C4 photosynthesis consists of morphological and biochemical novelties that create a CO2 pump that concentrates CO2 around Rubisco (Kanai and Edwards, 1999), which decreases photorespiration and the resulting energy waste. Consequently, C4 photosynthesis provides a competitive advantage in all conditions where photorespiration costs become important, especially at high temperatures and in arid and saline conditions (Sage, 2001). Despite being used by only 3% of extant angiosperm species (Sage, 2004), C4 plants account for one-fifth of global terrestrial primary production (Ehleringer et al., 1997). This is mainly due to the high productivity of C4 monocots, especially C4 grasses, which are the most speciose C4 group (Sage, 2004). The C4 grasses dominate most open subtropical and tropical habitats, and some, such as maize (Zea mays), sorghum (Sorghum bicolor), millets (e.g. Pennisetum glaucum, Setaria italica), and sugarcane (Saccharum officinarum), are used as crops and have direct importance for human food consumption and/or as livestock fodder (Table I).
Table I.
Characteristics of the C4 grass lineages
No., Lineage number; n, number of C4 species. PCK, Phosphoenolpyruvate carboxykinase.
| No.a | Name | Age Estimatesf | n | C3 Sister Group | C4 Subtype(s) | Cropsm | Habitatn |
|---|---|---|---|---|---|---|---|
| 1b | Stipagrostis | 15.1 (±4.6)–7.5 (±3.1) | 50 | Sartidiaa | NADP-ME | – | Deserts and semideserts |
| NA | |||||||
| 2b | Aristida | 28.8 (±5.2)–14.4 (±4.7) | 290 | Sartidiaa | NADP-ME | – | Large ecological range |
| [44.4 (±7.5)–present] | |||||||
| 3b | Core Chloridoideae | 32.0 (±4.4)–25.0 (±4.0) | 1,410 | Merxmuellera rangeiag | NAD-ME and PCK | Finger millet, teff | Large ecological range |
| [37.6 (±6.6)–22.5 (±5.7)] | |||||||
| 4b | Centropodia | 22.0 (±4.6)–11.3 (±5.5) | 4 | M. rangeiag | NAD-ME | – | Dry open habitats (semideserts) |
| NA | |||||||
| 5c | Eriachne | 11.5 (±3.6)–6.6 (±2.8) | 40 | Isachneagh | NADP-ME | – | Warm open habitats (savannah) |
| NA | |||||||
| 6b | Arundinelleae | 26.4 (±4.4)–7.9 (±3.4) | 95 | Centotheceae 2agh | NADP-ME | – | Large ecological range |
| [31.7 (±5.9)–present] | |||||||
| 7cd | Panicum/Urochloa/Setaria clade | 18.5 (±3.7)–16.4 (±3.6) | >530 | C3Neurachnea | NADP-ME, NAD-ME, and PCK | Foxtail, pearl, and proso millets | Large ecological range |
| [15.9 (±3.7)–13.1 (±3.2)] | |||||||
| 8c | Neurachne munroi | 4.4 (±3.3)–present | 1 | Neurachne tenuifoliaa | NADP-ME | – | Dry open habitats (steppes) |
| NA | |||||||
| 9c | Echinochloa | 13.8 (±3.5)–4.4 (±2.8) | 30–40 | Parodiophyllochloaai | NADP-ME | – | Warm open habitats |
| [20.6 (±4.5)–2.6 (±1.3)] | |||||||
| 10b | Alloteropsis | 15.3 (±3.5)–present | 4–7 | Forest shade cladeaj | NADP-ME and PCK | – | Warm open habitats (savannah) |
| NA | |||||||
| 11cd | Digitaria | 21.2 (±3.9)–8.1 (±3.4) | 220 | x = 9 Paniceaea | NADP-ME | Fonio | Various warm open habitats |
| [15.9 (±3.7)–5.4 (±2)] | |||||||
| 12b | Andropogoneae | 21.9 (±3.9)–17.1 (±4.1) | 1,085 | x = 10 Paniceaeak | NADP-ME | Maize, sorghum, sugarcane | Large ecological range |
| [24.3 (±4.9)–19.1 (±4.5)] | |||||||
| 13ab | Paspalum clade | 14.1 (±3.4)–8.5 (±3.1) | >345 | Streptostachys asperifoliaak | NADP-ME | Kodo millet | Warm open habitats (savannah) |
| [11.7 (±3.1)–present] | |||||||
| 13bc | Ophiochloa clade | 10.6 (±3.3)–2.8 (±1.9) | 115 | S. asperifoliaa | NADP-ME | – | Large ecological range |
| [13.7 (±3.5)–4.4 (±2.1)] | |||||||
