3;1,4)-β-d-glucan biosynthesis in the grasses, while powerful bioinformatic techniques are providing important clues and candidates for the enzymes that mediate in the biosynthesis of the other key wall polysaccharide of the grasses, namely the heteroxylans. Data generated in these studies have raised ancillary but fundamental questions about the subcellular location of wall polysaccharide synthesis in the grasses. Is the Golgi apparatus the only site for the complete synthesis of matrix phase polysaccharides of the wall, including the (1,3;1,4)-β-d-glucans and the heteroxylans, or are there other possibilities? Functional genomics analyses have also pointed to previously unsuspected roles for hydrolytic enzymes and transglycosylases in wall polysaccharide synthesis and remodeling in the grasses. In particular, the highly abundant xyloglucan transglycosylases/hydrolases (XTHs) might act not only as modulators of xyloglucan structure but also as heterotransglycosylating enzymes that covalently link different classes of polysaccharides in the wall. There have been hints in the literature for some time that wall polysaccharides might be covalently linked, but experimental evidence that had not previously been available is now starting to build.
While much of the new data have been highly informative with respect to the genes and enzymes involved in the biosynthesis of wall polysaccharides in the grasses, many of the additional questions mentioned above challenge the way in which wall polysaccharide biosynthesis and remodeling have traditionally been viewed. In this brief update, recent breakthroughs in the identification of genes that mediate wall synthesis in the grasses are discussed in the context of the complexities of chemical structures of the synthesized polysaccharides, their heterogeneity in structure and size, their physicochemical properties and functional imperatives, and their remodeling during growth and development.
PROPERTIES OF MAJOR NONCELLULOSIC CELL WALL POLYSACCHARIDES IN THE GRASSES
To fully appreciate the complexity of biochemical and cellular processes that lead to wall biosynthesis in the grasses, it is important to understand the chemical structures of the wall polysaccharides and the changes that occur to them during plant cell growth and development as well as how these structures are tailored to the functional needs of the wall. Therefore, structural characteristics of the two major wall polysaccharides of the grasses are briefly outlined below.
Heteroxylans
The heteroxylans that are abundant in walls of the grasses can be classified into two main types, namely the arabinoxylans and the glucuronoarabinoxylans. The arabinoxylans are the major noncellulosic polysaccharides in walls of starchy endosperm and aleurone layer cells in cereal grains, whereas the glucuronoarabinoxylans are characteristically found in walls of the pericarp seed coat tissues (Fincher and Stone, 2004).
Arabinoxylans of the grasses consist of a (1,4)-β-d-xylan backbone that has essentially the same conformation as a (1,4)-β-d-glucan, or cellulose. The β-d-xylopyranosyl (Xylp) units of the xylan backbone are substituted with single α-l-arabinofuranosyl (Araf) units, situated predominantly at C(O)3, but also at C(O)2 of the Xylp units, and in some cases at both C(O)3 and C(O)2 (Vietor et al., 1993; Fig. 1, A and B). The degree of Araf substitution depends on the species and wall type and is reflected in a wide range of Xylp-Araf ratios (Fincher and Stone, 2004). The distribution of Araf units along the xylan chain varies (Vietor et al., 1993). In some wheat arabinoxylans, isolated unsubstituted Xylp units are separated by one or two monosubstituted or disubstituted units. In others, Araf units linked at C(O)3 are clustered in discrete regions or there may be frequent areas of up to six or more contiguous, unsubstituted Xylp units. The latter might provide the potential for junction zone formation.
Figure 1.
Structural features of some common noncellulosic polysaccharides from cell walls of higher plants. A, Structure of a portion of a (1,4)-β-d-xylan backbone with a xylosyl residue substituted at C(O)3 with an α-l-arabinofuranosyl residue, which in turn is substituted at C(O)5 with a ferulic acid residue (Fincher and Stone, 2004). B, Substitution patterns of heteroxylans, which consist of a (1,4)-β-d-xylan backbone (blue) substituted at C(O)3 and C(O)2, and sometimes at both carbon atoms, with α-l-arabinofuranosyl residues (cyan). Some α-l-arabinofuranosyl residues are substituted with ferulic acid (red). In addition, the (1,4)-β-d-xylan backbone can be substituted with α-d-glucuronosyl residues and their methyl esters, while some xylosyl residues of cereal heteroxylans can be acetylated (Fincher and Stone, 2004). Some regions of the (1,4)-β-d-xylan backbone are unsubstituted. More details of heteroxylan structures are provided by York and O'Neill (2008). C, Diagrammatic representation of a xyloglucan, showing the (1,4)-β-d-glucan backbone (blue) substituted at C(O)6 with α-d-xylopyranosyl residues (red) or with short oligosaccharide chains of α-d-xylopyranosyl, β-d-galactopyranosyl (green), and α-l-fucopyranosyl residues. D, Diagrammatic representation of a (1,3;1,4)-β-d-glucan, showing (1,4)-β-d-glucosyl residues (blue) and (1,3)-β-d-glucosyl residues (red). The (1,3)-β-d-glucosyl residues introduce molecular kinks into the chain, and the irregular spacing of the (1,3)-β-d-glucosyl residues results in an asymmetric polysaccharide that can be soluble at high degrees of polymerization. It should be noted that while blocks of two or three adjacent (1,4)-β-d-glucosyl residues predominate, up to 10% of the polysaccharide chain consists of longer blocks of five to 20 adjacent (1,4)-β-d-glucosyl residues (Fincher and Stone, 2004).
The glucuronoarabinoxylans also contain substituents of d-GlcUA (GlcAp) and its 4-O-methyl ester, linked to the C(O)2 of Xylp units of the xylan backbone. A number of the Araf units in the arabinoxylans of grasses can be esterified with the hydroxycinnamic acids, ferulic acid and, to a lesser extent, its nonmethoxylated analog p-coumaric acid. The hydroxycinnamates are found at C(O)5 of Araf units that are linked to C(O)3 of the Xylp units (Fig. 1A).
Pena et al. (2007) demonstrated recently that glucuronoarabinoxylans from various dicotyledonous plants have an oligosaccharide consisting of 4-β-d-Xylp-(1,4)-β-d-Xylp-(1,3)-α-l-Rhap-(1,2)-α-d-GalpA-(1,4)-d-Xylp at their reducing termini, but attempts to identify a similar structure in arabinoxylans from grasses has so far been unsuccessful (W. York, personal communication).
