Abstract
The spikelet, which is a short branch bearing the florets, is the fundamental unit of grass inflorescence architecture. In most grasses, spikelets are borne singly on the inflorescence. However, paired spikelets are characteristic of the Andropogoneae, a tribe of 1,000 species including maize (Zea mays). The Suppressor of sessile spikelets1 (Sos1) mutant of maize produces single instead of paired spikelets in the inflorescence. Therefore, the sos1 gene may have been involved in the evolution of paired spikelets. In this article, we show that Sos1 is a semidominant, antimorph mutation. Sos1 mutants have fewer branches and spikelets for two reasons: (1) fewer spikelet pair meristems are produced due to defects in inflorescence meristem size and (2) the spikelet pair meristems that are produced make one instead of two spikelet meristems. The interaction of Sos1 with the ramosa mutants, which produce more branches and spikelets, was investigated. The results show that Sos1 has an epistatic interaction with ramosa1 (ra1), a synergistic interaction with ra2, and an additive interaction with ra3. Moreover, ra1 mRNA levels are reduced in Sos1 mutants, while ra2 and ra3 mRNA levels are unaffected. Based on these genetic and expression studies, we propose that sos1 functions in the ra1 branch of the ramosa pathway controlling meristem determinacy.
Organogenesis in plants is controlled by meristems (Steeves and Sussex, 1989). Organs are produced in the peripheral zone of the meristem, while the central zone retains a pool of cells that do not differentiate, enabling the meristem to maintain itself. Meristems are defined by their determinacy, identity, and position (McSteen et al., 2000). Determinate meristems produce a limited number of structures before terminating, while indeterminate meristems have the potential to continue producing organs indefinitely (Bortiri and Hake, 2007; Sablowski, 2007). Apical meristems are indeterminate in many species. The shoot apical meristem (SAM) gives rise to the vegetative shoot, while the inflorescence apical meristem gives rise to the flowering shoot. Axillary meristems, which form in the axils of leaf primordia, can be indeterminate and give rise to branches or can be determinate and give rise to flowers.
In maize (Zea mays) inflorescence development, there are multiple types of axillary meristems that differ in their determinacy, resulting in a highly branched inflorescence (Irish, 1997a; Bortiri and Hake, 2007; Barazesh and McSteen, 2008b). The mature male inflorescence consists of a main spike and several long lateral branches, which are covered by short branches called spikelet pairs (Fig. 1A; Kiesselbach, 1949). The spikelet is defined as a short branch with two leaf-like glumes enclosing the florets (Clifford, 1987). During inflorescence development, the apical inflorescence meristem (IM) produces axillary meristems, called branch meristems (BM), which are indeterminate and produce the branches. After several branches are made, the IM switches abruptly to producing spikelet pair meristems (SPM), which are determinate because they produce two spikelets. Spikelet meristems (SM) are also determinate, as they produce two floral meristems (FM) which then produce the floral organs. Although maize has separate male and female inflorescences, called the tassel and ear, respectively, early development is similar except that ears do not produce branches.
Figure 1.
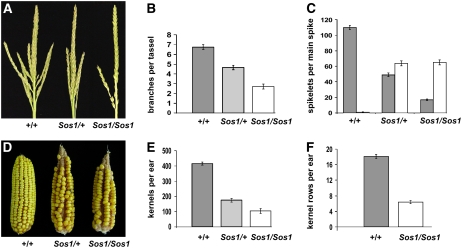
Sos1 tassel and ear phenotype. A, Photograph of mature tassels before anthesis. Normal tassels have long branches at the base of a main spike. Spikelet pairs cover the branches and the main spike. Sos1/+ mutants have a sparse appearance due to the production of fewer branches and spikelets. Sos1/Sos1 mutants have very few branches and single spikelets. B, Quantification of branch number in the tassel. C, Quantification of the number of paired (gray bar) versus single (white) spikelets on the tassel main spike. D, Photograph of mature ears after open pollination. Normal ears have paired rows of kernels. Sos1/+ and Sos1/Sos1 ears have fewer rows of kernels in the ear. E, Quantification of the number of kernels per ear. F, Quantification of the number of kernel rows per ear. Sos1/Sos1 ears have fewer than one-half the number of rows compared to normal siblings. All graphs are mean ± se. [See online article for color version of this figure.]
Two models had been proposed to explain how the SPM produces two SM and the SM produces two FM (Irish, 1997a, 1998; Chuck et al., 1998; Kaplinsky and Freeling, 2003). In the lateral branching model, the SPM produces two SM by lateral branching and a residual meristem remains between the two SM (Chuck et al., 1998). In the conversion model, the SPM initiates an SM, and then the remaining SPM converts to a SM (Irish, 1997a, 1998). Strong support for the conversion model was recently provided by experiments in which normal maize plants were treated with auxin transport inhibitors that inhibited SM initiation but did not inhibit the conversion of the SPM to a SM, resulting in the formation of single spikelets (Wu and McSteen, 2007).
The Suppressor of sessile spikelet1 (Sos1) mutant produces single, instead of paired, spikelets in the inflorescence (Doebley et al., 1995). In normal plants, one spikelet is attached to the inflorescence rachis by a pedicel and is called the pedicellate spikelet, while the other spikelet, which has no pedicel, is called the sessile spikelet. In Sos1 mutants, the sessile spikelet does not form, resulting in single pedicellate spikelets. Therefore, in Sos1 mutants, the SPM produces one instead of two SM and hence is more determinate than normal.
The determinacy of the SPM is positively regulated by the ramosa pathway (McSteen, 2006; Kellogg, 2007). ramosa1 (ra1) encodes a zinc finger transcription factor expressed at the base of the SPM (Vollbrecht et al., 2005). In ra1 mutants, the tassel and ear are highly branched, because SPM become indeterminate and grow out to produce extra spikelets (Gernart, 1912; Vollbrecht et al., 2005). ra1 acts downstream of ra2, which encodes a LOB domain transcription factor expressed in the anlagen of BMs, SPMs, and SMs (Bortiri et al., 2006a). ra1 also acts downstream of ra3, which encodes a trehalose-6-phosphate phosphatase (Satoh-Nagasawa et al., 2006). Interestingly, although ra2- and ra3-like genes are present in other grasses such as rice (Oryza sativa), ra1 has been identified only in the Andropogoneae, the clade to which maize belongs.
In the vast majority of grasses, including rice, barley (Hordeum vulgare), and wheat (Triticum aestivum), spikelets are produced singly (Clifford, 1987). The production of paired spikelets is characteristic of the Andropogoneae, a tribe of 1,000 species including maize, Miscanthus, sugarcane (Saccharum officinarum), and sorghum (Sorghum bicolor; Kellogg, 2000, 2001; Grass Phylogeny Working Group, 2001). In the Paniceae tribe, which is sister to the Andropogoneae, most species have single spikelets, but a few species produce paired spikelets and others produce spikelets that are paired with bristles, which are modified spikelets (Kellogg, 2000; Doust and Kellogg, 2002). Therefore, the paired spikelet is a derived trait, although it is unclear when this trait arose during the evolution of the Panicoideae. It has been suggested that ra1 may have been recruited for the evolution of the paired spikelet trait (Vollbrecht et al., 2005; McSteen, 2006). As Sos1 mutants produce single instead of paired spikelets, the sos1 gene may also have been involved in the evolution of the paired spikelet.
In this article, we use quantitative analysis, scanning electron microscopy (SEM), and histology to show that the Sos1 mutation causes defects in IM, BM, and SPM development in both the tassel and ear. We use dosage analysis to show that Sos1 is an antimorph, i.e. a dominant negative mutation. Moreover, genetic and expression analyses provide evidence that the sos1 gene acts in the ra1 branch of the ramosa pathway controlling meristem determinacy.
RESULTS
The Sos1-Reference allele arose spontaneously (Doebley et al., 1995). Seeds were obtained from the Maize Genetics Coop and backcrossed six times to B73. The mutant had previously been reported to map to the short arm of chromosome 4 (Doebley et al., 1995). To generate a mapping population, Sos1 (in the B73 background) was crossed to the Mo17 genetic background and backcrossed to Mo17. Simple sequence repeat (SSR) markers were used to further define the location of Sos1 to within 4 cM of umc1294 in bin 4.02. Because the umc1294 marker was polymorphic between B73 and the original background in which Sos1 arose, this marker was used for genotyping Sos1 in the B73 background. Genotyping combined with analysis of the phenotype determined that the Sos1 mutation was semidominant rather than dominant as previously reported (Fig. 1; Doebley et al., 1995).
