Abstract
Aims
Tanshinone IIA is an important ingredient in the herb danshen (Salvia miltiorrhiza), which has been used to treat cardiovascular diseases such as atherosclerosis and angina for hundreds of years in China. There are numerous reports that TIIA has antioxidant properties but the chemical structure indicates that TIIA is fully oxidized. Here, we test the hypothesis that TIIA alters the expression and/or activity of specific anti-oxidation enzymes to protect cells from oxidant damage.
Main Methods
We utilized J774 macrophages to model cellular responses to TIIA when challenged with H2O2. Expression and activity levels of several anti-oxidation enzymes were investigated and the only system modulated by TIIA was glutathione peroxidase (GPx).
Key findings
GPx-1 mRNA levels were significantly increased by TIIA but not the vitamin E analogue, Trolox. GPx activities were also significantly increased by TIIA. Mercaptosuccinic acid inhibited GPx activity and the protective effect of TIIA was attenuated. Thus, TIIA protects cultured macrophages from H2O2-induced cell death and protection is mediated in large part by TIIA induction of GPx gene expression and activity.
Significance
Because of the importance of GPx in health and because TIIA is able to modulate GPx activity to some extent in cell culture, we suggest that TIIA is a worthwhile candidate for further study in animal models of atherosclerosis and eventually in human prospective trials.
Keywords: Tanshinone, Macrophages, Cell death, Apoptosis, Glutathione peroxidase, Transcription, ROS, Herb
INTRODUCTION
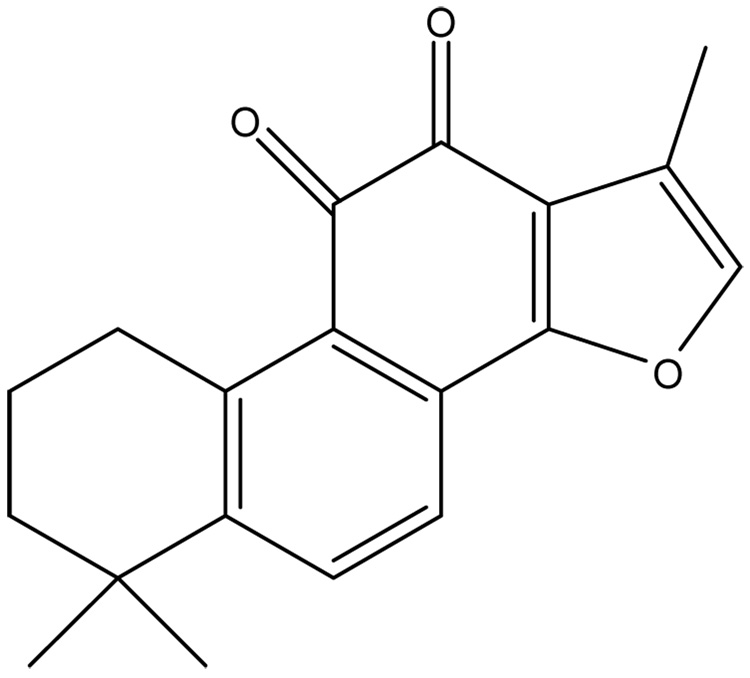
Danshen (Salvia miltiorrhiza) is an herbal supplement that has been used to treat cardiovascular disease for hundreds of years in China (Zhou et al. 2005). Danshen contains of a mixture of compounds but tanshinone IIA (TIIA) is considered to be the most important bioactive ingredient (Zhongguo 2005). The rationale for using danshen as a treatment is primarily based on observational studies in animals and cell culture, and data obtained from a few small clinical trials involving danshen or specific ingredients as performed in China (Janji et al. 2000). There are several pathways by which danshen or specific ingredients are thought to provide beneficial affects toward cardiovascular diseases. These are protecting vascular cells from damage and death through anti-oxidation pathways (Zhou et al. 2003), dampening thrombosis by reducing platelet aggregation and restenosis following vascular injury (Liu et al. 2002; Wang et al. 1989), and improving heart function by increasing collateral blood vessel formation and general myocyte function (Li and Tang 1991; Zhao et al. 1996). However, large scale prospective studies using well defined compounds with consistent end-points have yet to be performed to support claims that danshen is beneficial for cardiovascular diseases. Further, molecular mechanisms by which TIIA alters cell biology to effect protection are unclear. For instance, there are numerous reports that TIIA has anti-oxidant properties which prevent the oxidation of low-density lipoproteins (LDLs) (Niu et al. 2000), rescue PC-12 cells from hypoxia (He et al. 2001), and reduce cellular damage caused by free radicals (Zhao et al. 1996; Zhou et al. 1999; Wang et al. 2003). However, TIIA is a diterpene quinine which is fully oxidized (Figure 1). Thus, it is unlikely that TIIA acts directly as an anti-oxidant to prevent cell damage induced by reactive oxygen species (ROS).
Figure 1.
Chemical structure of tanshinone IIA (TIIA). This compound appears as a red crystal, has a molecular weight of 294.34 and is fully oxidized. It is hydrophobic and highly soluble in organic solvents such as ethanol.
In this report, we test the hypothesis that cells challenged with H2O2 are protected from cell damage and apoptosis by TIIA. We further test the hypothesis that TIIA effects such protection by altering the expression and/or activity of specific anti-oxidation enzymes. We utilize a macrophage cell culture system because macrophages play an important role in the pathogenesis of atherosclerosis. The main finding is that TIIA protects cultured macrophages from H2O2-induced cell death and protection is mediated in large part by the induction of glutathione peroxidase gene expression and activity.