The Araf and other substituents sterically inhibit aggregation of the (1,4)-β-d-xylan chains and lead to the formation of an extended, asymmetrical polysaccharide that has physicochemical properties suited to its function as a major matrix phase component of walls in grasses. The physicochemical properties of the arabinoxylans are similar to those of the xyloglucans and other wall polysaccharides, but they are achieved through different chemistries (Fig. 1C).
In summary, the heteroxylans from walls of the grasses have diverse chemical structures that are likely to be modified in response to changing functional requirements of the wall during growth and development. It is highly probable that synthesis of the heteroxylans involves the action of multiple polysaccharide synthase and/or glycosyl transferase enzymes. Progress toward the identification of these enzymes and the genes that encode them is summarized in the following sections.
(1,3;1,4)-β-d-Glucans
(1,3;1,4)-β-d-Glucans are unsubstituted, unbranched polysaccharides containing β-d-glucopyranosyl monomers polymerized through both (1,3)- and (1,4)-linkages. Within the grasses, barley, oat (Avena sativa), and rye (Secale cereale) grains are rich sources of (1,3;1,4)-β-d-glucans, while wheat, rice, and maize have lower concentrations of the polysaccharide (Fincher and Stone, 2004). The (1,3;1,4)-β-d-glucans are relatively minor components of walls in vegetative tissues of cereals and grasses. While it is often assumed that (1,3;1,4)-β-d-glucans are found only in the Poaceae, molecules with similar structures have been reported in species of angiosperms that are members of a major clade of the enlarged order Poales, which is referred to as the “core Poales” and includes the Poaceae and closely related families (Trethewey et al., 2005). In addition, (1,3;1,4)-β-d-glucans are found in lichens such as Iceland moss (Cetraria islandica; Olafsdottir and Ingolfsdottir, 2001), where they are located in walls of the mycobiont or fungal partner of the symbiosis (Honegger and Haisch, 2001), in certain fungal cell walls (Fontaine et al., 2000), in various algal species (Nevo and Sharon, 1969; Popper and Fry, 2003), in bryophytes (Popper and Fry, 2003), and in the cell walls of the horsetail (Equisetum species; Fry et al., 2008b; Sørensen et al., 2008).
The ratio of (1,4)- to (1,3)-linkages in the water-soluble (1,3;1,4)-β-d-glucan from barley ranges from 2.2:1 to 2.6:1. The two types of linkages are not arranged in regular, repeating sequences, but equally, they are not arranged at random. The (1,3)-β-d-glucosyl residues always occur as single residues between (1,4)-β-d-oligoglucosyl units; adjacent (1,3)-β-d-glucosyl residues are not present, at least in the barley (1,3;1,4)-β-d-glucan. The single (1,3)-β-d-glucosyl residues are separated by two or more (1,4)-β-d-oligoglucosyl units (Fig. 1D). The (1,4)-β-d-oligoglucosyl units generally consist of two or three adjacent (1,4)-β-d-glucosyl residues, but if one examines the sequence of these units along the polysaccharide chain, again there is no order in their arrangement of these units. Indeed, these higher level oligosaccharide units are arranged at random (Staudte et al., 1983; Woodward et al., 1983a; Buliga et al., 1986). Furthermore, up to 10% of the polysaccharide chain consists of longer blocks of five to 20 adjacent (1,4)-β-d-glucosyl residues. Thus, cereal (1,3;1,4)-β-d-glucans may be considered as (1,3)-β-linked copolymers of cellotriosyl units, cellotetraosyl units, and longer (1,4)-β-d-oligoglucosyl units, as underlined below:
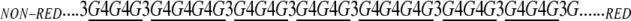 |
The ratio of cellotriosyl to cellotetraosyl units varies between species. In wheat, they range from 3.0:1 to 4.5:1, in barley from 2.9:1 to 3.4:1; in rye, the ratio is about 2.7:1, and in oats it is 1.8:1 to 2.3:1 (Fincher and Stone, 2004).
The net effect of these linkage arrangements is the irregular distribution of (1,3)-β-d-glucosyl residues along what would otherwise be a regular, “cellulosic” chain. The (1,3)-β-d-glucosyl residues cause molecular “kinks” in the chain, and the irregular occurrence of these kinks means not only that the overall shape of the polysaccharide is irregular but also that the molecules will not align over extended regions. (1,3;1,4)-β-d-Glucans with these types of structure, therefore, will remain in solution even when their degree of polymerization exceeds 1,000 (Woodward et al., 1983b). Within the wall, however, the longer blocks of adjacent (1,4)-β-d-glucosyl residues might provide the potential for alignment over limited regions of the polysaccharide and hence for junction zone formation between (1,3;1,4)-β-d-glucan chains and with other wall polysaccharides such as cellulose or arabinoxylans (Carpita et al., 2001; Fincher and Stone, 2004).
In aqueous media, barley (1,3;1,4)-β-d-glucans adopt an extended reptate conformation with an axial ratio (length-width) of about 100 (Woodward et al., 1983b). This asymmetrical conformation has presumably evolved to enable the polysaccharide to function as the gel-like matrix phase component of the wall. The gel-like structure allows the polysaccharide to provide some degree of structural support for the wall but to remain flexible, pliable, and sufficiently porous to permit the transfer of water, nutrients, and other small molecules across the wall during tissue growth and development. The same asymmetry is responsible for the high viscosities of (1,3;1,4)-β-d-glucans in aqueous media and hence for the undesirable characteristics attributed to (1,3;1,4)-β-d-glucans in animal feed formulations and in malting and brewing processes. On the other hand, the conformational asymmetry of wall polysaccharides in grasses is responsible for the beneficial effects of barley and other (1,3;1,4)-β-d-glucans on human health and nutrition (Brennan and Cleary, 2005).
As mentioned above, the reason why such considerations of (1,3;1,4)-β-d-glucan structure are important here is that molecular mechanisms proposed for their synthesis must take into account the chemical and physicochemical parameters that are observed in the final form of the polysaccharide in the cell wall. The proposed mechanisms, therefore, must explain how it is that only single (1,3)-β-d-glucosyl residues are inserted between blocks of adjacent (1,4)-β-d-glucosyl residues that are mostly three or four residues in length, but may be up to 15 (1,4)-β-d-glucosyl residues long, and how it is that the cellotriosyl and cellotetraosyl units are randomly distributed along the chain.
