One of the key events in plant development is the initiation of lateral organs from the flanks of the meristem. In grasses, the inflorescence meristem (IM) reiteratively initiates a series of lateral meristems with slightly different fates. Our understanding of the genes and networks that regulate grass inflorescence architecture has dramatically expanded due to significant advances in resources and tools. Many of the modules that regulate meristem fate in Arabidopsis (Arabidopsis thaliana) are also present in the grasses. Genetic networks that regulate IM size and floral organ fate are partially conserved between Arabidopsis and grasses, whereas genetic networks that regulate grass-specific meristems are either unique to grasses or have different functions in dicots.
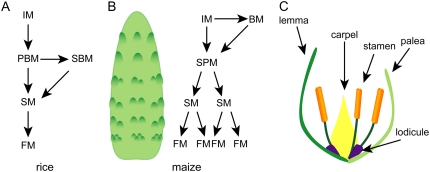
Grasses have complex inflorescence architecture, with multiple meristem types. Maize (Zea mays) and rice (Oryza sativa) are the most well-studied grasses, and mutants that affect discrete stages of inflorescence development have been identified. In rice, the IM produces several primary branch meristems (BMs) before terminating. These primary branches initiate secondary branches, and ultimately spikelet meristems (SMs), which will form florets (Fig. 1A). Maize has two inflorescences: the ear, which arises in the axil of a vegetative leaf, and the tassel, located at the apex of the plant. The tassel IM first initiates several lateral meristems that become long branches and later switches to producing spikelet pair meristems (SPMs). SPMs are transient and produce a pair of SMs. SMs are also transient and produce sterile leaves, called bracts, and two floral meristems (FMs). The ear is unbranched and does not produce BMs (Fig. 1B). The SM is a meristem type unique to all grasses (Clifford, 1987).
Figure 1.
Grass inflorescence development. A, Inflorescence development in rice. The IM initiates primary branch meristems (PBMs), which initiate SMs. Each SM initiates a single FM. PBMs also initiate secondary branch meristems (SBMs). The PMB is converted to a terminal spikelet meristem (TSM). B, Inflorescence development in maize. The IM initiates SPMs, which form two SMs. Each SM initiates two FMs. Early stages of development in the tassel and ear, except, in the tassel, the IM also initiates BMs. Left, Diagram of a developing ear; right, schematic of maize inflorescence development. C, Diagram of a grass floret.
MERISTEM IDENTITY AND MAINTENANCE
Meristem identity and maintenance are regulated by the CLAVATA (CLV) and KNOX gene pathways. In Arabidopsis, signaling through the CLV pathway restricts expression of WUSCHEL (WUS), which defines the stem cell niche (Laux et al., 1996). In the absence of CLV1, CLV2, or CLV3, WUS expression expands and meristem size is increased (Brand et al., 2000; Schoof et al., 2000). Similar regulation is likely to occur in maize and rice. Mutations in the maize genes thick tassel dwarf1 (td1) and fasciated ear2 (fea2), which encode orthologs of CLV1 and CLV2, respectively, have larger IMs and increased spikelet pair density on the main rachis of the tassel and in the ear. Male flowers also produce more stamens, indicating that these genes also likely regulate FM size. Interestingly, the regulation of meristem size in vegetative meristems is apparently different than in IMs; both td1 and fea2 make fewer leaves, suggesting that they positively regulate meristem size in vegetative development (Taguchi-Shiobara et al., 2001; Bommert et al., 2005). In rice, mutants in the CLV1 and CLV3 homologs, FLORAL ORGAN NUMBER1 (FON1) and FON2, respectively, yield similar phenotypes, although the defect is restricted to the FM (Suzaki et al., 2004, 2006). Another CLV3-like gene, FON2-LIKE CLE PROTEIN1 (FCP1), regulates vegetative meristem size, suggesting FON2 and FCP1 have functionally diversified to regulate different meristem types (Suzaki et al., 2008). WUS belongs to a large gene family with members in rice and maize; however, a WUS homolog with similar expression or function has not yet been identified in grasses (Nardmann and Werr, 2006).
In Arabidopsis, meristem maintenance requires SHOOTMERISTEMLESS (STM; Barton and Poethig, 1993). Strong stm alleles do not make an inflorescence, but weak alleles indicate that stm also functions in FMs (Lenhard et al., 2001; Kanrar et al., 2006; Scofield et al., 2007). A maize STM homolog with a similar function is knotted1 (kn1). Both STM and kn1 are expressed in all shoot meristems, but excluded from leaf primordia (Smith et al., 1992; Long et al., 1996). Maize kn1 mutant phenotypes are similar to Arabidopsis stm mutants; in some inbred backgrounds, kn1 mutants result in shootless seedlings, whereas in other inbreds, kn1 mutants result in decreased inflorescence branching (Vollbrecht et al., 1991, 2000; Kerstetter et al., 1997). OSH1 in rice is orthologous to maize kn1, with a similar expression pattern, but no known mutant phenotype.
