Abstract
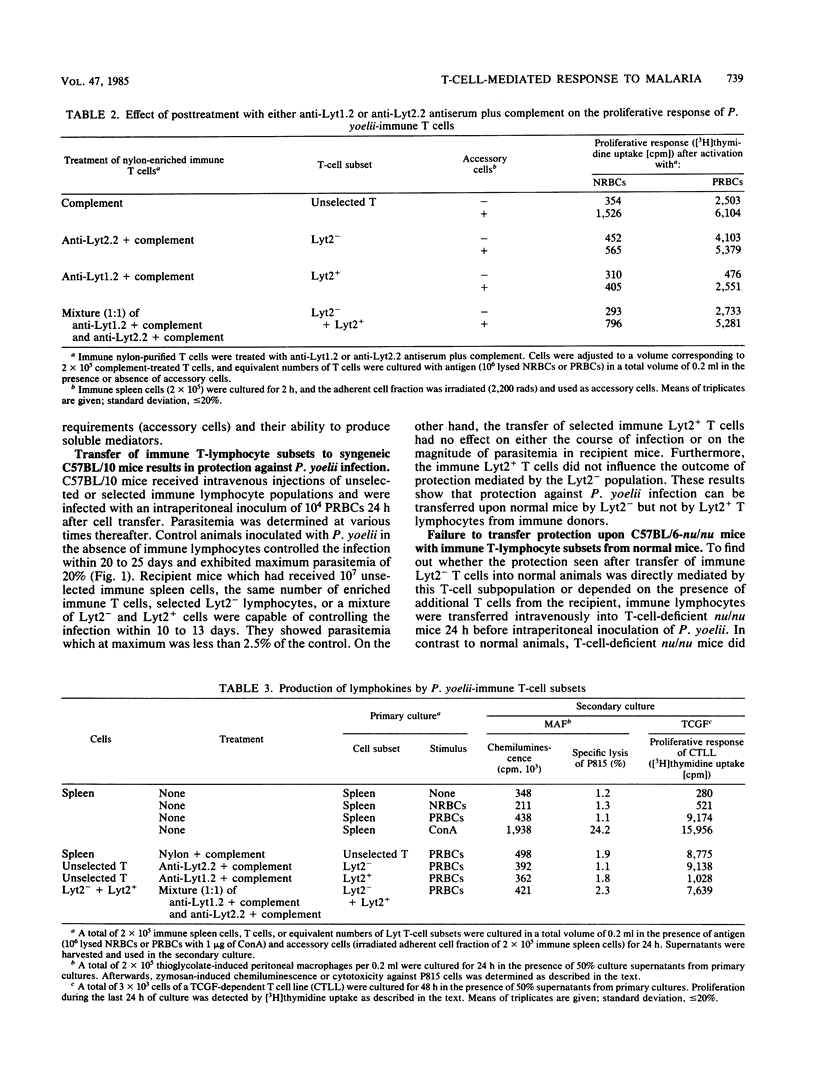
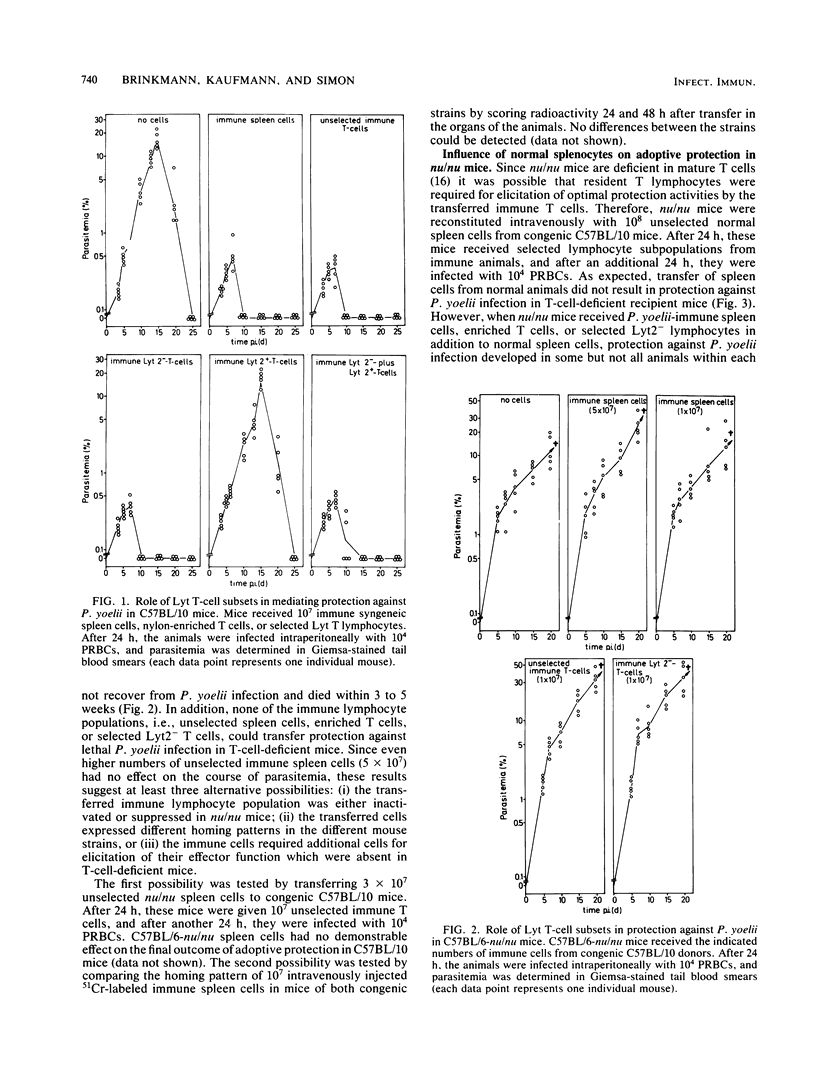
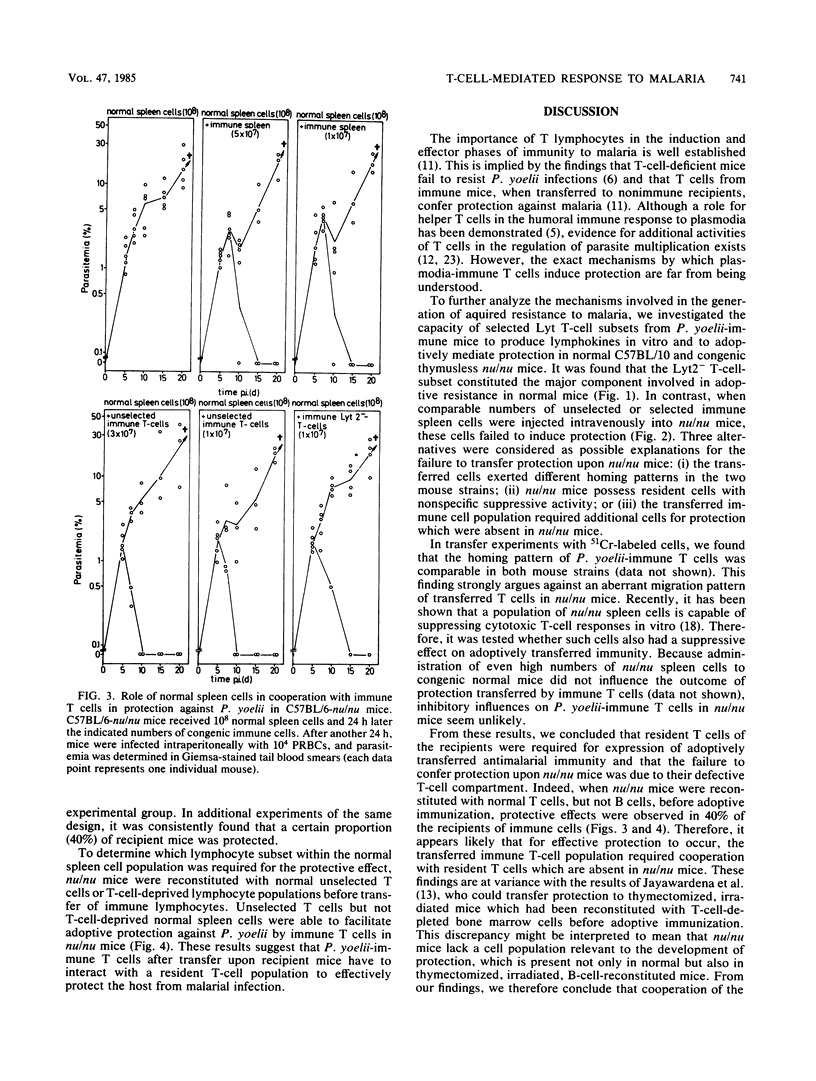
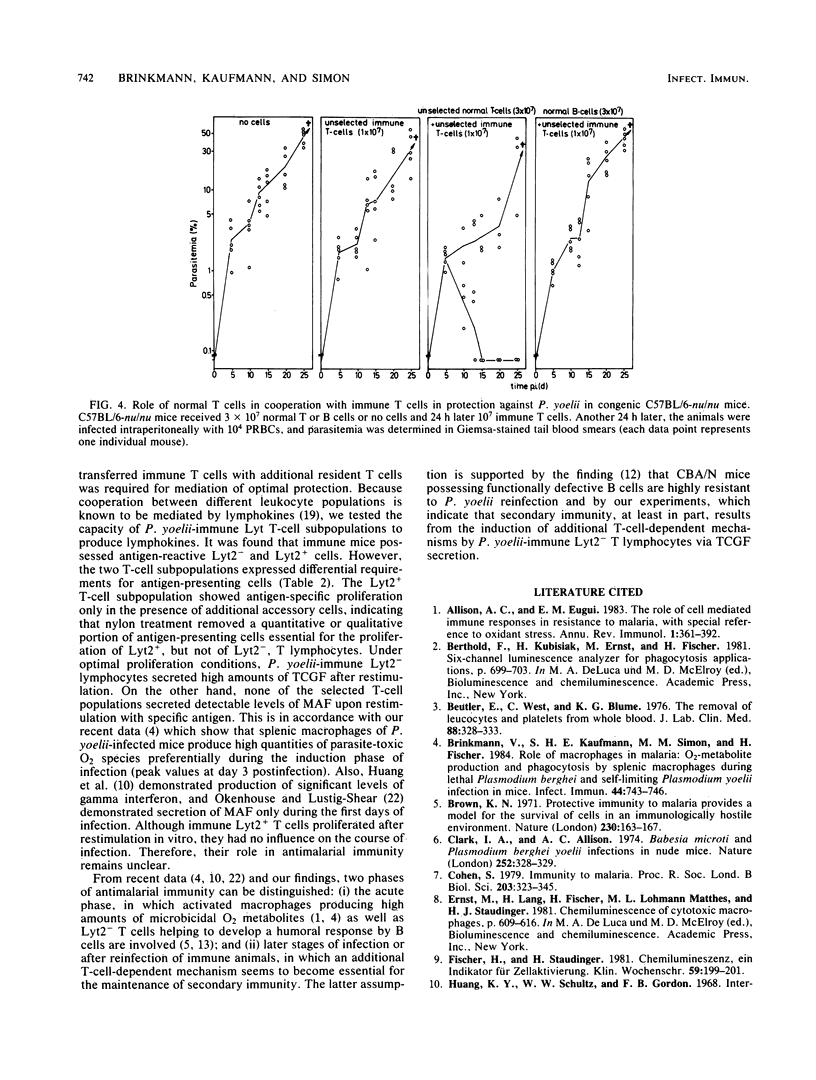
For analysis of the role of immune T cells in protective immunity against murine malaria, Plasmodium yoelii-immune Lyt T-cell subsets were functionally characterized in vitro and in vivo. Selected Lyt2- and Lyt2+ T cells from P. yoelii-immune C57BL/10 mice differed in their capability to proliferate in response to P. yoelii antigen in vitro. Only the Lyt2- T-cell population produced T-cell growth factor upon restimulation, and none of the selected T-cell subsets produced detectable amounts of macrophage activating factor. Lyt2- but not Lyt2+ lymphocytes were capable of transferring protection to normal C57BL/10 mice. When transferred into T-cell-deficient C57BL/6-nu/nu mice, adoptive resistance to P. yoelii by Lyt2- lymphocytes was only demonstrable after prior reconstitution of recipients with normal T cells. These results suggest an interaction between P. yoelii-immune Lyt2- T cells and normal T lymphocytes via T-cell growth factor