BIOSYNTHESIS OF (1,3;1,4)-β-d-GLUCANS IN THE GRASSES
For many years, biochemical approaches were taken in attempts to define the properties of enzymes required for arabinoxylan and (1,3;1,4)-β-d-glucan synthesis in walls of the grasses, and these usually involved the isolation of total microsomal fractions from homogenates of selected tissues and, in many cases, the enrichment of these for Golgi-derived membranes. Activity was subsequently measured as the incorporation by the membrane preparations of [14C]Glc from UDP-[14C]Glc into ethanol-insoluble polymeric material (Henry and Stone, 1982; Gibeaut and Carpita, 1993). However, the membrane-bound biosynthetic enzymes lost activity very quickly following lysis of cells, a variety of polysaccharides became labeled with the [14C]Glc, including (1,4)-, (1,3)-, and (1,3;1,4)-β-d-glucan, and overall incorporation of radiolabel into polysaccharide was usually low. As a result, there were no published reports of purified (1,3;1,4)-β-d-glucan synthases or xylan synthases from the grasses. Consequently, no amino acid sequence information could be generated using this approach, and the identities of the genes encoding the synthases, therefore, remained unknown.
However, new techniques of functional and comparative genomics have allowed novel approaches to be taken, and this is one area where significant progress in our understanding of wall polysaccharide biosynthesis in the grasses has been realized. Thus, the cellulose synthase-like CslF family of genes (Fig. 2) was recently implicated in (1,3;1,4)-β-d-glucan synthesis in the grasses (Burton et al., 2006, 2008), and it is likely that the genes that mediate xylan biosynthesis will soon be identified (Brown et al., 2007; Mitchell et al., 2007; Pena et al., 2007).
Figure 2.
Updated phylogenetic tree of cellulose synthase and cellulose synthase-like gene families in higher plants. The cellulose synthase (CesA) and Csl gene families from higher plants contain close to 50 members and have been classified into a number of subfamilies (Richmond and Somerville, 2000; Hazen et al., 2002; http://cellwall.stanford.edu/). Of these subfamilies, the CslF and CslH genes were shown to be “specific” for the Poaceae, while the CslB and CslG groups appear to be found only in eudicots (Hazen et al., 2002). The CslJ group is found in certain cereals, including barley, wheat, sorghum, and maize, but not in rice or Brachypodium. The gene name for the sorghum CslJ is Sb03g047220.1, and barley EST numbers for the HvCslJ genes are CA011599, CA002678, and CA000498. This phylogenetic tree was prepared by Andrew Harvey and is updated from a similar figure published previously (Farrokhi et al., 2006). It should be noted that the separation of clades can depend on software programs and settings used.
The experimental data that implicated the CslF genes as potential participants in (1,3;1,4)-β-d-glucan synthesis were based on comparative genomics in rice and barley. Molecular markers flanking a major quantitative trait locus for (1,3;1,4)-β-d-glucan content in barley grain (Han et al., 1995) allowed the syntenic region to be located on the rice genome. In that syntenic region of rice, a cluster of six rice OsCslF genes was detected, and these genes became prime candidates for a role in (1,3;1,4)-β-d-glucan synthesis (Burton et al., 2006). The rice genes were subsequently inserted into Arabidopsis (Arabidopsis thaliana), which does not have CslF genes or (1,3;1,4)-β-d-glucans in its walls. (1,3;1,4)-β-d-Glucans were deposited in the walls of the transgenic Arabidopsis lines (Burton et al., 2006). This suggested that the rice CslF genes were able to direct the synthesis of (1,3;1,4)-β-d-glucans, but it was also concluded that other genes would probably be necessary for (1,3;1,4)-β-d-glucans synthesis (Burton et al., 2006). Later, it was demonstrated that there are at least seven HvCslF genes in barley; these have been mapped and their transcription patterns defined (Burton et al., 2008).
It is noteworthy that although there appears to be only one wall polysaccharide that is “specific” for the grasses, namely the (1,3;1,4)-β-d-glucans, there are three “cereal-specific” groups of Csl genes, namely the CslFs, CslHs, and CslJs (Richmond and Somerville, 2000; Hazen et al., 2002; Fig. 2). The CslJ group was briefly mentioned by Farrokhi et al. (2006), but we would now propose that this group be formally recognized as a new subgroup of Csl genes and provide EST and gene accession numbers in support of this new classification (Fig. 2). The designation CslJ was chosen as the next letter in the alphabet (Hazen et al., 2002), after omitting CslI on the grounds that the letter I can be difficult to distinguish from the numeral 1. The CslJ group is only found in certain grasses, including barley, wheat, sorghum, and maize, but not in rice or Brachypodium. We have started to investigate the potential roles of CslH and CslJ genes in (1,3;1,4)-β-d-glucan synthesis in barley. Using similar methods to those used for the functional analysis of the rice CslF genes in Arabidopsis, it has been demonstrated that the single HvCslH gene can also mediate (1,3;1,4)-β-d-glucan synthesis in transgenic Arabidopsis (M. Doblin, S.M. Wilson, F. Pettolino, R.A. Burton, E.J. Newbigin, G.B. Fincher, and A. Bacic, unpublished data), and our preliminary association mapping data suggest that the HvCslJ genes could also be involved (D.E. Mather, R.A. Burton, and G.B. Fincher, unpublished data).
