Abstract
Chronic pain has been traditionally defined by pain duration, but this approach has limited empirical support; and does not account for chronic pain’s multidimensionality. This study compared duration-based and prospective approaches to defining chronic pain in terms of their ability to predict future pain course and outcomes for primary care patients with three common pain conditions: back pain (n = 971), headache (n = 1078), or orofacial pain (n = 455). At baseline, their chronic pain was classified retrospectively based on Pain Days in the prior six months and prospectively with a prognostic Risk Score identifying patients with “possible” or “probable” chronic pain. The 0–28 Risk Score was based on pain intensity, pain-related activity limitations, depressive symptoms, number of pain sites, and Pain Days. Pain and behavioral outcomes were assessed at six-month follow-up, and long-term opioid use was assessed two to five years after baseline. Risk Score consistently predicted clinically significant pain at six months better than did Pain Days alone (Area under the Curve of 0.74–0.78 for Risk Score vs. 0.63–0.73 for Pain Days). Risk Score was a stronger predictor of future SF-36 Physical Function, pain-related worry, unemployment, and long-term opioid use than Pain Days alone. Thus, for these three common pain conditions, a prognostic Risk Score had better predictive validity for pain outcomes than did pain duration alone. However, chronic pain appears to be a continuum rather than a distinct class, because long-term pain outcomes are highly variable and inherently uncertain.
Keywords: chronic pain, back pain, headache, orofacial pain, classification, epidemiology
1. Introduction
Patients seeking care for pain want to know whether their pain is likely to improve or run a chronic course, not only its cause and how it might be relieved and managed (13,24). Physicians’ abilities to provide guidance regarding pain’s likely course, as well as clinical and epidemiologic research, are hampered by lack of clear-cut, evidence-based operational criteria for classifying chronic pain (2,6,23,26).
The International Association for the Study of Pain defines chronic pain as: “…pain which persists past the normal time of healing…With nonmalignant pain, three months is the most convenient point of division between acute and chronic pain, but for research purposes six months will often be preferred (19).” Defining chronic pain solely by duration is based on the view that acute pain signals potential tissue damage, whereas chronic pain results from central and peripheral sensitization in which pain is sustained after nociceptive inputs have diminished (4). While conceptually appealing, this approach has not produced reliable or valid methods for differentiating acute from chronic pain for clinical or epidemiological research, nor has it led to practical operational criteria for identifying chronic pain in clinical practice. Defining chronic pain solely by duration does not indicate whether long-lasting pain is clinically significant, and duration-based definitions can be difficult to apply to recurrent pain (26). This approach is also contrary to the view that chronic pain is multi-dimensional (27). While several methods of assessing pain duration have been proposed (9,20,32), they have not been extensively evaluated. For these reasons, how chronic pain is defined and assessed merits reconsideration.
The term chronic pain is as much a prognostic statement as a description of pain history (32). Von Korff and Miglioretti (33) recently proposed defining chronic back pain prospectively, using a risk score to predict the likelihood that clinically significant pain is present at a future time point. A multivariate risk score was shown to predict probabilities of clinically significant back pain being present one to five years later. Based on these results, probable chronic back pain was defined by a risk score associated with ≥80% probability of having significant back pain at a future time. Possible chronic back pain was defined by a risk score predicting ≥50% probability of future significant pain (33). Dunn et al. subsequently replicated these results (8).
This paper extends prior work on a prospective approach to defining chronic back pain by comparing the predictive validity of a classification based on a risk score to one based on pain duration alone. Specifically, we directly compare prediction of pain and behavioral outcomes by a Risk Score (both including and excluding pain duration) to prediction of pain and behavioral outcomes by a measure of pain duration (pain days in the prior six months). To determine whether comparable predictive validity is observed across diverse pain conditions, we assessed the generalizability of using this prospective risk score among headache and orofacial pain patients, and we replicated the prospective approach in a new sample of back pain patients.
2. Methods
2.1. Setting and participants
This research was carried out at Group Health Cooperative, an integrated health plan in the Pacific Northwest. It was approved by Group Health’s Institutional Review Board. In Seattle area clinics, consecutive back pain, headache, and orofacial pain patients seeking care in 1994 to 1995 were eligible for enrollment. Following informed consent, patients age 18–75 completed a survey by either mail or telephone 2–4 weeks after their visit. Six months later, patients completing the baseline interview were re-interviewed by telephone.
The sample of back pain patients included in the analyses reported in this paper are a completely new and independent set of back pain patients from those included in the studies in which the chronic pain Risk Score was originally developed and evaluated (33) and from the back pain patients in the replication sample reported by Dunn et al. (8). While the study setting (Group Health primary care clinics) and the study measures are the same as the initial study (33), this research applies the chronic pain Risk Score to an entirely new set of back pain patients, as well as to new samples of headache and orofacial pain patients not assessed in prior research.
2.2. Questionnaire data
Pain status was assessed using the Chronic Pain Grade scale, which includes: 0–10 ratings of average, worst, and present pain intensity; 0–10 ratings of interference with usual activities, work/housework activities, and family/social activities; and days unable to carry out usual activities due to pain in the prior three months (34).
Pain duration was assessed by a question about Pain Days for the index pain in the prior six months (34,36). Patients were asked, “On about how many days have you had back/headache/facial pain in the last six months?” Although validity of recall of days with pain has not been extensively studied, several studies have shown a high correlation between retrospective recall and diary-based counts of days with pain, including a diary study covering a three month recall period which reported a correlation of 0.67 between retrospective recall and daily diary data (36).
We counted the number of pain conditions at anatomical sites where chronic pain is commonly reported, in addition to the index pain condition. In this research, we asked about five common pain conditions, including back, headache, chest, stomach, and orofacial pain. This limited set of pain conditions is not comprehensive and might be expanded in future research.