REGULATION OF INFLORESCENCE BRANCHING
A class of maize mutations, called ramosa (ra), increases branching in the tassel and ear. Typically, mutant spikelet pairs are replaced by long branches with multiple spikelets and display additional reiterations of branching. ra1 encodes a zinc-finger transcription factor that is expressed at the base of SPMs (Vollbrecht et al., 2005). ra3 encodes a trehalose-P phosphatase with an overlapping expression domain (Satoh-Nagasawa et al., 2006). ra2 encodes a LATERAL ORGAN BOUNDARY (LOB) transcription factor that is expressed prior to lateral meristem initiation and thus prior to both ra1 and ra3. ra2 is also expressed at the position where BMs and SMs initiate (Bortiri et al., 2006), suggesting that it is not unique to spikelet pairs. ra2 has a similar expression pattern in other grasses (Bortiri et al., 2006), but no known mutant phenotypes. ra1 expression is decreased in ra2 and ra3 mutants, suggesting that both genes function to regulate ra1. Given the uniqueness of the spikelet pair to maize (and other members of the Andropogoneae) and the lack of ra1 in rice, a species lacking spikelet pairs (Vollbrecht et al., 2005), it may be that LOB genes function to specify spikelet pairs due to the unique contribution of ra1.
In Arabidopsis, the timing of the IM to FM transition is critical to determine inflorescence architecture. The timing of this transition is controlled by the antagonistic activities of two genes, LEAFY (LFY), which promotes FM identity (Weigel et al., 1992), and TERMINAL FLOWER1 (TFL1), which promotes an indeterminate state (Bradley et al., 1997). LFY and TFL homologs also play a role in inflorescence architectures in grasses. RNAi knockdowns of the rice LFY homolog, RFL, severely decrease panicle branching (Rao et al., 2008). Similarly, maize double mutants in the LFY co-orthologs zfl1 and zfl2 exhibit reduced tassel branching. In addition, zfl1 and zfl2 are required to promote proper floral organ identity and phyllotaxy (Bomblies et al., 2003). In Arabidopsis, TFL1 overexpression increases inflorescence branching (Ratcliffe et al., 1998), and similar phenotypes are observed when the rice TFL1 homologs RCN1 and RCN2 are overexpressed in Arabidopsis or rice, indicating a conserved role in the regulation of inflorescence architecture (Nakagawa et al., 2002).
SM IDENTITY AND DETERMINACY
In grasses, the SM initiates two bracts, called glumes, and a variable number of FMs. In maize, the SM initiates two FMs, and in rice, the SM initiates a single FM. Two types of APETALA2 (AP2) domain-containing transcription factors, ERF and AP2, regulate SM identity and determinacy in maize and rice. The AP2 domain is a DNA-binding domain (Ohme-Takagi and Shinshi, 1995), and ERF proteins contain one AP2 domain, whereas AP2-like proteins contain two (Sakuma et al., 2002; Magnani et al., 2004). Mutants in orthologous ERF genes, branched silkless1 (bd1) in maize and FRIZZY PANICLE1 (FZP1) in rice, exhibit defects in SM identity and determinacy. bd1 mutants initiate extra spikelets in the tassel, and in the ear, SMs are replaced with BMs (Chuck et al., 2002). Similarly, mutants in rice FZP1 form ectopic branches in place of spikelets (Komatsu et al., 2003).
Little is known about how bd1 and FZP1 regulate SM identity. Interestingly, bd1 and FZP1 mRNA are not expressed in the SM itself, but instead are expressed in a semicircular pattern at the base of the SM (Chuck et al., 2002; Komatsu et al., 2003). How, then, do bd1 and FZP1 impart SM identity? One possibility is that a secondary signal, such as a hormone or sugar, is a mobile signal that moves into the SM from the base where bd1/FZP1 is expressed. Alternatively, FZP1 and bd1 might not affect SM identity per se, but rather repress the formation and outgrowth of ectopic axillary meristems in the axil of the glume (Chuck et al., 2002; Komatsu et al., 2003).
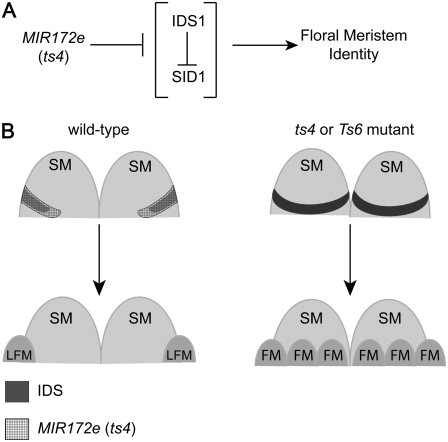
Regulation of SM determinacy by AP2 transcription factors in maize involves two related genes, indeterminate spikelet1 (ids1) and sister of ids1 (sid1; Fig. 2). ids1; sid1 double mutants do not make FMs, but instead initiate many bract-like organs, eventually terminating in an ovule-like structure that is probably not the product of a FM (Chuck et al., 2008). Thus, ids1 and sid1 are necessary for FM initiation. In rice, mutants in the ids1-like gene SUPERNUMERARY BRACT (SNB) resemble ids1; sid1 double mutants: They initiate extra bract-like organs and delay the transition of SM to FM (Lee et al., 2007). Notably, snb mutants do eventually make a FM.
Figure 2.
