Abstract
Context
Lofexidine is an alpha-2-A noradrenergic receptor agonist that is approved in the United Kingdom for the treatment of opioid withdrawal symptoms. Lofexidine has been reported to have more significant effects on decreasing opioid withdrawal symptoms with less hypotension than clonidine.
Objective
To demonstrate that lofexidine is well tolerated and effective in the alleviation of observationally-defined opioid withdrawal symptoms in opioid dependent individuals undergoing medically supervised opioid detoxification as compared to placebo.
Design
An inpatient, Phase 3, placebo-controlled, double blind, randomized multi-site trial with three phases: (1) Opioid Agonist Stabilization Phase (days 1–3), (2) Detoxification/Medication or Placebo Phase (days 4–8), and (3) Post Detoxification/Medication Phase (days 9–11).
Subjects
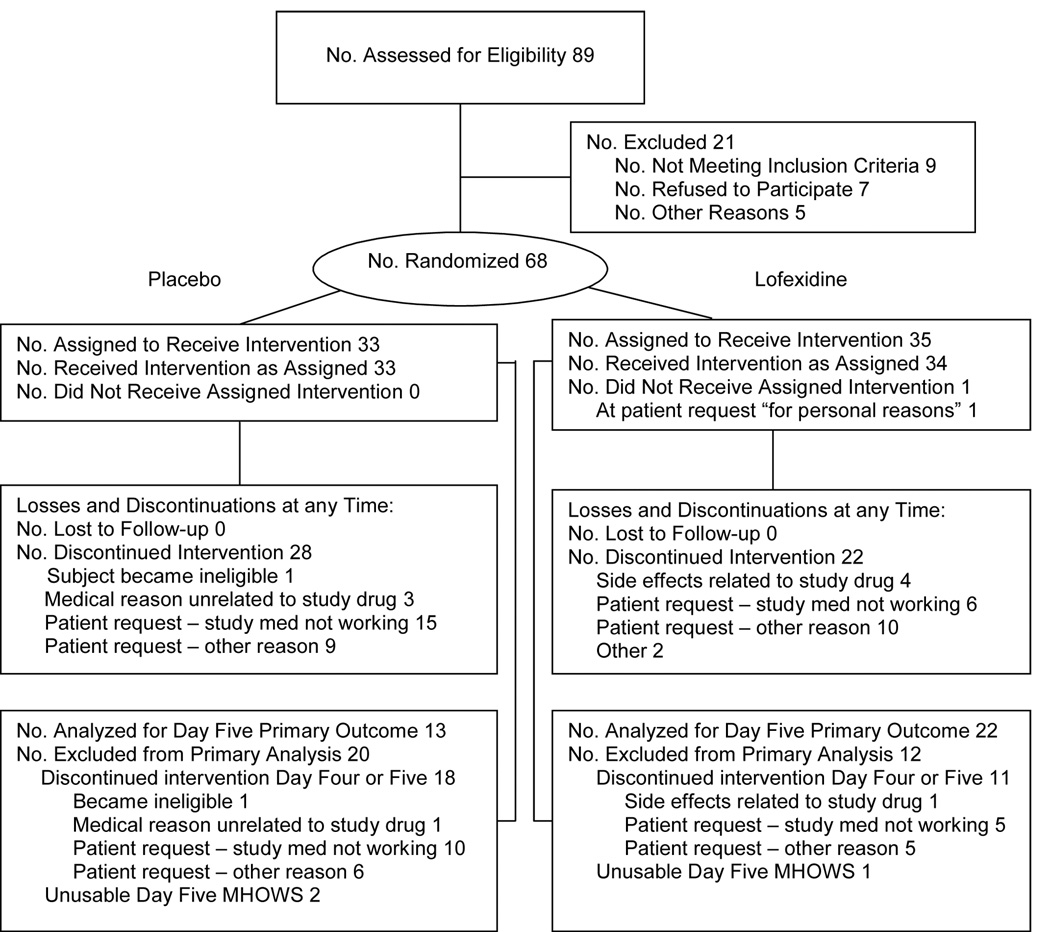
Sixty-eight opioid dependent subjects were enrolled at three sites with 35 randomized to lofexidine and 33 to placebo.
Main Outcome Measure
Modified Himmelsbach Opiate Withdrawal Scale (MHOWS) on study day 5 (2nd opioid detoxification treatment day).
Results
Due to significant findings, the study was terminated early. On the study day 5 MHOWS, subjects treated with lofexidine had significantly lower scores (equating to fewer/less severe withdrawal symptoms) than placebo subjects (Least squares means 19.5 ± 2.1 versus 30.9 ± 2.7; p=0.0019). Lofexidine subjects had significantly better retention in treatment than placebo subjects (38.2% versus 15.2%; Log rank test p=0.01).
Conclusions
Lofexidine is well tolerated and more efficacious than placebo for reducing opioid withdrawal symptoms in inpatients undergoing medically supervised opioid detoxification.
Trial Registration
trial registry name A Phase 3 Placebo-Controlled, Double-Blind Multi-Site Trial of Lofexidine for Opiate Withdrawal, registration number NCT00032942, URL for the registry http://clinicaltrials.gov/ct/show/NCT00032942?order=4.
Keywords: Lofexidine, Alpha 2 Agonist, Opioid Withdrawal Treatment, Phase 3, Placebo-Controlled, Double-Blind, Multi-Site Trail
1. Introduction
Opioid dependence is a medical condition associated with severe health and social consequences (Hser et al., 2001). The Office of National Drug Control Policy estimates the number of individuals addicted to heroin in the United States is between 750,000 and 1,000,000 users (Office of National Drug Control Policy, 2003). According to the 2006 National Survey on Drug Use and Health (NSDUH), approximately 3.8 million Americans aged 12 or older reported trying heroin at least once during their lifetimes, representing 1.5% of the population aged 12 or older. Approximately 560,000 (0.2%) reported past year heroin use and 338,000 (0.1%) reported past month heroin use. According to the NSDUH survey, across age groups, an estimated 5.2 million persons were current nonmedical users of prescription pain relievers in 2006, which is more than the estimated 4.7 million in 2005. The growing population of people with prescription opioid dependence increases the need for a range of evidence based treatments.
The medication treatment for opioid addiction can include short-term detoxification, longer-lasting opioid maintenance and the opioid relapse prevention therapy, such as naltrexone (Herman et al., 1995). The predominant treatment for opioid dependence is methadone for maintenance or detoxification. Another opioid, buprenorphine, has recently been approved for the same indication (Gonzalez et al., 2004). Some patients find maintanence or detoxification with an opioid unacceptable and prefer nonopioid treatment. No single treatment modality is currently effective, and opioid dependence requires adequate access to a wide range of options.
The alpha-2-adrenergic agonist clonidine is currently used “off label” for the treatment of withdrawal (Gossop, 1988). However, clonidine can produce problematic side effects, such as sedation and hypotension, generally restricting its use in the outpatient setting (Kleber et al., 1985; Preston and Bigelow, 1985). Lofexidine is an alpha-2-adrenergic agonist, structurally related to clonidine, which has been used in the United Kingdom primarily in an outpatient setting for opioid detoxification since 1992 under the label BritLofex® (Jarrott et al., 1983; Aigner and Schmidt, 1982). One advantage of lofexidine over clonidine is it is believed to have less hypotensive effects than clonidine (Kahn et al., 1997).
The preclinical neurobiology of the mechanism of alpha-2-adrenergic agonists in opioid withdrawal has been well studied. Early studies suggested that chronic opioid exposure leads to tonic inhibition of noradrenergic cells and cessation of opioid use results in disinhibition of noradrenergic cells in the locus coeruleus (Aghajanian, 1982; Aston-Jones et al., 1997). The therapeutic action of alpha-2-adrenergic agonists stemmed from the ability to reduce firing in the locus coeruleus (Freedman and Aghajanian, 1985; Aghajanian et al., 1978). In addition to the central actions of alpha-2-adrenergic agonists, a study by Buccafusco and Marshall also suggest spinal mediated anti-withdrawal effects (Buccafusco and Marshall, 1985).