If indeed the biosynthesis of (1,3;1,4)-β-d-glucan in grasses requires the action of multiple enzymes or involves a multienzyme complex in which an individual CslF isoenzyme represents just one component, then we are still a long way from describing the biosynthetic process in detail (Burton et al., 2008). Furthermore, and as noted above, the mechanisms for the arrangements of (1,3)-β-d-glucosyl and (1,4)-β-d-glucosyl residues along the chain have not yet been characterized at the biosynthetic level. An obvious initial question is whether specific CslF or CslH enzymes catalyze the synthesis of (1,3;1,4)-β-d-glucans with different structures. Of particular interest here will be the specificity of the HvCslF6 enzyme, which has a 54-amino acid insert in the cytosolic region (Burton et al., 2008). The complexity of the (1,3;1,4)-β-d-glucan structure would be expected to be reflected in a similar complexity in the enzymic mechanism for (1,3;1,4)-β-d-glucan biosynthesis. Buckeridge et al. (2001) present data in support of an intriguing model to explain the mechanisms for incorporating the structural variants of (1,3;1,4)-β-d-glucans in maize. Urbanowicz et al. (2004) propose that a cellulose synthase-like core catalytic domain makes up one component of a (1,3;1,4)-β-d-glucan synthase complex and synthesizes the cellotetraosyl units and larger even-numbered oligomeric units, while a separate glycosyl transferase is associated with the Csl component to add the glucosyl residues that complete the cellotriosyl and larger odd-numbered units. Burton et al. (2008) suggested that the hydrolytic (1,3;1,4)-β-d-glucanases might also participate in (1,3;1,4)-β-d-glucan synthesis and showed that the (1,3;1,4)-β-d-glucan endohydrolase gene that encodes isoenzyme EI (HvGlb1) is transcribed transiently in developing barley endosperm, where there is the net synthesis of large amounts of (1,3;1,4)-β-d-glucan. Finnie et al. (2006) also detected (1,3;1,4)-β-d-glucan endohydrolase isoenzyme EI during proteome profiling of developing barley grain. The hydrolases could function to trim or “edit” nascent (1,3;1,4)-β-d-glucan chains (Szyjanowicz et al., 2004) or to release newly synthesized chains from the biosynthetic enzymes (Farrokhi et al., 2006).
In some tissues, the levels of (1,3;1,4)-β-d-glucans in walls change dramatically during growth and development. For example, (1,3;1,4)-β-d-glucans increase to 10 mol % of walls during the elongation phase of growth in barley coleoptiles, but following the cessation of growth at about 5 d, (1,3;1,4)-β-d-glucan content rapidly decreases to 1 mol % (Gibeaut et al., 2005). McCann et al. (2007) also reported the transient nature of (1,3;1,4)-β-d-glucans in maize coleoptiles.
In related work, Roulin et al. (2002) reported that when barley seedlings were transferred into darkness, the (1,3;1,4)-β-d-glucan content of leaf cell walls decreased by about 30% and that this was associated with increased levels of (1,3;1,4)-β-d-glucan endohydrolase isoenzyme EI and β-d-glucan glucohydrolases. The authors concluded that cell wall (1,3;1,4)-β-d-glucans might be remobilized in nonelongating, dark-incubated leaves and the Glc so generated could serve as an energy source under conditions of sugar depletion (Roulin et al., 2002).
BIOSYNTHESIS AND REMODELING OF ARABINOXYLANS IN THE GRASSES
As with the biosynthesis of (1,3;1,4)-β-d-glucans, (1,4)-β-d-xylan synthase activity has been detected in membrane preparations from grasses over many years (Bailey and Hassid, 1966; Zeng et al., 2008), but the enzymes have not yet been purified to the extent that amino acid sequence information can be obtained. The genes and enzymes that are responsible for arabinoxylan synthesis in the grasses have not yet been identified unequivocally. Based on other polysaccharide biosynthetic systems, it might be expected that an integral, type I membrane-bound polysaccharide synthase with multiple transmembrane helices would mediate the synthesis of the (1,4)-β-d-xylan backbone. A type II arabinosyl transferase with a single transmembrane helix would be expected to be involved in the addition of α-l-arabinofuranosyl substituents, along with a different type II glucuronyl transferase for the addition of the α-d-glucuronopyranosyl substituents to the (1,4)-β-d-xylan backbone (Farrokhi et al., 2006).
On the basis of the chemical similarities between (1,4)-β-d-xylan and (1,4)-β-d-glucan chains, it might be predicted that a Csl enzyme or enzymes would mediate the synthesis of the (1,4)-β-d-xylan backbone. However, analyses of candidate genes from the various Csl gene subfamilies have not revealed any evidence that these genes are involved (Farrokhi et al., 2006). Indeed, recent analyses of mutant lines and bioinformatic information on transcript profiles during periods of arabinoxylan synthesis suggest an involvement of various type I glycosyl transferases (GT) from families GT8, GT43, and GT47 in the synthesis of heteroxylans, and it has been suggested that these type I glycosyl transferase enzymes might be responsible for the synthesis of the (1,4)-β-d-xylan backbone itself (Brown et al., 2007, Mitchell et al., 2007; Pena et al., 2007, Persson et al., 2007). More specifically, and based upon data available at the time for members of the Poaceae, Mitchell et al. (2007) concluded that genes in the GT43 families might encode (1,4)-β-d-xylan synthases, genes in the GT47 family encode xylan (1,2)-α- or (1,3)-α-l-arabinosyl transferases, and genes in the GT61 family encode feruloyl-arabinoxylan (1,2)-β-d-xylosyl transferases.
The presence of a reducing terminal oligosaccharide consisting of 4-β-d-Xylp-(1,4)-β-d-Xylp-(1,3)-α-l-Rhap-(1,2)-α-d-GalpA-(1,4)-d-Xylp (Pena et al., 2007) suggests that additional glycosyl transferases would be required for the synthesis of this portion of the polysaccharide, but so far there is no evidence that such an oligosaccharide is present in arabinoxylans from the grasses (W. York, personal communication). York and O'Neill (2008) note that the potential participation of GT enzymes and these other recent observations challenge traditional views regarding how heteroxylans in plants are assembled, and they offer a number of new models that are consistent with the current data.
As with the (1,3;1,4)-β-d-glucans, hydrolytic enzymes could participate in the biosynthesis of arabinoxylans in the grasses. There is good evidence that the fine structure of arabinoxylans changes after the initial deposition of the polysaccharide into walls. For example, in developing barley coleoptiles, the ratio of substituted to unsubstituted 4-linked xylosyl units changes from about 4:1 to 1:1 over about 3 d (Gibeaut et al., 2005). This indicates that about 80% of backbone xylopyranosyl residues in the newly synthesized arabinoxylan are substituted with arabinosyl residues but that the substituents are progressively removed during growth (Gibeaut et al., 2005) in a process that might be mediated by the action of arabinoxylan arabinofuranohydrolases (Lee et al., 2001). Similar changes have been reported in the arabinoxylans of developing maize coleoptiles (Carpita, 1984).
