Abstract
Aerobic exercise can serve as an alternative, non-drug reinforcer in laboratory animals and has been recommended as a potential intervention for substance abusing populations. Unfortunately, relatively little empirical data have been collected that specifically address the possible protective effects of voluntary, long-term exercise on measures of drug self-administration. The purpose of the present study was to examine the effects of chronic exercise on sensitivity to the positive-reinforcing effects of cocaine in the drug self-administration procedure. Female rats were obtained at weaning and immediately divided into two groups. Sedentary rats were housed individually in standard laboratory cages that permitted no exercise beyond normal cage ambulation; exercising rats were housed individually in modified cages equipped with a running wheel. After 6 weeks under these conditions, rats were surgically implanted with venous catheters and trained to self-administer cocaine on a fixed-ratio schedule of reinforcement. Once self-administration was acquired, cocaine was made available on a progressive ratio schedule and breakpoints were obtained for various doses of cocaine. Sedentary and exercising rats did not differ in the time to acquire cocaine self-administration or responding on the fixed-ratio schedule of reinforcement. However, on the progressive ratio schedule, breakpoints were significantly lower in exercising rats than sedentary rats when responding was maintained by both low (0.3 mg/kg/infusion) and high (1.0 mg/kg/infusion) doses of cocaine. In exercising rats, greater exercise output prior to catheter implantation was associated with lower breakpoints at the high dose of cocaine. These data indicate that chronic exercise decreases the positive-reinforcing effects of cocaine and support the possibility that exercise may be an effective intervention in drug abuse prevention and treatment programs.
Keywords: cocaine, exercise, progressive ratio, rat, self-administration
1. Introduction
Aerobic exercise produces a host of psychological effects that are negatively correlated with substance use and abuse. For instance, long-term voluntary exercise increases measures of self-esteem (Morgan, 1982; Waade 2004) and well-being (Norris et al., 1990; 1992; Muller et al., 2006) and decreases measures of depression (Veale et al., 1992; Dunn et al., 2005) and anxiety (Antunes et al., 2005 Manger and Motta, 2005). Not surprisingly, epidemiological studies report that participation in activities that promote physical fitness is associated with a lower incidence of tobacco and substance use among adolescent populations (Field et al., 2001; Kirkcaldy et al., 2002). Despite these promising epidemiological findings, remarkably little clinical and laboratory data exist that support a causal relationship between aerobic exercise and a decreased propensity to engage in drug-seeking behavior.
Studies using both human and animal subjects report that exercise produces interoceptive effects that are phenomenologically similar to those produced by addictive drugs. For instance, acute bouts of exercise increase subjective ratings of joy, pleasantness, and euphoria in human volunteers (Nowlis and Greenberg, 1979; Janal et al., 1984; Nabetani and Tokunaga, 2001). Similarly, pairing a distinctive environment with the aftereffects of running produces a conditioned place preference in laboratory animals (Lett et al., 2000; 2001; Belke and Wagner, 2005). Exercise can also serve as a positive reinforcer, as both laboratory animals (Iversen, 1993; Belke, 1997; 2000; Belke and Dunbar, 2001) and in-patient clinical populations (Schebendach et al., 2007) will perform an operant response that leads to the opportunity to exercise.
Although the neuroanatomical locus of exercise’s positive-reinforcing effects are not known, neurochemical data suggest that exercise activates the same reward pathways that are activated by addictive drugs. For instance, acute bouts of exercise increase central dopamine concentrations (Heyes et al., 1988; Hattori et al., 1994; Meeusen et al., 1997; Petzinger et al., 2007), and chronic exercise leads to sustained increases in dopamine concentrations and compensatory alterations in dopamine binding proteins (Gilliam et al., 1984; MacRae et al., 1987; Fisher et al., 2004). Given that many addictive drugs produce their positive-reinforcing effects by increasing dopamine transmission in mesolimbic and mesocortical pathways (Goeders and Smith, 1983; Caine and Koob, 1994; Wise et al., 1995; Pich et al., 1997), chronic exercise may produce functional changes in these pathways that leave an organism less susceptible to their positive-reinforcing effects.
The purpose of the present study was to determine whether long-term, voluntary exercise decreases the positive-reinforcing effects of cocaine in female rats responding on a progressive ratio (PR) schedule of reinforcement. In this schedule, the number of responses (i.e., lever presses) required to obtain a drug infusion progressively increases over the course of a session until a point is reached at which responding ceases. This point, known as the breakpoint, is taken as a measure of the reinforcing efficacy of a drug and can be compared across dosing conditions and subject populations (see reviews by Richardson and Roberts, 1996; Stafford et al., 1998). In the present study, cocaine-maintained breakpoints were compared in female rats reared under sedentary or exercising conditions for 6 weeks prior to drug exposure. Females were chosen for the study because they run significantly more than males when given free access to running wheels (Eikelboom and Mills, 1988; Boakes et al., 1999).