In vitro receptor binding studies have identified three subtypes of the alpha-2-adrenergic receptor: 2A, 2B, and 2C (Marjamaki et al., 1993; Uhlen and Wikberg, 1991). Clonidine is a nonspecific alpha-2-adrenergic receptor agonist with equal affinity for all three of these subtypes (cited in prior reference) while lofexidine appears to bind specifically to the subtype 2A alpha-2-adrenergic receptor (Marjamaki et al., 1993; Uhlen and Wikberg, 1991; Herman and O’Brien, 1997). Higher affinity 2A subtype agents have been shown in nonhuman primates to have less hypotensive effects and to have efficacy on processes such as memory enhancement in aged animals (Arnsten et al., 1988).
Results of clinical studies indicate that alpha-2-adrenergic agonists such as lofexidine are effective in the alleviation of opioid withdrawal. There have been a number of double-blind, controlled studies indicating that the efficacy of lofexidine is comparable to that of clonidine (Carnwath and Hardman, 1998; Kahn et al., 1997; Lin et al., 1997). All studies concluded that there was less problematic hypotension with lofexidine than with clonidine. Two controlled studies comparing lofexidine and short term methadone taper suggested no significant difference in the withdrawal intensity or blood pressure between the groups (Howells et al., 2002; Bearn et al., 1996). A recent report also indicated that the signs and symptoms of withdrawal occur and resolve earlier with treatment with an alpha-2-adrenergic agonist compared to methadone withdrawal treatment (Gowing et al., 2002). In addition, a large retrospective lofexidine use survey of patients and health care providers in the United Kingdom suggested a common ten-day outpatient treatment protocol without significant reports of adverse effects (Akhurst, 1999). As expected, lofexidine alleviates opioid withdrawal symptoms in the rat (Shearman et al., 1980).
Early open clinical studies supported the dose-dependent use of lofexidine as a treatment to decrease opioid withdrawal signs and symptoms (Gold et al., 1981; Washton et al., 1982; Gowing et al., 2004). The dose of lofexidine required to control withdrawal symptoms varies for each patient depending on the amount, frequency and duration of opioid used. In the U.K, lofexidine treatment is initiated at 0.2mg twice daily, increasing daily by 0.2mg - 0.4 mg with a recommended final dose of 2.4mg/day. An inpatient, dose-response analysis of the safety and efficacy of lofexidine in the U.S. evaluated four different divided doses: 1.6 mg/day, 2.4 mg/day, 3.2 mg/day and 4.0 mg/day groups. There was a dose-dependent decrease in objective opiate withdrawal symptoms using the Modified Himmelsbach Opiate Withdrawal Scale (MHOWS). However, transient orthostatic systolic blood pressure changes were more frequent in the higher dose groups (Yu, et al., 2001).
Here we report on the results of the first phase 3, inpatient, randomized, placebo-controlled, multi-site study designed to evaluate the efficacy of lofexidine for the treatment of opioid withdrawal.
2. Methods
2.1. Participants
All enrolled participants met the following inclusion criteria: (1) minimum 18 years of age; (2) current dependence on heroin, morphine, or hydromorphone according to DSM-IV criteria; (3) participants reported use of heroin, morphine, or hydromorphone for at least 21 of the past 30 days; and (4) a urine toxicology screen positive for opiates and negative for methadone, levo-alpha-acetylmethadol (LAAM), or buprenorphine at the time of screening. Exclusion criteria for potential participants were: (1) females who were pregnant or of child-bearing potential and did not agree to practice an effective form of birth control during the course of the study; (2) females currently nursing; (3) self-reported use of methadone, buprenorphine, or LAAM 14 days prior to admission; (4) history of seizures or receiving anticonvulsant therapy during the past 5 years; (5) history of pancreatic disease, liver disease, gastrointestinal or renal disease, neurological or psychiatric disorders; (6) positive tuberculosis (TB) skin test along with a clinical history and chest X-ray indicative of active TB; (7) an abnormal baseline cardiovascular exam; (8) undergoing treatment with a psychotropic, prescription analgesic, antihypertensive, antiarrhythmic, or antiretroviral medication; (9) current dependence on any psychoactive substance other than heroin, morphine, hydromorphone, cocaine, caffeine or nicotine that require detoxification; (10) symptomatic for HIV and CD4 T cell counts ≤ 200 cell per microliter; (11) blood donation within the past 8 weeks; (12) participation in an investigational drug study within the past 3 months; (13) veins that will not allow the collection of even single venipuncture needle sticks at the beginning and at the end of protocol; and (14) becoming overly sedated from the first dose of morphine.
Eighty-nine opiate-dependent participants, recruited through newspapers and walk-in clinics at three sites (University of Pennsylvania/Philadelphia Veterans Affairs Medical Center (VAMC), University of California Los Angeles/Long Beach VAMC, and New York State Psychiatric Institute/Columbia Presbyterian Medical Center) were screened for eligibility between May 2001 and April 2002 (Figure 1). Sixty-eight were enrolled. Of the 21 subjects not enrolled, seven declined participation and nine met study exclusion criteria as follows: AIDS (2 subjects), abnormal cardiovascular exam (2), liver disease (2), history of seizures (1), urine toxicology either negative for opiates or positive for methadone (1), and required excluded medication (1). Of the enrolled subjects, 35 were randomized to lofexidine and 33 to placebo. Only 17 enrolled subjects (12 lofexidine and 5 placebo) completed the full treatment phase. A completer was predefined in the protocol as someone who completed day 8, had a day 8 MHOWS, and received at least one dose of medication on day 8. Of those subjects terminating early, 41 were by subject request (17 lofexidine and 24 placebo). Four lofexidine subjects terminated because of adverse effects: one for continued hypertension, one for light-headedness, and two because of feelings of tiredness.
Figure 1. Profile of the randomized controlled trial for lofexidine.
2.2. Procedure and Medication
This double-blind, placebo-controlled trial was conducted at three different sites in the United States: Los Angeles, CA, New York, NY, and Philadelphia, PA. Each site’s individual Institutional Review Board and NIDA approved the study protocol and consent form. All participants gave written informed consent prior to study admission.
The lofexidine (Britlofex ®) and placebo were obtained from Britannia Pharmaceuticals, Ltd. Study medications were provided to the site pharmacists by the Cooperative Studies Program Clinical Research Pharmacy Coordinating Center (CSPCRPCC) in Albuquerque, NM in subject-specific kits for days 4 to 10. They were distributed to the three sites as 0.2 mg tablets of lofexidine or matching placebo. Placebo was used rather than an active control because the study was intended to support an FDA application. After tolerability is assured, the next objective required by the FDA is to prove the efficacy of a new molecular entity by showing that the medication is significantly more efficacious than placebo.
2.3. Randomization and Blinding
The Cooperative Studies Program Coordinating Center (CSPCC) in Perry Point, MD generated a randomization sequence for each site separately, in blocks of four, using non-sequential subject numbers. The site coordinator or investigator called the CSPCC just before the detoxification phase was to begin to randomize a subject to study treatment. After confirming eligibility, the CSPCC provided the site with a randomization number which corresponded to a specific drug therapy kit that had previously been shipped to the site by the Albuquerque CSPCRPCC. The study drug kits were labeled with the study name, a study drug name (the same on all kits), the number of tablets per card, the randomization number, and the address and 24-hour emergency phone number for the CSPCRPCC. In an emergency, the blind could be broken by calling the CSPCRPCC 24-hour emergency phone number.