SUBCELLULAR LOCATION OF (1,3;1,4)-β-d-GLUCAN SYNTHESIS IN THE GRASSES
It is generally accepted that the biosynthesis of matrix phase, noncellulosic polysaccharides of plant cell walls occurs in the Golgi apparatus and that newly synthesized polysaccharides are transported to the plasma membrane in Golgi-derived vesicles, where they are deposited into the extracellular space and ultimately incorporated into the wall (Gibeaut and Carpita, 1993). The exception to this notion has been (1,3)-β-d-glucan, or callose, for which biosynthesis is widely believed to occur at the plasma membrane. Wilson et al. (2006) used monoclonal antibodies against a range of wall polysaccharides to examine the spatial and temporal patterns of deposition of these polysaccharides into the wall in various barley tissues and organs and at the same time were able to observe the passage of newly synthesized polysaccharides through the cell. While they were able to detect arabinoxylans and heteromannans in the Golgi and Golgi-derived vesicles, they never detected (1,3;1,4)-β-d-glucan in the Golgi, in Golgi-derived vesicles, or anywhere else inside the cell. Similar results were obtained in developing barley endosperm, in elongating coleoptiles, and in suspension-cultured cells. Despite the absence of labeling in the Golgi or elsewhere in the cell, in each of these organs high concentrations of (1,3;1,4)-β-d-glucans were detected in the wall itself (Wilson et al., 2006; Fig. 3). Our unpublished findings further suggest that the Csl enzymes themselves are found in Golgi but not in the plasma membrane (M. Doblin, S.M. Wilson, R.A. Burton, G.B. Fincher, and A. Bacic, unpublished data). It is important to note here that although isolated Golgi membranes, whether or not they are completely free of plasma membrane or other cellular membranes, can synthesize some (1,3;1,4)-β-d-glucan when provided with UDP-Glc in vitro, Wilson et al. (2006) could not find (1,3;1,4)-β-d-glucans in intact tissues, and we know of no experimental evidence that the polysaccharide in fact exists in Golgi in intact tissues.
Figure 3.
Use of specific monoclonal antibodies to locate (1,3;1,4)-β-d-glucans and arabinoxylans in tissue sections. Sections of barley coleoptiles were probed with gold-labeled antibodies against (1,3;1,4)-β-d-glucans (left) and arabinoxylan (right). In the left panel, high levels of (1,3;1,4)-β-d-glucans can be seen in the coleoptile walls (cw), but none could be detected in the cytosol or associated with the Golgi apparatus (G). In contrast, in the right panel, antibodies against the arabinoxylan show that the polysaccharide is located both in the Golgi and in the wall. This pattern of polysaccharide distribution is seen in coleoptiles, developing endosperm, and suspension-cultured barley cells (Wilson et al., 2006). (Photographs generously provided by Sarah Wilson.)
To explain these apparent anomalies, Wilson et al. (2006) suggested that the (1,3;1,4)-β-d-glucan epitope within the Golgi or elsewhere in the barley cells might have been masked by substituents such as acetyl groups, which would prevent antibody binding. If these substituents were subsequently removed in the wall, then antibody binding would occur. One alternative explanation is that the (1,3;1,4)-β-d-glucans are synthesized sequentially at different sites in the cells. If, in the first phase of synthesis, (1,4)-β-d-oligoglucosides (or cellodextrins) were synthesized in the Golgi, the antibodies against the polymeric (1,3;1,4)-β-d-glucan would not bind to these oligosaccharides (Meikle et al., 1994). This would explain why we have been unable to detect (1,3;1,4)-β-d-glucans in the Golgi (Wilson et al., 2006). In the second phase, the newly synthesized (1,4)-β-d-oligoglucosides might be transported to the plasma membrane, where they could be assembled into polymeric (1,3;1,4)-β-d-glucans, whereafter they would be recognized by the antibodies. If this sequential assembly process indeed occurs, the population of (1,4)-β-d-oligoglucosides might be transported through the endomembrane system via a protein or lipid carrier. It is noteworthy that there have been suggestions that cellulose synthesis might occur through a lipid intermediate (Peng et al., 2002). Assembly of the oligosaccharides into the polysaccharide would require the action of a transferase enzyme. Could xyloglucan endotransglycosylases (XETs) or callose synthases perform this function? If the (1,4)-β-d-oligoglucosides synthesized in the Golgi were mainly cellotriosyl units but included longer cellodextrin units at decreasing abundance, random assembly of the (1,4)-β-d-oligoglucosides on the extracellular face of the plasma membrane would explain the fine structure of the final (1,3;1,4)-β-d-glucan found in the walls of grasses. It must be emphasized that while such a mode of assembly of the (1,3;1,4)-β-d-glucan would be consistent with all of our current data, it is also a speculative notion that has not yet been tested experimentally.
REMODELING OF WALL POLYSACCHARIDES
In addition to the hydrolytic modifications of arabinoxylans and other wall polysaccharides that occur after deposition of the nascent polysaccharide into the wall, another class of enzymes, known as XTHs, are widespread in higher plants and are believed to be involved in the remodeling of xyloglucans. The molecular sizes of xyloglucans can be altered after their deposition into the cell wall (Farkas et al., 2005), and this process is likely to be mediated by the XTHs. However, the XTHs can have XET activity or both xyloglucan endotransglycosylase and xyloglucan endohydrolase activities (Thompson and Fry, 2001). The XETs are abundant in the apoplastic space, they cleave the (1,4)-β-d-glucan backbone of xyloglucans, and they transfer the nonreducing fragment of the original substrate that remains bound to the enzyme directly onto the nonreducing terminus of another xyloglucan chain (Fig. 4). The action of XETs could lead to disproportionation of xyloglucan molecules, such that some will have increased molecular masses while others will have decreased molecular masses (Thompson and Fry, 2001).
Figure 4.
Proposed mechanism of action of XETs. Step 1, The so-called donor substrate (blue) is bound to the enzyme and a (1,4)-β-d-glucosyl linkage is cleaved by amino acid residues at the catalytic center (black arrow). Step 2, The reducing terminal portion of the cleaved donor substrate diffuses away from the enzyme surface, while the nonreducing portion of the substrate remains covalently linked to the enzyme, through the catalytic nucleophile. Step 3, The incoming acceptor substrate (orange) is bound to the subsites vacated by the departure of the reducing terminal portion of the cleaved donor substrate. The nonreducing terminal residue of the acceptor substrate is juxtaposed with the covalently bound, reducing end residue of the cleaved donor substrate. Step 4, The transglycosylation reaction results in the transfer of the donor substrate portion to the acceptor substrate. Step 5, The polysaccharide product diffuses away from the enzyme. There is evidence that XETs can use a range of donor and acceptor substrates in addition to xyloglucan substrates (Hrmova et al., 2007).