| 14b | Anthaenantiae | 14.3 (±3.5)–present | 1 | Steinchisma cladeak | NADP-ME | – | Warm open habitats (savannah) |
| [15 (±3.7)–present] | |||||||
| 15c | Streptostachys ramosa | 15.5 (±3.5)–present | 1 | Cyphonanthusl | NADP-ME | – | Warm open habitats (savannah) |
| [16.3 (±3.7)–present] | |||||||
| 16b | Panicum prionitis clade | 10.4 (±2.9)–6.3 (±2.7) | >5 | Arthropogon lanceolatusak | NADP-ME | – | Warm open habitats (savannah) |
| [11.2 (±2.9)–present] | |||||||
| 17c | Mesosetum clade | 12.3 (±3.2)–11.3 (±3.0) | 40 | Homolepisak | NADP-ME | Warm open habitats (savannah) | |
| [14.8 (±3.5)–13.9 (±3.4)] |
Independent origin confirmed by PEPC analyses (Christin et al., 2007).
Independent origin based on putative species relationships only.
Phylogeny from Vicentini et al. (2008) found Digitaria and the main x = 9 Paniceae C4 clade clustered together, suggesting a single C4 origin.
Previously named Leptocoryphium lanatum.
Christin et al. (2008) and Vicentini et al. (2008) into square brackets, ages are given in millions of years.
C3 subspecies of A. semialata could represent a reversion from C4 to C3 (Ibrahim et al., 2009).
Excluding fodders.
The biochemistry of the C4 pathway has been an active field of research over the last 40 years and is thus well described (Kanai and Edwards, 1999). However, many issues regarding C4 photosynthesis are still being investigated. A central problem has to do with the genetic regulation of C4 photosynthesis. The genetic mechanisms responsible for the transition from C3 to C4 remain poorly understood, despite extensive investigation on the part of numerous scientists (e.g. Covshoff et al., 2008; Lara et al., 2008). The evolution of the C4 pathway was previously thought to have involved relatively few key mutations (Ku et al., 1996), but recent studies showed that the C4 pathway of maize involves cell-specific expression for 18% of the genes (Sawers et al., 2007) and requires deep synchronization between mesophyll (M) and bundle sheath (BS) cells (Bailey et al., 2007). These transcriptional changes are likely to mediate, at least in part, the variation observed in BS and M plastid proteomes (Majeran et al., 2005, 2008). Rather than extensive changes in cis- and trans-acting regulatory elements, the segregation of enzymes between M and BS cells of C4 plants could have been acquired through changes in key regulatory elements changing M and BS cellular environments (Covshoff et al., 2008), leading to important differences in their transcriptomes (Sawers et al., 2007). In addition to the C4 enzymes, C4 photosynthesis evolution necessitated rearrangements of chloroplast envelope proteins (Bräutigam et al., 2008). Furthermore, transport of C4 intermediates between M and BS cells is probably not performed through simple diffusion, which suggests that other, unidentified, mechanisms exist (Sowinski et al., 2008), which may be yet another C4-specific adaptation.
Many of the enzymes that drive the carbon shuttle in C4 plants are also present in C3 plants but are involved in other aspects of plant growth and development (Monson, 2003). Tissue-specific regulation of C4 pathway enzymes appears to have been a crucial step in the evolution of C4 photosynthesis (Hibberd and Quick, 2002). One aspect of the pathway that remains poorly understood is the genetic components regulating the alteration of leaf anatomy (Kellogg, 1999). The developmental and genetic issues can be addressed with all C4 species, but the low number of model species used to date limits the generalization of the results.