2. Method
2.1. Animals
Female, Long-Evans rats were obtained at weaning (~21 days) and divided into two groups immediately upon arrival. Sedentary rats were housed individually in standard polycarbonate cages (interior dimensions: 50 × 28 × 20 cm) that permitted no exercise beyond normal cage ambulation. Exercising rats were housed in cages of equal dimensions but with a running wheel (35 cm diameter) affixed to the interior of the cage. Wheel revolutions were counted by magnetic switches and recorded weekly. Rats in both sedentary and exercising groups remained in their respective conditions for the duration of the study. Except during training and testing sessions, rats were kept in a colony room on a 12-hr light/dark cycle (lights on: 7:00 a.m.) with food and drinking water freely available in the home cage. All subjects were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Davidson College and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources, 1996). Estrous phase was allowed to cycle normally and was not monitored.
2.2. Surgery
Six weeks after arrival, each rat was anesthetized with a combination of ketamine (100 mg/kg, ip) and xylazine HCl (8.0 mg/kg. ip), and surgically implanted with a venous catheter (CamCaths, Cambridge, UK) that was inserted into the right jugular vein and exited the body on the dorsal surface of the scapulae. Rats were administered butorphanol HCl (1.0 mg/kg, sc) immediately after surgery as a post-operative analgesic and allowed to recover for 48 hours before beginning behavioral training. Beginning on the day immediately following surgery, a solution of 1.0 ml heparinized saline was infused through the catheter daily to maintain patency. Animals that lost catheter patency before completion of all behavioral tests were removed from the study and their data were not included in the statistical analysis. Four rats were removed from the study because of a loss of catheter patency (n = 2 sedentary rats; n = 2 exercise rats) A total of 17 rats (n = 8 sedentary; n = 9 exercise) completed all phases of the study.
2.3. Apparatus
Behavioral training and testing took place in polycarbonate and aluminum operant conditioning chambers (interior dimensions: 31 × 24 × 21 cm) from Med Associates, Inc. (St Albans, VT). Each chamber was equipped with two response levers located 10 cm above the chamber floor and a single houselight located on the ceiling above the rear wall. A white stimulus light located above the response lever signaled the availability of a drug infusion from a pump mounted outside the conditioning chamber. All infusions were delivered through a tygon tube attached to a steel swivel at the top of the chamber. Experimental events were programmed and data were collected through the use of software and interfacing supplied by Med Associates, Inc. The left lever was designated as the active lever for all rats. Responses on the right (inactive) lever were recorded but had no programmed consequences.
2.4. Behavioral Training
Training and testing sessions were conducted 7 days per week during the light phase of the light-dark cycle. All sessions began at 7:00 a.m. and lasted a maximum of 3 hr unless otherwise noted. For all sessions, rats were removed from their home cage, placed in the operant conditioning chambers, and connected to the infusion pump via tygon tubing. During initial training, all lever presses were reinforced on a fixed-ratio 1 (FR1) schedule of reinforcement. On this schedule, each lever press produced 1.0 mg/kg/infusion cocaine, with infusion duration varying between 3.5 and 5.0 s across rats based on individual body weight. Coincident with the beginning of each infusion, the stimulus light above the lever was turned off for 20 s to signal a time-out period in which cocaine was not available and responses had no programmed consequences. In this and all subsequent training sessions, the session was terminated automatically once 10 infusions were obtained. Once a subject obtained the maximum number of 10 infusions on the FR1 schedule during any two training days, contingencies were changed on the following day and responding was reinforced on an FR2 schedule of reinforcement. Once a subject obtained the maximum number of 10 infusions on the FR2 schedule during any one session, behavioral training was terminated and behavioral testing commenced on the following day. Thus, all rats completed a minimum of three training sessions before advancing to behavioral testing.
2.5. Behavioral Testing
Throughout behavioral testing, responding was reinforced on a PR schedule of reinforcement. On this schedule, the number of responses required for reinforcement incremented progressively through the following ratio values: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, 268, 346, 445, and 573 (for complete algorithm, see Suto et al., 2002). Each session continued until a breakpoint was reached, with breakpoint defined as the number of infusions obtained before one hour elapsed with no infusions. Daily PR sessions continued at a given dose until breakpoints were stable (i.e., 3 consecutive days during which the breakpoint varied by no more than 3 increments with no increasing or decreasing trends). Breakpoints were determined for 0.3 and 1.0 mg/kg/infusion cocaine, as well as for saline.
2.6. Data Analysis
The primary dependent measure of this study was breakpoint, which was defined as the number of infusions on the PR schedule of reinforcement. These data were analyzed via a two-way, mixed-factor ANOVA, with group serving as a between-subjects factor and dose serving as a repeated measure. Data on acquisition and from the FR schedules were analyzed via independent samples t-tests using group as a factor. Responses on the inactive lever were analyzed via a two-way, mixed-factor ANOVA, using group and dose as factors.
A Pearson product-moment correlation was used to compare exercise output and cocaine-maintained breakpoints in individual rats. Exercise output was defined as the mean number of revolutions per day (rev/day) and determined for each rat by dividing weekly wheel revolutions by 7. For all correlational analyses, exercise output before catheter implantation (i.e., 28 days immediately prior to surgery) was considered separately from exercise output after catheter implantation (days 2 through 15 after surgery). Correlation coefficients and statistical significance were determined with the aid of commercially available software (SPSS, v. 13.0). The alpha level was set at p < 0.05 for all statistical tests.