Sos1 Mutants Produce Fewer Branches and Spikelets
Sos1 mutants have defects in both the tassel and ear. In the tassel, Sos1 mutant plants produced fewer branches and spikelets (Fig. 1, A–C). Families segregating for Sos1 were genotyped, and the number of branches and spikelets were counted in normal siblings compared to plants heterozygous and homozygous for Sos1. These results showed that Sos1/+ and Sos1/Sos1 mutants produced fewer branches than normal (Fig. 1B). To analyze the spikelet defects, the number of paired versus single spikelets was counted separately. While normal plants had paired spikelets, Sos1/+ and Sos1/Sos1 mutants had more single than paired spikelets in the tassel (Fig. 1C).
The Sos1 mutation also affected the ear. Normal ears are not branched but they produce spikelets pairs (Kiesselbach, 1949). Each spikelet produces one floret (the lower floret aborts) which, when pollinated, gives rise to a single kernel (Cheng et al., 1983). The spikelets in the ear are sessile, so the pairing is not obvious, but it leads to an even number of rows of kernels in the mature ear. In Sos1 mutants, there were fewer kernels in the ear (Fig. 1D). Quantification showed that the total number of kernels was reduced in Sos1/+ and Sos1/Sos1 mutants relative to normal ears (Fig. 1E). There was also a reduction in the number of rows of kernels with Sos1/+ mutants producing a variable number of rows and Sos1 mutants producing less than one-half the number of rows of normal siblings (Fig. 1F).
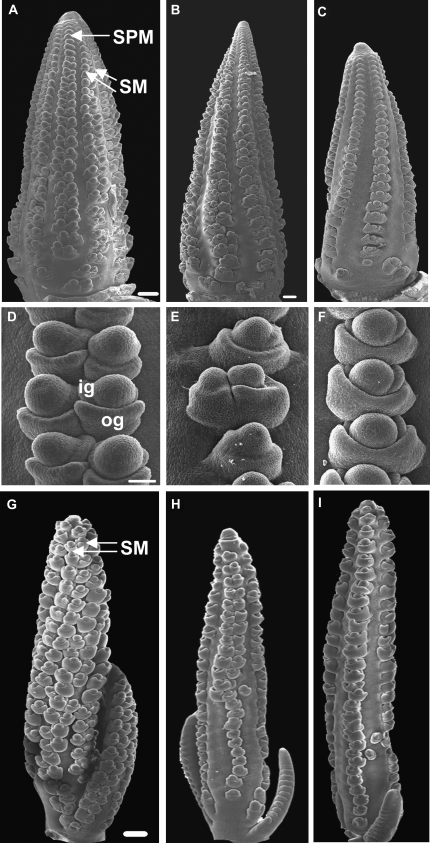
To determine the developmental basis for the production of fewer branches and spikelets in Sos1 mutants, SEM analysis was performed on developing tassels and ears. In normal inflorescences, SPM are produced from the IM at the tip of the inflorescence (Fig. 2A). The SPM then produces two SMs (Fig. 2, A and D). The identity of the meristem as an SM is indicated by the production of glumes, protective leaf-like organs, which are the first organs produced by the SM (Cheng et al., 1983). The outer glume is produced on the abaxial side of the SM, and the inner glume forms internal to the outer glume on the adaxial side of the SM (Fig. 2D). During ear development, Sos1/+ and Sos1/Sos1 mutants produced SPM as normal, except that there appeared to be fewer rows (Fig. 2, B and C). Later in ear development, when paired SMs were visible in normal ears, Sos/Sos1 mutants had single SMs and Sos1/+ mutants had a mixture of single and paired SMs (Fig. 2, D–F). Similarly, in the tassel, SPM of normal plants produced two SMs (Fig. 2G), while Sos/Sos1 mutants produced single SMs (Fig. 2I) and Sos1/+ mutants produced either one or two SMs (Fig. 2H). Hence, in Sos1 mutants, SPMs produce one instead of two SMs.
Figure 2.
SEM of Sos1 inflorescences. A to C, SEM of 8-week-old ears. A, Normal ear producing rows of SPM at the tip of the ear that transition to producing paired SM below the tip. B, Sos1/+ ear producing either single or paired SM. C, Sos1/Sos1 ear producing single SM. D to F, Higher magnification of developing SM in the ear. D, Normal ear with paired SM. The outer glume (og) and inner glume (ig) are the first organs to be produced by the SM. E, Sos1/+ ear producing either single or paired SM. F, Sos1/Sos1 ear with single SM. G, Normal tassel producing paired SM. H, Sos1/+ tassel producing paired SM at the top and single SM at the base. I, Sos1/Sos1 tassel producing rows of single SM. Scale bar = 100 μm in A to C and G to I and 50 μm in D to F.
Sos1 Mutants Have Defects in IM Size
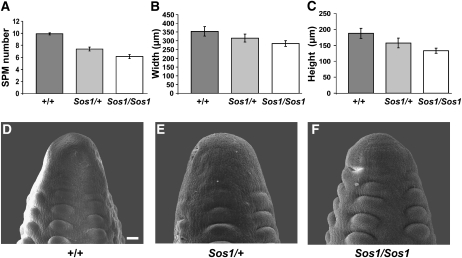
If the only defect in Sos1 mutants was the production of single instead of paired spikelets, then we would expect the mutants to produce exactly one-half the normal number of kernel rows. However, Sos1/Sos1 mutants produced less than one-half the normal number of kernel rows (Fig. 1F; Doebley et al., 1995). Furthermore, Sos1/Sos1 mutants produced less than one-half the normal number of spikelets in the tassel (Fig. 1C). This is indicative of an additional defect in SPM initiation. To quantify the defect in SPM initiation, SEM analysis was used to count the number of SPM that initiated around the circumference of the IM. This showed that normal ears produced either 9 or 10 SPM, Sos1/+ produced an average of 7.37 ± 0.33 SPM, while Sos1/Sos1 produced an average of 6.14 ± 0.34 SPM (Fig. 3A).
Figure 3.
Analysis of the IM of Sos1 mutants. A, Quantification of the number of SPM produced around the circumference of the tip of Sos1 ears. B, Measurement of the width of the IM. C, Measurement of the height of the IM. D to F, SEM of the IM of normal, Sos1/+, and Sos1/Sos1 ears. Scale bar = 50 μm.
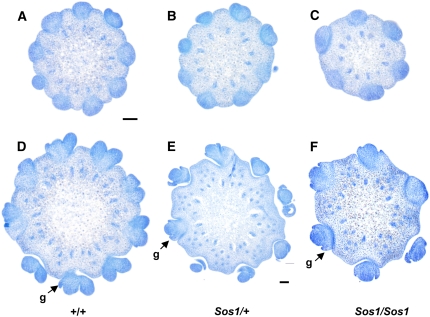
To further analyze the defects in SPM initiation, inflorescences were embedded in wax, sectioned, and stained with toluidine blue O (TBO). Near the tip of the ear, SPMs were visible in a ring around the circumference of the inflorescence. In the sections shown in Figure 4, the normal ear had initiated nine SPMs (Fig. 4A), the heterozygous ear had initiated eight SPMs (Fig. 4B), and the homozygous ear had initiated six SPMs (Fig. 4C). Later, when normal SPMs initiate two SMs (Fig. 4D), some of the SPMs in Sos1/+ mutants initiated two SMs (Fig. 4E), while very few of the SPMs in Sos1/Sos1 mutants initiated two SMs (Fig. 4F). Instead, SPMs converted directly to an SM as evidenced by the production of glumes (Fig. 4F).
Figure 4.
Cross sections of Sos1 ears stained with TBO. A to C, Cross sections of ears near the tip of the ear at the stage when SPM are being produced. A, Normal ear showing nine SPM, some of which are just beginning to form SM. B, Sos1/+ ear showing eight SPM. C, Sos1/Sos1 ears showing six SPM. D to F, Cross sections later in development when normal ears have produced paired SMs. D, Normal ear showing nine pairs of SMs. Note that the outer glume (g) is visible on some of the SMs. E, Sos1/+ ear in which two out of eight SPM are producing two SM. F, Sos1/Sos1 ear in which six single SMs are visible. All SM are producing glumes (g). Scale bar = 100 μm. [See online article for color version of this figure.]