Grasses have been the focus of much of the recent C4 research. For example, human-directed improvement of C3 grass crops, such as rice (Oryza sativa), barley (Hordeum vulgare), and wheat (Triticum aestivum), by introgression of C4 characteristics is receiving particular attention (Hibberd et al., 2008). Understanding the historical causes of C4 evolutionary and ecological success is another area of intense research activity (Cerling et al., 1997; Beerling and Osborne, 2006; Osborne and Beerling, 2006; Osborne, 2008). The ecological importance of grasses made this family a natural study system for investigating factors affecting the distribution and success of C4 plants (Taub, 2000; Carmo-Silva et al., 2007; Cabido et al., 2008; Edwards and Still, 2008). For instance, it has recently been shown that the oldest C4 origin in grasses is relatively young (approximately 30 million years old), and correlates with a marked decrease of atmospheric CO2 concentration (Christin et al., 2008; Vicentini et al., 2008). Since atmospheric CO2 concentration and air temperature both affect C4 plant success, the current changes in global climate will potentially trigger important perturbations in major ecosystems, and could affect the performance of extensively cultivated tropical cereals. Therefore, a complete understanding of C4 ecology and physiology is necessary for conservation biology and agriculture to face future climate changes (Sage and Kubien, 2003; Ainsworth et al., 2008).
Comparative analyses offer an attractive approach for both the study of genetic determinants of C4 photosynthesis (Christin et al., 2007) and the identification of attributes associated with it (Edwards et al., 2007; Edwards and Still, 2008). Such an approach requires comparing several independent origins of C4 plants to determine characteristics that are shared among them. Indeed, if two C4 species inherited the C4 trait from their common ancestor, they do not represent independent replicates. Ideally, comparative studies should consist of distinct C4 clades, known to represent distinct origins of the C4 pathway, as well as C3 sister groups to each of the C4 lineages. For this approach to work, species relationships have to be assessed by phylogenetic analyses, rendering the phylogenetic framework of systematic botany useful to evolutionary and physiological investigations.
C4 EVOLUTIONARY LINEAGES IN GRASSES
The grass family is composed of approximately 10,000 species, of which about 45% are C4 (Sage, 2004). Grass taxonomy recognizes between 12 and 13 main subfamilies but all C4 grasses belong to the PACMAD clade (Fig. 1; Duvall et al., 2007; or PACCMAD, Sánchez-Ken et al., 2007). Both the distribution of C4 grasses in distinct taxonomic groups and the high variability of their C4 syndrome led to the inference of multiple origins of the C4 pathway in this family (Sinha and Kellogg, 1996; Kellogg, 2001). Phylogenetic analyses of the subfamily Panicoideae further suggested that C4 photosynthesis appeared several times independently, although a single appearance followed by multiple reversions could not be excluded (Giussani et al., 2001; Duvall et al., 2003; Vicentini et al., 2008). The ancestral state reconstructions adopted in these studies are strongly dependent on species sampling and rely on statistical methods whose assumptions can produce different results. In addition, the transition rate from C3 to C4 could also change through time (Vicentini et al., 2008), for instance as a function of atmospheric CO2 levels (Christin et al., 2008) or after the acquisition of preadaptations to C4 photosynthesis (Sage, 2001). Finally, inferences of characters that affect the rates of speciation or extinction can yield erroneous conclusions if not carefully considered (Goldberg and Igić, 2008).
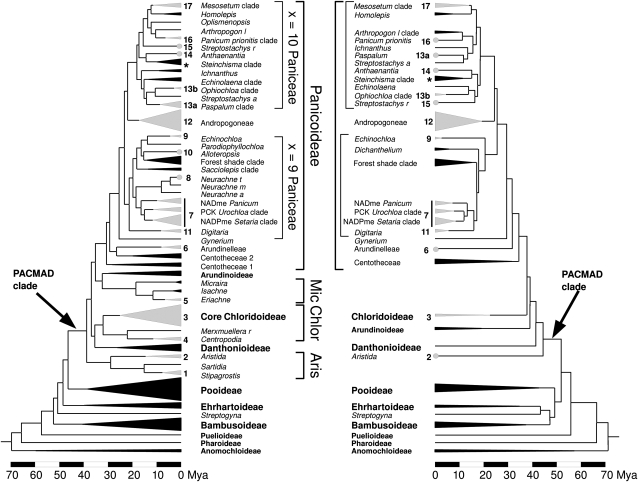
Figure 1.