in the development of protective immunity to malaria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Brinkmann V., Kaufmann S. H., Simon M. M., Fischer H. Role of macrophages in malaria: O2 metabolite production and phagocytosis by splenic macrophages during lethal Plasmodium berghei and self-limiting Plasmodium yoelii infection in mice. Infect Immun. 1984 Jun;44(3):743–746. doi: 10.1128/iai.44.3.743-746.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Fischer H., Staudinger H. Chemilumineszenz, ein Indikator für Zellaktivierung? Klin Wochenschr. 1981 Mar 2;59(5):199–201. doi: 10.1007/BF01476576. [DOI] [PubMed] [Google Scholar]
- Huang K. Y., Schultz W. W., Gordon F. B. Interferon induced by Plasmodium berghei. Science. 1968 Oct 4;162(3849):123–124. doi: 10.1126/science.162.3849.123. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Janeway C. A., Jr, Kemp J. D. Experimental malaria in the CBA/N mouse. J Immunol. 1979 Dec;123(6):2532–2539. [PubMed] [Google Scholar]
- Jayawardena A. N., Murphy D. B., Janeway C. A., Gershon R. K. T cell-mediated immunity in malaria. I. The Ly phenotype of T cells mediating resistance to Plasmodium yoelii. J Immunol. 1982 Jul;129(1):377–381. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Regulatory interactions between macrophages and T-cell subsets in Listeria monocytogenes-specific T-cell activation. Infect Immun. 1982 Dec;38(3):907–913. doi: 10.1128/iai.38.3.907-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindred B. Nude mice in immunology. Prog Allergy. 1979;26:137–238. [PubMed] [Google Scholar]
- Miller R. G. An immunological suppressor cell inactivating cytotoxic T-lymphocyte precursor cells recognizing it. Nature. 1980 Oct 9;287(5782):544–546. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. T-cell immunity to malaria in the B-cell deficient mouse. Am J Trop Med Hyg. 1979 Jan;28(1):1–3. doi: 10.4269/ajtmh.1979.28.1. [DOI] [PubMed] [Google Scholar]
- Tosta C. E., Sedegah M., Henderson D. C., Wedderburn N. Plasmodium yoelii and Plasmodium berghei: isolation of infected erythrocytes from blood by colloidal silica gradient centrifugation. Exp Parasitol. 1980 Aug;50(1):7–15. doi: 10.1016/0014-4894(80)90003-x. [DOI] [PubMed] [Google Scholar]