3. Results
3.1. Wheel Running and Body Weight
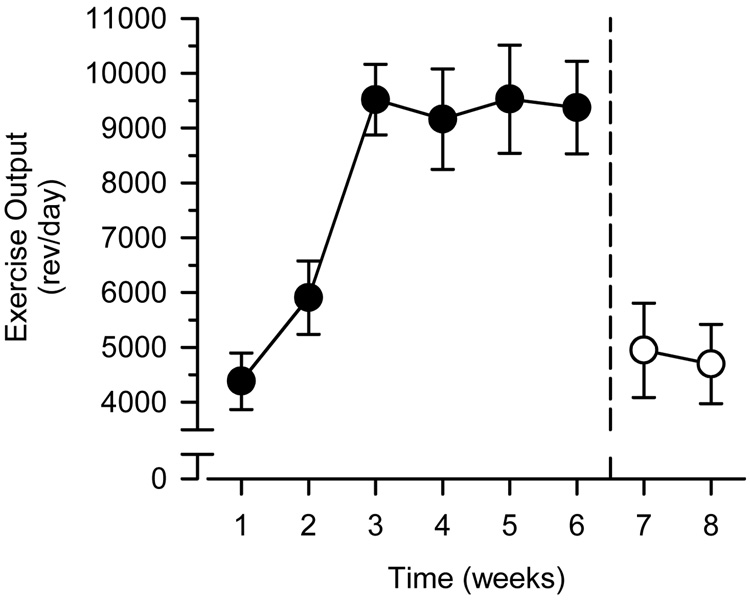
During the 6-week period between arrival and catheter implantation, exercising rats ran an average of 9203 rev/day (10,120 m/day), with a range across rats from 7381 rev/day (8116 m/day) to 11,316 rev/day (12,443 m/day). Exercise output steadily increased during the first 3 weeks of exposure to the running wheel before leveling out until catheter implantation and behavioral training (Figure 1). Exercise output declined sharply to an average of 4822 rev/day (5302 m/day) once self-administration was acquired and remained stable thereafter. Body weights did not differ between the two groups at arrival or during behavioral testing. At the outset of behavioral training, body weights averaged 248 g (range: 215 – 278 g) and 249 g (range: 206 – 288 g) in sedentary and exercising rats, respectively.
Figure 1.
Changes in exercise output before (filled circles) and after (open circles) catheter implantation and behavioral training. Left axis depicts exercise output expressed as the mean number of wheel revolutions per day (rev/day). Horizontal axis depicts time expressed in weeks. Vertical reference line after Week 6 indicates catheter implantation and the beginning of behavioral training. Vertical lines surrounding data points represent the SEM.
3.2. Behavioral Training and Fixed-Ratio Responding
Sedentary and exercising rats advanced to the PR schedule in a mean (SEM) of 6.0 (1.0) and 5.3 (0.8) days, respectively. Rates of responding on the initial FR1 and FR2 schedules of reinforcement were consistent across rats and similar between the two groups (Table 1). Independent samples t-tests did not reveal any significant differences between sedentary and exercising rats on days to acquisition or rates of responding on the FR1 and FR2 schedules of reinforcement.
Table 1.
Mean (SEM) inter-infusion intervala of sedentary and exercising rats responding on the FR1 and FR2 schedule of reinforcement.
| Schedule | Sedentary | Exercise |
|---|---|---|
| FR1 | 336 (63) | 248 (52) |
| FR2 | 411 (76) | 359 (93) |
all data expressed in seconds
3.3. Behavioral Testing and Progressive Ratio Responding
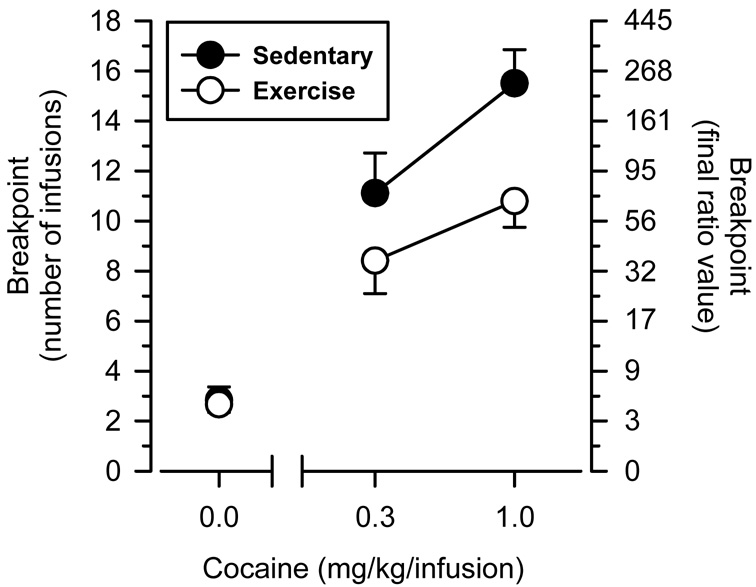
On the PR schedule, cocaine produced dose-dependent increases in breakpoints in both groups of rats (Figure 2). Breakpoints were greater in sedentary rats than exercising rats, and this effect was apparent at both the low (0.3 mg/kg/infusion) and high (1.0 mg/kg/infusion) dose of cocaine. Consistent with these observations, a two-way, mixed-factor ANOVA revealed main effects of dose (F [1, 15] = 97.169, p < 0.001) and group (F [1, 15] = 4.644, p = 0.048) on number of infusions obtained. Breakpoints maintained by saline were markedly lower than those maintained by cocaine and did not differ significantly between groups. Likewise, the number of inactive lever presses did not differ significantly as a function of group for any dosing condition (Table 2).