The production of fewer SPM could be due to a primary defect in IM size. Therefore, the height and width of the IM was measured in Sos1 and normal ears using SEM analysis (Fig. 3, D–F). This showed that Sos1/Sos1 IMs were 80% as wide as normal inflorescences, although the difference was on the border for statistical significance (Fig. 3B; P = 0.059). In addition, Sos1/Sos1 IMs were on average 71% of the height of normal IMs, and this difference was statistically significant (Fig. 3C; P = 0.014). Sos1/+ IMs were intermediate in size between normal and Sos1/Sos1 mutants. Therefore, Sos1 mutants produce fewer SPM, presumably due to a primary defect in IM size.
In summary, histological and SEM analyses show that Sos1 mutants produce fewer spikelets for two reasons: (1) there are fewer rows of SPMs; and (2) the SPMs convert directly to an SM without initiating a second SM, resulting in the formation of single instead of paired spikelets.
The Sos1 Mutation Is an Antimorph
Analysis of the Sos1 phenotype showed that plants homozygous for the Sos1 mutation had a more severe phenotype than plants heterozygous for Sos1, and hence the mutation is semidominant. Four types of dominant mutations have been defined (Muller, 1932). The two types of gain-of-function mutations are neomorph mutations, which confer a new function, and hypermorph mutations, which cause increased expression of the gene. Two types of loss-of-function mutations are hypomorph and antimorph. Hypomorph mutations are also called haplo-insufficient or dosage-sensitive mutations, because one wild-type copy of the gene is not sufficient for function. In antimorphs, which are also called dominant negative mutations, the mutant copy of the gene interferes with the wild-type gene function (Veitia, 2007).
To distinguish between these types of mutations, dosage analysis is used to vary the dose of the wild-type copy of the gene in the mutant background (Greene and Hake, 1994). In a neomorph, there would be no effect of varying dose (Freeling and Hake, 1985; Poethig, 1988), while in a hypermorph, increasing the number of wild-type copies would cause the phenotype to be more severe (Kessler et al., 2002). On the other hand, in hypo- and antimorphs, adding wild-type copies would cause the phenotype to be weaker (Poethig, 1988; Nelson et al., 2002). A hypomorph can be distinguished from an antimorph by looking at the effect of varying the wild-type copy number of the gene in the wild-type background. Removing one wild-type copy of the gene would cause a visible phenotype if the gene was haplo-insufficient and the mutation was a hypomorph, but not if the mutation was an antimorph.
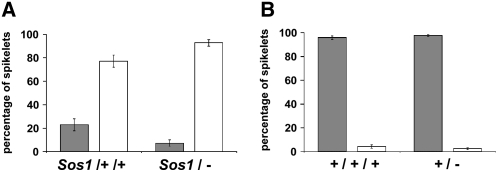
To determine the phenotypic effect of varying the wild-type dose of sos1, pollen from hyperploids of the B-A translocation line, TB-4Sa, was crossed onto normal plants or plants heterozygous for Sos1. The F1 of the cross was analyzed for ploidy level (by scoring pollen abortion; see “Materials and Methods”) and for severity of phenotype (by counting the percentage of single spikelets in the tassel and the number of kernels in the ear). These results showed that Sos1 plants with an extra copy of the short arm of chromosome 4 (Sos1/+/+, hyperploid) had a weaker phenotype than plants that were missing a normal copy of the short arm of chromosome 4 (Sos1/−, hypoploid), as they had a higher percentage of paired spikelets (Fig. 5A). Similar results were also observed in the ear (data not shown). These results show that Sos1 is not a neomorph, as there was an effect of varying gene dosage and not a hypermorph, as the hyperploid was not more severe than the hypoploid.
Figure 5.
Dosage analysis. A, The percentage of paired (gray bar) versus single (white) spikelets in Sos1 plants containing two copies of wild-type chromosome 4S (hyperploid) or missing the short arm of chromosome 4 (hypoploid). B, The percentage of paired (gray) versus single (white) spikelets in normal plants containing three wild-type copies of chromosome 4S (hyperploid) or one wild-type copy of chromosome 4S (hypoploid).
To distinguish if Sos1 was a hypomorph or an antimorph mutation, we generated a dosage series for chromosome 4S in a wild-type genetic background. Normal plants missing one copy of the short arm of chromosome 4 did not have a Sos1 phenotype. There were mostly paired spikelets in both the hypoploid (+/−) and the hyperploid (+/+/+) (Fig. 5B). This result indicates that the wild-type sos1 gene is not haplo-insufficient and therefore that the Sos1 mutation is not a hypomorph. Thus, the Sos1 mutation is likely an antimorph or dominant negative mutation.
Sos1 Suppresses the ra1 Mutant Phenotype
Sos1 mutants have single instead of paired spikelets, because SPMs produce one instead of two SM. Hence, the SPM is more determinate than normal in Sos1 mutants. Therefore, the sos1 gene could be considered a negative regulator of the determinacy of the SPM. In ra1 mutants, the SPM are indeterminate, resulting in the production of highly branched tassels and ears (Fig. 6, A and C; Gernart, 1912; Vollbrecht et al., 2005). The ra1 gene is interpreted as a positive regulator of SPM determinacy (Vollbrecht et al., 2005). Because Sos1 and ra1 mutants have opposite effects on SPM determinacy, we tested if the corresponding genes acted in the same pathway by constructing double mutants.
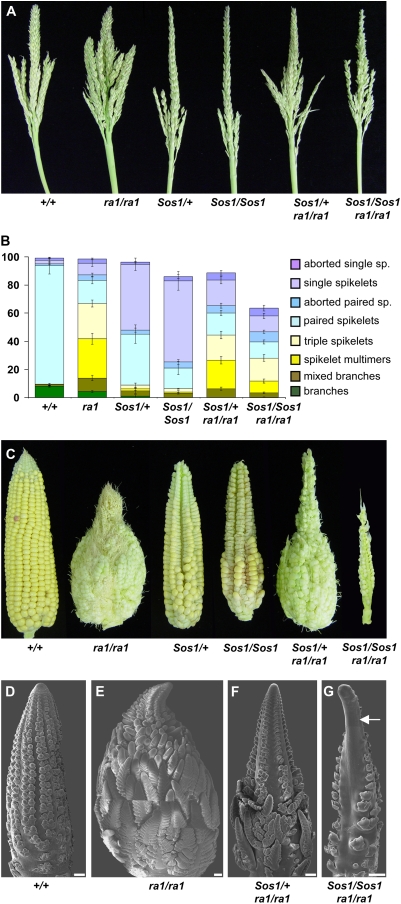
Figure 6.
Genetic interaction between Sos1 and ra1. A, Photograph of the mature tassel of all genetic classes of an F2 family segregating for Sos1 and ra1. B, Classification of the number of different types of axillary structures produced by the tassel. C, Photograph of mature ears of all genetic classes of an F2 family segregating for Sos1 and ra1. D to G, SEM of ear inflorescence. D, Normal ear showing rows of paired SM. E, ra1 ear that is highly branched as all SPM are converted to branches. F, Sos1/+; ra1/ra1 ear in which SPM produce extra SM but not as many as ra1. G, Sos1/Sos1; ra1/ra1 ear in which SPM mainly produce single spikelets. One side of the tip is bare with no SPMs (arrow). Scale bar = 100 μm.
In the tassel, Sos1/Sos1; ra1/ra1 double mutants were less branched than ra1 single mutants (Fig. 6A). To quantify the effects of both mutations, the branches on the tassels were removed, classified, and counted using a scheme similar to that used to analyze ra1 (Fig. 6B; Vollbrecht et al., 2005). Normal plants produce branches before switching abruptly to producing spikelet pairs. ra1 mutants produce several intermediates between branches and spikelet pairs (Vollbrecht et al., 2005). These intermediates include “mixed branches,” which are branches with a mixture of single and paired spikelets; “spikelet multimers,” which are branches with single spikelets; and “triple spikelets.” Our analysis confirmed that, similar to the previous report (Vollbrecht et al., 2005), ra1 mutants had a gradation in phenotype from branches through to spikelet pairs with a delayed switch to SPM identity compared to normal (Fig. 6B). The branches in Sos1 mutants were found to be mixed branches rather than true branches (Fig. 6B). Sos1 mutants produced fewer branch types overall and therefore had an earlier switch to SPM identity than normal. In the Sos1/Sos1; ra1/ra1 double mutants, there was a suppression of the ra1 phenotype. Branches were replaced by mixed branches, and there were fewer of all branch types compared to ra1 single mutants (Fig. 6B).