Depressive symptoms were evaluated using the Symptom Checklist-90-R (SCL-90-R) depression scale (7). To assess the patient’s level of concern about their pain condition, they were asked to rate worry about their pain condition on a 0–10 scale, where 0 was not at all worried and 10 extremely worried. Pain patients who make frequent health care visits have been found to have higher worry ratings than those who use health care infrequently (31). Disability was assessed by the Short Form-36 (SF-36) Physical Function subscale, on which lower scores indicate greater disability (38). Respondents were also asked if they were working, attending school, keeping house, retired, unemployed, or unable to work. Unemployment was then defined as being unemployed or unable to work, excluding persons who were retired or keeping house.
2.3. Opioid use
For study patients who remained enrolled in GHC for ≥270 days during 1997–1999, use of opioid analgesics was determined from GHC automated pharmacy data. Prior research has established that GHC data provide a high level of completeness for ascertaining use of prescription medications (25). Long-term use of opioids was defined as at least one episode of opioid use lasting ≥90 days with ≥10 prescriptions, ≥120 days supply of opioids dispensed, or both.
2.4. Prognostic variables
Based on our prior work (8,33), we estimated a 0– 28 Risk Score based on the following prognostic variables: pain intensity, interference, and activity limitation day ratings from the Graded Chronic Pain scale; SCL-90-R depression scale; Pain Days in the prior six months; and number of pain conditions at sites other than the index pain condition. We compared the prognostic value of the Risk Score to that of Pain Days alone.
2.5. Risk Score calculation
We calculated the Risk Score using scoring methods we developed and validated in prior research (8,33). As Table 1 shows, we calculated the Risk Score based on points allocated to item scores. Using this Risk Score, we defined possible chronic pain as a score of 16–21 and probable chronic pain as a score of 22–28. For some analyses, we calculated the Risk Score without including information concerning Pain Days.
Table 1.
Scoring values for prognostic chronic pain Risk Score (0–28)
| Item | Item value | Risk Score value* | |
|---|---|---|---|
| Average pain intensity (0–10 rating) | 0–3 | 0 | |
| 4–6 | 1 | ||
| 7–10 | 2 | ||
| Worst pain intensity (0–10 rating) | 0–4 | 0 | |
| 5–7 | 1 | ||
| 8–10 | 2 | ||
| Current pain intensity (0–10 rating) | 0–2 | 0 | |
| 3–4 | 1 | ||
| 5–10 | 2 | ||
| Interference with usual activities (0–10 rating) | 0–2 | 0 | |
| 3–4 | 1 | ||
| 5–10 | 2 | ||
| Interference with work/household activities (0–10 rating) | 0–2 | 0 | |
| 3–4 | 1 | ||
| 5–10 | 2 | ||
| Interference with family/social activities (0–10 rating) | 0–2 | 0 | |
| 3–4 | 1 | ||
| 5–10 | 2 | ||
| Days of activity limitation due to pain in prior three months | 0–2 | 0 | |
| 3–6 | 1 | ||
| 7–15 | 2 | ||
| 16–24 | 3 | ||
| 25–90 | 4 | ||
| SCL-90-R† Depression score | <0.50 | 0 | |
| 0.50–<1.0 | 1 | ||
| 1.0–<1.5 | 2 | ||
| 1.5–<2.0 | 3 | ||
| 2.0–4.0 | 4 | ||
| Number of other pain sites | 0 | 0 | |
| 1 | 1 | ||
| 2 | 2 | ||
| 3 | 3 | ||
| 4 | 4 | ||
| Number of days with index pain in prior six months | 0–30 | 0 | |
| 31–89 | 1 | ||
| 90–120 | 2 | ||
| 121–160 | 3 | ||
| 161–180 | 4 | ||
| Total Risk Score | 0–28 | ||
Risk Score values are summed across items.
Symptom Checklist-90-R.
This Risk Score algorithm has been placed in the Creative Commons and may be used without the authors’ permission with attribution.
2.6. Classification of chronic pain based on Pain Days
We divided patients into three groups based on Pain Days in the prior six months. Those reporting <90 Pain Days were classified as not having chronic pain. Those reporting ≥90 Pain Days in the prior six months were divided into two chronic pain groups: 90–149 Pain Days, and ≥150 Pain Days. For each of the three pain conditions, most in the latter group reported 180 Pain Days in the prior six months.
2.7. Clinically significant pain at follow-up
As in our prior research (8,33), we defined clinically significant pain at follow-up by Chronic Pain Grades 2, 3, or 4. We chose Chronic Pain Grades 2–4 as the criterion for clinically significant pain because these grades identify persons with intense pain generally accompanied by clinically significant pain dysfunction, whereas persons with Grade 1 pain have low levels of pain and dysfunction (34).
2.8. Analytic methods
We assessed agreement of the classification of chronic pain based on pain duration alone and the classification based on Risk Score. We measured the level of agreement of the 3×3 table using the weighted kappa statistic (17). We then assessed the ability of Risk Score and Pain Days to predict the percentage of patients with Grade 2–4 pain at six-month follow-up. The area under the curve (AUC) for predicting Grade 2–4 pain at follow-up was estimated from the C-statistic of a logistic regression model (21). Variables that perform no better than chance have an AUC of 0.50, whereas perfect prediction would yield an AUC of 1.0. Using logistic regression, we also assessed the extent to which the Risk Score calculated without Pain Days improved prediction of clinically significant pain at follow-up above and beyond prediction with Pain Days alone.
To assess prediction of diverse psychological and behavioral outcomes in addition to clinically significant pain, we examined prediction of three indicators at six months: SF-36 Physical Function score; ratings of worry about the pain condition; and percentage unemployed. We also assessed prediction of long-term use of opioid medications 2–5 years after the index visit. Using logistic regression to estimate adjusted odds ratios, we assessed: a) the ability of the Risk Score classification (calculated without including Pain Days) to predict unemployment and long-term use of opioid medications after controlling for Pain Days; and b) the ability of the Pain Days classification of chronic pain to predict these behavioral outcomes after controlling for Risk Score (calculated without including Pain Days).