Calibrated phylogenetic trees of the grass family. Phylogenetic trees are from independent studies by Christin et al. (2008; on the left; based on plastid markers) and Vicentini et al. (2008; on the right; based on one plastid and one nuclear marker). Branch lengths are proportional to elapsed time, in million years (Mya). All clades containing only C3 species are compressed (in black). Similarly, homogeneously C4 lineages are also compressed but in gray. C4 lineages represented by a single species are highlighted by a gray circle at the tip. C4 lineages are numbered according to Christin et al. (2008). Clade names and subfamilies are indicated between the two topologies. Asterisks indicate the position of the C3/C4 intermediate species S. hians. Mic, Micrairoideae; Chlor, Chloridoideae; Aris, Aristidoideae; PACMAD clade, subfamilies Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristidoideae, and Danthonioideae. x = 9 and x = 10 Paniceae identify two distinct groups of this tribe that differ according to their basic chromosome number (9 and 10, respectively; Giussani et al., 2001).
Some studies have thus focused on the evolutionary dynamics of specific key enzymes involved in the C4 pathway, in particular phosphoenolpyruvate carboxylase (PEPC). The use of PEPC for the atmospheric CO2 fixation is one of the rare characteristics common to all C4 plants (Sinha and Kellogg, 1996; Sage, 2004), and its recruitment is an important step in the integration and optimization of C4 biochemistry (Svensson et al., 2003) and can be considered as a critical event in the evolution into a C4 plant. The presence of a Ser at position 780 of PEPC (numbered based on the maize sequence) is required for C4 function (Svensson et al., 2003) and was accompanied by many other recurrent adaptive amino acid changes (Christin et al., 2007) that left reliable C4-specific genetic signatures. Because changes along a DNA sequence are amenable to statistical modeling, they can easily be traced on a PEPC phylogenetic tree. This technique was used to identify the grass lineages that likely evolved the C4 trait independently (Table I; Christin et al., 2007, 2008).
C4 MODEL SPECIES IN GRASSES
The grasses contain few examples of closely related C3/C4 pairs, and those that exist are not easily accessible. Alloteropsis semialata contains a C3 and a C4 subspecies, which are closely related (Ibrahim et al., 2009) but differ in chromosome number (Liebenberg and Fossey, 2001) and so are presumably intersterile. A recent phylogenetic study suggested that C3 subspecies of A. semialata could represent an evolutionary reversion from C4 to C3 photosynthesis (Ibrahim et al., 2009). The genus Neurachne includes both C3 and C4 species (Moore and Edwards, 1989); these are native to Australia and grow in relatively inaccessible parts of the continent and have not, to our knowledge, been cultivated. The C3/C4 intermediate Steinchisma hians (formerly Panicum milioides) is sister to a group of C3 species, and has been crossed with them (Brown et al., 1985). Steinchisma as currently circumscribed is mainly South American.
Historically much of the work on C4 grasses focused on the genus Panicum because it appeared to have species with all possible photosynthetic pathways. Unfortunately, this genus was an assemblage of unrelated species (Aliscioni et al., 2003) whose taxonomy is being completely redefined (Morrone et al., 2007, 2008; Sede et al., 2008). The name Panicum should be restricted to a set of species that are all C4 with the subtype using the NAD-malic enzyme (NAD-ME), including switchgrass (Panicum virgatum). C3 species of Panicum are not closely related to true Panicum (Aliscioni et al., 2003).
Future C4 research should consider additional C4 species systems since including other independent lineages would increase the power of comparative analyses. In particular, Aristida and Stipagrostis, as well as the subfamily Chloridoideae, represent interesting C4 lineages. These groups are ecologically important (Table I) and strongly differ from the Panicoideae C4 species in terms of ecological attributes, such as aridity tolerance (Taub, 2000; Sato and Kubota, 2004; Carmo-Silva et al., 2007). They are species rich and widely distributed, facilitating sampling for more detailed study.