MIR172 regulates ids1 and sid1 to control FM initiation. A, MIR172 negatively regulates ids1 and sid1, which promote FM fates. In addition, IDS1 negatively regulates sid1 mRNA accumulation. B, In wild type (left), MIR172 restricts IDS1 expression, and two FMs are formed. In Ts6 or ts4 mutants (right), IDS1 expression is increased and expanded, resulting in extra FMs. LFM, Lower floral meristem.
IDS1/SID1 is also sufficient to promote the SM to FM transition. Two mutants that increase IDS1 expression, tasselseed4 (ts4) and Ts6, initiate extra florets (Chuck et al., 2007). During normal development, MIR172 restricts the domain and level of IDS1/SID1 expression and thus restricts the number of FMs. ts4 encodes a MIR172 family member that negatively regulates AP2 genes, including ids1 and sid1 (Chuck et al., 2007, 2008). Ts6 harbors a mutation in the MIR172 complementarity site of ids1 mRNA, resulting in increased IDS1 expression (Chuck et al., 2007). Thus, up-regulation of IDS1, either by removing its negative regulator or rendering the ids1 mRNA immune to negative regulation, results in extra florets. Adding to the complexity, IDS1 negatively regulates sid1 mRNA accumulation, which might explain why ids1 single mutants initiate extra florets (Chuck et al., 2008). Interestingly, ts4 and Ts6 mutants also fail to abort carpels in the tassel, indicating that the sex determination and SM determinacy pathways intersect, although the relationship between these to pathways is unclear (see below).
FLORAL ORGAN IDENTITY AND PATTERNING
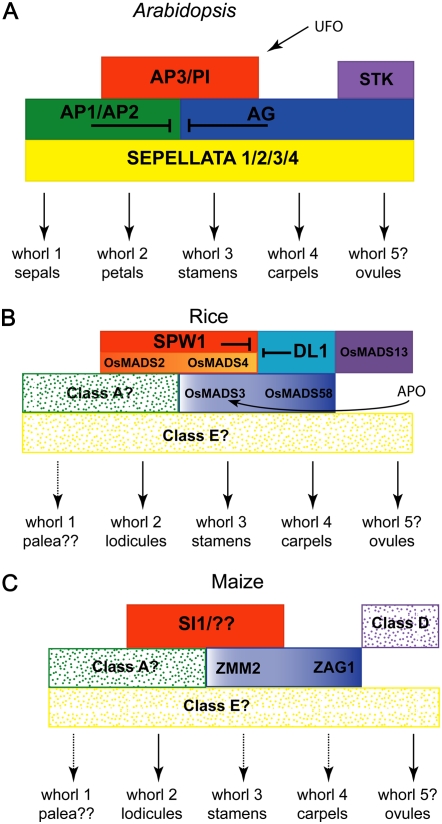
One of the most significant advances in the past 20 years in plant biology is the ABC model of floral development. This simple and elegant model posits that transcription factors act in a combinatorial manner to achieve floral patterning (Coen and Meyerowitz, 1991). The major class A, B, and C genes have been identified and, except for the class A gene AP2, encode members of the MIKC type of MADS-box transcription factors. The model has been expanded to include class D genes, which promote ovule development and class E, or SEPALLATA (SEP), genes, which act as cofactors to A, B, C, and D class genes (for review, see Theissen, 2001). The basic ABCDE model is depicted in Figure 3A. A major challenge for grass biologists is determining how this model applies to monocot flowers, which display unique floral morphologies compared to dicots.
Figure 3.
ABC model of floral development. The basic ABC model posits that class A genes specify whorl 1 organs, class A and B genes specify whorl 2 organs, class B and C genes specify whorl 3 organs, and class C genes specify whorl 4. The expanded ABCDE model includes class D genes that promote ovule identity and class E genes that act as cofactors for the class A, B, C, and D genes. Class A genes are depicted in green, class B in orange, class C in blue, class D in purple, and class E in yellow. Solid colors indicate functional data; shaded colors indicate that function is hypothesized based on expression data or phylogenetic analysis. Color gradients illustrate subfunctionalization of duplicated genes. A, Model of floral development in Arabidopsis. B, Model of floral development in rice. C, Model of floral development in maize. See text for details.
The grasses possess a unique floral structure, the floret (Fig. 1B). Florets contain carpels and stamens, like their dicot counterparts; however, they lack petals and sepals. Surrounding the sex organs are lodicules, and two bract-like organs, the palea and lemma. Lodicules are thought to correspond to petals in dicots (see below). The corresponding dicot organs to palea and lemma, however, remain controversial; palea and lemma may represent unique grass structures.
Forward and reverse genetic approaches have identified several genes required for floral development in grasses. Not surprisingly, some of the genes identified correspond to B, C, D, and E class genes in dicots. However, forward genetics has also identified a number of floral regulators that do not have a functional dicot counterpart and appear to have unique functions in grass floral development.