The reduction in the number of branch types in Sos1/Sos1; ra1/ra1 double mutants could potentially be explained by the fact that Sos1 mutants produce fewer SPM (Fig. 3A). So, we also estimated the percentage of branch types compared to the total number of axillary structures produced by the tassel. In this case, the Sos1 mutation still had a suppressive effect on the ra1 phenotype, as the percentage of all branch types was reduced from 41.8% in ra1 single mutants to 18.7% in Sos1/Sos1; ra1/ra1 double mutants. Therefore, even taking into account the production of fewer SPM by Sos1 mutants, branching in the double mutant tassel was suppressed.
The suppression of the ra1 phenotype by Sos1 was even more obvious in the ear than in the tassel. Ears of plants that were Sos1/+; ra1/ra1 were less branched than ra1 and even initiated some viable kernels, which happens very rarely in ra1 single mutants. Furthermore, in the Sos1/Sos1; ra1/ra1 double mutant ear, branching was almost completely suppressed. In the most extreme examples, the ears looked like Sos1, except that they were smaller and more barren (Fig. 6C).
To investigate the developmental basis for the suppression of ra1 by Sos1, SEM analysis was performed on the ears of families segregating for both mutations. In normal ears, SPM produce two SM (Fig. 6D), while in ra1 ears, each SPM becomes indeterminate and branches to continuously produce SM in a reiterative manner (Fig. 6E). Sos1/+; ra1/ra1 ears were suppressed compared to ra1 (Fig. 6, E and F). The tip resembled Sos1, but at the base of the ear, SPM branched to produce multiple SM, although not as many as in the ra1 mutants (Fig. 6F). In severe cases, Sos1/Sos1; ra1/ra1 produced ears that resembled Sos1 (Fig. 6G). Furthermore, there was sometimes a barren patch along the side of the ear in the double mutants (arrow, Fig. 6G), a phenotype that was not seen in either single mutant. This barren phenotype was also visible in the mature ear (Fig. 6C). Therefore, the Sos1 mutation suppressed the phenotype of the ra1 mutation in both the tassel and the ear.
sos1; ra2 Double Mutants Have a Synergistic Phenotype
ra2 also functions in SPM determinacy. In ra2 mutants, the SPM becomes indeterminate and produces extra branches and spikelets in the tassel (Fig. 7A; Kempton, 1923; Nickerson and Dale, 1955; Bortiri et al., 2006a). However, the ear is only mildly affected with irregular rowing and occasional branches (Fig. 7E). ra2 also plays unique roles in inflorescence development as, unlike ra1, tassel branches are upright and spikelet pedicels are elongated in ra2 mutants (Bortiri et al., 2006a). ra2 is proposed to act upstream of ra1 (Vollbrecht et al., 2005; Bortiri et al., 2006a).
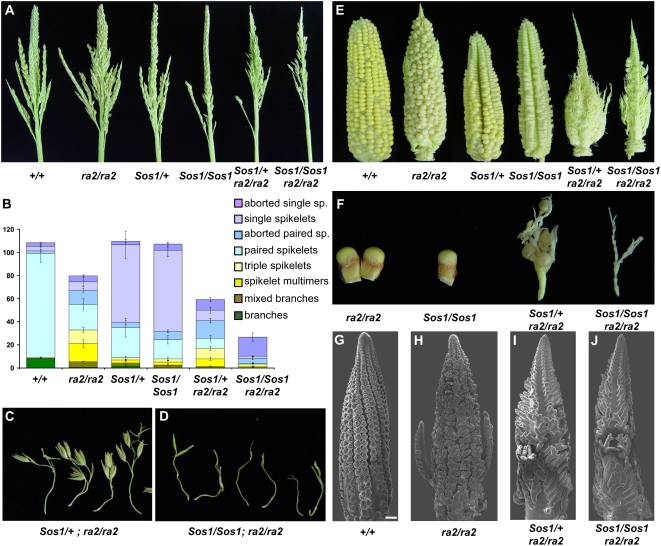
Figure 7.
Genetic interaction between Sos1 and ra2. A, Photographs of the mature tassel of all genetic classes of an F2 family segregating for Sos1 and ra2. B, Classification of the number of different types of axillary structures produced by the tassel. C, Close-up view of individual branches produced in Sos1/+; ra2/ra2 double mutant tassels. D, Close-up view of aborted spikelets produced in Sos1/Sos1; ra2/ra2 double mutant tassels. E, Photograph of mature ear of all genetic classes of an F2 family segregating for Sos1 and ra2. F, Close-up view of axillary structures produced in ears of double mutant family. Normal and ra2 produce two kernels per row, Sos1 mutants produce one kernel per row, Sos/+; ra2/ra2 produce long branches with multiple kernels, Sos1/Sos1; ra2/ra2 produce long sterile branches with no kernels. G to J, SEM of ear inflorescence. G, Normal ear showing rows of paired SM. H, ra2 ear in which SPM produce three SM or occasional branches. I, Sos1/+; ra2/ra2 ear in which SPM form elongated branches. J, Sos1/Sos1; ra2/ra2 ear in which SPM produce barren elongated branches. Scale bar = 100 μm.
To test if sos1 acted in the same pathway as ra2, double mutants were constructed. Surprisingly, Sos1 had a synergistic interaction with ra2 in both the tassel and the ear (Fig. 7). To determine the effect of the Sos1 mutation on the ra2 phenotype in the tassel, the branches were removed, classified, and quantified using the same classification system used previously for ra1 and ra2 (Vollbrecht et al., 2005; Bortiri et al., 2006a). ra2 mutants, like ra1, produced intermediates between branches and spikelets along the main spike, except the phenotype was weaker than ra1 (Fig. 7B; Vollbrecht et al., 2005; Bortiri et al., 2006a). Both Sos1 and ra2 single mutants had a small effect on the total number of axillary structures produced by the tassel (Fig. 7B; Bortiri et al., 2006a). Strikingly, there was a massive decrease (over 3-fold) in the total number of axillary structures produced by Sos1/Sos1; ra2/ra2 double mutants compared to either single mutant (Fig. 7B). Both Sos1 and ra2 single mutants produce a small number of rudimentary spikelets with 1 to 2 glumes and no florets (called aborted spikelets). The axillary structures that were produced in the Sos1/Sos1; ra2/ra2 double mutants consisted almost entirely of aborted spikelets (Fig. 7, B and D). We interpret these phenotypes as a synergistic enhancement of the ra2 defects.
In the Sos1; ra2 double mutant ear, an enhancement of the phenotype of ra2 was also apparent. The ears of ra2 mutants only occasionally produce branches (Vollbrecht et al., 2005; Bortiri et al., 2006a). However, Sos1/+; ra2/ra2 and Sos1/Sos1; ra2/ra2 ears were much more highly branched than ra2 ears (Fig. 7E). Sos1/+; ra2/ra2 produced branches with a few kernels on them, while Sos1/Sos1; ra2/ra2 branches were long and barren (Fig. 7F).
To understand the developmental basis for the enhanced branching and sterility of Sos1; ra2 double mutant ears, SEM analysis was performed on developing ears. In normal ears, SPMs produced two SM (Fig. 7G), while in the ra2 mutant, the SPM produced more than two SM and there were occasional branches (Fig. 7H). The Sos1/+; ra2/ra2 mutants were more highly branched than ra2 with each SPM branching multiple times (Fig. 7I). The branches were more elongated than ra2 and produced few SM (Fig. 7, H and I). In the Sos1/Sos1; ra2/ra2 double mutant, the phenotype was further enhanced with elongated branches in place of SPM and even fewer SM (Fig. 7J). Therefore, Sos1 enhanced the phenotype of the ra2 mutant in both the tassel and ear.