2.4. Design
This was an 11-day study that had three phases:
2.4.1. Opioid Agonist Stabilization Phase (days 1–3)
Because of the extensive differences in the amount of heroin that the study population was dependent upon, placing all subjects on a fixed dose of morphine decreased the variability of baseline opiate levels across subjects. Morphine sulfate was given subcutaneously to stabilize subjects on a fixed dose of opiate agonist. In this phase, participants received up to 100mg/day (25 mg at 06:30h, 11:00h, 16:30h, 22:00h) of subcutaneous morphine sulfate for three days. Secondly, the use of a stabilization phase was also suggested by the use of the MHOWS as the primary outcome measure. The choice of the MHOWS was based on discussions between the study sponsor, NIDA and the FDA. The 100mg/day dose was chosen since it is equivalent to 50 mg of oral methadone which is intermediate between the treatment initiation and maintenance average of 80 mg at the Philadelphia VAMC in 1997. This dose had previously been used in a lofexidine Phase 1 & 2 open tolerability study at Philadelphia VAMC and Los Angeles/Long Beach VAMC sites without subjects exhibiting intoxication, sedation or marked withdrawal and was well tolerated.
2.4.2. Detoxification/Medication or Placebo Phase (days 4–8)
On days 4–8, no morphine was administered. As described above, a call was placed to the Cooperative Studies Program Coordinating Center (CSPCC) for randomization. The first dose of lofexidine/placebo was administered at 0800h on day 4. Participants were medicated with 3.2 mg/day of lofexidine or placebo given as four 0.2 mg tablets of lofexidine or placebo to be taken at 0800h, 1300h, 1800h, and 2300h for four days. On day 8, participants were medicated with 1.6 mg/day of lofexidine or placebo (dosed with 2 tablets of lofexidine and 2 tablets of placebo or 4 tablets of placebo at 0800h, 1300h, 1800h and 2300h). The lofexidine dosage was reduced by 50% to minimize the risk of rebound hypertension. The placebo group remained on placebo from days 4 to 8.
2.4.3. Post Detoxification/Medication Phase (days 9–11)
On days 9 and 10, all participants received placebo QID. Day 11 was the discharge day and no study drug was given. Participants also received a physical exam on day 11.
2.5. Assessments and Data Collection
As described in the study protocol, the primary outcome measure was the Modified Himmelsbach Opiate Withdrawal Scale (MHOWS) including pupil diameter measures (Jasinski, 1977). The MHOWS was selected as the primary outcome measure because it is an objective assessment of the severity of opioid withdrawal signs without reference to subjective symptoms. It is performed by a rater according to a quantitative continuous scale with weighted values for 12 specific discontinuous and continuous signs of withdrawal. The original form was developed by Kolb and Himmelsbach (Kolb and Himmelsbach, 1938) to quantify the severity of observable signs of opioid withdrawal in humans. MHOWS data were collected during a 10-minute observation period conducted two hours after the first administration of morphine or study medication on days 1–10. The staff was trained prior to study commencement to ensure good interrater reliability within and between sites.
To calculate an MHOWS score, the discontinuous and continuous signs of withdrawal per subject must each be assigned a weighted point value. The 8 discontinuous signs of withdrawal were assigned points in the following manner: Yawning: 1 point, Lacrimation: 1 point, Rhinorrhea: 1 point, Perspiration: 1 point, Tremor: 3 points, Gooseflesh: 3 points, Anorexia: 3 points if appetite is coded as poor or none for any meal that day, Restlessness: 5 points, and Emesis: 0 points if no emesis on that day, 5 points if one emesis on that day, 10 points if 2 episodes of emesis on that day, 15 points if the number of episodes of emesis is 3 or more. The total number of emesis episodes in a 24-hour period was recorded daily.
The five continuous signs of withdrawal were assigned points in the following manner: MHOWS morphine agonist baseline evaluations for pupil dilation, temperature, respiratory rate and systolic blood pressure were the average of days 1 and 2. The baseline for weight was day 2. These baseline values were compared to study days 3 through 10 and assigned point values for each day as follows: Pupil dilation: 1 point for each 0.1 mm increase in pupil size, Temperature: 1 point for each 0.1 Celsius degree rise, Respiration: 1 point for each respiration per minute increase, Systolic BP: 1 point for each 2 mm Hg rise (up to 30 mm), and Weight: 1 point for each pound lost. Pupil diameter was measured from pictures taken using a Polaroid camera with a specially adapted lens. A pair of digital calipers was used to measure pupil diameter. A lower score on the MHOWS indicates fewer or less severe objective withdrawal signs.
The secondary outcome measures in this study included both objective and subjective measures to assess opiate withdrawal severity. The intention of utilizing both types of measures was to capture the physical attributes as well as the affective aspects of opiate withdrawal. These secondary measures included: dropout day, MHOWS peak effect, the Objective Opiate Withdrawal Scale (OOWS) (Handelsman et al., 1987), the Short Opiate Withdrawal Scale (SOWS-Gossop) (Gossop, 1990), the Modified Clinical Global Impressions Scale (MCGI), both the subject and rater forms, the Subjective Opiate Withdrawal Scale (SOWS-Handelsman) (Handelsman et al, 1987), a visual analog scale assessing the efficacy of the study medication for decreasing withdrawal sickness (VAS-E), and the number of concomitant medications used to treat opiate withdrawal symptoms and signs.
Those participants who smoked were also assessed for tobacco withdrawal symptoms daily (Hughes and Hatsukami, 1986). Participants had the option of either using nicotine patches or periodic smoking breaks.
2.6. Statistical Considerations
The study's original sample size of 96 subjects (48 per group) was based on being able to detect an 8-unit difference between the treatment groups on the primary outcome measure, the MHOWS score on study day 5. This parameter was to be analyzed using analysis of variance (ANOVA) techniques. Estimates of the minimum clinically meaningful difference between treatment groups of 8 units and a pooled standard deviation of 11.1 units were based on the results of a previous Phase 1 dose-response pilot study (Yu et al., 1999). A significance level of 0.05, power of 90, two-tailed t-test and a 15% loss rate were assumed. This estimate of 15% dropout rate was based on prior pilot studies and the time period (5 inpatient days) that subjects needed to be in the study.
Secondary efficacy measures and the safety monitoring variables were analyzed first using ANOVA and then ANCOVA, where the baseline (study day 3) value for the particular parameter being analyzed was used as the covariate (Snedecor and Cochran, 1967). Differences between the treatment groups for the adverse events were analyzed using Fisher's exact test. Time to early termination was analyzed using Kaplan-Meier curves and log rank statistics (Kalbfleisch and Prentice, 1980). While a significance level of p=0.05 was used for the primary outcome measure, a p-value of 0.01 was used to determine significance for the secondary outcome measures to guard against reporting chance findings. P-values between 0.01 and 0.05 were considered to show a trend towards significance.
The Data and Safety Monitoring Board for the study met every 12 months to review study progress and to determine whether there were any unacceptable subject risks. A single interim analysis was planned in the protocol which was to be performed after approximately half of the expected subjects had completed the study. Using the O’Brien-Fleming method (O’Brien and Fleming, 1979), this interim analysis was performed at a nominal p-value of 0.0035, while the final analysis was to be performed at p=0.0488. This ensured an overall significance level of 0.05.
3. Results
3.1. Demographics
Demographics did not differ significantly between the lofexidine and placebo groups (Table 1). All subjects were heroin users with the exception of one placebo subject who used hydromorphone. In addition to heroin, one lofexidine subject also used hydromorphone and one placebo subject also used morphine. One subject had a negative opiate urine toxicology on admission since he did not use for two days. Another subject had a positive urine toxicology for methadone on admission, but negative on screening. On average, subjects used opiates 29 out of the 30 days prior to entering the study, and they had been using opiates for approximately the past 12 years. Subjects averaged 41 years of age, were white (56%), black (29%) or hispanic (15%), were mostly male (87%), averaged a high school education (12.7 years), and worked at least part-time (68%). There were no significant differences between the treatment groups, except for marital status which showed a trend towards significance with placebo subjects more likely to be divorced (p<0.05).
Table 1.