In Arabidopsis, the class A genes AP1 and AP2 specify the outer two whorls, sepals and petals. AP1 has an additional role in promoting the transition to flowering. AP1 homologs have been identified in grasses, and, despite the lack of mutants, the available data do not support a role in floral patterning. AP1 homologs are expressed in the FM of diverse grass species, consistent with a function in transition to flowering, as in Arabidopsis. The general expression of AP1 throughout the spikelet is thought to be ancestral and thus is inconsistent with strict class A function (Preston and Kellogg, 2006). In addition, expression of a Lolium AP1 homolog in Arabidopsis ap1 mutants does not rescue ap1 organ identity defects (Gocal et al., 2001). Thus, AP1 genes do not appear to have strict class A function in grasses. However, true class A mutants have not been defined outside of Arabidopsis and the roles of AP1 and AP2 in floral organ identity may not be as clear as predicted by the ABC model (see Litt and Irish, 2003; Preston and Kellogg, 2006).
In Arabidopsis, the class B genes AP3 and PISTILLATA (PI) specify whorl 2 (with class A genes) and whorl 3 (with class C genes) organs. In contrast to class A genes, the function of at least one class B gene is clearly conserved between dicots and the grasses. Mutants in two AP3 homologs, silky1 (si1) in maize and SUPERWOMAN1 (SPW1) in rice, result in homeotic transformations of stamens to carpels and lodicules to palea-like organs (Ambrose et al., 2000; Nagasawa et al., 2003). Thus, AP3-like genes are required to promote whorl 2 and 3 identities in grasses. The homeotic transformation of lodicules to palea-like structures supports the hypothesis that lodicules correspond to whorl 2 organs or petals in dicots. Furthermore, the maize genes si1 (AP3) and zmm16 (PI) have similar biochemical activities as their Arabidopsis counterparts and can rescue the corresponding mutants in Arabidopsis, providing additional evidence of class B conservation (Whipple et al., 2004). Two rice genes, OsMADS2 and OsMADS4, are similar to the other class B gene, PI; however, OsMADS2 appears to be more important in whorl 2 and OsMADS4 in whorl 3. RNAi knockdowns of OsMADS2 affect lodicule development, but do not affect stamen development (Prasad and Vijayraghavan, 2003; Yadav et al., 2007). Furthermore, RNA expression patterns are consistent with OsMADS2 functioning in lodicule development, and OsMADS4 functioning in stamen development (Yadav et al., 2007).
A single class C gene, AGAMOUS (AG), specifies whorl 3 (with class B genes) and whorl 4 organs in Arabidopsis. In addition to its role in floral organ identity, AG also promotes FM determinacy. In grasses, the AG gene has been duplicated and the two class C functions have largely been subfunctionalized to separate genes. For example, rice contains two AG homologs, OsMADS3 and OsMADS58. OsMADS3 mutants transform stamens to lodicules, but have only minor defects in FM determinacy. In contrast, OsMADS58 RNAi knockdowns have only minor defects in floral organ identity, but greatly affect FM determinacy (Yamaguchi et al., 2006). Thus, OsMADS3 has a central role in floral organ identity, whereas OsMADS58 has a central role in FM determinacy. Similarly, mutants in the maize agamous homolog zag1 (for zea agamous1) lack FM determinacy, but do not affect floral organ identity (Mena et al., 1996). Mutants in the zag1 duplicate zmm2 have not been identified, but zmm2 expression patterns are consistent with class C function (Mena et al., 1996). Notably, available mutants do not support a role for grass class C genes in carpel identity.
Class D genes specify ovule identity. Cosuppression of FBP7 and FBP11 in Petunia transforms ovules into carpelloid structures (Angenent et al., 1995), and overexpression of FBP11 results in ectopic ovules on sepals and petals (Colombo et al., 1995). In Arabidopsis, shatterproof1 (shp1) shp2 seedstick1 (stk1) triple mutants also transform ovules into carpelloid structures (Pinyopich et al., 2003), indicating these genes function redundantly to promote ovule identity. SHP1 and SHP2 are more closely related to AG, and STK1 groups with the class D genes of Petunia. Rice contains two putative class D genes, OsMADS13 and OsMADS21. osmads21 mutants do not have a mutant phenotype; however, osmads13 mutants convert ovules into carpelloid structures, indicating that class D function is in part conserved between dicots and the grasses. osmads13 mutants also make excess carpels, indicating that OsMADS13 also plays a role in FM determinacy (Dreni et al., 2007).
In Arabidopsis, SEP1 to SEP4 function redundantly as class E genes. Class E genes function as cofactors with class A, B, and C genes, and in the absence of all four SEP genes, floral organs are transformed into leaf-like structures (Ditta et al., 2004). leafy hull sterile1 (lhs1), which encodes the SEP-like gene, OsMADS1, is the only reported class E mutant in grasses. lhs1 mutants produce fewer stamens and transform lemma, palea, and lodicules into leaf-like structures (Jeon et al., 2000). OsMADS1 RNAi knockdowns have a more severe phenotype in which floral organs in all four whorls are transformed into leaf-like structures (Prasad et al., 2005). The grass SEP lineage is complex and gene expression patterns are variable, suggesting that grass SEP-like genes potentially fulfill more diverse developmental functions than in Arabidopsis (Malcomber and Kellogg, 2004).