Sos1; ra3 Double Mutants Have an Additive Phenotype
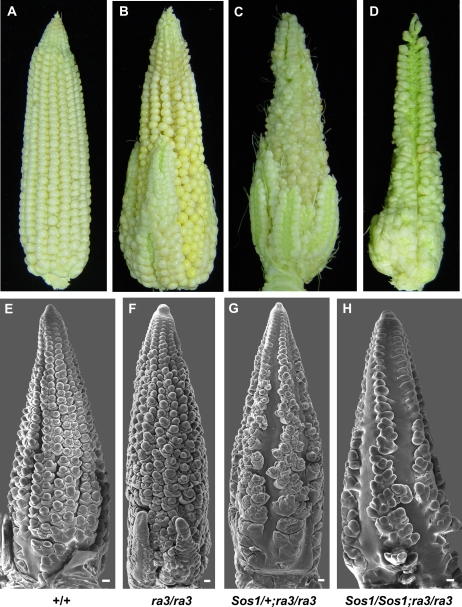
ra3 also functions in meristem determinacy, though the phenotype is weaker than ra1 and ra2 (Satoh-Nagasawa et al., 2006). In the tassel, ra3 mutants produce a few extra long branches but do not have the determinacy defects seen in ra1 and ra2 mutants (data not shown). In the ear, ra3 mutants have irregular rows and branches at the base of the ear (Fig. 8B; Satoh-Nagasawa et al., 2006). These defects are caused by the conversion of SPMs to branches and SMs producing additional structures, including additional FMs (Fig. 8F; Satoh-Nagasawa et al., 2006). In Sos1/+; ra3/ra3 double mutant ears, branches were produced at the base of the ear, and single spikelets were produced at the tip of the ear (Fig. 8C). Furthermore, similar to ra3 single mutants, Sos1/+; ra3/ra3 spikelets produced additional FM (Fig. 8G). Sos1/Sos1; ra3/ra3 double mutant ears had a similar phenotype to Sos1/+; ra3/ra3, except that the Sos1 phenotype was apparent along a greater extent of the ear (Fig. 8D). SEM analysis showed that indeterminate SPM were produced at the base of the ear (Fig. 8H). Thus, the double mutants had mostly additive defects with single rows of spikelets caused by the Sos1 mutation at the tip and additional structures caused by the ra3 mutation at the base of the ear.
Figure 8.
Genetic interaction between Sos1 and ra3. A to D, Photographs of mature ears from a Sos1; ra3 segregating family. E to H, SEM analysis of ears from a Sos1; ra3 segregating family. Scale bar = 100 μm.
Expression of ra1, ra2, and ra3 in Sos1 Mutants
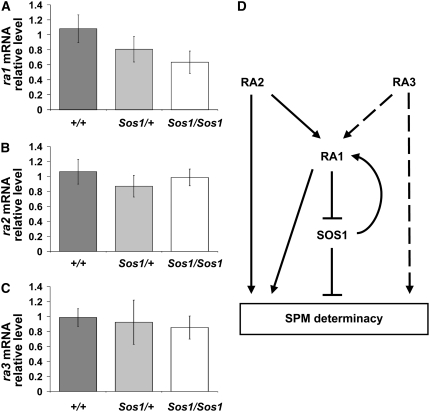
To further test the role of sos1 in the ramosa pathway, the relative mRNA expression level of ra1, ra2, and ra3 was tested in Sos1 mutants using quantitative real-time reverse transcription (RT)-PCR. The results showed that ra1 mRNA levels were reduced in Sos1/Sos1 mutants (Fig. 9A). As ra1 mRNA levels are regulated by ra2 and ra3 (Vollbrecht et al., 2005; Satoh-Nagasawa et al., 2006), we tested whether the reduction in ra1 expression was due to a defect in ra2 or ra3 expression. However, ra2 and ra3 mRNA levels were not affected in Sos1/Sos1 mutants (Fig. 9, B and C). Therefore, the Sos1 mutation specifically affected ra1 expression levels.
Figure 9.
Expression of ra1, ra2, and ra3 in Sos1 mutants and model for the role of sos1 in SPM determinacy. A to C, Real-time RT-PCR experiments showing the relative expression level of (A) ra1, (B) ra2, and (C) ra3 in Sos/+ and Sos/Sos1 mutants. C, Model for the interaction between Sos1 and the ramosa genes. ra1, ra2, and ra3 are required to promote SPM determinacy. We propose that Sos1 is a negative regulator of SPM determinacy. ra2 and ra3 act upstream of ra1 as well as having roles independent of ra1. As Sos1; ra1 double mutants resemble Sos1 single mutants, sos1 is placed downstream of ra1. As Sos1; ra2 double mutants have an enhanced phenotype similar to ra1; ra2 double mutants, sos1 is proposed to positively regulate ra1. Sos1; ra3 double mutants have an additive phenotype, indicating that ra3 acts in an independent pathway. The model is supported by expression studies showing that ra1 is reduced, but ra2 and ra3 are unchanged in Sos1 mutants.
DISCUSSION
The most striking defect in Sos1 mutants is that the SPM initiates one instead of two SMs. In addition, Sos1 mutants have defects in SPM initiation, which we propose is due to the overall reduction in apical IM size. Sos1 mutants also produce fewer branches. However, the branches that are produced are not normal branches but are mixed branches, which are more determinate than normal. Once SMs are produced in Sos1 mutants, they usually produce two florets. However, a small percentage of aborted spikelets (spikelets with no florets) are apparent later in development. Rudimentary spikelets were also reported by Doebley et al. (1995). Therefore, the Sos1 mutation affects the determinacy of all meristems produced during inflorescence development.
The Role of sos1 in the ramosa Pathway for Meristem Determinacy
ra1 and ra2 mutants are highly branched because the SPM are indeterminate. Therefore, the role of the ra1 and ra2 genes is to impose determinacy on the SPM (Vollbrecht et al., 2005; Bortiri et al., 2006a), represented by a solid arrow in the model shown in Figure 9D. ra2 is proposed to act upstream of ra1, because ra1 mRNA levels are reduced in ra2 mutants, and double mutants between ra2 and a weak allele of ra1 have an enhanced phenotype (Vollbrecht et al., 2005; Bortiri et al., 2006a; Satoh-Nagasawa et al., 2006). Furthermore, ra2 is proposed to have additional roles, independent of ra1 (Bortiri et al., 2006a).
In Sos1 mutants, the SPM are more determinate than normal, producing one instead of two SM. The Sos1 mutation is an antimorph, which is a type of dominant loss-of-function mutation (more correctly an antagonist of wild-type function). Therefore, in Sos1 mutants, the absence of the normal function of the sos1 gene causes an increase in determinacy. One interpretation of the wild-type function of the sos1 gene is to oppose SPM determinacy, represented as a bar in Figure 9D. Another way of describing this is that the sos1 gene confers indeterminacy on the SPM, but as the SPM is normally determinate, we propose that sos1 inhibits determinacy. As the sos1 gene inhibits SPM determinacy, while the ramosa genes promote SPM determinacy, double mutants were constructed to test the genetic interaction between sos1 and the ramosa genes. Surprisingly, we found a difference in the interaction between sos1 and ra1, ra2, and ra3.
We propose the model shown in Figure 9D to account for all of the genetic interaction and expression data. Sos1; ra1 double mutants resemble Sos1 single mutants. As the Sos1 and ra1 single mutants have opposite phenotypes, we interpret the double mutant result to mean that the wild-type sos1 gene functions downstream of ra1 (Fig. 9D; Avery and Wasserman, 1992). Therefore, ra1 could confer SPM determinacy by negatively regulating the sos1 gene. Unexpectedly, Sos1; ra2 double mutants had an enhanced phenotype compared to ra2 single mutants. The Sos1; ra2 double mutant looks strikingly similar to the double mutant between ra2 and a weak allele of ra1 (Vollbrecht et al., 2005). One hypothesis to account for the enhancement of the ra2 phenotype by the Sos1 mutation would be if the sos1 gene positively regulated the ra1 gene. In support of this hypothesis, ra1 mRNA levels are reduced in Sos1 mutants. Therefore, we propose that sos1 acts in the ra1 branch of the ramosa pathway, providing a negative feedback loop to control SPM determinacy (Fig. 9D).
ra3 is also proposed to act upstream of ra1, although as ra3 encodes a trehalose-6-phosphate phosphatase, this interaction may not be direct (Satoh-Nagasawa et al., 2006). ra3 mutants have weak defects in SPM determinacy, indicating that ra3 also plays a role in SPM determinacy (Satoh-Nagasawa et al., 2006). Sos1; ra3 double mutants had mostly additive defects, implying that sos1 and ra3 act in independent pathways.
The Sos1 mutation had a more suppressive effect on the ra1 phenotype in the ear than the tassel. An alternative interpretation of the Sos1;ra1 tassel phenotype would be that the Sos1 and ra1 mutations have an additive effect, indicating that sos1 acts independently of the ra1 pathway. However, the reduction of expression of ra1 in Sos1 mutants does not support an independent interaction. Furthermore, as there is sometimes a complete suppression of the ra1 phenotype in the Sos1; ra1 ear, we favor the hypothesis that the difference between the tassel and ear double mutant phenotypes is due to differences in modifying factors between the tassel and the ear. Differences in severity of tassel versus ear phenotypes are common in maize inflorescence determinacy mutants (Irish, 1997a, 1997b; Laudencia-Chingcuanco and Hake, 2002; Kaplinsky and Freeling, 2003; Bortiri et al., 2006a). One explanation is that the tassel forms in an apical position and the ear forms in an axillary position, so their hormonal environments likely differ. Furthermore, subfunctionalization of duplicate genes between tassel and ear has been demonstrated (Mena et al., 1996). In addition, the extent of branching is affected by environmental conditions, indicating that additional modifying factors can influence branching.