Figure 2.
Cocaine-maintained breakpoints in sedentary and exercising rats. Left axis depicts the number of infusions obtained; right axis depicts the final ratio value completed. Horizontal axis depicts dose of cocaine in mg/kg/infusion. Points above 0.0 reflect the effects of saline. Vertical lines surrounding data points represent the SEM.
Table 2.
Mean (SEM) number of inactive lever presses of sedentary and exercising rats for each dose condition on the progressive ratio schedule.
| Condition | Sedentary | Exercise |
|---|---|---|
| 0.0 mg/kg/inf | 3.1 (1.1) | 3.3 (1.4) |
| 0.3 mg/kg/inf | 65.1 (49.7) | 23.0 (11.9) |
| 1.0 mg/kg/inf | 68.4 (45.7) | 36.0 (10.5) |
3.4. Correlation between Exercise Output and Cocaine-Maintained Breakpoints
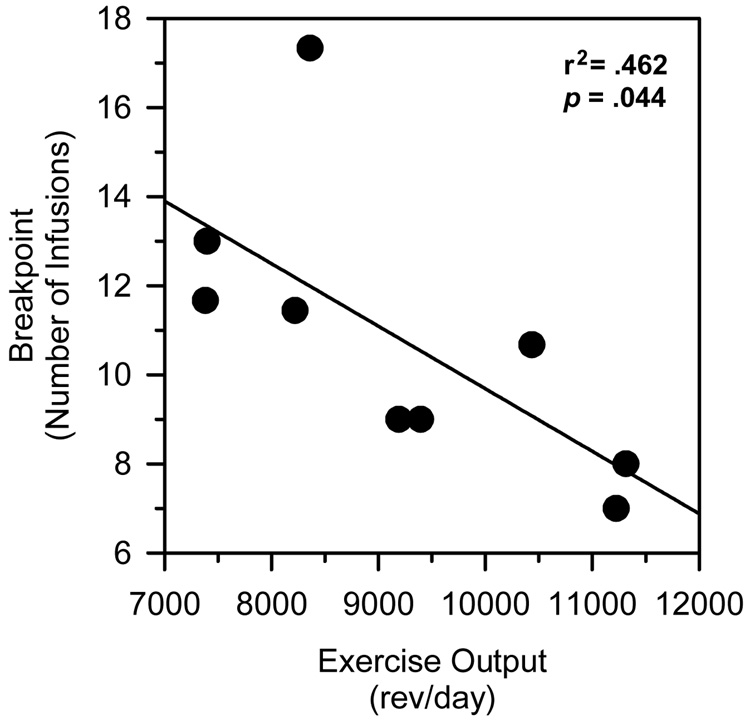
The correlation between exercise output (rev/day) and cocaine-maintained breakpoints was examined for both doses of cocaine. Exercise output prior to catheter implantation was inversely correlated with cocaine-maintained breakpoints for the high dose (Figure 3; r = −0.684; p = 0.042), but not for the low dose (r = 0.083; p = 0.831). Exercise output after catheter implantation was not correlated with breakpoints maintained by either the low (r = 0.529; p = 0.170) or high (r = 0.326; p = 0.395) dose of cocaine. Breakpoints maintained by the two doses of cocaine were positively correlated with one another, but this effect failed to reach statistical significance (r = 0.646; p = 0.060).
Figure 3.
Correlation between exercise output and breakpoints maintained by a high dose (1.0 mg/kg/infusion) of cocaine. Vertical axis reflects the number of infusions obtained. Horizontal axis reflects exercise output expressed as the mean number of wheel revolutions per day (rev/day).
4. Discussion
The principal finding of this study is that long-term voluntary exercise decreases sensitivity to the positive-reinforcing effects of cocaine in female rats. Breakpoints maintained by cocaine on the PR schedule of reinforcement were significantly lower in exercising rats than sedentary rats, and this effect was apparent at both low and high doses of cocaine. In the high-dose condition, exercising rats reached a final ratio value that was less than one third the ratio value reached by sedentary rats (70 vs. 240). When these values are considered in terms of total number of responses per session, exercising rats emitted approximately 600 fewer responses per session than sedentary rats (270 vs. 1000). Importantly, these reductions in responding translated into a lower number of cocaine infusions (11 vs. 16) and a lower amount of cocaine intake (3.0 mg vs. 4.5 mg) during each self-administration session. Collectively, these data suggest the exercise may have “protective effects” on cocaine-seeking behavior, possibly by reducing the motivation to engage in behaviors that lead to cocaine self-administration.