INTEGRATING PHYSIOLOGICAL STUDIES IN A PHYLOGENETIC CONTEXT
Understanding C4-specific growth, survival, and reproductive success, as well as the environmental conditions that influence these traits, is of prime ecological, agricultural, and evolutionary importance. Assessment of plant physiological traits, such as photosynthetic activity and efficiency, is time consuming, especially when performed under a range of environmental conditions. Therefore, physiological studies typically consider only a limited number of species. Unfortunately, due to the strong variations of the C4 pathway (Sinha and Kellogg, 1996), all C4 plants are far from being equivalent. Species sampling for physiological investigations is crucial to ensure the generalization of conclusions. As noted above, taxa that inherited their C4 trait from a common ancestor do not represent independent replicates. Their common ancestry can potentially lead to spurious correlations, which in turn can entangle characteristics due to the C4 trait and those resulting from a close phylogenetic relationship (Taub, 2000). A sound phylogenetic framework showed that a low carbonic anhydrase activity, previously attributed to C4 grasses (Gillon and Yakir, 2001), characterizes the whole PACMAD clade and is not linked to the C4 trait (Edwards et al., 2007). Thanks to its highly convergent nature, the C4 trait is present in numerous natural replicates. Species sampling for C4 physiological studies can take advantage of this by comparing species from independent C4 lineages, as well as each C4 clade with its C3 sister group (Table I). Therefore, species relationships deduced from molecular markers should serve as a guide for species sampling.
As a C4 study system, the grass family allows combining physiological, ecological, genomic, and evolutionary approaches, which are all necessary for a complete understanding of C4 photosynthesis. Integration of the wide knowledge we are gaining about C4 grasses to reach a full picture requires incorporation of evolutionary history by using phylogenetic information. Important efforts have led to a reasonably well-resolved phylogenetic tree for the grass family (e.g. Grass Phylogeny Working Group, 2001; Aliscioni et al., 2003; Duvall et al., 2007; Christin et al., 2008; Vicentini et al., 2008) but conflicts between plastid and nuclear markers (Fig. 1) still need to be resolved. Recent analyses of C4 genes have identified grass lineages that evolved the C4 pathway independently (Christin et al., 2007, 2008). These correspond to more than 15 independent replicates (Fig. 1), enabling wide-scale comparative studies to sort general attributes of C4 plants as well as particular ones. By taking advantage of the convergent nature of C4 photosynthesis, multidisciplinary studies in the grasses could bring a complete view of the selective pressures and genetic mechanisms responsible for the evolution of C4 photosynthesis and the factors that control the current distribution and success of C4 plants. C4 photosynthesis in grasses could become a model of macroevolution process when completely elucidated, from the selective pressures to the genetic mechanisms that led to its appearances.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Pascal-Antoine Christin (pascal-antoine.christin@unil.ch).
References
- Ainsworth EA, Rogers A, Leakey ADB (2008) Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol 147 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliscioni SS, Giussani LM, Zuloaga FO, Kellogg EA (2003) A molecular phylogeny of Panicum (Poaceae: Paniceae): tests of monophyly and phylogenetic placement within the Panicoideae. Am J Bot 90 796–821 [DOI] [PubMed] [Google Scholar]
- Bailey KJ, Gray JE, Walker RP, Leegood RC (2007) Coordinate regulation of phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase by light and CO2 during C4 photosynthesis. Plant Physiol 144 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerling DJ, Osborne CP (2006) The origin of the savanna biome. Glob Change Biol 12 2023–2031 [Google Scholar]