The Sos1; ra2 double mutant had a synergistic effect on branching in the ear. An alternative interpretation is that the Sos1; ra2 double mutant phenotype could be considered additive, if, for example, the ra2 mutation caused the SPM to become indeterminate but the SPM were unable to initiate sessile SMs due to the Sos1 mutation. However, as the SPMs in the Sos1; ra2 double mutant are more indeterminate than ra2 single mutants, we conclude that the interaction between Sos1 and ra2 is not additive in the ear. Furthermore, the effect of the Sos1 mutation on the ra2 phenotype in the tassel is not additive.
Therefore, although other interpretations can be envisioned, we favor the model presented in Figure 9D, because it explains all of the single and double mutant phenotypes as well as the expression studies. Dominant negative mutations can be caused by mutations in transcription factors that, for example, can dimerize but not bind DNA or can bind DNA but not activate transcription (Veitia, 2007). Based on the Sos1 mutant phenotype, genetic interactions with the ra mutants and the decrease in ra1 expression in Sos1 mutants, we hypothesize that sos1 may encode a transcription factor that interacts with ra1 or ra2.
Additional Roles of sos1 and the ramosa Genes in Inflorescence Development
Besides the effect on determinacy of the SPM, the Sos1; ra1 and Sos; ra2 double mutants had additional defects in inflorescence development. This implies that the corresponding genes play additional roles in development that had not previously been discovered. Sos1; ra1 double mutants produced barren patches in the ear that were not seen in either single mutant. This synergistic interaction could be explained by the function of both genes in the SPM. As ra1 and ra2 are not expressed in the apical IM, we propose that this is due to a defect in the SPM itself. We infer that as the genes have opposing functions in the SPM, in their absence the SPM sometimes fails to initiate. This effect was also seen in the tassel, as there was an overall reduction in the number of axillary structures produced in the Sos1; ra1 double mutant. In Sos1; ra2 double mutants, there was an even more severe reduction in the number of axillary structures produced in the tassel. These results indicate that the sos1, ra1, and ra2 genes play overlapping roles in the production of SPM in both the tassel and ear.
In the single Sos1, ra1, and ra2 mutants, a small number of aborted spikelets were produced in the tassel, with ra2 mutants having the strongest effect. In Sos1; ra1 double mutants, there was a somewhat additive increase in the number of aborted spikelets in the tassel compared to either single mutant. However, in Sos1; ra2 double mutants, there was a synergistic increase in the number of aborted spikelets. In fact, almost all of the spikelets produced in the double mutant tassel were aborted. Therefore, we propose that sos1, ra1, and ra2 also function in SMs. However, SMs are still indeterminate in Sos1;ra3 double mutants similar to ra3 single mutants, indicating that the role of sos1 is independent of ra3 in SMs.
Role of sos1 in Regulating IM Size
Sos1 mutants have a smaller apical IM and a reduced number of SPM. We argue that the defects in SPM initiation are due to the defects in IM size, as there are other examples where an increase or decrease in meristem size affected SPM initiation. For example, knotted1 (kn1) loss-of-function mutants, which also produce fewer branches and spikelets in the inflorescence, have reduced SAM size in some genetic backgrounds, although a difference in the size of the IM has not been demonstrated (Kerstetter et al., 1997; Vollbrecht et al., 2000). The opposite phenotype is seen in thick tassel dwarf1 (td1) and fasciated ear2 (fea2) mutants, which have a larger IM and produce more SPM (Taguchi-Shiobara et al., 2001; Bommert et al., 2005). Interestingly, one of the ra3 alleles, ra3-fae1, was originally identified as having a fasciated ear phenotype (Satoh-Nagasawa et al., 2006), indicating that the ramosa pathway may have indirect effects on the IM. abphyl1 (abph1) mutants, which affect leaf phyllotaxy, also have a larger SAM (Jackson and Hake, 1999). abph1 encodes a cytokinin response regulator that negatively regulates cytokinin signaling (Giulini et al., 2004). In addition, mutations that affect cytokinin levels in rice also affect spikelet number (Ashikari et al., 2005; Kurakawa et al., 2007). The defects in kn1, td1, and fea2 mutants have been suggested to be due to a primary defect in cytokinin levels (Barazesh and McSteen, 2008b), as levels of cytokinin are correlated with meristem size in eudicots (Sakakibara, 2006; Kyozuka, 2007). Whether Sos1 mutants have defects in cytokinin levels or signaling remains to be determined. As Sos1 mutants affect the size of the IM and the determinacy of the BM and SPM, we propose that there is a similar underlying defect in maintenance or determinacy of both apical and axillary meristems.
Does Auxin Play a Role in sos1 Function?
Single pedicellate spikelets are also characteristic of mutations affecting auxin transport. For example, Barren inflorescence1 (Bif1) and bif2 mutants, which are defective in auxin transport, produce single spikelets (McSteen and Hake, 2001; McSteen et al., 2007; Barazesh and McSteen, 2008a). barren stalk1 (ba1), which is proposed to act both upstream and downstream of auxin transport, also produces single spikelets when mutated (Ritter et al., 2002; Gallavotti et al., 2004, 2008; Wu and McSteen, 2007; Skirpan et al., 2008). Furthermore, treatment of normal maize inflorescences with auxin transport inhibitors later in development results in the production of single spikelets (Wu and McSteen, 2007). Therefore, auxin transport is required for sessile spikelet initiation. Genetic analysis shows Sos1; bif2 double mutants look similar to bif2 (Supplemental Fig. S1). Similarly, ba1 is epistatic to Sos1 (Supplemental Fig. S1). This could be interpreted to mean that sos1 acts in the same pathway as bif2 and ba1, as Sos1 does not appear to enhance the phenotype of bif2 or ba1. However, the bif2 and ba1 phenotypes are so severe that it would be difficult to see an enhancement. Thus, it is not clear if sos1 plays a role in enabling auxin, directly or indirectly, to initiate the sessile SM.
To conclude, we are currently fine mapping Sos1 to identify the gene using positional cloning (Bortiri et al., 2006b). Cloning the sos1 gene will elucidate the molecular basis for its interaction with the ramosa pathway. Furthermore, the cloning of sos1 will provide insight into its predicted role in the evolution of the paired spikelet. Because ra1 has only been identified in the Andropogoneae, it would be interesting to determine if sos1 was co-opted at the same timepoint during the diversification of the grasses.
MATERIALS AND METHODS
Plant Growth and Mature Phenotype Characterization
The Sos1-Reference allele was obtained from the Maize Genetics Cooperation Stock Center (stock 427I) and backcrossed six times into the B73 genetic background of maize (Zea mays). Analysis of mature phenotype was carried out on plants grown at Rock Springs, PA, during the summer. The plants were genotyped as Sos1 heterozygotes or homozygotes using SSR marker umc1294, which is the closest genetic marker identified so far (umc1294F, 5′-GCC GTC AAC GGG CTT AAA CT-3′ and umc1294R, 5′-GCC TCC ACG TCT CTC GTC TCT T-3′). For phenotype characterization, tassels and ears were collected at maturity from normal Sos1/+ and Sos1/Sos1 plants from segregating families. Branch number and the number of paired versus single spikelets on the main spike were counted on the tassels after anthesis. The sample size was 28 normal, 35 Sos1/+, and 33 Sos1/Sos1 in the data presented in Figure 1, B and C. Kernel number was counted on open pollinated ears. The sample size was 16 normal, 13 Sos1/+, and 10 Sos1/Sos1 in the data presented in Figure 1, E and F.
SEM and Histology
Immature ears (5–20 mm) were collected from plants grown for 8 weeks during the summer at Rock Springs, PA. Immature tassels (3–6 mm) were obtained from 5-week-old plants grown in the spring in the greenhouse with supplemental lighting. Both tassels and ears were fixed and prepared for SEM as described (Wu and McSteen, 2007). The number of SPM around the circumference of the apical IM was determined by mounting the top of the ear on an SEM stub and viewing the samples from above (n = 11 normal, 8 Sos1/+, and 7 Sos1/Sos1). For measurements of IM size, ear samples were viewed from the side, and the width of the IM was measured across the meristem immediately above the point where bract primordia were visible and the height was measured perpendicular from this line to the tip of the inflorescence (n = 7 normal, 7 Sos1/+, and 6 Sos1/Sos1). In addition, tassels and ears were embedded in wax and sectioned for TBO staining as described (Wu and McSteen, 2007).