Baseline Demographics
| Demographic | Lofexidine (N = 35) |
Placebo (N = 33) |
Total (N = 68) |
P-value |
|---|---|---|---|---|
| Age at Randomization (Mean ± SD) | 42.0 ± 8.3 | 40.5 ± 10.2 | 41.3 ± 9.2 | 0.38 |
| Race (N, %) | ||||
| White | 21 (60.0) | 17 (51.5) | 38 (55.9) | 0.84 |
| Black | 9 (25.7) | 11 (33.3) | 20 (29.4) | |
| Native American | 0 (0) | 0 (0) | 0 (0) | |
| Asian/Pacific Islander | 0 (0) | 0 (0) | 0 (0) | |
| Hispanic | 5 (14.3) | 5 (15.2) | 10 (14.7) | |
| Sex (N, %) | ||||
| Male | 31 (88.6) | 28 (84.8) | 59 (86.8) | 0.73 |
| Female | 4 (11.4) | 5 (15.2) | 9 (13.2) | |
| Marital Status (N, %) | ||||
| Married | 8 (22.9) | 4 (12.1) | 12 (17.5) | 0.04 |
| Widowed | 2 (5.7) | 0 (0) | 2 (2.9) | |
| Separated | 5 (14.3) | 2 (6.1) | 7 (10.3) | |
| Divorced | 2 (5.7) | 10 (30.3) | 12 (17.7) | |
| Never Married | 18 (51.4) | 17 (51.5) | 35 (51.5) | |
| Yrs of Education Completed (Mean ± SD) | 12.7 ± 1.7 | 12.7 ± 2.8 | 12.7 ± 2.3 | 0.93 |
| Employment Pattern last 3 Yrs (N, %) | ||||
| Full-time | 11 (31.4) | 14 (42.4) | 25 (36.8) | 0.26 |
| Part-time, regular | 3 (8.6) | 2 (6.1) | 5 (7.4) | |
| Part-time, irregular | 11 (31.4) | 5 (15.2) | 16 (23.5) | |
| Student | 1 (2.9) | 1 (3.0) | 2 (2.9) | |
| Retired/disability | 0 (0) | 3 (9.1) | 3 (4.4) | |
| Unemployed | 7 (20.0) | 8 (24.2) | 15 (22.1) | |
| In controlled environment | 2 (5.7) | 0 (0) | 2 (2.9) | |
| Currently Smoking Cigarettes (%) | 82.9 | 84.9 | 83.8 | >0.99 |
| Urine Toxicology (% Positive) | ||||
| Amphetamines | 5.7 | 6.1 | 5.9 | >0.99 |
| Cocaine | 31.4 | 45.5 | 38.2 | 0.32 |
| Barbiturates | 2.9 | 3.0 | 2.9 | >0.99 |
| Opiates | 97.1 | 100.0 | 98.5 | >0.99 |
| Benzodiazepines | 8.6 | 9.1 | 8.8 | >0.99 |
| Cannabinoids | 25.7 | 24.2 | 25.0 | >0.99 |
| Methadone | 2.9 | 9.1 | 5.9 | 0.35 |
| Route of Opiate Administration(N, %) | ||||
| Oral/Nasal/Smoking | 11 (31.4) | 11 (33.3) | 22 (32.4) | >0.99 |
| IV | 24 (68.6) | 22 (66.7) | 46 (67.6) | |
| Days of Opiate Use in Last 30 Days(Mean ± SD) | 29.3 ± 1.7 | 29.1 ± 2.0 | 29.2 ± 1.8 | 0.57 |
| Years of Opiate Use (Mean ± SD) | 13.2 ± 8.3 | 11.4 ± 8.7 | 12.3 ± 8.5 | 0.39 |
3.2. Primary Outcome Measures
While reviewing the results of the single planned interim analyses which were done after 68 subjects completed treatment, the Data and Safety Monitoring Board stopped the study due to a significant difference in favor of lofexidine versus placebo on the study's primary outcome measure, the MHOWS. The Board believed that continuing subjects on placebo would not be ethical given these findings.
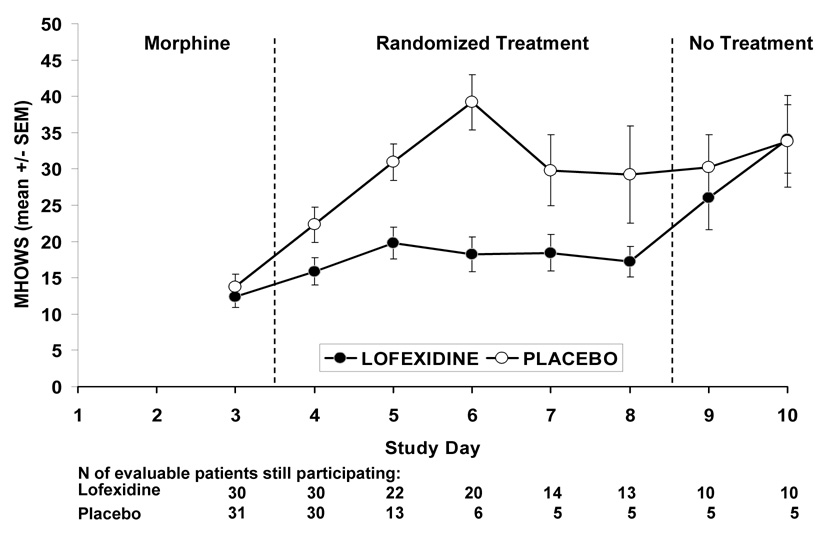
For the primary outcome measure of MHOWS scores on study day 5 (2nd opioid detoxification treatment day), lofexidine scores were significantly lower than placebo scores (LSM = 19.5 ± 2.1 versus 30.9 ± 2.7; p=0.0019). A confirmatory analysis of covariance (ANCOVA) using the study day 3 score as a baseline covariate also indicated significantly lower study day 5 MHOWS scores for lofexidine versus placebo subjects (p=0.0002) with a significance level well below the predetermined nominal p-value of 0.0035. Only 35 subjects with usable MHOWS scores were included in these analyses. Figure 2 shows the MHOWS scores over time by treatment group. The two groups were very similar at baseline (study day 3) with the peak difference occurring on study day 6. However, it should be noted that retention dropped off sharply in the placebo group after study day 5. In addition, Figure 2 also shows that the MHOWS scores for the lofexidine subjects increase to the level of the placebo subjects MHOWS scores after the drug is stopped indicating that lofexidine’s benefits do not continue after the drug is stopped.
Figure 2. Effects of placebo versus lofexidine on MHOWS scores.
Effects of placebo (original n=31) as compared with lofexidine (3.2 mg/day, original n=30) on MHOWS (Modified Himmelsbach Opiate Withdrawal Scale) scores for evaluable patients still participating on each study day. Shown are the three phases of the study: Morphine Baseline Phase (study day 1–3), Randomized Treatment Phase (study day 4–8), and No Treatment Phase (study day 9–11). MHOWS was the a priori primary outcome measure of the study.
3.3. Secondary Outcome Measures
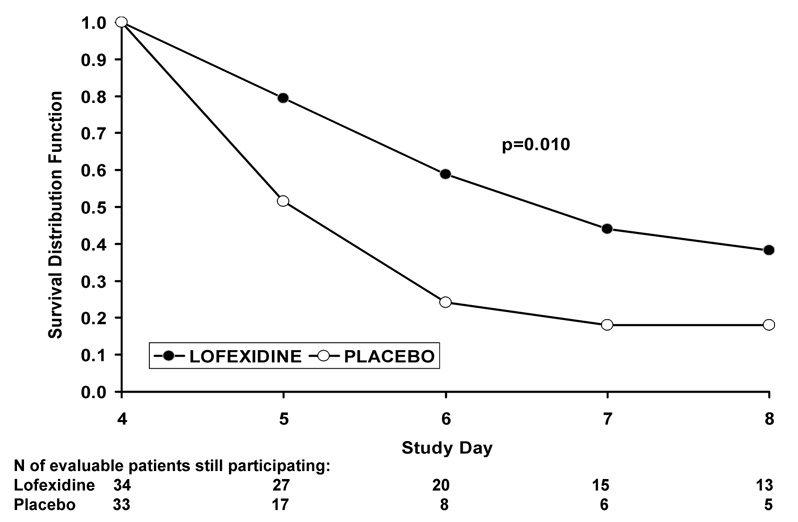
Time to early termination by treatment group is shown in Figure 3 as Kaplan-Meier curves. The lofexidine subjects had a trend towards significantly fewer (log rank test = 6.62, p=0.01) early terminations than the placebo subjects. The largest difference between the two groups occurs at study day 6, the third day of study treatment.