EXPERIMENTAL PROCEDURES
Materials
The J774 mouse macrophage cell line was purchased from ATCC (Manassas, VA). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), L-glutamine, penicillin and streptomycin, and SYBR Green I nucleic acid gel stain (SYBR-I) were purchased from Invitrogen (Carlsbad, CA). Hydrogen peroxide, JC-1 dye, 3-amino-1,2,4-triazole (AZT), mercaptosuccinic acid (MS) and DMSO were purchased from Sigma (St. Louis, MO). TUNEL reagents were obtained from Roche Applied Science (Indianapolis, IN). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), Caspase-3 Cellular Activity Assay Kit (#235419) and Caspase-9 Assay Kit (#218824) were purchased from EMD Biosciences (San Diego, CA). The Caspase-8 Assay Kit (#BV-K113-2) was purchased from MBL International (Watertown, MA). The Glutathione Peroxidase Assay Kit (#703102) and the Catalase activity kit (#707002) were purchased from Cayman Chemical (Ann Arbor, MI). Tanshinone IIA was obtained from LKT labs (#T0154; St. Paul, MN) and purity certification given as 98.5% based on HPLC analyses. RNeasy Mini Kit was obtained from Qiagen (Chatsworth, CA) and the Micro BCA reagents for protein concentration determinations were purchased from Pierce (Rockford, IL).
Cell Culture, Drug Pretreatments and Oxidative Model
J774 macrophages were maintained in 150 cm2 culture flasks in DMEM supplemented with 10% FBS, 2mM L-glutamine and 100 U/ml penicillin and streptomycin in humidified 95% air and 5% CO2 at 37°C before subculture. Trypan blue exclusion was used to examine cell viability which was higher than 90%.
To determine conditions needed for H2O2 cytotoxicity and rescue by Trolox, cells were planted in chamber slides filled with FBS-free DMEM and then H2O2 was added in increasing concentrations per slide (50 µM, 100 µM, 200 µM, 300 µM and 400 µM) and for different times (0.5, 1, 2, and 4 hours). Over 90% cell death was achieved by 4 hours of treatment with 300 µM to 400 µM H2O2 (data not shown) and this condition (4 hours treatment with 300 µM H2O2) was selected for further studies.
In order to test the ability of Trolox and TIIA to protect cells from H2O2-mediated cell death, cells were incubated in DMEM supplemented with 1% FBS, 2mM L-glutamine, 100 U/ml penicillin and streptomycin. Trolox and TIIA were dissolved in 100% ethanol and added into the medium to achieve final concentrations of either 1 mM (Trolox), 0.6 µM (TIIA), or 3.0 µM (TIIA) and 1 µg/ml ethanol (vehicle). Cells were then incubated for 18 hours prior to cell viability and other assays. In a separate set of control studies, ethanol treatment alone failed to lead to cell death and did not protect cells from H2O2 mediated cell death (data not shown). In all cases, studies were performed in quadruplicate, and in most cases, complete repetitions of studies were performed.
Caspase Activities, DNA Fragmentation and Mitochondrial Membrane Potential Change
To evaluate activities for caspase-3, 8 and 9, cells were lysed, centrifuged and cellular debris was discarded. Protein concentrations were quantified using Micro BCA reagents and activities were measured using commercial kits as described in Materials. DNA fragmentation was assayed by using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick-end labeling (TUNEL) method and the procedures were followed as given by the manufacturer. The mitochondrial membrane potential (Ψm) was assayed by using JC-1 dye (Reers et al. 1991). After treatments, cells were incubated in the dark for 15 min at 37 °C in DMEM containing the JC-1 d ye. In healthy cells, the dye accumulates and aggregates in mitochondria, emitting a bright red fluorescence (λem=585–590nm). In apoptotic and necrotic cells with altered Ψm, the dye remains in the cytoplasm in its monomeric form and the fluorescence is green (λem=527–530nm). Fluorescence intensity was measured by fluorescence-activated cell sorting (FACS) using a Beckman Coulter FC500. Each condition was conducted in triplication and five thousand events were counted in one sample.
Real-Time Reverse-Transcribed Polymerase Chain Reaction (RT-PCR)
Total RNA samples were isolated from J774 cells using the RNeasy Mini Kit and quantified by spectrometry. First-strand cDNAs were synthesized from 2.0 µg of total RNA with M-MLV reverse transcriptase. The fluorescent reporter dye used in real-time RT-PCR was SYBR-I. The primer pairs were purchased from IDT (Coralville, IA) and the sequences are listed in Table 1. Sequences for SOD1-2, GPx-3-4 were designed by using Primer Express 3.0 (Applied Biosystem, Foster City, CA) and sequences for catalase, GPx-1-2 were adopted from literature sources (Esworthy et al. 2005; Fujita et al. 2005; Kwei et al. 2004). All primer sequences were examined by using the NCBI Basic Local Alignment Search Tool (BLAST) (Altschul et al. 1990) to validate sequences and specificity. PCR assays were carried out using the Taqman Universal Master Mix and an ABI Prism 7500 Fast Sequence Detection System (Applied Biosystem, Foster City, CA) under the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute.
Table 1.
Primer sequences of 18S, SOD1, SOD2, Catalase, GPx-1-4
| Forward Primer | Reverse Primer | Reference | |
|---|---|---|---|
| 18S | 5’-CGCCGCTAGAGGTGAAATTC-3’ | 5’-TTGGCAAATGCTTTCGCTC-3’ | |
| SOD1 | 5’-TTTTTTTGCGCGGTCCTTT-3’ | 5’-ACCAGAGAGAGCAAGACGAGAAG-3’ | |
| SOD2 | 5’-CCTGCTCTAATCAGGACCCATT-3’ | 5’-CGTGCTCCCACACGTCAAT-3’ | |
| Catalase | 5’-CCGACCAGGGCATCAAAA-3’ | 5’-GAGGCCATAATCCGGATCTTC-3’ | Esworthy et al. 2005 |
| GPx-1 | 5’-TCAGTTCGGACACCAGGAGAA-3’ | 5’-CTCACCATTCACTTCGCACTTC-3’ | Kwei et al. 2004 |
| GPx-2 | 5’-CCAGCTCAATGAGCTGCAATG-3’ | 5’-CCCCCAGGTCGGACATACTT-3’ | Fujita et al. 2005 |
| GPx-3 | 5’-GCCCTCCCACTGCAGAACT-3’ | 5’-GGATCTTCATGGGTTCCCAAA-3’ | |
| GPx-4 | 5’-GCATCCCGCGATGATTG-3’ | 5’-TCGATGTCCTTGGCTGAGAAT-3’ |
Glutathione Peroxidase (GPx) and Catalase Activities
After treatments, J774 cells were collected by centrifugation and pellets were homogenized in a cell lysis buffer containing 1mM Tris base, 15mM NaCl, and 1% Triton X-100 and proteasase inhibitor cocktail. After centrifugation at 10,000 × g for 15 minutes at 4°C, the supernatant was stored at −80°C until further analysis. The protein concentration was measured using Micro BCA reagents. Cellular glutathione peroxidase and catalase activities were measured as described in the manufacturer’s instructions. Briefly, for GPx, cell lysates were mixed with co-substrate containing NADPH, glutathione, and glutathione reductase in a 96-well plate. The reaction was initiated by cumene hydroxide supplied in the kit. The absorbance was read every minute at 340nm using a plate reader at 25°C for 5 minutes. The GPx activity was then calculated by comparisons to pure GPX enzyme supplied in the kit and reported as nmol/min/ml. For catalase activity, 20µl cell lysates were added to 100µl assay buffer containing 100mM potassium phosphate and 30µl methanol. The reaction was initiated by adding 20µl hydrogen peroxide and incubated for 20minutes at room temperature. After adding 30µl potassium hydroxide and 30µl Purpald (4-amino-3-hydazino-5-mercapto-1,2,4-triazole which is used as a chromagen for this colorimetric assay) for 10 minutes at room temperature, 10ul potassium periodate was added and the plate was read at 540nm.