Recently, a more radical function was advanced for the XETs of grasses, namely that XETs could link different polysaccharides in vivo and hence influence cell wall strength, flexibility, and porosity. Strohmeier et al. (2004) first suggested that some of the XETs from monocots, which are encoded by large families of genes that were often transcribed at very high levels in both growing and senescent cells, might be active on the more abundant matrix phase polysaccharides of cell walls in barley, namely the arabinoxylans and the (1,3;1,4)-β-d-glucans, rather than on xyloglucans alone. Hrmova et al. (2007) subsequently showed that highly purified barley HvXET5, a member of the GH16 group of glycoside hydrolases, catalyzed the formation of covalent linkages between xyloglucans and celluloses at quite reasonable rates, and between xyloglucans and (1,3;1,4)-β-d-glucans at relatively low rates, at least in vitro (Hrmova et al., 2007). The HvXET5 activity proceeds via a non-Leloir type of reaction, because the energy required for the formation of the new glycosidic linkage is provided from an existing glycosidic linkage rather than from an activated sugar nucleotide donor.
The suggestion of Hrmova et al. (2007) that the XETs of barley might remodel (1,3;1,4)-β-d-glucans and/or arabinoxylans was consistent with the work of Mohand and Farkas (2006), who demonstrated “heterotransglycosylating” activity in extracts from nasturtium (Tropaeolum majus), and with the recent observation that unpurified extracts from Equisetum (horsetail) stems and from charophytic algae contain a transglycosylase enzyme that transfers donor molecules from (1,3;1,4)-β-d-glucans to oligoxyloglucoside acceptors (Fry et al., 2008a). The same authors concluded that the so-called (1,3;1,4)-β-d-glucan:xyloglucan endotransglycosylase from Equisetum species has a preference for transferring (1,3;1,4)-β-d-glucans to xyloglucans, but they also concluded that cereals lack this activity (Fry et al., 2008a).
Remodeling of fungal cell walls during spore formation and under stress has been attributed to a group of glycosylphosphatidylinositol-anchored transferase enzymes of family GH16, which might link different polysaccharides such as β-d-glucans and chitin in the fungal wall and thereby reinforce the walls (Gómez-Esquer et al., 2004). There have also been suggestions that pectic polysaccharides might be covalently linked with xyloglucans in plant cell walls (Keegstra et al., 1973; Thompson and Fry, 2000; Cumming et al., 2005; Popper and Fry, 2005). However, the HvXET5 enzyme purified by Hrmova et al. (2007) did not link pectin-related polysaccharides such as polygalacturonan or β-d-galactans to xyloglucan, nor did it link arabinoxylans to xyloglucans, despite suggestions based on molecular modeling (Strohmeier et al., 2004) that this was a possibility. It nevertheless remains possible that other XET isoforms might prefer different donor and acceptor substrates, including arabinoxylans and (1,3;1,4)-β-d-glucans. Given the large size of the XET gene families in the grasses, this will involve the heterologous expression and/or purification of different isoenzymes, followed by the detailed analysis of donor and acceptor substrate specificities.
Molecular interactions between different classes of wall polysaccharides in higher plants have generally been assumed to be noncovalent in nature. The work summarized above provides at least circumstantial evidence that covalent linkages between different classes of wall polysaccharides might occur in plants, including the grasses, in vivo, although unequivocal evidence for this has yet to be presented.
CONCLUSION
Key challenges to our understanding of cell wall biogenesis in the grasses are presented by recent indications that while Csl genes are involved in the biosynthesis of some noncellulosic wall polysaccharides, it is not known if individual isoenzymes synthesize (1,3;1,4)-β-d-glucans with different fine structures, especially with respect to the length, relative abundance, and distribution of the (1,4)-β-d-oligoglucoside units along the chain. It is not known if Csl enzymes participate in heteroxylan biosynthesis, and indeed rigorous proof of function is still required for the various candidate glycosyl transferase genes and enzymes that have recently been proposed for the synthesis of the (1,4)-β-d-xylan backbone of wall arabinoxylans in grasses. Here, it has been suggested that the (1,3;1,4)-β-d-glucans of walls of grasses might be synthesized through a previously unknown series of cellular and enzymic events and that XET enzymes might catalyze the formation of covalent linkages between different types of wall polysaccharides in the grasses. To further investigate and develop these ideas, it will be necessary to take both biochemical and cell biological approaches to these questions. For example, it will be necessary to dissect the subcellular locations and enzymic mechanisms that are associated with (1,3;1,4)-β-d-glucan biosynthesis. It will also be necessary to characterize highly purified XET preparations from grasses to precisely define their substrate preferences and, at the same time, to seek chemical evidence for the existence of junction regions between covalently linked polysaccharides of different types within the cell wall itself. If such covalent linkages do exist between different polysaccharides in plant cell walls, this will not only change the way we view cell wall biology in plants in general terms but will also have important implications for wall rigidity, strength, and porosity. A thorough understanding of any covalent linkages between wall polysaccharides would also provide opportunities to genetically manipulate agroindustrial processes such as paper production, food quality and texture, malting and brewing, bioethanol production, dietary fiber, and ruminant digestibility.
Acknowledgments
Much of the work described here was undertaken by an enthusiastic group of hard-working colleagues, including in particular Rachel Burton, Monika Doblin, Andrew Harvey, Maria Hrmova, Sarah Wilson, and Tony Bacic.
This work was supported by the Australian Research Council, the Grains Research and Development Corporation, and the CSIRO Flagship Collaboration Fund.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Geoffrey B. Fincher (geoff.fincher@adelaide.edu.au).