Figure 3. Effects of placebo versus lofexidine on subject retention.
Effects of placebo (original n=34) as compared with lofexidine (3.2 mg/day, original n=33) on retention in the study (drop out rate) for all patients still participating on day 4 at the start of the randomized treatment phase (days 4–8).
Table 2 summarizes the effects of lofexidine versus placebo on the secondary efficacy measures on study day 5, and on the peak scores during days 4 to 8. These results indicate that lofexidine versus placebo had trends toward decreased objective scores on the following measures: MHOWS (peak) lofexidine = 26.1 ± 2.0, placebo = 32.8 ± 2.4; OOWS (day 5) lofexidine = 2.4 ± 0.4, placebo = 4.1 ± 0.6; OOWS (peak) lofexidine = 3.3 ± 0.5, placebo = 4.7 ± 0.5 (p’s <0.05). In addition, the VAS-E (peak), a subjective scale of opiate withdrawal symptoms, showed that lofexidine relieved subjects’ withdrawal symptoms significantly more than placebo (55.5 ± 6.3 versus 27.7 ± 6.2; p<0.003). The following subjective symptom scales failed to show significant differences between lofexidine versus placebo: SOWS-Gossop, SOWS-Handelsman, MCGI-Subject, MCGI-Rater, and VAS-E (day 6). Finally, the lofexidine group was given a greater number of medications than the placebo group, although this effect failed to achieve significance (p=0.07).
Table 2.
Secondary Efficacy Measures during days 4 to 8
| Variable | Rating Period | Lofexidine | Placebo | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| n | x | SE | n | x | SE | |||
| MHOWS | Peak | 30 | 26.1 | 2.0 | 31 | 32.8 | 2.4 | 0.036 |
| OOWS | Day 5 | 24 | 2.4 | 0.4 | 16 | 4.1 | 0.6 | 0.026 |
| Peak | 30 | 3.3 | 0.5 | 31 | 4.7 | 0.5 | 0.048 | |
| SOWS-Gossop | Day 5 | 22 | 10.7 | 1.7 | 15 | 14.7 | 2.1 | 0.15 |
| Peak | 30 | 13.8 | 1.4 | 31 | 16.2 | 1.4 | 0.23 | |
| SOWS-Handelsman | Day 5 | 21 | 20.0 | 3.3 | 14 | 24.6 | 4.1 | 0.38 |
| Peak | 30 | 24.7 | 2.4 | 30 | 29.7 | 2.4 | 0.23 | |
| MCGI-Subject(Severity) | Day 5 | 22 | 3.5 | 0.4 | 15 | 4.3 | 0.5 | 0.20 |
| Peak | 30 | 4.4 | 0.3 | 31 | 4.5 | 0.3 | 0.67 | |
| MCGI-Rater(Severity) | Day 5 | 24 | 1.7 | 0.2 | 16 | 2.3 | 0.3 | 0.08 |
| Peak | 30 | 2.0 | 0.2 | 31 | 2.5 | 0.2 | 0.11 | |
| VAS-E | Day 6 | 19 | 59.7 | 7.5 | 7 | 32.1 | 12.3 | 0.07 |
| Peak | 30 | 55.5 | 6.3 | 31 | 27.7 | 6.2 | 0.003 | |
| # Concomitant Meds Given (All) | Days 4–8 | 30 | 3.9 | 0.6 | 31 | 2.2 | 0.6 | 0.07 |
3.4. Safety Monitoring Variables
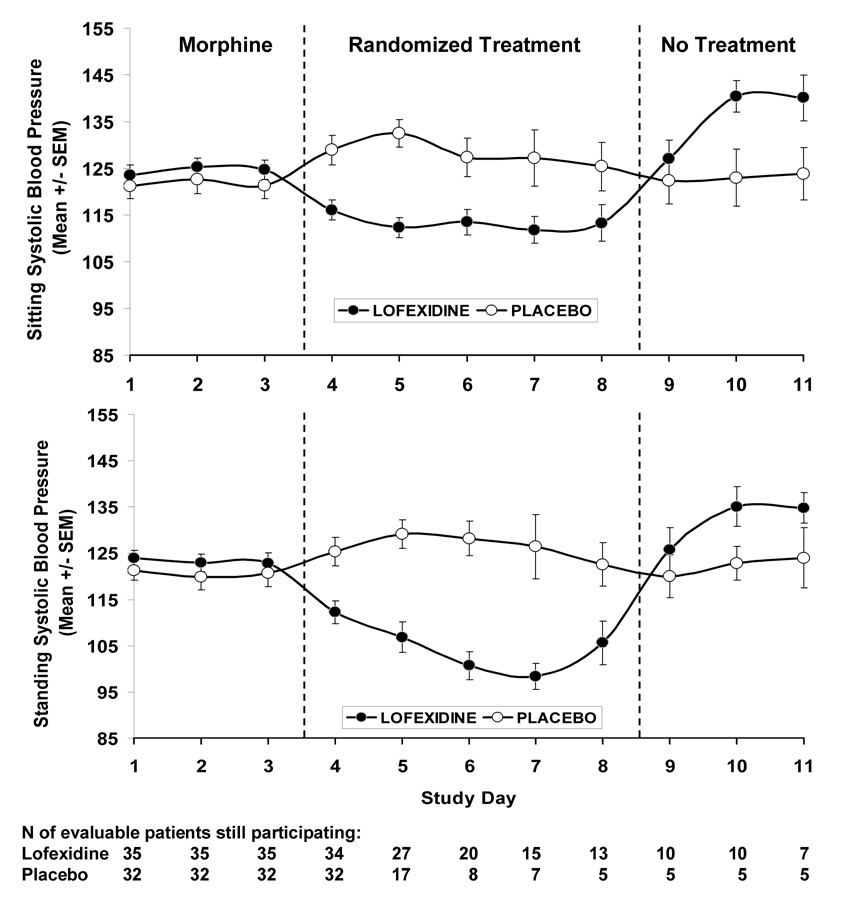
Systolic blood pressure and heart rate were considered safety monitoring variables and were measured daily. Figure 4 shows the daily systolic blood pressure measurements by treatment group for sitting and standing measurements, respectively. As shown in Figure 4, there was no statistically significant difference on sitting systolic blood pressure between the treatment groups at baseline (study day 3) (124.7 ± 2.0 versus 121.3 ± 2.7, p =0.31). However, for study days 4 (lofexidine 116.1 ± 2.2 versus placebo 128.9 ± 3.1, p=0.001), 5 (112.3 ± 2.1 versus 132.5 ± 2.9, p=0.001), 6 (113.5 ± 2.7 versus 127.3 ± 4.1, p=0.01), and 7 (111.8 ± 2.9 versus 127.2 ± 6.0, p=0.02), lofexidine either significantly decreased or had a trend towards decreasing blood pressure as compared with placebo. As shown, a similar result was also obtained for standing systolic blood pressure where systolic blood pressure decreased to about 95 mm Hg. Figure 4 shows that, after the treatment period is over, the lofexidine group experienced an elevation in systolic blood pressure compared to the placebo group. This indicates that lofexidine subjects may have had some rebound hypertension after treatment is stopped.
Figure 4. Effects of placebo versus lofexidine on sitting and standing systolic blood pressure.
Effects of placebo (original n=32) as compared with lofexidine (3.2 mg/day, original n=35) on sitting systolic and standing systolic blood pressure as a function of study day. Sitting systolic and standing systolic blood pressure were side effects variables in the study.