Statistics
Values are reported as the mean ± SEM. ANOVA analyses were applied to assess differences between averages. In some cases, the Student’s t-test was used to compare independent means.
RESULTS
TIIA and Trolox protect against H2O2-mediated toxicity in J774 macrophages
We utilized Trolox as a positive control for assays used throughout this study. Trolox is a water soluble anti-oxidant vitamin E analog which has been shown to have scavenging properties for a wide range of ROS (Penn et al. 1997; Salgo and Pryor 1996). Trolox is a powerful inhibitor of membrane damage (Forrest et al. 1994), and is known to reduce H2O2 induced damage to a variety of cell types including reducing apoptosis (Salgo and Pryor 1996; Forrest et al. 1994). Thus, Trolox provides a useful reference to which to compare the antioxidant capacity of TIIA.
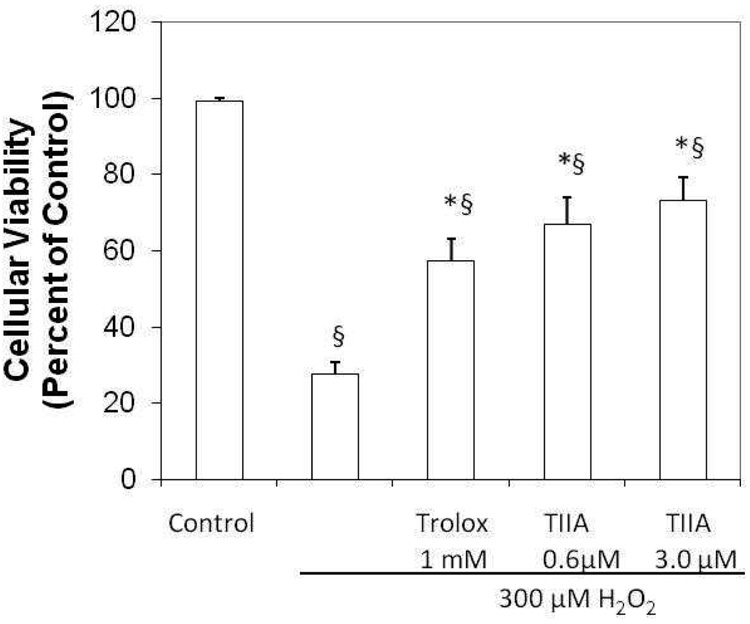
The effects of H2O2 on cell death were first determined using the TUNEL assay. Cytotoxicity was negligible for control cells (viability near 100%), but significant death (73%) was seen for cells treated with H2O2 (300 µM) (Figure 2). Pretreatment with Trolox significantly attenuated the cytotoxicity mediated by H2O2 (relative viability 57%, p<0.001). TIIA was also able to maintain cell viability at levels comparable to or better than Trolox (67% at 0.6 µM TIIA, p<0.001 and 73% in 3 µM TIIA, p<0.007).
Figure 2.
Protective effect of Trolox and TIIA against H2O2 mediated cytotoxicity. J774 macrophages were pretreated for 18 hours with ethanol (vehicle), 1 mM Trolox, 0.6 µM TIIA or 3 µM TIIA. Except for a control group, cells were then treated with 300 µM H2O2 for 4 hours. Cell death was evaluated by TUNEL staining, using DAPI (Blue) for cellular nuclei. Cell death was nearly absent in the control group (vehicle treatment only) for which cell viability was set to 100%. Positive TUNEL staining was evident in cells treated with H2O2 with significantly less staining seen for cells pretreated with Trolox or TIIA. TUNEL positive cells were evaluated in five different microscope fields with n=4 per group and data presented as mean ± SEM; *p<0.007 versus H2O2; § p<0.005 versus control.