Non-MADS-box genes also play key roles in floral development. Two rice mutants, drooping leaf1 (dl1) and aberrant panicle organization1 (apo1), resemble class C mutants, suggesting that they regulate class C genes or that they have some class C function. DL1 is a candidate carpel identity gene in rice; dl1 mutants convert carpels to stamens (Nagasawa et al., 2003). dl1 encodes a YABBY transcription factor, most similar to CRABSCLAW (CRC) in Arabidopsis (Yamaguchi et al., 2004). CRC also has a role in carpel and ovule development, but, unlike DL1, does not play the central role in carpel identity (Bowman and Smyth, 1999). DL1 and the class B gene, SPW, are mutually antagonistic, and this antagonism is critical to set up the boundary between whorls 3 and 4. No carpel identity genes have been identified in maize.
The apo1 mutant also phenotypically resembles class C mutants. apo1 mutants make extra lodicules at the expense of stamens, suggesting stamens are converted to lodicules. In addition, apo1 mutants make extra carpels, implicating apo1 in FM determinacy, another class C function. Consistent with this phenotype, expression of the class C gene, OsMADS3, is reduced in apo1 mutants, indicating that APO1 positively regulates class C gene expression (Ikeda et al., 2005, 2007). APO1 encodes an F-box protein, similar to UNUSUAL FLORAL ORGANS (UFO) in Arabidopsis (Ikeda et al., 2007). In contrast to APO1, UFO is required to activate class B genes (Lee et al., 1997). Thus, whereas UFO and APO both play key roles in floral development and are likely to have similar biochemical functions, their roles in the floral regulatory network are distinct.
SEX DETERMINATION
Grasses exhibit a variety of sexual systems, including bisexual and unisexual flowers. Plants that make unisexual flowers are most commonly monoecious (male and female flowers on the same plant, but separate inflorescences) or dioecious (male and female flowers on separate plants). Maize is monoecious and the only grass for which significant genetic and molecular data on sex determination exist.
In maize (and in other grasses with unisexual flowers), flowers are initially bisexual, but carpel and stamen primordia arrest in male and female flowers, respectively. The rich history of maize genetics has produced a collection of sex determination mutants, giving insight into the molecular regulation of this process. In ts1, ts2, Ts3, ts4, Ts5, and Ts6, carpels do not abort in the tassel (Veit et al., 1993; Irish et al., 1994). Mutants that affect GA synthesis, including anther ear1 and the dwarf mutants, do not abort stamens in the ear (Emerson and Emerson, 1922; Bensen et al., 1995). Finally, carpels inappropriately abort in ears of silkless1 (sk1) mutants (Jones, 1925).
ts2, ts4, and Ts6 have been cloned. ts2 encodes a short-chain dehydrogenase (DeLong et al., 1993), but the substrate for this enzyme and how it functions in the molecular regulation of sex determination are unknown. As discussed above, ts4 encodes MIR172, and Ts6 harbors a mutation in the MIR172 complementarity site of ids1. ts1 is necessary for ts2 expression, and sk1 is thought to protect carpel primordia in the ear from ts2-mediated cell death (Calderon-Urrea and Dellaporta, 1999). Malcomber and Kellogg (2006) identified ts2 homologs in various grasses and asked whether specific variants were associated with unisexual floral development. However, they found that ts2 homologs likely have a broader role in programmed cell death and are not specific to sex determination. Indeed, no single sex-determining gene is likely to be responsible for the variety of sexual systems observed in the grasses, and experiments will have to be conducted in multiple species.
Interestingly, several ts mutants affect branching as well as sex determination, including Ts3, ts4, and Ts6. While we have some insight into the role of ts4 and Ts6 in branching (Chuck et al., 2007), we do not know downstream targets that are important in sex determination. GA treatment mimics the ts phenotype (Nickerson, 1959) and is also critical for stamen arrest in the ear. A more complete understanding of sex determination will require cloning additional sex determination genes, and a challenge for the future is understanding how these how these modules link together into a coherent regulatory network to regulate sex determination.
REGULATORY NETWORKS AND INFLORESCENCE ARCHITECTURE
Developmental biology has long focused on identifying individual genes and studying their role in development. Genome-scale experiments in models such as Arabidopsis permit us to assemble these genes into pathways and even networks that control developmental processes. Recently, large-scale experiments have yielded similar data in other species, such as maize and rice, which have distinct inflorescence morphologies. Inflorescence morphology is determined by the architecture of the underlying gene regulatory network and differences in morphologies reflect the differences in the network architecture (Prusinkiewicz et al., 2007). Taking into consideration the broader themes of network biology provides insight into inflorescence development and the evolution of different morphologies.
A network is defined as the connections between nodes (Barabasi and Oltvai, 2004); nodes can be genes, cells, systems, etc. Cellular networks are scale free; that is, a few nodes have many connections, called hubs, but most nodes have only a few connections (Barabasi and Oltvai, 2004). Hubs are more likely to appear early in the history of the network (Barabasi and Albert, 1999). Thus, hubs are likely to be conserved between species. One implication of scale-free networks is that disruption of most nodes does not perturb the network; however, disruption of a hub greatly perturbs the network. Forward genetic screens are likely to uncover hub genes that are conserved in the regulatory networks of different species. Because the hubs are likely to be the same, morphological diversity must be rooted in the connections between hubs and other nodes.