3. Results
3.1. Study samples
Of the eligible patients, 64% of those with back pain, 71% with headache, and 76% with orofacial pain completed the baseline questionnaire. Response rates for the six-month follow-up were: 90% for back pain (n = 971); 94% for headache (n = 1078), and 91% for orofacial pain (n = 455). Among study participants, 792 with back pain, 877 with headache, and 384 with orofacial pain remained enrolled in Group Health in 1997–1999 and were included in analyses of long-term opioid use.
Effects of mode of data collection
Among the respondents with six month follow-up data included in the analyses reported in this paper, the percent of respondents who provided baseline data by mail survey was 59% for back pain; 63% for headache; and 67% for orofacial pain. At six month follow-up, all respondents were interviewed by telephone. For each pain condition, we used t-tests to compare mail and telephone respondents on mean pain intensity, interference with activities related to their pain condition, number of pain days, SCL-90-R depression, and number of pain conditions (other than the index condition). We also assessed whether there was a difference in the percent with Grade II-IV chronic pain at six month follow-up for persons who provided baseline data by mail survey versus telephone interview with a chi-square test. For orofacial pain, there were no significant differences (as defined by p<0.05 for a two-sided test) by mode of data collection for any of the six comparisons. For back pain, only one of the six comparisons was significant. Mail respondents reported more Pain Days than telephone respondents (p=0.045), but the difference was small (102 vs. 93 back pain days). Headache respondents showed significant differences by mode of administration for characteristic pain intensity (59.3 for mail vs. 53.6 for phone, p<0.0001); pain interference ratings (58.9 for mail vs. 49.9 for phone, p<0.0001); for SCL-90-R depression (1.11 for mail vs. 1.00 for phone, p=0.03) and for number of pain sites (2.0 for mail vs. 1.6 for phone, p<0.0001). The greater severity of headache patients who responded by mail may reflect higher motivation to participate, prompting them to return the mail survey before being called for the phone interview.
Missing data
Rates of missing data for variables used in the analyses reported in this paper were low, generally in the vicinity of 1% and not exceeding 2.5%.
3.2. Agreement of pain duration and Risk Score classifications
The weighted kappa for agreement of the 3×3 classification of chronic pain based on Pain Days and Risk Score was 0.40 for back pain, 0.35 for headache, and 0.24 for orofacial pain. Weighted kappas between 0.20 and 0.40 are characterized as less than moderate agreement (17).
3.3. Prevalence of chronic pain
As Table 2a shows, when classified solely by pain duration, chronic pain was quite common among the general healthcare patients in this sample, as 55% of patients with back pain, 32% with headache, and 65% with orofacial pain reported ≥90 Pain Days in the prior six months at baseline. More than one third of patients with back or orofacial pain, but only one in eight with headache, reported ≥150 Pain Days. When defined solely by pain duration, chronic pain was more common among orofacial pain patients than among back pain patients. Table 2b shows that when chronic pain was determined by Risk Score, probable chronic pain was less common, with 8.3% to 15.5% of patients having a risk score of ≥22. However, an additional 17% to 31% met criteria for possible chronic pain (a Risk Score of 16–21). Based on Risk Score classification, chronic pain was most common among patients with back pain, and least common among those with orofacial pain. While classifications by both Risk Score and Pain Days identify a broad spectrum of chronic pain, the two methods differ in their depiction of the prevalence of chronic pain across pain conditions. Previous research has shown that in both the general population and patient samples, back pain is typically associated with greater impact than orofacial pain (30,34), so defining chronic pain solely by duration may provide a misleading portrait of the relative burden of chronic pain across pain conditions.
Table 2.
Pain profile at baseline for chronic pain classification based on Pain Days in the prior six months (Table 2a) and on prospective Risk Score (Table 2b): Patients with back pain (n = 971), headache (n = 1078), or orofacial pain (n = 455).
| Table 2a Classification of chronic pain based on pain days in the six months before baseline | |||
|---|---|---|---|
| Type of pain | Not chronic: 1–89 Pain Days |
Chronic: 90–149 Pain Days |
Chronic: ≥150 Pain Days |
| Patients with: | |||
| Back pain | 45.2% | 18.1% | 36.7% |
| Headache | 68.5% | 18.9% | 12.6% |
| Orofacial pain | 35.0% | 21.5% | 43.5% |
| Average pain intensity, mean (SD)* | |||
| Back pain | 5.3 (2.1) | 5.4 (1.8) | 5.9 (1.9) |
| Headache | 6.3 (2.1) | 6.3 (1.9) | 6.4 (1.7) |
| Orofacial pain | 4.6 (2.2) | 4.8 (1.8) | 5.6 (2.1) |
| Pain Days, mean (SD) | |||
| Back pain | 32.7 (22.3) | 100.8 (14.4) | 177.2 (8.3) |
| Headache | 29.7 (21.9) | 103.9 (15.2) | 171.3 12.4) |
| Orofacial pain | 35.7 (21.8) | 101.5 (16.1) | 175.7 (9.9) |
| Table 2b Classification of chronic pain based on prospective Risk Score at baseline | |||
|---|---|---|---|
| Type of pain | Lower risk (0–15) |
Possible chronic pain (16–21) |
Probable chronic pain (22–28) |
| Patients with: | |||
| Back pain | 55.6% | 29.0% | 15.5% |
| Headache | 60.4% | 31.4% | 8.3% |
| Orofacial pain | 72.5% | 17.4% | 10.1% |
| Average pain intensity, mean (SD) | |||
| Back pain | 4.8 (1.9) | 6.1 (1.7) | 7.3 (1.5) |
| Headache | 5.8 (2.1) | 7.1 (1.6) | 7.6 (1.4) |
| Orofacial pain | 4.4 (2.0) | 6.2 (1.8) | 7.2 (1.5) |
| Pain Days, mean (SD) | |||
| Back pain | 67.5 (60.6) | 122.3 (57.1) | 160.7 (39.7) |
| Headache | 43.1 (44.1) | 78.0 (54.1) | 131.7 (43.6) |
| Orofacial pain | 95.5 (63.4) | 142.3 (49.2) | 165.8 (33.1) |
Standard deviation.