TIIA does not prevent mitochondrial membrane potential changes or reduce caspase activities following H2O2 treatment
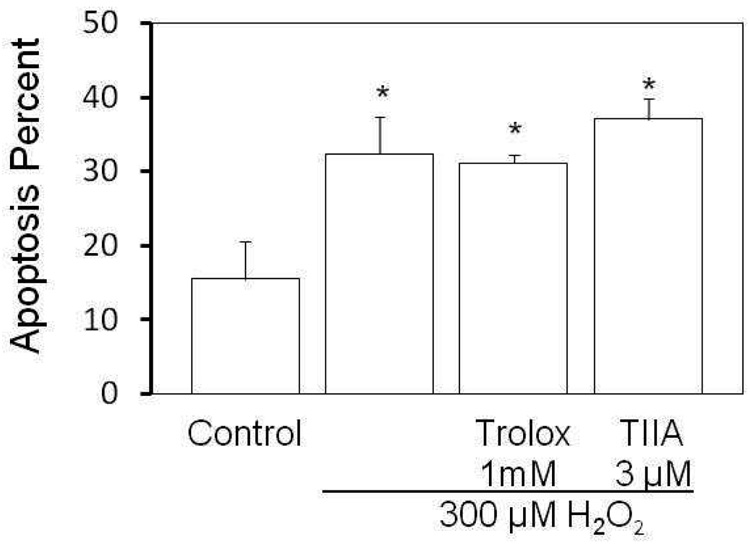
We tested whether TIIA protects cells through processes often associated with apoptosis. To evaluate the ability of TIIA to preserve the mitochondrial membrane potential, J774 cells were incubated with JC-1, a fluorescent indicator of mitochondrial membrane potential, following treatment of cells with H2O2. Cells with healthy mitochondria emit red fluorescence, whereas cells with unhealthy mitochondria emit green fluorescence. FACS was used to quantify the extent of red or green fluorescence in cells and results are shown in Figure 3. H2O2 treatment induced marked increased green to red fluorescence intensity as indicated by an apoptosis percentage of 32.4 ± 4.9% (p=0.006 vs control). No significant changes from the H2O2 treatment were seen for the TIIA group suggesting that TIIA does not prevent collapse of the mitochondrial electrochemical gradient as elicited by H2O2.
Figure 3.
Mitochondrial potential is not altered by TIIA. H2O2-induced apoptosis in J774 cells was analyzed by flow cytometry. J774 cells receiving vehicle, 1mM Trolox or 3µM TIIA pretreatment as in Figure 2 were incubated with 300 µM H2O2 for 4 hours. The mitochondrial membrane potential was assessed using the JC-1 dye (as described in Methods). Cells emitting green fluorescence were considered apoptotic. Five thousands cells were counted in one sample. The apoptosis percentage was calculated as: cells emitting green fluorescence/ total cells, presented as mean ± SEM and n = 3 in each group; *p<0.006 versus control.
We tested whether TIIA protects cells from H2O2-mediated death by altering the activities of caspase enzymes. Caspase-3, -8, and -9 are members of separate subfamilies of caspase enzymes which act at different stages of cell death and cell protection (Kuranaga and Miura 2007). Caspase-3 has been implicated in macrophage apoptosis (Asmis and Begley 2003) and is activated by caspase-9. Caspase-8 is activated through an extrinsic pathway involving the tumor necrosis factor receptor and Fas receptor (Kuranaga and Miura 2007). Cell lysates from untreated cells (control) and cells treated with H2O2 (300µM), Trolox (1mM) and TIIA (3.0 µM) were collected and assayed for caspase-3, -8 and -9 activities (Table 2). As expected, H2O2 activated all three cellular caspase activities significantly as compared to control cells. Trolox and TIIA did not reduce caspase-3 and -9 activities due to pretreatment of cells with H2O2. While Trolox significantly reduced caspase-8 activity as compared to the H2O2 levels (22% reduction, p< 0.05), TIIA did not reduce caspase-8 activity. Trolox is known to protect against apoptosis likely because of its radical scavenging potential which may have reduced cellular ROS concentrations. Overall, it is unlikely that TIIA mediates protective effects against cell death through caspase pathways.
Table 2.
TIIA did not affect caspase activities. J774 cells received no pretreatment (control and H2O2 group), 1mM Trolox (Trolox group) and 3µM TIIA (TIIA group) for 18 hours. Then, H2O2, Trolox and TIIA groups were incubated with 300µM H2O2, whereas the control group was kept in regular medium. After 4 hours, cells were collected and cell lysates were used for measuring caspase activities as described under “Experimental Procedures.” H2O2 increased all three caspases activities. Trolox only reduced caspase-8 activity, not caspase-9 or caspase-3. TIIA, however, did not inhibit any of these caspases.
| Pretreatment | H2O2 | Caspase-9 (OD/µg protein) | Caspase-3 (pmol/min/µg protein) | Caspase-8 (OD/µg protein) | |
|---|---|---|---|---|---|
| Control | None | − | 2.05±0.39 | 0.21±0.01 | 1.73±0.22 |