References
- Bailey RW, Hassid WZ (1966) Xylan synthesis from uridine-diphosphate-D-xylose by particulate preparations from immature corncobs. Proc Natl Acad Sci USA 56 574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten JT, Wood PJ, Scott FW, Wolynetz MS, Lowe MK, Bradley-White P, Collins MW (1994) Oat beta-glucan reduces blood cholesterol concentration in hypercholesterolemic subjects. Eur J Clin Nutr 48 465–474 [PubMed] [Google Scholar]
- Brennan CS, Cleary LJ (2005) The potential use of (1→3,1→4)-β-D-glucans as functional food ingredients. J Cereal Sci 42 1–13 [Google Scholar]
- Brown DM, Goubet F, Vicky WWA, Goodacre R, Stephens E, Dupree P, Turner SR (2007) Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis. Plant J 52 1154–1168 [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Vergara CE, Carpita NC (2001) Insight into multi-site mechanisms of glycosyl transfer in (1→4)-β-D-glycans provided by the cereal mixed-linkage (1→3),(1→4)-β-D-glucan synthase. Phytochemistry 57 1045–1053 [DOI] [PubMed] [Google Scholar]
- Buliga GS, Brandt DA, Fincher GB (1986) The sequence statistics and solution conformation of a barley (1→3,1→4)-β-D-glucan. Carbohydr Res 157 139–156 [DOI] [PubMed] [Google Scholar]
- Burton RA, Jobling SA, Harvey AJ, Shirley NJ, Mather DE, Bacic A, Fincher GB (2008) The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley (Hordeum vulgare L.). Plant Physiol 146 1821–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A, Stone BA, Newbigin EJ, Bacic A, Fincher GB (2006) Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-d-glucans. Science 311 1940–1942 [DOI] [PubMed] [Google Scholar]
- Carpita NC (1984) Cell wall development in maize coleoptiles. Plant Physiol 76 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Defernez M, Findlay K, Wells B, Shoue DA, Catchpole G, Wilson RH, McCann MC (2001) Cell wall architecture of the elongating maize coleoptile. Plant Physiol 127 551–565 [PMC free article] [PubMed] [Google Scholar]
- Cumming CM, Rizkallah HD, McKendrick KA, Abdel-Massih RM, Baydoun EA, Brett CT (2005) Biosynthesis and cell-wall deposition of a pectin-xyloglucan complex in pea. Planta 222 546–555 [DOI] [PubMed] [Google Scholar]
- Farkas V, Ait-Mohand F, Stratilová E (2005) Sensitive detection of transglycosylating activity of xyloglucan endotransglycosylase/hydrolase (XTH) after isoelectric focusing in polyacrylamide gels. Plant Physiol Biochem 43 431–435 [DOI] [PubMed] [Google Scholar]
- Farrokhi N, Burton RA, Brownfield L, Hrmova M, Wilson SM, Bacic A, Fincher GB (2006) Plant cell wall biosynthesis: genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnol J 4 145–167 [DOI] [PubMed] [Google Scholar]
- Fincher GB, Stone BA (2004) Chemistry of nonstarch polysaccharides. In C Wrigley, H Corke, CE Walker, eds, Encyclopedia of Grain Science. Elsevier, Oxford, pp 206–223
- Finnie C, Bak-Jensen KS, Laugesen S, Roepstorff P, Svensson B (2006) Differential appearance of isoforms and cultivar variation in protein temporal profiles revealed in the maturing barley grain proteome. Plant Sci 170 808–821 [Google Scholar]
- Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, Vorgias CE, Diaquin M, Latgé JP (2000) Molecular organization of the alkali-insoluble fraction of Aspergillus fumigates cell wall. J Biol Chem 275 27594–27607 [DOI] [PubMed] [Google Scholar]
- Fry SC, Mohler KE, Nesselrode BH, Franková L (2008. a) Mixed-linkage beta-glucan:xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. Plant J 55 240–252 [DOI] [PubMed] [Google Scholar]
- Fry SC, Nesselrode BH, Miller JG, Mewburn BR (2008. b) Mixed-linkage (1→3,1→4)-β-D-glucan is a major hemicellulose of Equisetum (horsetail) cell walls. New Phytol 179 104–115 [DOI] [PubMed] [Google Scholar]
- Gaut BS (2002) Evolutionary dynamics of grass genomes. New Phytol 154 15–28 [Google Scholar]
- Gibeaut DM, Carpita NC (1993) Synthesis of (1→3),(1→4)-beta-D-glucan in the Golgi apparatus of maize coleoptiles. Proc Natl Acad Sci USA 90 3850–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Pauly M, Bacic A, Fincher GB (2005) Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta 221 729–738 [DOI] [PubMed] [Google Scholar]
- Gómez-Esquer F, Rodríguez-Peña JM, Díaz G, Rodriguez E, Briza P, Nombela C, Arroyo J (2004) CRR1, a gene encoding a putative transglycosidase, is required for proper spore wall assembly in Saccharomyces cerevisiae. Microbiology 150 3269–3280 [DOI] [PubMed] [Google Scholar]
- Han F, Ullrich S, Chirat S, Menteur S, Jestin L, Sarrafi A, Hayes P, Jones B, Blake T, Wesenberg D, et al (1995) Mapping of beta-glucan content and beta-glucanase activity loci in barley grain and malt. Theor Appl Genet 91 921–927 [DOI] [PubMed] [Google Scholar]
- Hazen SP, Scott-Craig JS, Walton JD (2002) Cellulose synthase-like genes of rice. Plant Physiol 128 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RJ, Stone BA (1982) Solubilization of β-glucan synthases from the membranes of cultured ryegrass endosperm cells. Biochem J 203 629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger R, Haisch A (2001) Immunocytochemical location of the (1,3)(1,4)-beta-glucan lichenin in the lichen-forming ascomycete Cetraria islandica (Icelandic moss). New Phytol 150 739–746 [Google Scholar]
- Hrmova M, Farkas V, Lahnstein J, Fincher GB (2007) A barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates and (1,3;1,4)-β-D-glucans. J Biol Chem 282 12951–12962 [DOI] [PubMed] [Google Scholar]
- Keegstra K, Talmadge KW, Bauer WD, Albersheim P (1973) The structure of plant cell walls. III. A model of the walls of suspension-cultured sycamore cells based on the interconnections of the macromolecular components. Plant Physiol 51 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz RJ, Bamforth CW (2007) Time course for the development of enzymes in barley. J Inst Brew 113 196–205 [Google Scholar]