Dosage Analysis
Dosage analysis was conducted by crossing known hyperploids of the B-A translocation line, TB-4Sa (marked with sugary1, Coop stock 421D) as males to normal plants or plants heterozygous for Sos1. TB-4Sa is a reciprocal translocation of the short arm of chromosome 4 with the supernumery B chromosome, which due to a very high rate of non-disjunction during the second mitotic division of pollen development gives rise to pollen with either two or zero copies of chromosome 4S (Roman, 1947; Beckett, 1978, 1994). The ploidy of the F1 progeny can be determined by the rate of pollen abortion (Roman, 1947; Carlson, 1994). Pollen abortion was scored using a pocket microscope on consecutive mornings as the anthers started to shed fresh pollen (Phillips, 1994). As had been observed previously for TB4Sa, there was 50% pollen abortion in hypoploid plants and 2% to 10% pollen abortion in hyperploid plants (Carlson, 1994; D. Auger, South Dakota State University, personal communication). The tassels and ears of hypoploids and hyperploids were collected at maturity for phenotype analysis. The percentage of paired and single spikelets was counted on a 15-cm region of the tassel main spike after anthesis. For the analysis shown in Figure 4, the samples sizes were 13 Sos1/+/+, 6 Sos1/−, 8 +/+/+, and 4 +/− tassels.
Double Mutant Analysis
All double mutant segregating families were generated in the B73 genetic background and were planted during the summer at Rock Springs, PA, for two field seasons. At least 360 plants were planted for each double mutant combination each year. All plants were genotyped for Sos1 with the SSR marker umc1294 as described above. Each double mutant family was scored, and chi-square analysis failed to reject the segregation ratio expected (data not shown).
Families segregating for Sos1; bif2 and Sos1; ba1 were genotyped for the bif2 and ba1 mutations as described (Skirpan et al., 2008). At maturity, tassel branch and spikelet number on the main spike was counted. For the analysis shown in Supplemental Figure S1B, the sample size was 2 normal, 4 Sos1/+, 11 Sos1/Sos1, 17 bif2/bif2, 13 Sos1/+;bif2/bif2, and 13 Sos1/Sos1; bif2/bif2. For the analysis shown in Supplemental Figure S1D, the sample size was 2 normal, 5 Sos1/+, 7 Sos1/Sos1, 11 ba1/ba1, 11 Sos1/+;ba1/ba1, and 8 Sos1/Sos1; ba1/ba1.
Families segregating Sos1; ra1 and Sos1; ra2 were scored and the tassel and ear phenotype analyzed at maturity (9 weeks old). Branches were removed from the tassels and classified according to Vollbrecht et al. (2005) and Bortiri et al. (2006a). For the analysis shown in Figure 6B, the sample size was 4 normal, 4 Sos1/+, 3 Sos1/Sos1, 9 ra1/ra1, 10 Sos1/+; ra1/ra1, and 10 Sos1/Sos1; ra1/ra1. For the analysis shown in Figure 7B, the sample size was 4 normal, 4 Sos1/+, 6 Sos1/Sos1, 6 ra2/ra2, 8 Sos1/+; ra2/ra2, and 9 Sos1/Sos1; ra2/ra2. Families segregating for Sos1; ra3 were scored for ear phenotype only as the ra3 tassel phenotype was very weak.
All graphs depict mean ± se of the mean. Probability values were determined from Student's two-tailed t tests performed in Microsoft Excel 2003.
Real-Time RT-PCR
Total RNA was extracted from 4- to 5-mm tassels from normal, Sos1/+, and Sos1/Sos1 plants with the NucleoSpin RNA Plant kit (Macherey-Nagel GmbH & Co.). Four to six samples from each class were used as biological replicates, three technical replicates for each biological replicate were performed, and the entire experiment was repeated three times. Synthesis of cDNA, real-time RT-PCR, and analysis were performed as described (Barazesh and McSteen, 2008a). To detect ra1 expression, the Taqman probe was (FAM-5′-ATC CAC AGG CTG GAC AGG GCC A-3′-BHQ) and the RT-PCR primers were ra1F (5′-GCT GGG AGG CCA CAT GAA-3′) and ra1R (5′-GTG AAG TGT ACT GTT GGT GGA TCA G-3′). To detect ra2 expression, the Taqman probe was (FAM-5′-TCG TCA TTA GTA GCT CCC CAG GCG C-3′-BHQ) and the RT-PCR primers were ra2F (5′-TGC TAC TTC ATG CGG AAC CA-3′) and ra2R (5′-TTA GCC ACG GAA GCG TAA GG-3′). To detect ra3 expression, the Taqman probe was (FAM-5′-CCG TCC ACT TCC GCT GCG TCC-3′-BHQ) and the RT-PCR primers were ra3F (5′-CCA GGG TGG AGC ACA ACA A-3′) and ra3R (5′-CTC GTT CAC GGC ATT CCA T). The internal control for amplification was ubiquitin (ubq). To detect ubq expression, the Taqman probe was (5′-FAM-AAA TCC ACC CGT CGG CAC CTC C-3′ BHQ) and the RT-PCR primers were ubqF (5′-CTC TTT CCC CAA CCT CGT GTT-3′) and ubqR (5′-ACG AGC GGC GTA CCT TGA-3′).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Genetic interaction of Sos1 with bif2 and ba1.
Supplementary Material
Acknowledgments
We thank Erik Vollbrecht for pointing out the similarity between Sos1; ra2 and ra1; ra2 double mutants, David Barnes and Sarah Hake for first discovering the Sos1; ra2 interaction, and two anonymous reviewers for their helpful suggestions. We thank Deb Grove of the Huck Institutes Nucleic Acid Facility for conducting the real-time RT-PCR experiments, Missy Hazen of the Huck Institutes Electron Microscopy Facility for training on the SEM, and Jeffrey Buterbaugh, Jason Hoar, Chris Cook, Kim Phillips, Matt Davis, and Chris Hudson for help in the field and with Sos1 genotyping. We thank the Maize Coop for Sos1 seed, Frank Baker for seed of TB4Sa hyperploids, and Don Auger for advice on TB dosage analysis. We thank Tony Omeis and Scott Harkcom for plant care. We thank members of the McSteen and Braun labs for discussion and comments on the manuscript.