| H2O2 | None | + | 7.88±0.04 | 1.13±0.05 | 6.30±0.22 |
| Trolox | Trolox | + | 7.04±0.89 | 1.32±0.12 | 4.92±0.44* |
| TIIA | TIIA | + | 9.37±1.23 | 1.55±0.26 | 6.47±0.54 |
p-value < 0.05 versus H2O2 group
Tanshinone IIA induces GPx-1 mRNA
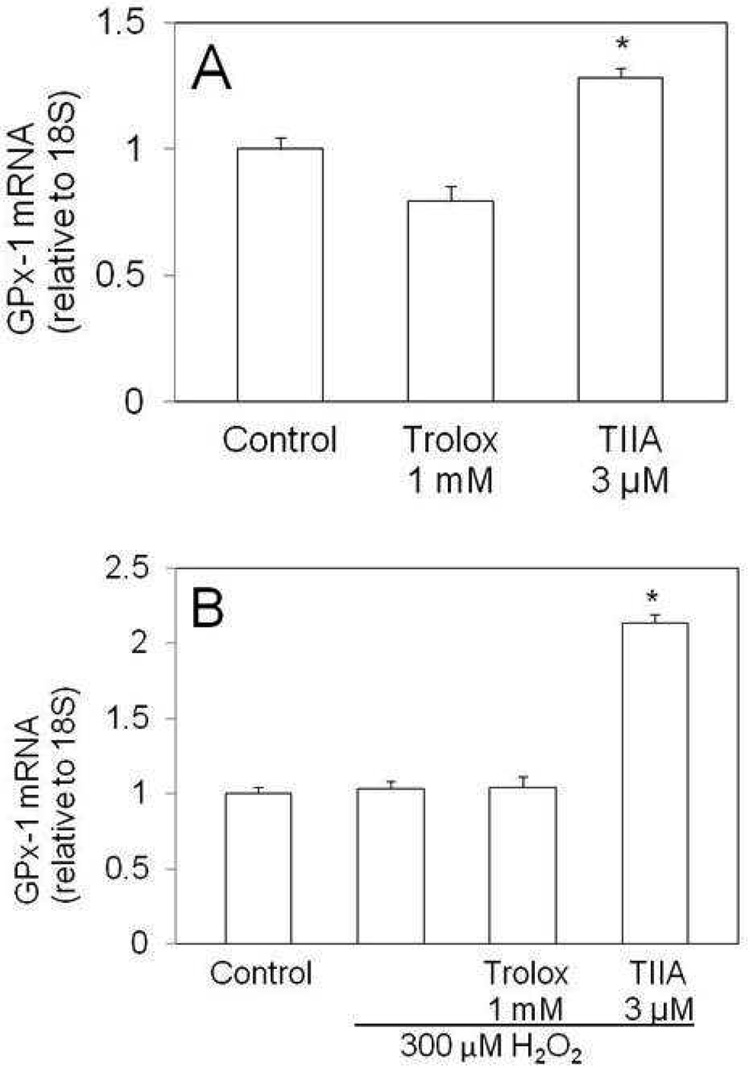
Several reports implicate tanshinone derivatives as able to scavenge oxidative radicals and protect cells, tissue and low density lipoproteins from oxidative damage (Zhao et al 1996; Niu et al. 2000; He et al. 2001; Zhou et al. 1999; Wang et al. 2003). Since TIIA is fully oxidized, it is unlikely that TIIA acts directly as an antioxidant. However, it is possible that TIIA induces antioxidant defenses through increasing the expression of one or more antioxidant enzymes. We tested this concept by examining gene expression and in some cases, enzymatic activity for seven antioxidant enzymes (SOD1, SOD2, catalase, GPx-1, GPx-2, GPx-3 and GPx-4). Gene expression was initially examined in the absence of H2O2 treatment to test for direct effects of TIIA. Trolox treatment resulted in a 2.7-fold increase in catalase mRNA level as compared to levels in untreated cells (p=0.02), but no other differences were seen among the genes tested (data not shown). For cells treated with TIIA, the only alteration in mRNA level among the genes tested was for GPx-1 for which mRNA levels were elevated by 30% over levels seen for untreated cells (Figure 4A; p=0.01). GPx-1 mRNA levels were additionally and markedly elevated (100%; p<0.001) for TIIA treated cells which were also challenged with H2O2 (Figure 4B).
Figure 4.
TIIA treated cells contain increased levels of GPx-1. J774 cells received vehicle, Trolox or TIIA for 18 hours. After this pretreatment, cells were treated with H2O2 for 4 hours. GPx-1 mRNA levels were measured after pretreatment (A) and after treatment (B) with H2O2. Data is presented as mean ± SEM and three replicates per group; *: p< 0.01 versus control.
Mercaptosuccinic acid inhibits GPx activity and eliminates the protective effect of TIIA against H2O2
The detoxification of H2O2 in cells is predominantly mediate by the antioxidation enzyme activities of catalase and glutathione peroxidase (GPx) (Dringen et al. 2005; Liddell et al. 2006). We tested whether TIIA could modulate enzymatic activities for catalase and GPx.
TIIA failed to significantly alter catalase enzyme activity (data not shown). We tested for TIIA contributions to catalase activity in studies of J774 cells treated and untreated with H2O2. Thus, TIIA is unlikely to provide cytoprotection by influencing the expression or activity of catalase.
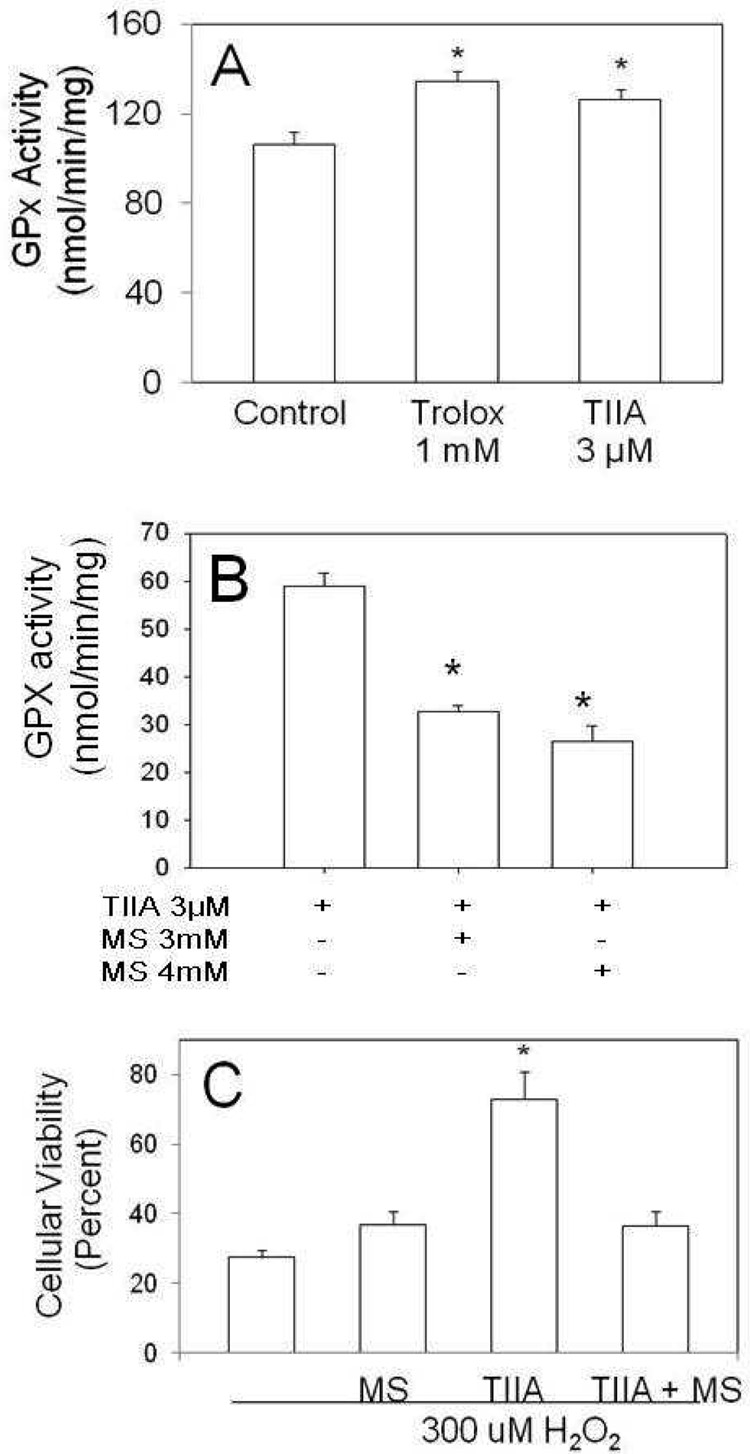
GPx enzyme activity in J774 cells was increased modestly (26% for Trolox and 18% for TIIA) but significantly (p=0.03) by TIIA and Trolox as compared to untreated control cells (Figure 5A). To further evaluate the contribution of GPx activity in the mediation of cell survival by TIIA, we utilized an inhibitor of overall GPx activity, mercaptosuccinic acid (MS). We treated cells with TIIA and either 3 mM or 4 mM MS. Treatment with MS significantly decreased the TIIA enhancing effects on GPx activity (by 45%, p=0.004; Figure 5B). The functional relevance of reduced GPx activity was tested by quantifying the extent of cell death using TUNEL staining. The extent of cell death induced following treatment of J774 cells with 300 uM H2O2 (approximately 70%) did not change significantly when cells were also treated with 3 mM MS (Figure 5C). Importantly, the protection from H2O2 mediated cell death provided by TIIA was ameliorated when 3mM MS was present (Figure 5C). Taken together, these data suggest that the induction of GPx provides an important mechanism by which TIIA mediates its protective effects.