- Lee RC, Burton RA, Hrmova M, Fincher GB (2001) Barley arabinoxylan arabinofuranohydrolases: purification, characterization and determination of primary structures from cDNA clones. Biochem J 356 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MC, Defernez M, Urbanowicz BR, Tewari JC, Langewisch T, Olek A, Wells B, Wilson RH, Carpita NC (2007) Neural network analyses of infrared spectra for classifying cell wall architectures. Plant Physiol 143 1314–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren JS (2005) Crop biotechnology provides an opportunity to develop a sustainable future. Trends Biotechnol 23 339–342 [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Hoogenraad NJ, Bonig I, Clarke AE, Stone BA (1994) A (1,3;1,4)-beta-glucan-specific monoclonal antibody and its use in the quantitation and immunocytochemical location of (1,3;1,4)- beta-glucans. Plant J 5 1–9 [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Dupree P, Shewry PR (2007) A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol 144 43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohand FA, Farkas V (2006) Screening for hetero-transglycosylating activities in extracts from nasturtium (Tropaeolum majus). Carbohydr Res 341 577–581 [DOI] [PubMed] [Google Scholar]
- Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11 266–277 [DOI] [PubMed] [Google Scholar]
- Nevo Z, Sharon N (1969) The cell wall of Peridinium westii, a non cellulosic glucan. Biochim Biophys Acta 173 161–175 [DOI] [PubMed] [Google Scholar]
- Olafsdottir ES, Ingolfsdottir K (2001) Polysaccharides from lichens: structural characteristics and biological activity. Planta Med 67 199–208 [DOI] [PubMed] [Google Scholar]
- Pena MJ, Zhong R, Zhou GK, Richardson EA, O'Neill MA, Darvill AG, York WS, Ye ZH (2007) Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. Plant Cell 19 549–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Kawagoe Y, Hogan P, Delmer D (2002) Sitosterol-beta-glucoside as primer for cellulose synthesis in plants. Science 295 147–150 [DOI] [PubMed] [Google Scholar]
- Persson S, Caffall KH, Freshour G, Hilley MT, Bauer S, Poindexter P, Hahn MG, Mohnen D, Somerville C (2007) The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. Plant Cell 19 237–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Fry SC (2003) Primary cell wall composition of bryophytes and charophytes. Ann Bot (Lond) 91 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Fry SC (2005) Widespread occurrence of a covalent linkage between xyloglucan and acidic polysaccharides in suspension-cultured angiosperm cells. Ann Bot (Lond) 96 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T, Somerville C (2000) The cellulose synthase superfamily. Plant Physiol 124 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulin S, Buchala AJ, Fincher GB (2002) Induction of (1,3;1,4)-β-d-glucan hydrolases in leaves of dark-incubated barley seedlings. Planta 215 51–59 [DOI] [PubMed] [Google Scholar]
- Sørensen I, Pettolino FA, Wilson SM, Doblin MS, Johansen B, Bacic A, Willats WG (2008) Mixed-linkage (1→3),(1→4)-β-d-glucan is not unique to the Poales and is an abundant component of Equisetum arvense cell walls. Plant J 54 510–521 [DOI] [PubMed] [Google Scholar]
- Staudte RG, Woodward JR, Fincher GB, Stone BA (1983) Water-soluble (1→3),(1→4)-β-D-glucans from barley (Hordeum vulgare) endosperm. III. Distribution of cellotriosyl and cellotetraosyl residues. Carbohydr Polym 3 299–312 [Google Scholar]
- Strohmeier M, Hrmova M, Fischer M, Harvey AJ, Fincher GB, Pleiss J (2004) Molecular modelling of family GH16 glycoside hydrolases: potential roles for xyloglucan endotransglucosylases/hydrolases in cell wall modification in the Poaceae. Protein Sci 13 3200–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyjanowicz PM, McKinnon I, Taylor NG, Gardiner J, Jarvis MC, Turner SR (2004) The irregular xylem 2 mutant is an allele of korrigan that affects the secondary cell wall of Arabidopsis thaliana. Plant J 37 730–740 [DOI] [PubMed] [Google Scholar]
- Thompson JE, Fry SC (2000) Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta 211 275–286 [DOI] [PubMed] [Google Scholar]
- Thompson JE, Fry SC (2001) Restructuring of wall-bound xyloglucan by transglycosylation in living plant cells. Plant J 26 23–34 [DOI] [PubMed] [Google Scholar]
- Trethewey JAK, Campbell LM, Harris PJ (2005) (1→3),(1→4)-β-d-Glucans in the cell walls of the Poales (sensu lato): an immunogold labeling study using a monoclonal antibody. Am J Bot 92 1669–1683 [DOI] [PubMed] [Google Scholar]
- Urbanowicz BR, Rayon C, Carpita NC (2004) Topology of the maize mixed linkage (1→3),(1→4)-β-d-glucan synthase at the Golgi membrane. Plant Physiol 134 758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vietor RJ, Angelino SAGF, Voragen AGJ (1993) Structural features of arabinoxylans from barley and malt cell wall material. J Cereal Sci 15 213–222 [Google Scholar]
- Wilson SM, Burton RA, Doblin MS, Stone BA, Newbigin E, Fincher GB, Bacic A (2006) Temporal and spatial appearance of wall polysaccharides during cellularization of barley (Hordeum vulgare) endosperm. Planta 224 655–667 [DOI] [PubMed] [Google Scholar]
- Woodward JR, Fincher GB, Stone BA (1983. a) Water-soluble (1→3),(1→4)-β-d-glucans from barley (Hordeum vulgare) endosperm. II. Fine structure. Carbohydr Polym 3 207–225 [Google Scholar]
- Woodward JR, Phillips DR, Fincher GB (1983. b) Water-soluble (1→3)(1→4)-β-d-glucans from barley (Hordeum vulgare) endosperm. I. Physicochemical properties. Carbohydr Polym 3 143–156 [Google Scholar]
- Ye ZH, York WS, Darvill AG (2006) Important new players in secondary wall synthesis. Trends Plant Sci 11 162–164 [DOI] [PubMed] [Google Scholar]
- York WS, O'Neill MA (2008) Biochemical control of xylan biosynthesis: which end is up? Curr Opin Plant Biol 11 258–265 [DOI] [PubMed] [Google Scholar]
- Zabotina OA, van de Ven WT, Freshour G, Drakakaki G, Cavalier D, Mouille G, Hahn MG, Keegstra K, Raikhel NV (2008) Arabidopsis XXT5 gene encodes a putative alpha-1,6-xylosyltransferase that is involved in xyloglucan biosynthesis. Plant J 56 101–115 [DOI] [PubMed] [Google Scholar]
- Zeng W, Chatterjee M, Faik A (2008) UDP-xylose-stimulated glucuronyltransferase activity in wheat microsomal membranes: characterization and role in glucurono(arabino)xylan biosynthesis. Plant Physiol 147 78–91 [DOI] [PMC free article] [PubMed] [Google Scholar]