Supporting the possibility that exercise was protective in the present investigation, there was a significant negative correlation between exercise output prior to catheter implantation and cocaine-maintained breakpoints after catheter implantation. In other words, those rats that ran the most prior to catheter implantation self-administered significantly less cocaine when it was later made available on the PR schedule. These data suggest that exercise may produce an output-dependent effect on those neuronal substrates that mediate the positive-reinforcing effects of cocaine. If this is correct, then greater levels of physical activity may result in greater degrees of protection from cocaine’s positive-reinforcing effects. Such a conclusion must be viewed with caution, however, as a significant correlation was not observed in the low dose condition, nor were significant correlations observed when exercise output after catheter implantation was used in the regression analysis.
As reported previously (Smith and Yancey, 2003; Smith and Lyle, 2006), exercise output gradually increased over the first 3 weeks of wheel exposure before leveling out until behavioral testing commenced. Exercise output decreased in all rats by approximately 50% with the acquisition of self-administration and then remained consistent until the end of behavioral testing. It is unlikely that these reductions were due to catheter implantation per se, as exercise output typically returns to baseline levels within 48 to 72 hours in the absence of drug administration (personal observations). It is also unlikely that these reductions can be attributed to the fact that rats were prevented from running during the self-administration sessions. Microanalysis studies of female rats reveal that over 95% of wheel running occurs during the dark phase of the light/dark cycle and less than 1% occurs during the first 3 hours of the light phase of the cycle (Eikelboom and Mills, 1988), the period of time in which self-administration sessions were conducted. Such reductions in wheel running reported in the present study may be due to depletion of central dopamine stores induced by repeated exposure to high doses of cocaine. Consistent with this possibility, a number of studies report that dopamine depletion induced by 6-hydroxydopamine lesions (Derevenco et al., 1986; Isobe and Nishino 2001), 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine lesions (Leng et al., 2004; Nakajima and Minematsu, 2006), and repeated amphetamine administration (Serwatkiewicz et al., 2000) markedly reduces exercise output in laboratory rats and mice.
In order to preserve the ecological validity of the study, no attempt was made to control or manipulate the estrous cycle. Previous studies report that hormonal fluctuations due to the estrous cycle can influence day-to-day variability in both running wheel activity (Steiner et al., 1982; Kent et al., 1991) and cocaine self-administration (Roberts et al., 1989; Feltenstein and See, 2007); however, it is unlikely that such day-to-day fluctuations could have accounted for the present results. For instance, on the PR schedule, all rats remained at a given dose until a stable breakpoint was reached. Based on our operational definition, a stable breakpoint could only be obtained after a minimum of 3 days, and we were able to obtain stable breakpoints in all rats at both doses of cocaine. Furthermore, all correlational analyses were conducted using exercise output data that were averaged over several weeks of data collection, thus masking any day-to-day fluctuations in running wheel activity. Although forced-exercise procedures serve as behavioral stressors and disrupt normal estrous cycling (Chatterton et al., 1990; Caston et al., 1995), voluntary exercise procedures do not produce estrous cycle disruptions or hormonal abnormalities (Dixon et al., 2003; Mathes and Kanarek, 2001). Although estrous cycle was not monitored in the present study, gonadal hormones were likely within the normal range of variability for both sedentary and exercising subjects.
Although it was not the aim of this study to determine the mechanism by which exercise alters the reinforcing efficacy of cocaine, a few possibilities deserve attention. One potential explanation for the decreased responding in exercising rats involves behavioral fatigue induced by wheel running. Although this is perhaps the most parsimonious explanation for the observed differences in cocaine-maintained responding, several pieces of data argue against this possibility. For instance, behavioral fatigue would be expected to affect all measures of operant responding. Although differences were observed in cocaine-maintained responding, no significant differences were observed in responding maintained by saline or in the number of inactive lever presses. Also, if wheel running immediately prior to the experimental session led to behavioral fatigue during the session, then exercise output during the period of time in which behavioral testing took place should be inversely related to responding. As noted above, this was not the case, and only exercise output prior to catheter implantation was predictive of cocaine self-administration.
Another possible explanation for our findings involves potential pharmacokinetic differences in the absorption, distribution, and metabolism of cocaine between sedentary and exercising subjects. Previous studies report that exercising rats have lower body weights, less adipose tissue, and smaller livers than sedentary rats (Pitts and Bull, 1977), any of which could alter the bioavailability of cocaine. Studies that have specifically compared plasma concentrations of cocaine in sedentary and exercising subjects have typically reported that exercise increases its bioavailability. For instance, Han et al. (1996) reported that plasma concentrations of cocaine were 69% greater in a group of forced exercise rats than in a group of rested control rats. Such increases in plasma concentrations would be expected to increase, not decrease, the reinforcing efficacy of cocaine in exercising subjects, thus making pharmacokinetic differences an unlikely explanation for our findings.
A third potential explanation for our findings involves exercise serving as an alternative, non-drug reinforcer to decrease cocaine self-administration. Although exercise can serve as an alternative, non-drug reinforcer when both are concurrently available (Kanarek et al., 1995, Cosgrove et al., 2002), a running wheel was never available during the self-administration sessions in the present study, and test sessions were scheduled at a time of day in which wheel running was a low-probability behavior. A previous study examining the circadian control of running reports that wheel activity is low during the first 3 hours of the light phase of the light/dark cycle (i.e., when all test sessions took place), and is virtually absent between the third and ninth hour of the light phase (Eikelboom and Mills, 1988). Consequently, the effects of exercise on cocaine-maintained breakpoints cannot be attributed to its ability to function as an alternative reinforcer either during or immediately after the self-administration sessions.