The effects of lofexidine on daily mean sitting and standing heart rates were analyzed. There was a trend towards significance (lofexidine 69.0 ± 1.8 versus placebo 74.6 ± 1.8, p=0.03) in the sitting heart rate at baseline with lofexidine subjects having lower baseline heart rates than placebo subjects. This difference persisted and appeared to increase over part of the treatment period with significant differences or a trend towards significance seen at study days 4 (lofexidine 70.1 ± 2.2 versus 79.9 ± 2.2, p=0.003), 5 (74.0 ± 2.8 versus 85.2 ± 4.0, p=0.02), and 7 (64.3 ± 2.9 versus 77.2 ± 5.7, p=0.04). ANCOVA using the baseline sitting heart rate as the covariate indicated a trend towards a lower sitting heart rate for lofexidine subjects at study day 4 (lofexidine 72.7 ± 1.4 versus 77.1 ± 1.4, p=0.03). Lofexidine subjects also had a trend towards significantly lower standing heart rates at study day 4 (82.7 ± 3.1 versus 92.7 ± 2.6, p=0.02) and 7 (79.6 ± 3.2 versus 91.5 ± 4.1, p=0.04) than placebo subjects. When an ANCOVA using baseline standing heart rate as the covariate was performed, there were no differences seen between treatment groups on any treatment study day for standing heart rate.
3.5. Adverse Effects
Table 3 illustrates the number of subjects experiencing any adverse event during study days 4 to 8 when study medication was administered. Out of 42 Costart-coded adverse events, four showed a trend towards a significant difference between lofexidine versus placebo subjects respectively: asthenia (loss of strength) 61% versus 32%, dizziness 39% versus 12%, hypotension (systolic < 80 mmHg) 18% versus 0%, and insomnia 79% versus 47% (p’s<0.05), while somnolence 42% versus 9% (p=0.002) was significant. It should be kept in mind that, due to the small sample size, only adverse events with large differences were able to be detected.
Table 3.
Number of subjects experiencing any Adverse Event during study days 4 to 8
| Adverse Event Costart Term | Lofexidine (N=33) |
Placebo (N=34) |
Total (N=67) |
Fisher’s p-value |
|---|---|---|---|---|
| N (%) of subjects | ||||
| Anorexia | 12 (36%) | 11 (32%) | 23 (34%) | 0.80 |
| Anxiety | 11 (33%) | 12 (35%) | 23 (34%) | >0.99 |
| Arthralgia | 5 (15%) | 3 (9%) | 8 (12%) | 0.48 |
| Asthenia | 20 (61%) | 11 (32%) | 31 (46%) | 0.028 |
| Bradycardia | 1 (3%) | 1 (3%) | 2 (3%) | >0.99 |
| Bradycardia sinus | 0 (0%) | 1 (3%) | 1 (1%) | >0.99 |
| Chills | 4 (12%) | 4 (12%) | 8 (12%) | >0.99 |
| Constipation | 4 (12%) | 3 (9%) | 7 (10%) | 0.71 |
| Cramps leg | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Depression psychotic | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Diarrhea | 10 (30%) | 9 (26%) | 19 (28%) | 0.79 |
| Dizziness | 13 (39%) | 4 (12%) | 17 (25%) | 0.012 |
| Dry mouth | 6 (18%) | 2 (6%) | 8 (12%) | 0.15 |
| Dyspepsia | 8 (24%) | 7 (21%) | 15 (22%) | 0.78 |
| GI disease | 1 (3%) | 1 (3%) | 2 (3%) | >0.99 |
| Hair disease | 0 (0%) | 2 (6%) | 2 (3%) | 0.49 |
| Headache | 8 (24%) | 4 (12%) | 12 (18%) | 0.22 |
| Hyperglycemia | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Hypertension | 1 (3%) | 2 (6%) | 3 (4%) | >0.99 |
| Hypotension (systolic blood pressure <80mmHg) | 6 (18%) | 0 (0%) | 6 (9%) | 0.011 |
| Injury acid | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Insomnia | 26 (79%) | 16 (47%) | 42 (63%) | 0.011 |
| Lacrimation dis | 7 (21%) | 5 (15%) | 12 (18%) | 0.54 |
| Myalgia | 16 (48%) | 19 (56%) | 35 (52%) | 0.63 |
| Nausea | 10 (30%) | 15 (44%) | 25 (37%) | 0.31 |
| Nervousness | 8 (24%) | 9 (26%) | 17 (25%) | >0.99 |
| Pain | 1 (3%) | 1 (3%) | 2 (3%) | >0.99 |
| Pain abdominal | 9 (27%) | 14 (41%) | 23 (34%) | 0.31 |
| Paresthesia | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Pruritus | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Rash | 0 (0%) | 1 (3%) | 1 (1%) | >0.99 |
| Rhinitis | 17 (52%) | 20 (59%) | 37 (55%) | 0.63 |
| Somnolence | 14 (42%) | 3 (9%) | 17 (25%) | 0.002 |
| Sweat | 12 (36%) | 13 (38%) | 25 (37%) | >0.99 |
| Syncope | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Tachycardia | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Tremor | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Twitch | 2 (6%) | 0 (0%) | 2 (3%) | 0.24 |
| Vasodilation | 3 (9%) | 0 (0%) | 3 (4%) | 0.11 |
| Vertigo | 1 (3%) | 0 (0%) | 1 (1%) | 0.49 |
| Vomit | 5 (15%) | 11 (32%) | 16 (24%) | 0.15 |
The most serious side effect associated with hypotension is syncope. There was one incidence of syncope in the lofexidine group and none in the placebo group. In addition, there was one incidence of vertigo in the lofexidine group and none in the placebo group. Insomnia was the most frequent adverse event associated with lofexidine (p<0.05), however the high rate of this symptom in the placebo group suggests that at least a portion of this effect is attributable to a placebo effect.
4. Discussion
This is the first published placebo-controlled (PC), double-blind (DB), randomized clinical trial (RCT) evaluating the tolerability and the efficacy of lofexidine in the treatment of opioid withdrawal symptoms. The study demonstrates that lofexidine (3.2 mg/day, p.o.) compared with placebo significantly decreases the signs and symptoms of opioid withdrawal in opioid dependent individuals in an inpatient setting. Moreover, the retention of the lofexidine subjects was significantly greater than the retention of the placebo subjects during treatment. Lofexidine decreased both systolic and diastolic blood pressure, and one lofexidine treated subject had a syncopal episode.
Were the decreases in MHOWS scores related to or independent of the hypotensive effects of lofexidine? Since lofexidine induced significant reductions in both sitting and standing blood pressure, the two previously mentioned effects may be inextricably related. Aside from the present study, there have been a total of six controlled studies comparing lofexidine to clonidine or to methadone (Carnwath and Hardman, 1998; Lin et al., 1997; Howells et al., 2002; Bearn et al., 1996; Gowing et al., 2002; Bearn et al., 1998). All of these studies were RCTs, with one being an open trial (Bearn et al., 1998). The first study was a double-blind RCT comparing lofexidine with methadone as a detoxification agent (Bearn et al., 1996). Results of this study indicated that lofexidine was significantly less efficacious than methadone in decreasing opioid withdrawal symptoms during days 3 to 10, but thereafter both groups showed a similar decline. Finally, there were no significant differences between lofexidine versus methadone on daily systolic or diastolic blood pressure. Another double-blind RCT comparing lofexidine with methadone (Howells et al. 2002) found no statistical difference between the severity of withdrawal and also no difference in sitting blood pressures between lofexidine and methadone. An open study by Bearn (Bearn et al., 1998) compared an accelerated 5-day lofexidine regimen with a 10-day lofexidine and methadone treatment for opioid withdrawal in 61 polysubstance-abusing opioid addicts. The authors of this study concluded that an accelerated 5-day lofexidine regimen attenuates withdrawal symptoms significantly more rapidly than conventional 10-day lofexidine or methadone treatment schedules. Subjects in both lofexidine groups could receive a maximum of 2.4 mg/d and there was no significant systolic or diastolic blood pressure difference between either lofexidine group. Five lofexidine subjects did experience symptoms of decreased blood pressure that resolved with dose reduction. Similar symptoms of decreased blood pressure were seen in our current study that resolved with the reduction in lofexidine dose.