- Bräutigam A, Hofmann-Benning S, Weber APM (2008) Comparative proteomics of chloroplasts envelopes from C3 and C4 plants reveals specific adaptations of the plastid envelope to C4 photosynthesis and candidate proteins required for maintaining C4 metabolite fluxes. Plant Physiol 148 568–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, Bouton JH, Evans PT, Malter HE, Rigsby LL (1985) Photosynthesis, morphology, leaf anatomy, and cytogenetics of hybrids between C3 and C3/C4 Panicum species. Plant Physiol 77 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabido M, Pons E, Cantero JJ, Lewis JP, Anton A (2008) Photosynthetic pathway variation among C4 grasses along a precipitation gradient in Argentina. J Biogeogr 35 131–140 [Google Scholar]
- Carmo-Silva AE, Soares AS, Marques da Silva J, Bernardes da Silva A, Keys AJ, Arrabaça MC (2007) Photosynthetic responses of three C4 grasses of different metabolic subtypes to water deficit. Funct Plant Biol 34 204–213 [DOI] [PubMed] [Google Scholar]
- Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR (1997) Global vegetation change through the Miocene/Pliocene boundary. Nature 389 153–158 [Google Scholar]
- Christin PA, Besnard G, Samaritani E, Duvall MR, Hodkinson TR, Savolainen V, Salamin N (2008) Oligocene CO2 decline promoted C4 photosynthesis in grasses. Curr Biol 18 37–43 [DOI] [PubMed] [Google Scholar]
- Christin PA, Salamin N, Savolainen V, Duvall MR, Besnard G (2007) C4 photosynthesis evolved in grasses via parallel adaptive genetic changes. Curr Biol 17 1241–1247 [DOI] [PubMed] [Google Scholar]
- Covshoff S, Majeran W, Liu P, Kolkman JM, van Wijk KJ, Brutnell TP (2008) Deregulation of maize C4 photosynthetic development in a mesophyll cell-defective mutant. Plant Physiol 146 1469–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall MR, Davis JI, Clark LG, Noll JD, Goldman DH, Sánchez-Ken JG (2007) Phylogeny of the grasses (Poaceae) revisited. Aliso 23 237–247 [Google Scholar]
- Duvall MR, Saar DE, Grayburn WS, Holbrook GP (2003) Complex transitions between C3 and C4 photosynthesis during the evolution of Paniceae: a phylogenetic case study emphasizing the position of Steinchisma hians (Poaceae), a C3-C4 intermediate. Int J Plant Sci 164 949–958 [Google Scholar]
- Edwards EJ, Still CJ (2008) Climate, phylogeny and the ecological distribution of C4 grasses. Ecol Lett 11 266–276 [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Still CJ, Donoghue MJ (2007) The relevance of phylogeny to studies of global change. Trends Ecol Evol 22 243–249 [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112 285–299 [DOI] [PubMed] [Google Scholar]
- Gillon JS, Yakir D (2001) Influence of carbonic anhydrase activity in terrestrial vegetation on the 18O content of atmospheric CO2. Science 291 2584–2587 [DOI] [PubMed] [Google Scholar]
- Giussani LM, Cota-Sanchez JH, Zuloaga FO, Kellogg EA (2001) A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of C4 photosynthesis. Am J Bot 88 1993–2012 [PubMed] [Google Scholar]
- Goldberg EE, Igić B (2008) On phylogenetic tests of irreversible evolution. Evolution Int J Org Evolution 62 2727–2741 [DOI] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group (2001) Phylogeny and subfamilial classification of the grasses (Poaceae). Ann Mo Bot Gard 88 373–457 [Google Scholar]
- Hibberd JM, Quick WP (2002) Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415 451–454 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Sheehy JE, Langdale JA (2008) Using C4 photosynthesis to increase the yield of rice: rationale and feasibility. Curr Opin Plant Biol 11 228–231 [DOI] [PubMed] [Google Scholar]
- Ibrahim DG, Burke T, Ripley BS, Osborne CP (2009) A molecular phylogeny of the genus Alloteropsis (Panicoideae, Poaceae) suggests an evolutionary reversion from C4 to C3 photosynthesis. Ann Bot (Lond) 103 doi/10.1093/aob/mcn204 [DOI] [PMC free article] [PubMed]
- Kanai R, Edwards GE (1999) The biochemistry of C4 photosynthesis. In RF Sage, RK Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 49–87
- Kellogg EA (1999) Phylogenetic aspects of the evolution of C4 photosynthesis. In RF Sage, RK Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 411–444