Comparing Tables 2a and 2b reveals similarities and differences between the Pain Days and Risk Score classifications of chronic pain in their profiles of average pain intensity and Pain Days in the prior six months. By definition of the Risk Score, patients with possible and probable chronic pain differed from lower-risk patients in reporting pain that was more intense and of longer duration. In contrast, patients classified as having chronic pain based solely on duration differed markedly on Pain Days, but differences in pain intensity ratings were modest.
3.4. Predictive validity for clinically significant pain
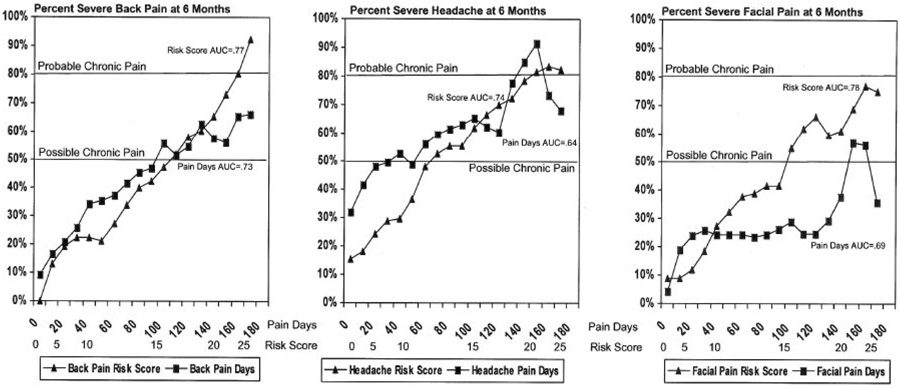
Figure 1 shows smoothed probability plots of the proportion with clinically significant pain at six months as a function of Risk Score and Pain Days for back pain, headache, and orofacial pain. The area under the curve (AUC) estimates were consistently higher for Risk Score than for Pain Days, indicating improved prediction: 0.77 vs. 0.73 for back pain; 0.74 vs. 0.64 for headache; and 0.78 vs. 0.69 for orofacial pain. The AUC estimates for the chronic pain Risk Score are higher than some other prognostic indicators evaluated for back pain (14), comparable to other screening and prognostic tests used in clinical medicine (1,3,12,28), but lower than highly specific diagnostic tests (22,29). This is consistent with viewing chronic pain as a prognostic evaluation associated with uncertainty regarding long-term outcome (32).
Figure 1.
Prediction of severe pain at six months from baseline Risk Score and Pain Days.
The probability plots for predicting clinically significant pain at follow-up as a function of Risk Score increased monotonically in a generally linear trend (see Figure 1). For back pain and orofacial pain, Risk Score surpassed the 50% threshold for possible chronic pain at around the originally proposed cut-point of 15/16, while headache surpassed the 50% threshold at a lower Risk Score cut-point of 12/13. The lower Risk Score threshold for possible chronic pain for headache may reflect the higher proportion of headache patients with clinically significant pain at six months relative to back pain and orofacial pain patients. Patients with back pain or headache surpassed the 80% probability threshold for probable chronic pain near the originally proposed 21/22 cutpoint (back pain at 22/23 and headache at 20/21). While the orofacial pain patients approached the 80% probability threshold, the probability plot reached a maximum of 77% at a Risk Score in the 23–25 range.
The prediction of clinically significant pain at follow-up from Pain Days alone was better than one might expect for a single item. However, the probability plots did not approach the 80% threshold for probable chronic pain for either back pain or orofacial pain. While the headache probability plot did surpass the 80% threshold, headache patients with ≥150 Pain Days had only about a 70% probability of having clinically significant pain at six-month follow-up. Using Pain Days alone was especially ineffective in identifying orofacial pain patients at high risk of clinically significant pain at follow-up. Patients with the most persistent orofacial pain had <60% risk of having clinically significant pain at follow-up. In general, the probability plots for Pain Days did not increase consistently with increasing Pain Days, possibly reflecting lower reliability of prediction from a single test item than from a multivariate Risk Score.
When the ability of Risk Score (excluding Pain Days) to predict clinically significant pain at six month follow-up was compared to Pain Days, Risk Score (excluding Pain Days) was a consistently better predictor of future severe pain than Pain Days. Using logistic regression to control for Pain Days, Risk Score (with Pain Days excluded) was a highly significant predictor of Grade II-IV pain for back pain, for headache, and for orofacial pain (p<0.0001 for each of the three pain conditions). The adjusted Chi-square values for these predictions (with one degree of freedom each) were: 90.6 for Risk Score vs. 78.2 for Pain Days for back pain; 114.2 for Risk Score vs. 20.2 for Pain Days for headache; and 51.1 for Risk Score vs. 14.8 for Pain Days for orofacial Pain. While it is not surprising that a risk score including measures of pain severity would be a better predictor of future pain severity than a measure of pain duration alone, these findings suggest the importance of considering pain severity in the definition and classification of chronic pain.
3.5. Predictive validity for specific pain and disability outcomes
As Table 3 shows, baseline Risk Score predicted both SF-36 Physical Function scores and pain-related worry ratings at six-month follow-up more strongly than did baseline Pain Days alone. Across all three pain conditions, these measures were consistently correlated more strongly with Risk Score than with Pain Days.
Table 3.