One additional explanation for the differences reported in the present study involves pharmacodynamic changes in those neuronal pathways that contribute to the positive reinforcing effects of cocaine. Several pieces of evidence suggest that acute bouts of exercise produce effects that are neurochemically similar to those produced by cocaine and other psychomotor stimulants. For instance, like cocaine, exercise increases central dopamine concentrations (Heyes et al., 1988; Hattori et al., 1994; Meeusen et al., 1997; Petzinger et al., 2007), and these increases are positively correlated with exercise output (Freed and Yamamoto, 1985). Importantly, chronic, long-term exercise leads to sustained increases in dopamine concentrations (Bauer et al., 1989) and compensatory changes in dopamine binding proteins (Fisher et al., 2004). Studies focusing specifically on the dopamine D2 receptor have typically reported an increase in D2 receptor density following chronic exercise (Gilliam et al., 1984; MacRae et al., 1987). The D2 receptor plays an important modulatory role in cocaine’s reinforcing effects (Nader et al., 1999; Caine et al., 2000; Khroyan et al., 2000), and there is an increasing body of evidence that the reinforcing effects of psychomotor stimulants are inversely related to D2 receptor density. For instance, in humans, the psychomotor stimulant methylphenidate is rated as less pleasurable and more aversive in people with high D2 receptor density than in people with low D2 receptor density (Volkow et al., 1999). In studies with non-human primates, social housing increases D2 receptor density in dominate males while simultaneously decreasing their propensity to self-administer cocaine (Morgan et al., 2002). It is possible that exercise produces its protective effects on cocaine self-administration via similar mechanisms; specifically, by producing functional alterations in those dopamine binding proteins that are critical for psychomotor stimulant reward.
Although exercise is not a standard component of most drug abuse prevention and treatment programs, those that do employ a physical fitness component have generally reported positive effects. For instance, a 12-week training program targeting adolescents and focusing on learning values and life skills through exercise reported a significant decrease in several risk factors associated with substance abuse and a concomitant reduction in the percentage of individuals who use cigarettes, smokeless tobacco, and alcohol (Collingwood et al., 2000). Similarly, a drug intervention program targeting at-risk adolescents and including an 8-week structured exercise class reported a significant decrease in anxiety, depression, and substance use in those participants exhibiting an improvement in physical fitness (Collingwood et al., 1991). Studies examining the efficacy of physical fitness programs in inpatient treatment facilities have also reported that exercise decreases depression and anxiety risk factors that are associated with relapse (Frankel and Murphy, 1974; Palmer et al., 1988). In a residential correctional facility for federal drug offenders, a wellness program that emphasized physical fitness produced improvements in several areas related to psychological well-being, including self-esteem, health awareness, healthy lifestyle adoption, and relapse prevention skills (Peterson and Johnstone, 1995). Finally, in one of the few studies that examined relapse to substance use after the termination of active treatment, a thrice weekly exercise program significantly increased abstinence rates in recovering alcoholics from 38% to 69% after 3 months (Sinyor et al., 1982). Such findings, coupled with the present data, suggest that aerobic exercise is an effective intervention for substance abuse and warrants an expanded role in prevention and treatment programs. Importantly, this particular intervention is inexpensive, widely available, easy to execute, and feasible for use in diverse patient populations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antunes HK, Stella SG, Santos RF, Bueno OF, de Mello MT. Depression, anxiety and quality of life scores in seniors after an endurance exercise program. Rev. Bras. Psiquiatr. 2005;27:266–271. doi: 10.1590/s1516-44462005000400003. [DOI] [PubMed] [Google Scholar]
- Bauer BA, Rogers PJ, Miller TD, Bove AA, Tyce GM. Exercise training produces changes in free and conjugated catecholamines. Med. Sci. Sports Exerc. 1989;21:558–562. [PubMed] [Google Scholar]
- Belke TW. Running and responding reinforced by the opportunity to run: effect of reinforcer duration. J. Exp. Anal. Beh. 1997;67:337–351. doi: 10.1901/jeab.1997.67-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW. Varying wheel-running reinforcer duration within a session: effect on the revolution-postreinforcement pause relation. J. Exp. Anal. Beh. 2000;73:225–239. doi: 10.1901/jeab.2000.73-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW, Dunbar MJ. Effects of cocaine on fixed-interval responding reinforced by the opportunity to run. J. Exp. Anal. Beh. 2001;75:77–91. doi: 10.1901/jeab.2001.75-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav. Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav. Neurosci. 1999;113:1080–1089. [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of mesolimbic dopamine depletion on responding maintained by cocaine and food. J. Exp. Anal. Behav. 1994;61:213–221. doi: 10.1901/jeab.1994.61-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Effects of dopamine D(1-like) and D(2-like) agonists on cocaine self-administration in rhesus monkeys: rapid assessment of cocaine dose-effect functions. Psychopharmacology. 2000;148:41–51. doi: 10.1007/s002130050023. [DOI] [PubMed] [Google Scholar]