This work was supported by The Pennsylvania State University (start-up funds to P.M. and a Biology Department, Henry W. Popp graduate assistantship to X.W.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Paula McSteen (pcm11@psu.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Ashikari M, Sakakibara H, Lin SY, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005) Cytokinin oxidase regulates rice grain production. Science 309 741–745 [DOI] [PubMed] [Google Scholar]
- Avery L, Wasserman S (1992) Ordering gene function: the interpretation of epistasis in regulatory hierarchies. Trends Genet 8 312–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazesh S, McSteen P (2008. a) barren inflorescence1 functions in organogenesis during vegetative and inflorescence development in maize. Genetics 179 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazesh S, McSteen P (2008. b) Hormonal control of grass inflorescence development. Trends Plant Sci 13 656–662 [DOI] [PubMed] [Google Scholar]
- Beckett JB (1978) B-A translocations in maize. I. Use in locating genes by chromosome arms. J Hered 69 27–36 [Google Scholar]
- Beckett JB (1994) Locating recessive genes to chromosome arm with B-A translocations. In M Freeling, V Walbot, eds, The Maize Handbook. Springer-Verlag, New York, pp 315–327
- Bommert P, Lunde C, Nardmann J, Vollbrecht E, Running M, Jackson D, Hake S, Werr W (2005) thick tassel dwarf1 encodes a putative maize ortholog of the Arabidopsis CLAVATA1 leucine-rich repeat receptor-like kinase. Development 132 1235–1245 [DOI] [PubMed] [Google Scholar]
- Bortiri E, Chuck G, Vollbrecht E, Rocheford T, Martienssen R, Hake S (2006. a) ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18 574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortiri E, Hake S (2007) Flowering and determinacy in maize. J Exp Bot 58 909–916 [DOI] [PubMed] [Google Scholar]
- Bortiri E, Jackson D, Hake S (2006. b) Advances in maize genomics: the emergence of positional cloning. Curr Opin Plant Biol 9 164–171 [DOI] [PubMed] [Google Scholar]
- Carlson WR (1994) B-A translocation manipulation. In M Freeling, V Walbot, eds, The Maize Handbook. Springer-Verlag, New York, pp 308–314
- Cheng PC, Greyson RI, Walden DB (1983) Organ initiation and the development of unisexual flowers in the tassel and ear of Zea mays. Am J Bot 70 450–462 [Google Scholar]
- Chuck G, Meeley RB, Hake S (1998) The control of maize spikelet meristem fate by the APETALA2- like gene indeterminate spikelet1. Genes Dev 12 1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford HT (1987) Spikelet and floral morphology. In TR Soderstrom, KW Hilu, CS Campbell, ME Barkworth, eds, Grass Systematics and Evolution. Smithsonian Institution Press, Washington, DC, pp 21–30
- Doebley J, Stec A, Kent B (1995) Suppressor of sessile spikelets1 (Sos1): a dominant mutant affecting inflorescence development in maize. Am J Bot 82 571–577 [Google Scholar]
- Doust AN, Kellogg EA (2002) Inflorescence diversification in the panicoid “bristle grass” clade (Paniceae, Poaceae): evidence from molecular phylogenies and developmental morphology. Am J Bot 89 1203–1222 [DOI] [PubMed] [Google Scholar]
- Freeling M, Hake S (1985) Developmental genetics of mutants that specify knotted leaves in maize. Genetics 11 617–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallavotti A, Yang Y, Schmidt RJ, Jackson D (2008) The relationship between auxin transport and maize branching. Plant Physiol 147 1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallavotti A, Zhao Q, Kyozuka J, Meeley RB, Ritter M, Doebley JF, Pe ME, Schmidt RJ (2004) The role of barren stalk1 in the architecture of maize. Nature 432 630–635 [DOI] [PubMed] [Google Scholar]
- Gernart W (1912) A new subspecies of Zea mays L. Am Nat 46 616–622 [Google Scholar]
- Giulini A, Wang J, Jackson D (2004) Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature 430 1031–1034 [DOI] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group (2001) Phylogeny and subfamilial classification of the grasses (Poaceae). Ann Miss Bot Gard 88 373–457 [Google Scholar]
- Greene B, Hake S (1994) Use of segmental aneuploids for mutant analysis. In M Freeling, V Walbot, eds, The Maize Handbook. Springer-Verlag, New York, pp 270–273
- Irish EE (1997. a) Class II tassel seed mutations provide evidence for multiple types of inflorescence meristems in maize (Poaceae). Am J Bot 84 1502–1515 [PubMed] [Google Scholar]
- Irish EE (1997. b) Experimental analysis of tassel development in the maize mutant Tassel seed 6. Plant Physiol 114 817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish EE (1998) Grass spikelets: a thorny problem. Bioessays 20 789–793 [Google Scholar]
- Jackson D, Hake S (1999) Control of phyllotaxy in maize by the abphyl1 gene. Development 126 315–323 [DOI] [PubMed] [Google Scholar]
- Kaplinsky NJ, Freeling M (2003) Combinatorial control of meristem identity in maize inflorescences. Development 130 1149–1158 [DOI] [PubMed] [Google Scholar]
- Kellogg EA (2000) Molecular and morphological evolution in Andropogoneae. In SWL Jacobs, JE Everett, eds, Grasses: Systematics and Evolution. CSIRO, Collingwood, Australia, pp 149–158
- Kellogg EA (2001) Evolutionary history of the grasses. Plant Physiol 125 1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA (2007) Floral displays: genetic control of grass inflorescences. Curr Opin Plant Biol 10 26–31 [DOI] [PubMed] [Google Scholar]
- Kempton JH (1923) Heritable characters of maize XIV: branched ears. J Hered 14 243–252 [Google Scholar]
- Kerstetter RA, Laudencia-Chingcuanco D, Smith LG, Hake S (1997) Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development 124 3045–3054 [DOI] [PubMed] [Google Scholar]
- Kessler S, Seiki S, Sinha N (2002) Xcl1 causes delayed oblique periclinal cell divisions in developing maize leaves, leading to cellular differentiation by lineage instead of position. Development 129 1859–1869 [DOI] [PubMed] [Google Scholar]
- Kiesselbach TA (1949) The Structure and Reproduction of Corn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 37–63
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J (2007) Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445 652–655 [DOI] [PubMed] [Google Scholar]
- Kyozuka J (2007) Control of shoot and root meristem function by cytokinin. Curr Opin Plant Biol 10 442–446 [DOI] [PubMed] [Google Scholar]
- Laudencia-Chingcuanco D, Hake S (2002) The indeterminate floral apex1 gene regulates meristem determinacy and identity in the maize inflorescence. Development 129 2629–2638 [DOI] [PubMed] [Google Scholar]
- McSteen P (2006) Branching out: the ramosa pathway and the evolution of grass inflorescence morphology. Plant Cell 18 518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSteen P, Hake S (2001) barren inflorescence2 regulates axillary meristem development in the maize inflorescence. Development 128 2881–2891 [DOI] [PubMed] [Google Scholar]
- McSteen P, Laudencia-Chingcuanco D, Colasanti J (2000) A floret by any other name: control of meristem identity in maize. Trends Plant Sci 5 61–66 [DOI] [PubMed] [Google Scholar]
- McSteen P, Malcomber S, Skirpan A, Lunde C, Wu X, Kellogg E, Hake S (2007) barren inflorescence2 encodes a co-ortholog of the PINOID serine/threonine kinase and is required for organogenesis during inflorescence and vegetative development in maize. Plant Physiol 144 1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena M, Ambrose BA, Meeley RB, Briggs SP, Yanofsky MF, Schmidt RJ (1996) Diversification of C-function activity in maize flower development. Science 274 1537–1540 [DOI] [PubMed] [Google Scholar]
- Muller HJ (1932) Further studies on the nature and causes of gene mutations. In DF Jones, WI Menasha, eds, Proceedings of the Sixth International Congress of Genetics. Brooklyn Botanical Gardens, Brooklyn, NY, pp 213–255
- Nelson JM, Lane B, Freeling M (2002) Expression of a mutant maize gene in the ventral leaf epidermis is sufficient to signal a switch of the leaf dorsoventral axis. Development 129 4581–4589 [DOI] [PubMed] [Google Scholar]
- Nickerson NH, Dale EE (1955) Tassel modifications in Zea mays. Ann Miss Bot Gard 42 195–211 [Google Scholar]
- Phillips RL (1994) Classification of pollen abortion in the field. In M Freeling, V Walbot, eds, The Maize Handbook. Springer-Verlag, New York, pp 297–298
- Poethig RS (1988) Heterochronic mutations affecting shoot development in maize. Genetics 119 959–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter MK, Padilla CM, Schmidt RJ (2002) The maize mutant barren stalk1 is defective in axillary meristem development. Am J Bot 89 203–210 [DOI] [PubMed] [Google Scholar]
- Roman H (1947) Mitotic nondisjunction in the case of interchanges involving the B-type chromosome in maize. Genetics 32 391–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R (2007) Flowering and determinacy in Arabidopsis. J Exp Bot 58 899–907 [DOI] [PubMed] [Google Scholar]
- Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57 431–449 [DOI] [PubMed] [Google Scholar]
- Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H, Jackson D (2006) A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441 227–230 [DOI] [PubMed] [Google Scholar]
- Skirpan A, Wu X, McSteen P (2008) Genetic and physical interaction suggest that barren stalk1 is a target of barren inflorescence2 in maize inflorescence development. Plant J 55 81–91 [DOI] [PubMed] [Google Scholar]
- Steeves T, Sussex I (1989) Patterns in Plant Development. Cambridge University Press, Cambridge, UK
- Taguchi-Shiobara F, Yuan Z, Hake S, Jackson D (2001) The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes Dev 15 2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitia RA (2007) Exploring the molecular etiology of dominant-negative mutations. Plant Cell 19 3843–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht E, Reiser L, Hake S (2000) Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted1. Development 127 3161–3172 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Springer PS, Goh L, Buckler IVES, Martienssen R (2005) Architecture of floral branch systems in maize and related grasses. Nature 436 1119–1126 [DOI] [PubMed] [Google Scholar]
- Wu X, McSteen P (2007) The role of auxin transport during inflorescence development in maize, Zea mays (Poaceae). Am J Bot 11 1745–1755 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.