The other three studies compared the efficacy and tolerability of lofexidine to clonidine, another alpha-2-adrenergic agonist. Kahn (Kahn et al., 1997) conducted a double-blind study showing that lofexidine versus clonidine was associated with similar withdrawal symptoms from methadone. However, the overall frequency of postural hypotension was significantly lower with lofexidine than clonidine. Using a RCT, double-blind design, Carnworth (Carnworth and Hardman, 1998) compared the degree of withdrawal in subjects detoxifying from methadone that were treated with lofexidine versus clonidine. These medications were found to be broadly equivalent on the SOWS (Short Opiate Withdrawal Scale), while lofexidine had significantly less hypotensive effects in comparison to clonidine. Finally, a third study by Lin (Lin et al., 1997) reported on results of a randomized double-blind comparison of lofexidine (1.6 mg/day) versus clonidine (0.6 mg/day) in the treatment of heroin withdrawal. Both medications were similarly efficacious in the treatment of opioid withdrawal symptoms. In contrast, significantly more hypotensive problems were associated with clonidine than lofexidine. Thus, while indicating the broad equivalency of lofexidine versus clonidine in the treatment of withdrawal symptoms, these three studies showed the significant advantage of lofexidine versus clonidine in decreasing hypotensive effects.
These results are consistent with the overall review of this area by Gowing (Gowing et al., 2002) in suggesting that the efficacy of lofexidine is in general comparable to that of clonidine. Direct comparisons of clonidine with lofexidine suggest that both alpha-2-adrenergic agonists are effective in reducing opioid withdrawal symptoms in humans, and that lofexidine would appear to have a slight advantage of producing a smaller hypotensive effect than clonidine. Clonidine given chronically as an antihypertensive has shown rebound hypertension upon abrupt termination and a taper is recommended. Since lofexidine is also aalpha-2-adrenergic agonist, a taper should also be considered after chronic administration to prevent the possibility of rebound hypertension. Rebound hypertension has not been demonstrated to be a problem with lofexidine detoxifications in the United Kingdom (Akhurst, 1999).
Buprenorphine and lofexidine have been compared in an open-label RCT by Raistrick (Raistrick et al., 2005). Buprenorphine was found to be at least as effective as lofexidine detoxification in 210 subjects. In an open-label comparison of lofexidine and buprenorphine (White et al., 2001), it was found that buprenorphine subjects had a less severe withdrawal syndrome in 69 subjects.
There was good agreement between the results of lofexidine on the primary outcome measures (MHOWS) and the results on retention and the other secondary outcome measures that are frequently used by other investigators in the assessment of the potency of a medication on the alleviation of opiate withdrawal symptoms (Table 2). As shown, over the entire expanse of medication treatment days, lofexidine significantly alleviated opiate withdrawal signs as assessed by the MHOWS, the OOWS (both objective withdrawal measures), and the VAS-E (peak), which is a subjective opiate withdrawal scale. However, other subjective scales (SOWS-Gossop, SOWS-Handelsman, MCGI-Subject, and MCGI-Rater) failed to show a significant treatment difference probably indicating the lack of relative sensitivity of these tests. This broadens our findings concerning the efficacy of lofexidine in significantly decreasing MHOWS scores (an objective measure) to those of the VAS-E (a subjective measure), providing evidence of the robust efficacy of this medication in the alleviation of opiate withdrawal symptoms.
It should be noted that only 35 of the 68 randomized subjects with usable MHOWS scores were included in the primary outcome measure analyses. In addition, there was a differential loss rate for the treatment groups. With the assumption that losses were due to subjects doing poorly, the study day 5 MHOWS scores given are most likely underestimating the true scores that subjects can expect to have on day 5 (the second day of the detoxification/medication phase). The reported day 5 MHOWS scores would only apply to subjects who can be maintained on treatment until day 5. The differential loss rate, with fewer placebo subjects being retained until day 5, would indicate that the difference noted between the lofexidine and placebo groups is probably larger than indicated. Finally, the smaller than planned recruitment (68 instead of 96) and the larger than expected drop-out rate have resulted in reduced statistical power to find differences in the baseline characteristics, secondary outcome measures, safety data, and adverse events.
As previously noted, there were relatively few adverse effects (AEs) reported in this study in subjects receiving lofexidine or placebo (Table 3) although the small size and the use of a p = 0.01 significance level has most likely made the detection of some adverse effects unlikely. This speaks well for the relative safety and tolerability of lofexidine when administered to heroin addicts undergoing opiate detoxification with lofexidine. As with any alpha-adrenergic agonist, physicians should be aware of the side effects including orthostatic hypotension and syncope and insure that the subject is well hydrated when lofexidine is initiated. If subjects demonstrate orthostatic changes, then the dose should be decreased.
As conceptualized by Herman (Herman et al., 1995), there are three primary medication treatment phases for opiate addiction. These phases are 1) the short-term detoxification phase, 2) the longer-lasting opioid maintenance phase, and 3) the opioid relapse prevention therapy phase. It is the combination of detoxification, maintenance and relapse treatment that represents the ideal program of treatment for opiate addiction. However, there is a paucity of medications that are approved by the FDA for treating detoxification or relapse prevention, especially non-opioid treatments. As a detoxification agent, lofexidine would represent a considerable advance over other detoxification medications currently approved for this use (e.g. methadone and buprenorphine) because it is not a narcotic and is not considered to be an addictive drug. This would offer the opportunity for patients to safely self-medicate with lofexidine on an outpatient basis per the instructions of their physician rather than in an outpatient clinic setting.