- Caston AL, Farrell PA, Deaver DR. Exercise training-induced changes in anterior pituitary gonadotrope of the female rat. J. Appl. Physiol. 1995;79:194–201. doi: 10.1152/jappl.1995.79.1.194. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Hartman AL, Lynn DE, Hickson RC. Exercise-induced ovarian dysfunction in the rat. Proc. Soc. Exp. Biol. Med. 1990;193:220–224. doi: 10.3181/00379727-193-43029. [DOI] [PubMed] [Google Scholar]
- Collingwood TR, Reynolds R, Kohl HW, Smith W, Sloan S. Physical fitness effects on substance abuse risk factors and use patterns. J. Drug Educ. 1991;21:73–84. doi: 10.2190/HV5J-4EYN-GPP7-Y3QG. [DOI] [PubMed] [Google Scholar]
- Collingwood TR, Sunderlin J, Reynolds R, Kohl HW., 3rd Physical training as a substance abuse prevention intervention for youth. J. Drug Educ. 2000;30:435–451. doi: 10.2190/RVUE-9XW7-TYRQ-EJR8. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol. Biochem. Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Derevenco P, Stoica N, Sovrea I, Imreh S. Central and peripheral effects of 6-hydroxydopamine on exercise performance in rats. Psychoneuroendocrinology. 1986;11:141–153. doi: 10.1016/0306-4530(86)90049-1. [DOI] [PubMed] [Google Scholar]
- Dixon DP, Ackert AM, Eckel LA. Development of, and recovery from, activity-based anorexia in female rats. Physiol. Behav. 2003;80:273–279. doi: 10.1016/j.physbeh.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am. J. Prev. Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol. Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Sanders CE. Exercise is positively related to adolescents' relationships and academics. Adolescence. 2001;36:105–110. [PubMed] [Google Scholar]
- Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J. Neurosci. Res. 2004;77:378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- Frankel A, Murphy J. Physical fitness and personality in alcoholism. Canonical analysis of measures before and after treatment. Q. J. Stud. Alcohol. 1974;35:1272–1278. [PubMed] [Google Scholar]
- Freed CR, Yamamoto BK. Regional brain dopamine metabolism: a marker for the speed, direction, and posture of moving animals. Science. 1985;229:62–65. doi: 10.1126/science.4012312. [DOI] [PubMed] [Google Scholar]
- Gilliam PE, Spirduso WW, Martin TP, Walters TJ, Wilcox RE, Farrar RP. The effects of exercise training on [3H]-spiperone binding in rat striatum. Pharmacol. Biochem. Behav. 1984;20:863–867. doi: 10.1016/0091-3057(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Han DH, Kelly KP, Fellingham GW, Conlee RK. Cocaine and exercise: temporal changes in plasma levels of catecholamines, lactate, glucose, and cocaine. Am. J. Physiol. 1996;270:E438–E444. doi: 10.1152/ajpendo.1996.270.3.E438. [DOI] [PubMed] [Google Scholar]
- Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain Res. Bull. 1994;35:41–49. doi: 10.1016/0361-9230(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Garnett ES, Coates G. Nigrostriatal dopaminergic activity is increased during exhaustive exercise stress in rats. Life Sci. 1988;42:1537–1542. doi: 10.1016/0024-3205(88)90011-2. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Isobe Y, Nishino H. Circadian rhythm of drinking and running-wheel activity in rats with 6-hydroxydopamine lesions of the ventral tegmental area. Brain Res. 2001;899:187–192. doi: 10.1016/s0006-8993(01)02223-5. [DOI] [PubMed] [Google Scholar]
- Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J. Exp. Anal. Beh. 1993;60:219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1984;19:13–25. doi: 10.1016/0304-3959(84)90061-7. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Marks-Kaufman R, D'Anci KE, Przypek J. Exercise attenuates oral intake of amphetamine in rats. Pharmacol. Biochem. Behav. 1995;51:725–729. doi: 10.1016/0091-3057(95)00022-o. [DOI] [PubMed] [Google Scholar]
- Kent S, Hurd M, Satinoff E. Interactions between body temperature and wheel running over the estrous cycle in rats. Physiol. Behav. 1991;49:1079–1084. doi: 10.1016/0031-9384(91)90334-k. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J. Pharmacol. Exp. Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Kirkcaldy BD, Shephard RJ, Siefen RG. The relationship between physical activity and self-image and problem behaviour among adolescents. Soc. Psychiatry Psychiatr. Epidemiol. 2002;37:544–550. doi: 10.1007/s00127-002-0554-7. [DOI] [PubMed] [Google Scholar]