Transcriptional control of floral development by MADS-box proteins provides an example of how different interactions between nodes can lead to morphological diversity. The protein-protein interactions of MADS-box proteins have been intensively studied in Arabidopsis (de Folter et al., 2005). While a similar endeavor has not been undertaken in a grass, data suggest that some interactions are conserved, while others are novel (see Malcomber and Kellogg, 2005). The composition of specific protein complexes determine DNA-binding specificity and downstream target genes. A key to understanding different floral morphologies will be determining the protein interaction network and how it maps onto an underlying transcriptional network.
Another key aspect of networks is modularity. Regulatory modules from one part of the network can be moved to another part of the network where they fulfill novel functions. An excellent example in the inflorescence is MIR172 regulation of AP2 genes. In Arabidopsis, this regulatory module is required for floral organ identity and FM size (Zhao et al., 2007), whereas in maize it is critical for sex determination and SPM determinacy (Chuck et al., 2007, 2008).
Maize has recently undergone whole-genome duplication (Swigonova et al., 2004) and thus provides a unique opportunity to study how gene duplication events affect regulatory networks. Duplicated genes, or nodes, provide redundancy and therefore network robustness. For example, in maize, ids1 and sid1 function redundantly to promote the SM to FM transition. In addition to redundancy, node (gene) duplication can also lead to subfunctionalization. These duplicated nodes will eventually form unique connections to other nodes. In Arabidopsis, the class C gene, AG, provides both class C functions, whereas in maize and rice, AG has been duplicated and no single gene provides all class C function.
Experiments in Arabidopsis have provided a framework to understanding developmental processes in other species. However, in different species, new modules arise for new structures (e.g. the ra genes), modules attain new functions, and nodes form new connections. Indeed, regulatory networks vary even within species, as evidenced by the dramatic phenotypic variations of some maize mutants (e.g. kn1) in different inbred backgrounds. Ultimately, comparisons of regulatory networks both within and between species will aid in our understanding of evolution of different morphologies.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Beth E. Thompson (bethompson@berkeley.edu).
References
- Ambrose BA, Lerner DR, Ciceri P, Padilla CM, Yanofsky MF, Schmidt RJ (2000) Molecular and genetic analyses of the silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Mol Cell 5 569–579 [DOI] [PubMed] [Google Scholar]
- Angenent GC, Franken J, Busscher M, van Dijken A, van Went JL, Dons HJ, van Tunen AJ (1995) A novel class of MADS box genes is involved in ovule development in petunia. Plant Cell 7 1569–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabasi AL, Albert R (1999) Emergence of scaling in random networks. Science 286 509–512 [DOI] [PubMed] [Google Scholar]
- Barabasi AL, Oltvai ZN (2004) Network biology: understanding the cell's functional organization. Nat Rev Genet 5 101–113 [DOI] [PubMed] [Google Scholar]
- Barton MK, Poethig RS (1993) Formation of the shoot apical meristem in Arabidopsis thaliana—an analysis of development in the wild-type and in the shoot meristemless mutant. Development 119 823–831 [Google Scholar]
- Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP (1995) Cloning and characterization of the maize An1 gene. Plant Cell 7 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies K, Wang RL, Ambrose BA, Schmidt RJ, Meeley RB, Doebley J (2003) Duplicate FLORICAULA/LEAFY homologs zfl1 and zfl2 control inflorescence architecture and flower patterning in maize. Development 130 2385–2395 [DOI] [PubMed] [Google Scholar]
- Bommert P, Lunde C, Nardmann J, Vollbrecht E, Running M, Jackson D, Hake S, Werr W (2005) thick tassel dwarf1 encodes a putative maize ortholog of the Arabidopsis CLAVATA1 leucine-rich repeat receptor-like kinase. Development 132 1235–1245 [DOI] [PubMed] [Google Scholar]
- Bortiri E, Chuck G, Vollbrecht E, Rocheford T, Martienssen R, Hake S (2006) ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18 574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR (1999) CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development 126 2387–2396 [DOI] [PubMed] [Google Scholar]
- Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E (1997) Inflorescence commitment and architecture in Arabidopsis. Science 275 80–83 [DOI] [PubMed] [Google Scholar]
- Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R (2000) Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289 617–619 [DOI] [PubMed] [Google Scholar]
- Calderon-Urrea A, Dellaporta SL (1999) Cell death and cell protection genes determine the fate of pistils in maize. Development 126 435–441 [DOI] [PubMed] [Google Scholar]
- Chuck G, Meeley R, Hake S (2008) Floral meristem initiation and meristem cell fate are regulated by the maize AP2 genes ids1 and sid1. Development 135 3013–3019 [DOI] [PubMed] [Google Scholar]
- Chuck G, Meeley R, Irish E, Sakai H, Hake S (2007) The maize tasselseed4 microRNA controls sex determination and meristem cell fate by targeting tasselseed6/indeterminate spikelet1. Nat Genet 39 1517–1521 [DOI] [PubMed] [Google Scholar]