Worry about pain and physical disability for back pain (n = 971), headache (n = 1078), or orofacial pain (n = 455) patients at 6-month follow-up (mean and standard deviation) by baseline Pain Days (Table 3a) and Risk Score (Table 3b)*
| Table 3a Chronic pain classification based on Pain Days in the six months before baseline | ||||
|---|---|---|---|---|
| Pain and disability status at 6 months | Not chronic: 1–89 Pain Days |
Chronic: 90–149 Pain Days |
Chronic: ≥150 Pain Days |
Correlation with Pain Days |
| Worry about pain (0–10 rating) | ||||
| Back pain | 3.8 (3.1) | 5.0 (3.0) | 5.7 (3.2) | 0.29 |
| Headache | 4.1 (3.1) | 4.9 (3.0) | 5.8 (3.4) | 0.22 |
| Orofacial pain | 3.3 (2.7) | 3.6 (2.8) | 4.9 (3.2) | 0.25 |
| SF-36†Physical Function at 6 months, mean (SD)‡ | ||||
| Back pain | 80.3 (20.6) | 68.2 (23.7) | 56.9 (26.9) | −0.42 |
| Headache | 82.6 (21.8) | 77.1 (23.6) | 70.4 (26.0) | −0.21 |
| Orofacial pain | 83.5 (23.4) | 81.6 (22.9) | 76.0 (26.5) | −0.15 |
| Table 3b Chronic pain classification based on prospective Risk Score at baseline | ||||
|---|---|---|---|---|
| Pain and disability status at 6 months | Lower risk (0–15) |
Possible chronic pain (16–21) |
Probable chronic pain (22–28) |
Correlation with Risk Score |
| Worry about pain (0–10 rating) | ||||
| Back pain | 3.6 (2.9) | 5.5 (3.2) | 7.1 (2.8) | 0.46 |
| Headache | 3.6 (2.9) | 5.4 (3.1) | 6.6 (3.1) | 0.40 |
| Orofacial pain | 3.7 (2.9) | 4.4 (3.0) | 6.0 (3.5) | 0.33 |
| SF-36 Physical Function at 6 months, mean (SD) | ||||
| Back pain | 80.2 (18.7) | 62.3 (25.9) | 44.0 (25.6) | −0.53 |
| Headache | 85.3 (19.4) | 73.3 (25.5) | 64.1 (27.1) | −0.33 |
| Orofacial pain | 84.6 (21.2) | 70.8 (29.1) | 61.2 (27.2) | −0.36 |
All correlations in Tables 3a and 3b are significant at p <.0001.
Short Form-36. Correlations are negative because a lower SF-36 score indicates greater disability.
Standard deviation.
3.6. Predictive validity for behavioral outcomes
Risk Score was also a stronger predictor of unemployment at six-month follow-up and long-term opioid use 2–5 years after baseline than Pain Days (see Table 4).
Table 4.
Percentage of patients unemployed or unable to work at six-month follow-up and percentage with long-term opioid Use in 1997–1999 by baseline Pain Days (Table 4a) and prospective Risk Score (Table 4b)
| Table 4a Chronic pain classification based on Pain Days in the six months before baseline | ||||
|---|---|---|---|---|
| Percent of patients: | Not chronic: 1–89 Pain Days | Chronic: 90–149 Pain Days | Chronic: ≥150 Pain Days | Test statistics |
| Unemployed/unable to work at 6 months (%) | ||||
| Back pain | 2.6% | 6.9% | 17.1% | X2= 41.9 p <.001 |
| Headache | 5.1% | 10.3% | 10.3% | X2= 7.6 p = .02 |
| Orofacial pain | 5.7% | 5.3% | 11.6% | X2= 4.2 p = .12 |
| Long-term opioid use in 1997–1999 (%) | ||||
| Back pain | 10.0% | 14.4% | 23.4% | X2= 22.1 p <.001 |
| Headache | 15.0% | 23.5% | 28.6% | X2= 14.6 p <.001 |
| Orofacial pain | 7.0% | 8.8% | 16.2% | X2= 6.4 p = .04 |
| Table 4b. Chronic pain classification based on prospective Risk Score at baseline | ||||
|---|---|---|---|---|
| Percent of patients: | Lower risk (0–15) | Possible chronic pain (16–21) | Probable chronic pain (22–28) | Test statistics |
| Unemployed/unable to work at 6 months (%) | ||||
| Back pain | 1.8% | 8.7% | 33.9% | X2= 118.0 p <.001 |
| Headache | 3.8% | 9.9% | 17.5% | X2= 22.9 p <.001 |
| Orofacial pain | 6.8% | 7.7% | 22.9% | X2= 10.3 p = .006 |
| Long-term opioid use in 1997–1999 (%) | ||||
| Back pain | 9.3% | 21.5% | 30.3 % | X2= 38.5 p <.001 |
| Headache | 12.5% | 25.3% | 35.3 % | X2= 34.0 p <.001 |
| Orofacial pain | 6.9% | 17.6% | 30.0 % | X2= 22.0 p <.001 |
In logistic regression analyses, we assessed the prediction of unemployment/inability to work at six months and long-term opioid use in 1997–1999 from Risk Score (excluding Pain Days) after controlling for Pain Days. We also assessed the prediction of these behavioral outcomes from Pain Days after controlling for Risk Score (excluding Pain Days). These comparative analyses are presented in Table 5. Comparing the adjusted odds ratios in Tables 5b to those in Table 5a, Risk Score (excluding Pain Days) was a consistently stronger independent predictor of unemployment and of long-term opioid use two to five years after baseline than Pain Days. For headache and orofacial pain, Pain Days was not a significant predictor of unemployment/inability to work at six month follow-up after controlling for Risk Score. The increase in risk of both unemployment and long-term opioid use among persons with 90–149 Pain Days relative to those with fewer Pain Days was non-significant (i.e. the confidence intervals of the odds ratios included 1.0) for all three pain conditions (see Table 5a). In contrast, after controlling for Pain Days, Risk Score (excluding Pain Days) was a significant predictor of both unemployment/inability to work at six months and long-term opioid use at two to five years after baseline for all three pain conditions (see Table 5b). Except among orofacial pain patients with possible chronic pain, possible chronic pain was associated with a two- to three-fold increase in risk of these behavioral outcomes relative to persons with low Risk Scores, while probable chronic pain was associated with a three- to 14-fold increase in risk of these behavioral outcomes relative to persons with low Risk Scores.