- Leng A, Mura A, Hengerer B, Feldon J, Ferger B. Effects of blocking the dopamine biosynthesis and of neurotoxic dopamine depletion with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on voluntary wheel running in mice. Behav. Brain Res. 2004;154:375–383. doi: 10.1016/j.bbr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol. Behav. 2001;72:355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- MacRae PG, Spirduso WW, Walters TJ, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology. 1987;92:236–240. doi: 10.1007/BF00177922. [DOI] [PubMed] [Google Scholar]
- Manger TA, Motta RW. The impact of an exercise program on posttraumatic stress disorder, anxiety, and depression. Int. J. Emerg. Ment. Health. 2005;7:49–57. [PubMed] [Google Scholar]
- Mathes WF, Kanarek RB. Wheel running attenuates the antinociceptive properties of morphine and its metabolite, morphine-6-glucuronide, in rats. Physiol. Behav. 2001;74:245–251. doi: 10.1016/s0031-9384(01)00577-7. [DOI] [PubMed] [Google Scholar]
- Meeusen R, Smolders I, Sarre S, de Meirleir K, Keizer H, Serneels M, Ebinger G, Michotte Y. Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta. Physiol. Scand. 1997;159:335–341. doi: 10.1046/j.1365-201X.1997.00118.x. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morgan WP. Psychological effects of exercise. Behav. Med. Update. 1982;4:25–30. [Google Scholar]
- Muller SM, Dennis DL, Gorrow T. Emotional well-being of college students in health courses with and without an exercise component. Percept. Mot. Skills. 2006;103:717–725. doi: 10.2466/pms.103.3.717-725. [DOI] [PubMed] [Google Scholar]
- Nabetani T, Tokunaga M. The effect of short-term (10- and 15-min) running at self-selected intensity on mood alteration. J. Physiol. Anthropol. Appl. Human Sci. 2001;20:231–239. doi: 10.2114/jpa.20.233. [DOI] [PubMed] [Google Scholar]
- Nader MA, Green KL, Luedtke RR, Mach RH. The effects of benzamide analogues on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1999;147:143–152. doi: 10.1007/s002130051154. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Minematsu M. Ameliorating effect of dimethylsulfoniopropionate on the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease of mice. J. Nutr. Sci. Vitaminol. 2006;52:70–74. doi: 10.3177/jnsv.52.70. [DOI] [PubMed] [Google Scholar]
- Norris R, Carroll D, Cochrane R. The effects of aerobic and anaerobic training on fitness, blood pressure, and psychological stress and well-being. J. Psychosom. Res. 1990;34:367–375. doi: 10.1016/0022-3999(90)90060-h. [DOI] [PubMed] [Google Scholar]
- Norris R, Carroll D, Cochrane R. The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. J. Psychosom. Res. 1992;36:55–65. doi: 10.1016/0022-3999(92)90114-h. [DOI] [PubMed] [Google Scholar]
- Nowlis DP, Greenberg N. Empirical description of effects of exercise on mood. Percept. Mot. Skills. 1979;49:1001–1002. doi: 10.2466/pms.1979.49.3.1001. [DOI] [PubMed] [Google Scholar]
- Palmer J, Vacc N, Epstein J. Adult inpatient alcoholics: physical exercise as a treatment intervention. J. Stud. Alcohol. 1988;49:418–421. doi: 10.15288/jsa.1988.49.418. [DOI] [PubMed] [Google Scholar]
- Peterson M, Johnstone BM. The Atwood Hall Health Promotion Program, Federal Medical Center, Lexington, KY. Effects on drug-involved federal offenders. J. Subst. Abuse Treat. 1995;12:43–48. [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Pitts GC, Bull LS. Exercise, dietary obesity, and growth in the rat. Am. J. Physiol. 1977;232:R38–R44. doi: 10.1152/ajpregu.1977.232.1.R38. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Schebendach JE, Klein DA, Foltin RW, Devlin MJ, Walsh BT. Relative reinforcing value of exercise in inpatients with anorexia nervosa: model development and pilot data. Int. J. Eat. Disord. 2007;40:446–453. doi: 10.1002/eat.20392. [DOI] [PubMed] [Google Scholar]
- Serwatkiewicz C, Limebeer C, Eikelboom R. Sensitization of amphetamine-induced wheel running suppression in rats: dose and context factors. Psychopharmacology. 2000;151:219–225. doi: 10.1007/s002130000446. [DOI] [PubMed] [Google Scholar]
- Sinyor D, Brown T, Rostant L, Seraganian P. The role of a physical fitness program in the treatment of alcoholism. J. Stud. Alcohol. 1982;43:380–386. doi: 10.15288/jsa.1982.43.380. [DOI] [PubMed] [Google Scholar]
- Smith MA, Lyle MA. Chronic exercise decreases sensitivity to mu opioids in female rats: correlation with exercise output. Pharmacol. Biochem. Behav. 2006;85:12–22. doi: 10.1016/j.pbb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology. 2003;168:426–434. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Steiner M, Katz RJ, Carroll BJ. Detailed analysis of estrous-related changes in wheel running and self-stimulation. Physiol. Behav. 1982;28:201–204. doi: 10.1016/0031-9384(82)90127-5. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology. 2002;27:970–979. doi: 10.1016/S0893-133X(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Veale D, Le Fevre K, Pantelis C, de Souza V, Mann A, Sargeant A. Aerobic exercise in the adjunctive treatment of depression: a randomized controlled trial. J. R. Soc. Med. 1992;85:541–544. doi: 10.1177/014107689208500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am. J. Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Waade NR. Exercise improves self-esteem in children and young people. Aust. J. Physiother. 2004;50:117. doi: 10.1016/s0004-9514(14)60106-9. [DOI] [PubMed] [Google Scholar]
- Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]