Figure 5.
GPx activity and cytotoxicity evaluated in J774 macrophage cells. (A) J774 cells were incubated with vehicle, Trolox (1mM) or TIIA (3µM) for 18 hours. Cell lysates were then assayed for total GPx activity. (B) J774 cells were incubated with 3 uM TIIA, or with TIIA plus 3 mM or 4 mM of MS for 18 hours. (C) J774 cells were pretreated for 18 hours with ethanol (vehicle), 3 mM MS, 3 uM TIIA or 3 uM TIIA with 3 mM MS. Cells were then treated with 300 uM H2O2 for 4 hours. Cell death was evaluated by TUNEL stainig, using DPI (Blue) for cellular nuclei and data is presented with respect to untreated cells which were set at 100% viability (not included in this figure). Data is presented as mean ± SEM. For panels A and B, * p< 0.03 versus control; n=3 per group. For panel C, *p<0.02 versus all other conditions; n=3 or 4 per group.
DISCUSSION
Oxidative stress has been implicated in pathological processes associated with atherosclerosis and other cardiovascular diseases. In the present report, we tested the hypotheses that TIIA, an ingredient of the herbal supplement danshen, is cytoprotective following ROS challenge and that protective effects are mediated through the altered expression or activity of specific antioxidant enzymes. The primary findings were that TIIA protects cultured macrophages from H2O2-induced cell death and protection is mediated in part by induction of glutathione peroxidase activity.
TIIA is a diterpene thought to be a major bioactive molecule in danshen, a dietary supplement derived from the root of Salvia miltiorrhiza, widely used in China and now available in health food stores in the United States. In China, danshen is commonly used to treat heart disease and cancer but large scale prospective studies have not been performed to test its efficacy. Nonetheless, several ingredients of danshen such as tanshinone derivatives are being investigated as possible drug treatments (Geng et al. 2004; Min et al. 2002). In most cases, studies utilize mixtures of danshen products or crude extracts from the Salvia miltiorrhiza root and thus, it is difficult to attribute protective or damaging effects to one danshen component. Our study is significant because we utilize a purified product to identify mechanisms by which TIIA can alter cell biology.
We chose a system of cell death mediated by a peroxide product, H2O2, produced from the reduction of superoxide, in a cell culture system modeling macrophages as found in vascular diseases such as atherosclerosis. H2O2 is known to induce cell death in many types of cells (Chaube et al. 2005; Park et al. 2005; Jiang et al. 2005) by promoting changes in mitochondrial permeability and transmembrane potential, leading to release of cytochrome c, activation of caspase-3, and apoptosis (M’Bemba-Meka et al. 2005; Lee et al. 2005; Simizu et al. 1998). In our study, J774 cells exhibited increases in mitochondrial potential and in the activities of caspase-3, -9, and-8 with H2O2 treatment. Although these events were not reversed by TIIA pretreatment, TIIA did promote cell viability (Figure 2) suggesting that TIIA cytoprotection was mediated via other pathways.
TIIA has been implicated as an antioxidant because it has been found to reduce the oxidation of LDL particles challenged with peroxynitrite (Niu et al. 2000). TIIA has also been shown to maintain the activity of SOD in human umbilical vein endothelial cells challenged with H2O2 (Lin et al. 2006). However, TIIA is a fully oxidized diterpene quinine (Figure 1) and is unlikely to participate directly in antioxidation pathways. We suggest that TIIA triggers protection from oxidative stress by altering the expression of antioxidation defense systems. In this report, we tested this concept at the level of mRNA for seven anti-oxidation genes (SOD1, SOD2, catalase, GPx-1, GPx-2, GPx-3 and GPx-4) and enzyme activity for catalase and GPx, two enzymes known to inactivate H2O2. mRNA for catalase and other genes were not influenced by TIIA treatment except for GPx-1 for which mRNA levels were markedly (2-fold) elevated as compared to control, H2O2, and Trolox + H2O2 treated cells (Figure 4B). Further, overall GPx enzymatic activity was increased by 20% with TIIA treatment as compared with control cells. Finally, reducing GPx activity by introducing the generalized GPx inhibitor MS markedly reduced the protective effect of TIIA toward H2O2-induced cell death, suggesting that TIIA provides cytoprotection from H2O2 challenge in part via modulation of GPx activity.