Table 5.
Odds ratios estimated from multivariate (logistic regression) prediction of unemployment/inability to work at six-month follow-up and long-term opioid Use in 1997–1999 by baseline Pain Days (Table 5a) and prospective Risk Score excluding Pain Days(Table 5b)
| Table 5a Odds ratios for prediction of behavioral outcomes from baseline Pain Days adjusted for baseline Risk Score (excluding Pain Days) | ||||
|---|---|---|---|---|
| Odds ratios (95% confidence intervals): | Not chronic: 1–89 Pain Days (reference group) | Chronic: 90–149 Pain Days | Chronic: ≥150 Pain Days | Test statistics |
| Unemployed/unable to work at 6 months | ||||
| Back pain | 1.0 | 2.0 (0.7, 5.2) | 4.0 (1.8, 8.6) | X2=13.3 df=2, p=0.0013 |
| Headache | 1.0 | 1.5 (0.8, 3.0) | 1.4 (0.6, 3.1) | X2=1.9 df=2, NS |
| Orofacial pain | 1.0 | 0.8 (0.2, 2.7) | 1.4 (0.5, 3.8) | X2=1.3 df=2, NS |
| Long-term opioid use in 1997–1999 | ||||
| Back pain | 1.0 | 1.3 (0.7, 2.4) | 2.1 (1.3, 3.3) | X2=9.8 df=2, p=0.0074 |
| Headache | 1.0 | 1.4 (0.9, 2.2) | 1.8 (1.1, 2.9) | X2=5.8 df=2, p=0.054 |
| Orofacial pain | 1.0 | 0.9 (0.3, 2.7) | 1.6 (0.7, 3.7) | X2=1.8 df=2, NS |
| Table 5b. Odds ratios for prediction of behavioral outcomes from baseline Risk Score (excluding Pain Days) adjusted for baseline Pain Days | ||||
|---|---|---|---|---|
| Percentage of patients: | Lower risk 0–15 (Reference Group) | Possible chronic pain 16–21 | Probable chronic pain 22–28 | Test statistics |
| Unemployed/unable to work at 6 months | ||||
| Back pain | 1.0 | 3.1 (1.3, 7.7) | 14.5 (5.9, 35.8) | X2=41.4 df=2, p<0.0001 |
| Headache | 1.0 | 2.7 (1.4, 5.1) | 4.5 (1.7, 11.5) | X2=12.7 df=2, p<0.0018 |
| Orofacial pain | 1.0 | 1.1 (0.3, 3.5) | 3.4 (1.2, 9.8) | X2=5.8 df=2, p=0.055 |
| Long-term opioid use in 1997–1999 | ||||
| Back pain | 1.0 | 2.3 (1.4, 3.8) | 3.3 (1.8, 5.9) | X2=17.53 df=2, p=0.0002 |
| Headache | 1.0 | 2.0 (1.4, 3.0) | 2.7 (1.5, 5.2) | X2 =15.7 df=2, p=0.0004 |
| Orofacial pain | 1.0 | 2.5 (1.1, 5.6) | 4.5 (1.9, 11.0) | X2 =11.8 df=2, p=0.0028 |
4. Discussion
Across three disparate pain conditions (back pain, headache, and orofacial pain), a prospective classification of chronic pain based on a multivariate Risk Score consistently outperformed a classification of chronic pain based solely on pain duration. These results suggest that defining chronic pain by pain duration alone is less than optimal.
Defining chronic pain by pain duration has not yielded evidence-based methods for assessing and classifying chronic pain in clinical practice, nor in clinical nor epidemiological research (2,5,6,23,26). Viewing chronic pain as multi-dimensional has been more fruitful (15,26,27). The Risk Score approach suggests that chronic pain should be defined by the likelihood that clinically significant pain will continue in the future, not only by how long pain has lasted. We found this approach to be valid for headache, orofacial pain, and for back pain.
4.1. Clinical implications
It may seem intuitive that chronic pain should be defined solely by pain duration, as indeed it traditionally has been. However, as many practicing clinicians recognize, predicting whether pain will run a chronic course is not simply a matter of determining pain’s duration. A patient who limits activities and reports depressive symptoms and pain at diffuse anatomical locations may fit a clinician’s intuitive chronic pain profile better than a patient with long-lasting pain not accompanied by other unfavorable prognostic indicators.
Pain outcomes are highly variable over time and between individuals (6,10,11,18,35). A prognostic approach discards the notion that “chronic” means unlikely to change. In this research, we observed a continuum of chronic pain, with no distinct class of chronic pain patients. No clear demarcation distinguished persons with possible or probable chronic pain from those with less significant and enduring pain. Chronic pain should be viewed as a condition whose future implications are inherently uncertain and mutable, rather than as a fixed trait identifying patients with intractable pain. The potential for change, indeed the likelihood of change, is an important and oft-neglected feature of chronic pain. For these reasons, the terms possible and probable chronic pain, defining chronic pain in probabilistic terms, appropriately emphasize the inherent uncertainty of long-term pain outcomes—with improvement always possible (32). This approach shifts the focus from the potentially stigmatizing labeling of “chronic pain patients” to the likelihood that clinically significant pain will continue and, by extension, to steps that might reduce future risks of significant pain and dysfunction.