- Chuck G, Muszynski M, Kellogg E, Hake S, Schmidt RJ (2002) The control of spikelet meristem identity by the branched silkless1 gene in maize. Science 298 1238–1241 [DOI] [PubMed] [Google Scholar]
- Clifford HT (1987) Spikelet and floral morphology. In TR Soderstrom, KW Hilu, CS Campbell, ME Barkworth, eds, Grass Systematics and Evolution. Smithsonian Institution Press, Washington, DC, pp 21–30
- Coen ES, Meyerowitz EM (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353 31–37 [DOI] [PubMed] [Google Scholar]
- Colombo L, Franken J, Koetje E, van Went J, Dons HJ, Angenent GC, van Tunen AJ (1995) The petunia MADS box gene FBP11 determines ovule identity. Plant Cell 7 1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Folter S, Immink RG, Kieffer M, Parenicova L, Henz SR, Weigel D, Busscher M, Kooiker M, Colombo L, Kater MM, et al (2005) Comprehensive interaction map of the Arabidopsis MADS box transcription factors. Plant Cell 17 1424–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong A, Calderon-Urrea A, Dellaporta SL (1993) Sex determination gene TASSELSEED2 of maize encodes a short-chain alcohol dehydrogenase required for stage-specific floral organ abortion. Cell 74 757–768 [DOI] [PubMed] [Google Scholar]
- Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF (2004) The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol 14 1935–1940 [DOI] [PubMed] [Google Scholar]
- Dreni L, Jacchia S, Fornara F, Fornari M, Ouwerkerk PB, An G, Colombo L, Kater MM (2007) The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J 52 690–699 [DOI] [PubMed] [Google Scholar]
- Emerson RA, Emerson SH (1922) Genetic interrelations of two andromonoecious types of maize, dwarf and anther ear. Genetics 7 203–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocal GF, King RW, Blundell CA, Schwartz OM, Andersen CH, Weigel D (2001) Evolution of floral meristem identity genes. Analysis of Lolium temulentum genes related to APETALA1 and LEAFY of Arabidopsis. Plant Physiol 125 1788–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ito M, Nagasawa N, Kyozuka J, Nagato Y (2007) Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F-box protein, regulates meristem fate. Plant J 51 1030–1040 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Nagasawa N, Nagato Y (2005) ABERRANT PANICLE ORGANIZATION 1 temporally regulates meristem identity in rice. Dev Biol 282 349–360 [DOI] [PubMed] [Google Scholar]
- Irish EE, Langdale JA, Nelson TM (1994) Interactions between tassel seed genes and other sex-determining genes in maize. Dev Genet 15 155–171 [Google Scholar]
- Jeon JS, Jang S, Lee S, Nam J, Kim C, Lee SH, Chung YY, Kim SR, Lee YH, Cho YG, et al (2000) leafy hull sterile1 is a homeotic mutation in a rice MADS box gene affecting rice flower development. Plant Cell 12 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DF (1925) Heritable characters of maize XXIII—silkless. J Hered 16 339–341 [Google Scholar]
- Kanrar S, Onguka O, Smith HM (2006) Arabidopsis inflorescence architecture requires the activities of KNOX-BELL homeodomain heterodimers. Planta 224 1163–1173 [DOI] [PubMed] [Google Scholar]
- Kerstetter RA, Laudencia-Chingcuanco D, Smith LG, Hake S (1997) Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development 124 3045–3054 [DOI] [PubMed] [Google Scholar]
- Komatsu M, Chujo A, Nagato Y, Shimamoto K, Kyozuka J (2003) FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development 130 3841–3850 [DOI] [PubMed] [Google Scholar]
- Laux T, Mayer KF, Berger J, Jurgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122 87–96 [DOI] [PubMed] [Google Scholar]
- Lee DY, Lee J, Moon S, Park SY, An G (2007) The rice heterochronic gene SUPERNUMERARY BRACT regulates the transition from spikelet meristem to floral meristem. Plant J 49 64–78 [DOI] [PubMed] [Google Scholar]
- Lee I, Wolfe DS, Nilsson O, Weigel D (1997) A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr Biol 7 95–104 [DOI] [PubMed] [Google Scholar]
- Lenhard M, Bohnert A, Jurgens G, Laux T (2001) Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 105 805–814 [DOI] [PubMed] [Google Scholar]
- Litt A, Irish VF (2003) Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: implications for the evolution of floral development. Genetics 165 821–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379 66–69 [DOI] [PubMed] [Google Scholar]
- Magnani E, Sjolander K, Hake S (2004) From endonucleases to transcription factors: evolution of the AP2 DNA binding domain in plants. Plant Cell 16 2265–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcomber ST, Kellogg EA (2004) Heterogeneous expression patterns and separate roles of the SEPALLATA gene LEAFY HULL STERILE1 in grasses. Plant Cell 16 1692–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcomber ST, Kellogg EA (2005) SEPALLATA gene diversification: brave new whorls. Trends Plant Sci 10 427–435 [DOI] [PubMed] [Google Scholar]
- Malcomber ST, Kellogg EA (2006) Evolution of unisexual flowers in grasses (Poaceae) and the putative sex-determination gene, TASSELSEED2 (TS2). New Phytol 170 885–899 [DOI] [PubMed] [Google Scholar]
- Mena M, Ambrose BA, Meeley RB, Briggs SP, Yanofsky MF, Schmidt RJ (1996) Diversification of C-function activity in maize flower development. Science 274 1537–1540 [DOI] [PubMed] [Google Scholar]
- Nagasawa N, Miyoshi M, Sano Y, Satoh H, Hirano H, Sakai H, Nagato Y (2003) SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice. Development 130 705–718 [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Shimamoto K, Kyozuka J (2002) Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J 29 743–750 [DOI] [PubMed] [Google Scholar]