Glutathione peroxidases are selenoproteins involved in multiple cellular defense mechanisms including inflammation, oxidation, and cytokine production (Drevet 2006; Brigelius-Flohe 2006). Macrophages express GPx-1 as well as other GPx forms such the phospholipid hydroperoxide GPx (GPx-4) (Straif et al. 2000; Fu et al. 2001). We showed that mRNA for GPx-1 but not other GPx forms are increased with TIIA treatment. However, the GPx assay used is not specific for GPx-1 and so, additional studies are required to rigorously test the role of specific enzymes which can detoxify H2O2. However, it is of interest that mRNA and activity levels of catalase were not modulated by TIIA treatment suggesting specificity of action by TIIA toward glutathione peroxidases.
The importance of at least one GPx, GPx-1, to health is highlighted by multiple reports showing that risk of cardiovascular diseases vary inversely with GPx-1 levels in erythrocytes (Blankenberg et al. 2003; Espinola-Klein et al. 2007). Genetic variation in the GPx-1 gene is a determinant for coronary artery calcification among type 2 diabetic patients (Nemoto et al. 2007). In hyperlipidemic mice, reduction in macrophage glutathione content, controlled in large part by GPx-1 activity, is associated with increased lesion development (Rosenblat et al. 2002) and Gpx-1 deficient mice show an acceleration of atherosclerosis with altered cellular contents (Torzewski et al. 2007).
CONCLUSION
Because of the importance of GPx in health and because TIIA is able to modulate GPx activity to some extent in cell culture, we suggest that TIIA is a worthwhile candidate for further study in animal models of atherosclerosis and eventually in human prospective trials.
Acknowledgements
This project is supported by grants from the National Institute of Health (HL52848 and HL79382).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Asmis R, Begley JG. Oxidized LDL promotes peroxide-mediated mitochondrial dysfunction and cell death in human macrophages: a caspase-3-independent pathway. Circulation Research. 2003;92(1):e20–e29. doi: 10.1161/01.res.0000051886.43510.90. [DOI] [PubMed] [Google Scholar]
- 3.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. New England Journal of Medicine. 2003;349(17):1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 4.Brigelius-Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biological Chemistry. 2006;387(10–11):1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- 5.Chaube SK, Prasad PV, Thakur SC, Shrivastav TG. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis. 2005;10(4):863–874. doi: 10.1007/s10495-005-0367-8. [DOI] [PubMed] [Google Scholar]
- 6.Drevet JR. The antioxidant glutathione peroxidase family and spermatozoa: a complex story. Molecular and Cellular Endocrinology. 2006;250(1–2):70–79. doi: 10.1016/j.mce.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. Journal of Neuroscience Research. 2005;79(1–2):157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- 8.Espinola-Klein C, rupprecht HJ, Bickel C, Schnabel R, Genth-Zotz S, Torzewski M, Lackner K, Munzel T, Blankenberg S. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. American Journal of Cardiology. 2007;99(6):808–812. doi: 10.1016/j.amjcard.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Esworthy RS, Yang L, Frankel PH, Chu FF. Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. Journal of Nutrition. 2005;135(4):740–745. doi: 10.1093/jn/135.4.740. [DOI] [PubMed] [Google Scholar]
- 10.Forrest VJ, Kang YH, McClain DE, Robinson DH, Ramakrishnan N. Oxidative stress-induced apoptosis prevented by Trolox. Free Radical Biology & Medicine. 1994;16(6):675–684. doi: 10.1016/0891-5849(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, McCormick CC, Roneker C, Lei XG. Lipopolysaccharide and interferon-gamma-induced nitric oxide production and protein oxidation in mouse peritoneal macrophages are affected by glutathione peroxidase-1 gene knockout. Free Radical Biology & Medicine. 2001;31(4):450–459. doi: 10.1016/s0891-5849(01)00607-4. [DOI] [PubMed] [Google Scholar]
- 12.Fujita A, Sasaki H, Ogawa K, Okamoto K, Matsuno S, Matsumoto E, Furuta H, Nishi M, Nakao T, Tsuno T, Taniguchi H, Nanjo K. Increased gene expression of antioxidant enzymes in KKAy diabetic mice but not in STZ diabetic mice. Diabetes Research Clinical Practice. 2005;69(2):113–119. doi: 10.1016/j.diabres.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Geng QX, Zhu XL, Zhang XH. Effect of combined therapy of shenmai and compound danshen injection on myocardial reperfusion injury after percutaneous coronary intervention in patients with acute myocardial infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(6):496–499. [PubMed] [Google Scholar]
- 14.He LN, Yang J, Jiang Y, Wang J, Liu C, He SB. Protective effect of tanshinone on injured cultured PC12 cells in vitro. Zhongguo Zhong Yao Za Zhi. 2001;26(6):413–416. [PubMed] [Google Scholar]
- 15.Janji B, Melchior C, Vallar L, Kieffer N. Cloning of an isoform of integrin-linked kinase (ILK) that is upregulated in HT-144 melanoma cells following TGF-beta 1 stimulation. Oncogene. 2000;19(27):3069–3077. doi: 10.1038/sj.onc.1203640. [DOI] [PubMed] [Google Scholar]
- 16.Jiang B, Xiao W, Shi Y, Liu M, Xiao X. role of Smac/DIABLO in hydrogen peroxide-induced apoptosis in C2C12 myogenic cells. Free Radical Biology & Medicine. 2005;39(5):658–667. doi: 10.1016/j.freeradbiomed.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Kuranaga E, Miura M. Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends in Cell Biology. 2007;17(3):135–144. doi: 10.1016/j.tcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Kwei Ka, finch JS, Thompson EJ, Bowden GT. Transcriptional repression of catalase in mouse skin tumor progression. Neoplasia. 2004;6(5):440–448. doi: 10.1593/neo.04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CS, Kim YJ, Ko HH, Han ES. Synergistic effects of hydrogen peroxide and ethanol on cell viability los in PC12 cells by increase in mitochondrial permeability transition. Biochemical Pharmacology. 2005;70(2):317–325. doi: 10.1016/j.bcp.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Li XH, Tang RY. Relationship between inhibitory action of tanshinone on neutrophil function and its prophylactic effects on myocardial infarction. Zhongguo Yao Li Xue Bao. 1991;12(3):269–272. [PubMed] [Google Scholar]