Defining chronic pain prospectively by a risk score is consonant with the view that chronic pain has multiple attributes, including psychological and behavioral components, in addition to pain severity and duration (27). A multi-factorial Risk Score emphasizes factors other than pain (e.g., activity limitation and depression), suggesting avenues for improving patient outcomes in addition to controlling pain per se (e.g., increasing activity levels and treating depression). By broadening the defining features of chronic pain to include factors other than pain duration, both clinicians and patients may become more aware of opportunities to improve outcomes when pain continues past the normal time of healing. Increasing activity levels and addressing emotional distress may be perceived more readily as viable options for reducing risks of pain running a chronic course, along with biomedical interventions addressing underlying physical causes of pain and palliative interventions that seek to control pain per se.
4.2. Research implications
Our results support the feasibility of using risk scoring methods for diverse applications—across populations, settings, and pain conditions—to identify cases likely to have enduring clinically significant chronic pain in clinical and epidemiological research. The Risk Score may provide an empirical basis for integrating several themes in chronic pain assessment, including multivariate approaches to assessing chronic pain (27) and identifying “yellow flags” marking increased risks of a poor functional outcome (16). It extends prior work on grading chronic pain by validating a measure of chronic pain status not only by its correlation with concurrent measures of pain dysfunction, but also by prediction of future pain and dysfunction (34,36). The Risk Score could provide a common metric quantifying both current severity and risk that clinically significant pain will persist. Using a common metric could potentially alleviate difficulties in comparing results across epidemiological and clinical studies of chronic pain.
4.3.Limitations
Despite predictive advantages, determining a patient’s Risk Score requires asking more questions than assessing pain duration, as well as calculating the Risk Score, which are limitations for clinical practice. The time required to complete the Risk Score questions would vary depending on the patient and on whether the questions were asked in interview or self-administered format. It should generally take less than 10 minutes to complete the Risk Score questions if they are self-administered. All the Risk Score questions are suitable for self-administration via a computer-based assessment, and could be readily scored by a computer algorithm. The Risk Score approach could be readily adapted to use different questions than the specific items and scales employed in this study. Apart from their potential value for classifying chronic pain, clinicians should be aware that pain intensity, interference with activities, depressive symptoms, and number of pain sites have prognostic value above and beyond information about pain duration. We have not optimized the ability of the Risk Score to predict pain outcomes. Additional predictor variables and use of empirically derived weights might improve prediction. Since the focus of this paper was not on prognostic factors per se, we did not consider the extent to which the different variables included in the Risk Score independently contributed to prediction of pain outcomes. Baseline pain status was assessed 2–4 weeks after the index pain visit. Therefore, although the results reported in this paper can be applied to assessment at follow-up visits, the prognostic value of the Risk Score if assessed at an initial pain visit deserves further study.
4.4. Conclusions
Our results provide empirical support for a prognostic approach to defining chronic pain for back pain, headache, and orofacial pain. The predictive value of a multi-factorial Risk Score for pain, psychological, and behavioral outcomes consistently exceeded that of pain duration alone. We observed a continuum of chronic pain, rather than a distinct class of chronic pain patients. Because pain outcomes are highly variable across persons and over time, chronic pain should be viewed as having an inherently uncertain prognosis, not as a static trait that identifies patients whose pain is intractable. The term chronic pain carries negative connotations for patients and clinicians alike. By shifting the focus from pain duration to prognosis, and by defining chronic pain in probabilistic terms, the assessment of chronic pain may be refocused from labeling patients as having intractable pain to focusing on steps that might be taken to reduce risks of an unfavorable outcome.
Acknowledgements
NIDCR grant P01 DE08773 and NIDA grant R01 DA022557 supported this research. We gratefully acknowledge Kathleen Saunders’s contributions to this research and Rebecca Hughes’s editorial suggestions. The Risk Score algorithm reported in this study has been placed in the Creative Commons and may be used without the authors’ permission with attribution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amir E, Evans DG, Shenton A, Lalloo F, Moran A, Boggis C, Wilson M, Howell A. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genetics. 2003;40:807–814. doi: 10.1136/jmg.40.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson GB. Epidemiological features of low back pain. Lancet. 1999;14:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. Development of a Ventilatory Classification System in patients with heart failure. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.107.686576. In press. [DOI] [PubMed] [Google Scholar]
- 4.Bonica JJ. General considerations of chronic pain. In: Bonica JJ, editor. The Management of Pain. 2nd Edition. Philadelphia: Lea and Febiger; 1990. [Google Scholar]
- 5.Cedraschi C, Nordin M, Nachemson AL, Vischer TL. Health care providers should use a common language in relation to low back pain patients. Bailleres Clin Rheumatol. 1998;12:1–8. doi: 10.1016/s0950-3579(98)80003-4. [DOI] [PubMed] [Google Scholar]
- 6.Cedraschi C, Robert J, Goerg D, Perrin E, Fischer W, Vischer TL. Is chronic non-specific low back pain chronic? Definitions of a problem and problems of a definition. Br J Gen Pract. 1999;49:358–362. [PMC free article] [PubMed] [Google Scholar]