Our study adds to the existing literature on the tolerability and efficacy of lofexidine as a detoxification agent for opioid addiction. Using an objective scale of opioid withdrawal measures (MHOWS), significant reductions in withdrawal signs were obtained with lofexidine (dose = 3.2 mg/day) versus placebo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK. Central noradrenergic neurons: a locus for the functional interplay between alpha-2 adrenoceptors and opiate receptors. J Clin Psychiatry. 1982;43(6 Pt 2):20–24. [PubMed] [Google Scholar]
- Aghajanian GK. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276(5684):186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Aigner A, Schmidt U. Lofexidine, a new, antihypertensive imidazoline derivative. Clinical profile of action with single-drug treatment and in combination with hydrochlorothiazide. Arzneimittelforschung. 1982;32(8a):976–983. [PubMed] [Google Scholar]
- Akhurst JS. The use of lofexidine by drug dependency units in the United Kingdom. Eur Addict Res. 1999;5(1):43–49. doi: 10.1159/000018962. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J. Neurosci. 1998;8(11):4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Hirata H, Akaoka H. Local opiate withdrawal in locus coeruleus in vivo. Brain Res. 1997;765(2):331–336. doi: 10.1016/s0006-8993(97)00682-3. [DOI] [PubMed] [Google Scholar]
- Bearn J, Gossop M, Strang J. Randomised double-blind comparison of lofexidine and methadone in the in-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1996;43(1–2):87–91. doi: 10.1016/s0376-8716(96)01289-6. [DOI] [PubMed] [Google Scholar]
- Bearn J, Gossop M, Strang J. Accelerated lofexidine treatment regimen compared with conventional lofexidine and methadone treatment for in-patient opiate detoxification. Drug Alcohol Depend. 1998;50:227–232. doi: 10.1016/s0376-8716(98)00030-1. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Marshall DC. Dorsal root lesions block the expression of morphine withdrawal elicited from the rat spinal cord. Neurosci Lett. 1985;59(3):319–324. doi: 10.1016/0304-3940(85)90152-1. [DOI] [PubMed] [Google Scholar]
- Carnwath T, Hardman J. Randomised double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251–254. doi: 10.1016/s0376-8716(98)00040-4. [DOI] [PubMed] [Google Scholar]
- Freedman JE, Aghajanian GK. Opiate and alpha 2-adrenoceptor responses of rat amygdaloid neurons: co-localization and interactions during withdrawal. J Neurosci. 1985;5(11):3016–3024. doi: 10.1523/JNEUROSCI.05-11-03016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Pottash AC, Sweeney DR, Extein I, Annitto WJ. Opiate detoxification with lofexidine. Drug Alcohol Depend. 1981;8(4):307–315. doi: 10.1016/0376-8716(81)90040-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Expert Opin Pharmacother. 2004;5(4):713–725. doi: 10.1517/14656566.5.4.713. [DOI] [PubMed] [Google Scholar]
- Gossop M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug Alcohol Depend. 1988;21(3):253–259. doi: 10.1016/0376-8716(88)90078-6. [DOI] [PubMed] [Google Scholar]
- Gossop M. The development of a short opiate withdrawal scale. Addict Behav. 1990;15(5):487–490. doi: 10.1016/0306-4603(90)90036-w. [DOI] [PubMed] [Google Scholar]
- Gowing LR, Farrell M, Ali RL, White JM. Alpha 2-adrenergic agonists in opioid withdrawal. Addiction. 2002;97(1):49–58. doi: 10.1046/j.1360-0443.2002.00037.x. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Ali R, White J. Alpha2 adrenergic agonists for the management of opioid withdrawal. The Cochrane Database of Systematic Reviews CD002024. 2004 doi: 10.1002/14651858.CD002024.pub2. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13(3):293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Herman BH, Vocci F, Bridge P. The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal: medication development issues for opiate addiction. Neuropsychopharmacology. 1995;13:269–294. doi: 10.1016/0893-133X(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Herman BH, O'Brien CP. Clinical medications development for opiate addiction: Focus on nonopioids and opioid antagonists for the amelioration of opiate withdrawal symptoms and relapse prevention. Seminars in Neuroscience. 1997;9:158–172. [Google Scholar]
- Howells C, Allen S, Gupta J, Stillwell G, Marsden J, Farrell M. Prison based detoxification for opioid dependence: a randomised double blind controlled trial of lofexidine and methadone. Drug Alcohol Depend. 2002;67(2):169–176. doi: 10.1016/s0376-8716(02)00024-8. [DOI] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry. 2001;58(5):503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jarrott B, Louis WJ, Summers RJ. Characterization of central alpha-adrenoceptors using 3H-clonidine and its derivatives. Chest. 1983;83 Suppl 2:S339–S340. [PubMed] [Google Scholar]
- Jasinski DR. Assessment of the abuse potentiality of morphine-like drugs (methods used in man) In: Martin WR, editor. Handbook of Experimental Pharmacology. Vol 45. Berlin: Springer-Verlag; 1977. pp. 197–258. [Google Scholar]
- Kahn A, Mumford JP, Rogers GA, Beckford H. Double-blind study of lofexidine and clonidine in the detoxification of opiate addicts in hospital. Drug Alcohol Depend. 1997;44(1):57–61. doi: 10.1016/s0376-8716(96)01316-6. [DOI] [PubMed] [Google Scholar]
- Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons, Inc.; 1980. [Google Scholar]
- Kleber HD, Riordan CE, Rounsaville B, Kosten T, Charney D, Gaspari J, Hogan I, O'Connor C. Clonidine in outpatient detoxification from methadone maintenance. Arch Gen Psychiatry. 1985;42(4):391–394. doi: 10.1001/archpsyc.1985.01790270081009. [DOI] [PubMed] [Google Scholar]
- Kolb MR, Himmelsbach CK. Clinical studies of drug addiction. III. A critical review of the withdrawal treatments with method of evaluating abstinence syndromes. Amer. J. Psychiatry. 1938;94:759–797. [Google Scholar]
- Lin SK, Strang J, Su LW, Tsai CJ, Hu WH. Double-blind randomised controlled trial of lofexidine versus clonidine in the treatment of heroin withdrawal. Drug Alcohol Depend. 1997;48(2):127–133. doi: 10.1016/s0376-8716(97)00116-6. [DOI] [PubMed] [Google Scholar]
- Marjamaki A, Luomala K, Ala-Uotila S, Scheinen M. Use of recombinant human alpha 2-adrenoceptors to characterize subtype selectively of antagonist binding. Eur J Pharmacol. 1993;246(3):219–226. doi: 10.1016/0922-4106(93)90034-7. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health. [accessed on April 10, 2008];2006 doi: 10.1177/0886260513506279. Availabel at: http://www.oas.samhsa.gov/nsduh/2k6nsduh/2k6Results.cfm. [DOI] [PMC free article] [PubMed]
- O’Brien PC, Fleming TR. A multiple testing procedure for clinical trails. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- Office of National Drug Control Policy. Heroin Fact Sheet. [Accessed February 6, 2006];2003 Available at: http://www.whitehousedrugpolicy.gov/publications/factsht/heroin/index.html.
- Preston KL, Bigelow GE. Pharmacological advances in addiction treatment. Int J Addict. 1985;20(6–7):845–867. doi: 10.3109/10826088509047756. [DOI] [PubMed] [Google Scholar]
- Raistrick D, West D, Finnegan O, Thistlethwaite G, Brearley R, Banbery J. A comparison of buprenorphine annd lofexidine for community opiate detoxification: results from a randomized controlled trail. Addict. 2005;100(12):1860–1867. doi: 10.1111/j.1360-0443.2005.01273.x. [DOI] [PubMed] [Google Scholar]
- Shearman GT, Lal H, Ursillo RC. Effectiveness of lofexidine in blocking morphine-withdrawal signs in the rat. Pharmacol Biochem Behav. 1980;12(4):573–575. doi: 10.1016/0091-3057(80)90191-4. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, IA: Iowa State University Press; 1967. [Google Scholar]
- Substance Abuse and Mental Health Data Archive. [Accessed February 6, 2006];National Household Survey on Drug Abuse (NHSDA) series. 2003 Available at http://www.icpsr.umich.edu/SAMHDA/ and http://webapp.icpsr.umich.edu/cocoon/SAMHDA-SERIES/00064.xml.
- Uhlen S, Wikberg JE. Delineation of three pharmacological subtypes of alpha 2-adrenoceptor in the rat kidney. Br J Pharmacol. 1991;104(3):657–6419. doi: 10.1111/j.1476-5381.1991.tb12485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ. Opiates and addiction. In: Sibley DR, Hanin I, Kuhar M, Skolnick P, editors. Handbook of Contemporary Neuropharmacology Volume 2. John Wiley and Sons publisher; 2007. pp. 691–705. [Google Scholar]
- Washton AM, Resnick RB, Perzel JF, Garwood J. Opiate detoxification using lofexidine. NIDA Res Monogr. 1982;41:261–263. [PubMed] [Google Scholar]
- White R, Alcorn R, Feinmann C. Two methods of community detoxification from opiates: an open-label comparison of lofexidine and buprenorphine. Drug Alcohol Depend. 2001;65(1):77–83. doi: 10.1016/s0376-8716(01)00149-1. [DOI] [PubMed] [Google Scholar]
- Yu E, Herman BH, Miotto K, Montgomery A, Fudala PJ, Chiang CN, Fisher C, Kampman K, Dhopesh V, Cornish J, Walsh B, Davies K, Vocci F, Bridge P, Ling W, O’Brien CP. NIDA Research Monograph. Rockville: DHHS/NIH/NIDA; 1999. In-patient safety evaluation of lofexidine (alpha-2 adrenergic agonist) for opiate withdrawal; p. 227. [Google Scholar]
- Yu E, Herman BH, Miotto K, Montgomery A, Fudala PJ, Chiang CN, Fisher C, Kampman K, Dhopesh V, Cornish J, Walsh B, Davies K, Vocci F, Bridge P, Ling W, O’Brien CP. In-patient safety evaluation of lofexidine (alpha-2 adrenergic agonist) for opiate detoxification. Drug Alcohol Depend. 2001;63:S175. [Google Scholar]