- Nardmann J, Werr W (2006) The shoot stem cell niche in angiosperms: expression patterns of WUS orthologues in rice and maize imply major modifications in the course of mono- and dicot evolution. Mol Biol Evol 23 2492–2504 [DOI] [PubMed] [Google Scholar]
- Nickerson NH (1959) Sustained treatment with gibberellin acid of five different kinds of maize. Ann Mo Bot Gard 47 19–37 [Google Scholar]
- Ohme-Takagi M, Shinshi H (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 2 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF (2003) Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424 85–88 [DOI] [PubMed] [Google Scholar]
- Prasad K, Parameswaran S, Vijayraghavan U (2005) OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs. Plant J 43 915–928 [DOI] [PubMed] [Google Scholar]
- Prasad K, Vijayraghavan U (2003) Double-stranded RNA interference of a rice PI/GLO paralog, OsMADS2, uncovers its second-whorl-specific function in floral organ patterning. Genetics 165 2301–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JC, Kellogg EA (2006) Reconstructing the evolutionary history of paralogous APETALA1/FRUITFULL-like genes in grasses (Poaceae). Genetics 174 421–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E (2007) Evolution and development of inflorescence architectures. Science 316 1452–1456 [DOI] [PubMed] [Google Scholar]
- Rao NN, Prasad K, Kumar PR, Vijayraghavan U (2008) Distinct regulatory role for RFL, the rice LFY homolog, in determining flowering time and plant architecture. Proc Natl Acad Sci USA 105 3646–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ (1998) A common mechanism controls the life cycle and architecture of plants. Development 125 1609–1615 [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290 998–1009 [DOI] [PubMed] [Google Scholar]
- Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H, Jackson D (2006) A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441 227–230 [DOI] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jurgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100 635–644 [DOI] [PubMed] [Google Scholar]
- Scofield S, Dewitte W, Murray JA (2007) The KNOX gene SHOOT MERISTEMLESS is required for the development of reproductive meristematic tissues in Arabidopsis. Plant J 50 767–781 [DOI] [PubMed] [Google Scholar]
- Smith LG, Greene B, Veit B, Hake S (1992) A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development 116 21–30 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Sato M, Ashikari M, Miyoshi M, Nagato Y, Hirano HY (2004) The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 131 5649–5657 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Toriba T, Fujimoto M, Tsutsumi N, Kitano H, Hirano HY (2006) Conservation and diversification of meristem maintenance mechanism in Oryza sativa: Function of the FLORAL ORGAN NUMBER2 gene. Plant Cell Physiol 47 1591–1602 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Yoshida A, Hirano HY (2008) Functional diversification of CLAVATA3-related CLE proteins in meristem maintenance in rice. Plant Cell 20 2049–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigonova Z, Lai J, Ma J, Ramakrishna W, Llaca V, Bennetzen JL, Messing J (2004) Close split of sorghum and maize genome progenitors. Genome Res 14 1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi-Shiobara F, Yuan Z, Hake S, Jackson D (2001) The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes Dev 15 2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen G (2001) Development of floral organ identity: stories from the MADS house. Curr Opin Plant Biol 4 75–85 [DOI] [PubMed] [Google Scholar]
- Veit B, Schmidt RJ, Hake S, Yanofsky MF (1993) Maize floral development: new genes and old mutants. Plant Cell 5 1205–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht E, Reiser L, Hake S (2000) Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted1. Development 127 3161–3172 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Springer PS, Goh L, Buckler ES IV, Martienssen R (2005) Architecture of floral branch systems in maize and related grasses. Nature 436 1119–1126 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Veit B, Sinha N, Hake S (1991) The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350 241–243 [DOI] [PubMed] [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 69 843–859 [DOI] [PubMed] [Google Scholar]
- Whipple CJ, Ciceri P, Padilla CM, Ambrose BA, Bandong SL, Schmidt RJ (2004) Conservation of B-class floral homeotic gene function between maize and Arabidopsis. Development 131 6083–6091 [DOI] [PubMed] [Google Scholar]
- Yadav SR, Prasad K, Vijayraghavan U (2007) Divergent regulatory OsMADS2 functions control size, shape and differentiation of the highly derived rice floret second-whorl organ. Genetics 176 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Lee DY, Miyao A, Hirochika H, An G, Hirano HY (2006) Functional diversification of the two C-class MADS box genes OSMADS3 and OSMADS58 in Oryza sativa. Plant Cell 18 15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano HY (2004) The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell 16 500–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Kim Y, Dinh TT, Chen X (2007) miR172 regulates stem cell fate and defines the inner boundary of APETALA3 and PISTILLATA expression domain in Arabidopsis floral meristems. Plant J 51 840–849 [DOI] [PMC free article] [PubMed] [Google Scholar]