- Kellogg EA (2001) Evolutionary history of the grasses. Plant Physiol 125 1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku MSB, Kano-Murakami Y, Matsuoka M (1996) Evolution and expression of C4 photosynthesis genes. Plant Physiol 111 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara MV, Offermann S, Smith M, Okita TW, Andreo CS, Edwards GE (2008) Leaf development in the single-cell C4 system in Bienertia sinuspersici: expression of genes and peptide levels for C4 metabolism in relation to chlorenchyma structure under different light conditions. Plant Physiol 148 593–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenberg EJL, Fossey A (2001) Comparative cytogenetic investigation of the two subspecies of the grass Alloteropsis semialata (Poaceae). Bot J Linn Soc 137 243–248 [Google Scholar]
- Majeran W, Cai Y, Sun Q, van Wijk KJ (2005) Functional differentiation of bundle sheath and mesophyll maize chloroplasts determined by comparative proteomics. Plant Cell 17 3111–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W, Zybailov B, Ytterberg AJ, Dunsmore J, Sun Q, van Wijk KJ (2008) Consequences of C4 differentiation for chloroplast membrane proteomes in maize mesophyll and bundle sheath cells. Mol Cell Proteomics 7 1609–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK (2003) Gene duplication, neofunctionalization, and the evolution of C4 photosynthesis. Int J Plant Sci 164 S43–S54 [Google Scholar]
- Moore BD, Edwards GE (1989) Metabolism of 14CO2 by leaves of different photosynthetic types of Neurachne species. Plant Sci 60 155–161 [Google Scholar]
- Morrone O, Denham SS, Aliscioni SS, Zuloaga FO (2008) Parodiophyllochloa, a new genus segregated from Panicum (Paniceae, Poaceae) based on morphological and molecular data. Syst Bot 33 66–76 [Google Scholar]
- Morrone O, Scataglini MA, Zuloaga FO (2007) Cyphonanthus, a new genus segregated from Panicum (Poaceae: Panicoideae: Paniceae) based on morphological, anatomical and molecular data. Taxon 56 521–532 [Google Scholar]
- Osborne CP (2008) Atmosphere, ecology and evolution: what drove the Miocene expansion of C4 grasslands? J Ecol 96 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Beerling DJ (2006) Nature's green revolution: the remarkable evolutionary rise of C4 plants. Philos Trans R Soc Lond B Biol Sci 361 173–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF (2001) Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthetic syndrome. Plant Biol 3 202–213 [Google Scholar]
- Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161 341–370 [DOI] [PubMed] [Google Scholar]
- Sage RF, Kubien DS (2003) Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynth Res 77 209–225 [DOI] [PubMed] [Google Scholar]
- Sánchez-Ken JG, Clark LG, Kellogg EA, Kay EE (2007) Reinstatement and emendation of subfamily Micrairoideae (Poaceae). Syst Bot 32 71–80 [Google Scholar]
- Sato A, Kubota F (2004) Specific difference in photorespiration activity in C4 subtype plants and its relationship with drought tolerance of leaf photosynthesis. J Fac Agr Kyushu Univ 49 25–32 [Google Scholar]
- Sawers RJH, Liu P, Anufrikova K, Hwang JTG, Brutnell TP (2007) A multi-treatment experimental system to examine photosynthetic differentiation in the maize leaf. BMC Genomics 8 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sede SM, Morrone O, Giussani LM, Zuloaga FO (2008) Phylogenetic studies in the Paniceae (Poaceae): a realignment of section Lorea of Panicum. Syst Bot 33 284–300 [Google Scholar]
- Sinha NR, Kellogg EA (1996) Parallelism and diversity in multiple origins of C4 photosynthesis in the grass family. Am J Bot 83 1458–1470 [Google Scholar]
- Sowinski P, Szczepanik J, Minchin PEH (2008) On the mechanism of C4 photosynthesis intermediate exchange between Kranz mesophyll and bundle sheath cells in grasses. J Exp Bot 59 1137–1147 [DOI] [PubMed] [Google Scholar]
- Svensson P, Bläsing OE, Westhoff P (2003) Evolution of C4 phosphoenolpyruvate carboxylase. Arch Biochem Biophys 414 180–188 [DOI] [PubMed] [Google Scholar]
- Taub DR (2000) Climate and the US distribution of C4 grass subfamilies and decarboxylation variants of C4 photosynthesis. Am J Bot 87 1211–1215 [PubMed] [Google Scholar]
- Vicentini A, Barber JC, Aliscioni SS, Giussani LM, Kellogg EA (2008) The age of the grasses and clusters of origins of C4 photosynthesis. Glob Change Biol 14 2963–2977 [Google Scholar]
- Watson L, Dallwitz MJ (1992) Grass genera of the world: descriptions, illustrations, identification, and information retrieval. CAB International. http://biodiversity.uno.edu/delta/ (August 1, 2008)