- 21.Liddell JR, Dringen R, Crack PJ, Robinson SR. Glutathione peroxidae 1 and a high cellular glutathione concentration are essential for effective organic hydroperoxide detoxification in astrocytes. Glia. 2006;54(8):873–879. doi: 10.1002/glia.20433. [DOI] [PubMed] [Google Scholar]
- 22.Lin R, Wang WR, Liu JT, Yang GD, Han CJ. Protective effect of tanshinone IIA on human umbilical vein endothelial cell injured by hydrogen peroxide and its mechanism. Journal of Ethnopharmacology. 2006;108(2):217–222. doi: 10.1016/j.jep.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Liu T, Qin CL, Zhang Y, Kang LY, Sun YF, Zhang BL. Effect of dan-shen, san-qi of different proportion on platelet aggregation and adhesion in normal rabbits. Zhongguo Zhong Yao Za Zhi. 2002;27(8):609–611. [PubMed] [Google Scholar]
- 24.M’Bemba-Meka P, Lemieux N, Chakrabarti SK. Role of oxidative stress, mitochondrial membrane potential, and calcium homeostasis in nickel sulfate-induced human lymphocyte death in vitro. Chemico-Biological Interactions. 2005;156(1):69–80. doi: 10.1016/j.cbi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Min LQ, Dang LY, Ma WY. Clinical study on effect and therapeutical mechanism of composite Salvia injection on acute cerebral infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22(5):353–355. [PubMed] [Google Scholar]
- 26.Nemoto M, Nishimura R, Sasaki T, Hiki Y, Miyashita Y, Nishioka M, Fujimoto K, Sakuma T, Ohashi T, Fukuda K, Eto Y, Tajima N. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: a case control study with multi-slice computed tomography. Cardiovascular Diabetology. 2007;6(Sept 7):23. doi: 10.1186/1475-2840-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu XL, Ichimori K, Yang X, Hirota Y, Hoshiai K, Li M, Nakazawa H. Tanshinone II-A inhibits low density lipoprotein oxidation in vitro. Free Radical Research. 2000;33(3):305–312. doi: 10.1080/10715760000301471. [DOI] [PubMed] [Google Scholar]
- 28.Park BG, Yoo CI, Kim HT, Kwon CH, Kim YK. Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells. Toxicology. 2005;215(1–2):115–125. doi: 10.1016/j.tox.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Penn JS, Tolman BL, Bullard LE. Effect of a water-soluble vitamin E analog, trolox C, on retinal vacular development in an animal model of retinopathy of prematurity. Free Radical Biology & Medicine. 1997;22(6):977–984. doi: 10.1016/s0891-5849(96)00479-0. [DOI] [PubMed] [Google Scholar]
- 30.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30(18):4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 31.Rosenblat M, Coleman R, Aviram M. Increased macrophage glutathione content reduces cell-mediated oxidation of LDL and atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2002;163(1):17–28. doi: 10.1016/s0021-9150(01)00744-4. [DOI] [PubMed] [Google Scholar]
- 32.Salgo MG, Pryor WA. Trolox inhibits peroxynitrite-mediated oxidative stress and apoptosis in rat thymocytes. Archives of Biochemistry and Biophysics. 1996;33392:482–488. doi: 10.1006/abbi.1996.0418. [DOI] [PubMed] [Google Scholar]
- 33.Simizu S, Takada M, Umezawa K, Imoto M. Requirement of caspase-3(-like) protease-mediated hydrogen peroxide production for apoptosis induced by varius anticancer drugs. Journal of Biological Chemistry. 1998;273(41):26900–26907. doi: 10.1074/jbc.273.41.26900. [DOI] [PubMed] [Google Scholar]
- 34.Straif D, Werz O, Kellner R, Bahr U, Steinhilber D. Glutathione peroxidase-1 but not-4 is involved in the regulation of cellular 5-lipoxygenase activity in monocytic cells. The Biochemical Journal. 2000;349(Pt 2):455–461. doi: 10.1042/0264-6021:3490455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr HA, Blankenberg S, Forstermann U, Munzel T, Laackner KJ. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arteriosclerosis Thrombosis and Vascular Biology. 2007;27(4):850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 36.Wang AM, Sha SH, Lesniak W, Schacht J. Tanshinone (Salviae miltiorrhizae extract) preparations attenuate aminoglycoside-induced free redical formation in vitro and ototoxicity in vivo. Antimicrobial Agents Chemotherapy. 2003;47(6):2836–1841. doi: 10.1128/AAC.47.6.1836-1841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang N, Luo HW, Niwa M, Ji J. A new platelet aggregation inhibitor from Salvia miltiorrhiza. Planta Medica. 1989;55(4):390–391. doi: 10.1055/s-2006-962037. [DOI] [PubMed] [Google Scholar]
- 38.Zhao BL, Jiang W, Zhao Y, Hou JW, Xin WJ. Scavenging effects of salvia miltiorrhiza on free radicals and its protection for myocardial mitochondrial membranes from ischemia-reperfusion injury. Biochemistry & Molecular Biology International. 1996;38(6):1171–1182. [PubMed] [Google Scholar]
- 39.Zhongguo . Pharmacopoeia of the People’s Republic of China. Beijing: People’s Medical Publishing House; 2005. [Google Scholar]
- 40.Zhou G, Jiang W, Zhao Y, Ma G, Xin W, Yin J, Zhao B. Sodium tanshinone IIA sulfonate mediates electron transfer reaction in rat hear mitochondria. Biochemical Pharmacology. 2003;65(1):51–57. doi: 10.1016/s0006-2952(02)01447-8. [DOI] [PubMed] [Google Scholar]
- 41.Zhou GY, Zhao BL, Hou JW, Ma GE, Xin WJ. Protective effects of sodium tanshinone IIA sulphonate against adriamycin-induced lipid peroxidation in mice hearts in vivo and in vitro. Pharmacological Research. 1999;40(6):487–491. doi: 10.1006/phrs.1999.0545. [DOI] [PubMed] [Google Scholar]
- 42.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. Journal of Clinical Pharmacology. 2005;45(12):1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]