- 7.Derogatis LR. SCL-90-R: Administration, Scoring and Procedures Manual-II. Towson, MD: Clinical Psychometric Research; 1983. [Google Scholar]
- 8.Dunn KM, Croft PR, Main CJ, Von Korff M. A prognostic approach to defining chronic pain: replication in a UK primary care low back pain population. Pain. 2007 doi: 10.1016/j.pain.2007.05.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Dunn KM, Croft PR. The importance of symptom duration in determining prognosis. Pain. 2006;121:126–132. doi: 10.1016/j.pain.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Dunn KM, Croft PR. Repeat assessment improves the prediction of prognosis in patients with low back pain in primary care. Pain. 2006;126(1–3):10–15. doi: 10.1016/j.pain.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Dunn KM, Jordan K, Croft PR. Characterizing the course of low back pain: a latent class analysis. Am J Epidemiol. 2006;163(8):754–761. doi: 10.1093/aje/kwj100. [DOI] [PubMed] [Google Scholar]
- 12.Gould MK, Ananth L, Barnett PG. Veteran’s Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodule. Chest. 2007;131:383–388. doi: 10.1378/chest.06-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellström C, Carlsson SG. The long-lasting now: disorganization in subjective time in long-standing pain. Scand J Psychol. 1996;37:416–423. doi: 10.1111/j.1467-9450.1996.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 14.Jellema P, van der Windt DA, van der Horst HE, Stalman WA, Bouter LM. Prediction of an unfavorable course of low back pain in general practice: comparison of four instruments. Br J Gen Practice. 2007;57:15–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen JP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. Second Edition. New York: Guilford Press; 2001. [Google Scholar]
- 16.Kendall NAS, Linton SJ, Main CJ. Guide to assessing psychosocial yellow flags in acute low back pain: risk factors for long-term disability and work loss. Wellington, New Zealand: Accident Rehabilitation & Compensation Insurance Corporation of New Zealand and the National Health Committee; 1997. [Google Scholar]
- 17.Landis RJ, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 18.McGorry RW, Webster BS, Snook SH, Hsiang SM. The relation between pain intensity, disability, and the episodic nature of chronic and recurrent low back pain. Spine. 2000;25:834–841. doi: 10.1097/00007632-200004010-00012. [DOI] [PubMed] [Google Scholar]
- 19.Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Seattle: IASP Press; 1994. [Google Scholar]
- 20.Olesen J, Goadsby P, Steiner T. The International Classification of Headache Disorders: 2nd edition. Lancet Neurol. 2003;2:720. [Google Scholar]
- 21.Pepe MS. Receiver operating characteristic methodology. J Am Stat Assn. 2000;95:308–311. [Google Scholar]
- 22.Purkayastha S, Athanasiou T, Tekkis PP, Constantinides V, Teare J, Darzi AW. Magnetic resonance colonography vs. computed tomography colonography for the diagnosis of colorectal cancer: an indirect comparison. Colorectal Dis. 2007;9:100–111. doi: 10.1111/j.1463-1318.2006.01126.x. [DOI] [PubMed] [Google Scholar]
- 23.Raspe H, Huppe A, Matthis C. Theories and models of chronicity: on the way to a broader definition of chronic pain [Article in German] Schmerz. 2003;17:359–366. doi: 10.1007/s00482-003-0233-y. [DOI] [PubMed] [Google Scholar]
- 24.Richardson JC, Ong BN, Sim J. Remaking the future: contemplating a life with chronic widespread pain. Chronic Illness. 2006;2:209–218. doi: 10.1177/17423953060020030201. [DOI] [PubMed] [Google Scholar]
- 25.Saunders KW, Davis RL, Stergachis A. Pharmacoepidemiology. Fourth Edition. West Sussex, England: John Wiley and Sons; 2005. Group Health Cooperative. [Google Scholar]
- 26.Turk DC, Melzack R. The measurement of pain and the assessment of people experiencing pain. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment Second Edition. New York: Guilford Press; 2001. [Google Scholar]
- 27.Turk DC, Rudy TE. Toward an empirically derived taxonomy of chronic pain patients: Integration of psychological assessment data. J Consult Clin Psychol. 1988;56:233–238. doi: 10.1037//0022-006x.56.2.233. [DOI] [PubMed] [Google Scholar]
- 28.Ueda T, Takeyama Y, Yasuda T, Matsumura N, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kurado Y. Simple scoring system for the prediction of the prognosis of severe acute pancreatitis. Surgery. 2007;141:51–58. doi: 10.1016/j.surg.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Van Calster B, Timmerman D, Lu C, Suykens JA, Valentin L, Van Holsbeke C, Amant F, Vergote I, Van Huffel S. Preoperative diagnosis of ovarian tumors using Bayesian kernel-based methods. Ultrasound Obstet Gynecol. 2007;29:496–504. doi: 10.1002/uog.3996. [DOI] [PubMed] [Google Scholar]
- 30.Von Korff M, Dworkin SF, Le Resche L, Kruger A. An epidemiologic comparison of pain complaints. Pain. 1988;32:173–183. doi: 10.1016/0304-3959(88)90066-8. [DOI] [PubMed] [Google Scholar]
- 31.Von Korff M, Lin EHB, Fenton JJ, Saunders K. Frequency and priority of pain patients’ health care use. Clinical J Pain. 2007;23:400–408. doi: 10.1097/AJP.0b013e31804ac020. [DOI] [PubMed] [Google Scholar]
- 32.Von Korff M, Miglioretti D. A prospective approach to defining chronic pain. In: Flor H, editor. Proceedings of the 11th World Congress on Pain of the International Association for the Study of Pain. IASP Press; 2006. pp. 761–769. [Google Scholar]
- 33.Von Korff M, Miglioretti DL. A prognostic approach to defining chronic pain. Pain. 2005;117:304–313. doi: 10.1016/j.pain.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Von Korff M, Ormel J, Keefe F, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 35.Von Korff M, Saunders K. The course of back pain in primary care. Spine. 1996;21:2833–2837. doi: 10.1097/00007632-199612150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Von Korff M. Epidemiological and survey methods: assessment of chronic pain. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. Second Edition. New York: Guilford Press; 2001. [Google Scholar]
- 37.Von Korff M. Studying the natural history of back pain. Spine. 1994;19:2041S–2046S. doi: 10.1097/00007632-199409151-00005. [DOI] [PubMed] [Google Scholar]
- 38.Ware J, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 Health Profile and Summary Measures: Summary of the results from the Medical Outcomes Study. Med Care. 1995;33:AS264–AS279. [PubMed] [Google Scholar]