Abstract
Whole cell patch-clamp recordings were obtained from dissociated mouse lumbar dorsal root ganglion (DRG) neurons. Recordings were made from control neurons and neurons axotomized by transection of the corresponding spinal nerve 1–2 days prior to dissociation. Medium to large muscle and cutaneous afferent neurons were identified by retrograde transport of True Blue or Fluoro-Gold injected into the corresponding peripheral tissue. Action potentials were classified as non-inflected spikes (A0) and inflected spikes (Ainf). High-frequency, low-amplitude subthreshold membrane potential oscillations were observed in 8% of control A0 neurons, but their incidence increased to 31% in the nerve injury group. Fifty percent of axotomized muscle afferent A0 cells displayed oscillations, while 26% of axotomized cutaneous afferents exhibited oscillations. Lower-frequency oscillations were also observed in a small fraction (4%) of Ainf neurons on strong depolarization. Their numbers were increased after the nerve injury, but the difference was not statistically significant. The oscillations often triggered burst firing in distinct patterns of action potential activity. These results indicate that injury-induced membrane oscillations of DRG neurons, previously observed in whole DRG of rats, are present in dissociated DRG neurons of the adult mouse. Moreover, these observations indicate that both muscle and cutaneous afferents in the Aβ size range give rise to injury-induced membrane oscillations, with muscle afferents being more prone to develop oscillations.
INTRODUCTION
The cell bodies of primary afferent neurons in the dorsal root ganglia (DRGs) are a significant source of ectopic afferent discharge after peripheral nerve injury (Amir et al. 1999; Burchiel 1984; Kajander et al. 1992; Kirk 1974; Liu et al. 2000b; Wall and Devor 1983). This spontaneous discharge is thought to induce ongoing paresthesia and pain, and it may also contribute to central sensitization and hence tactile hypersensitivity (Devor and Seltzer 1999; Gracely et al. 1992; Han et al. 2000; Kocsis and Devor 2000; Liu et al. 2000b; Rowbotham and Fields 1996; Sheen and Chung 1993; Woolf and Thompson 1991; Yoon et al. 1996). Our previous studies demonstrated that the ability of DRG neurons to generate repetitive impulse activity depends on the resonance properties of the neuronal membrane (Amir et al. 1999; Liu et al. 2000a). Specifically, in a whole excised rat DRG preparation, we identified a small subclass of fast-conducting DRG neurons that show subthreshold oscillations at resting membrane potential (Vr) or when depolarized. When the amplitude of an oscillation sinusoid reaches threshold, action potentials are evoked. Interestingly, axotomy increases the proportion of neurons that display subthreshold oscillations at Vr and on depolarization. This injury-induced increase in membrane oscillations, and the consequent increase in burst firing, correlates well with ectopic discharge patterns seen in vivo (Amir et al. 1999; Lee et al. 1999; Liu et al. 2000b; Study and Kral 1996; Zhang et al. 1997), supporting the suggestion that the oscillations made a fundamental contribution to neuropathic dysesthesia and pain (Devor and Seltzer 1999).
The precise subpopulation of afferent neurons that develop subthreshold oscillations and ectopic impulse activity after a peripheral nerve lesion has not been well characterized. However, experiments in vivo using teased fiber recordings from whole DRGs, in which specific muscle or cutaneous nerves were injured, implicated muscle afferents as the predominant source of ectopic electrogenesis (Michaelis et al. 2000; Tal et al. 1999). Oscillatory behavior has not been studied in dissociated DRG neurons where greater biophysical and pharmacological control can be achieved for the detailed examination of the oscillatory mechanism.
Here, we report that membrane potential oscillations are indeed generated in dissociated mouse DRG neurons and are enhanced following nerve injury. Virtually all neurons that display membrane oscillations have narrow noninflected (A0) action potentials. While oscillations are present in both cutaneous and muscle afferents following axotomy, the predominant functional class of neurons showing oscillations following nerve injury are medium-sized muscle afferents in the Aβ size range. The results have implications for the mechanism underlying sensory abnormalities, such as pain and paresthesia, associated with nerve injury.
METHODS
Animals
Adult C57BL6 mice of both sexes (19 –35 g) were used (n = 60). The surgical procedure was in concordance with the recommendations of the International Association for the Study of Pain (IASP) and was approved by the Institutional Animal Care and Use Committee of Yale University.
Spinal nerve injury
The animals were subjected to transection of the L5 spinal nerve as previously described in rats by Kim and Chung (1992). Briefly, under pentobarbital sodium (Nembutal) anesthesia (50 mg/kg ip), the left paraspinal muscles were separated from the spinous processes at the L3–S2 levels. In seven mice, the L5 transverse processes were removed and the L5 spinal nerve was then transected with fine scissors with care taken not to injure the adjacent L4 spinal nerve. In 37 animals, the L4 spinal nerve was also cut. Two mice underwent sham surgery that involved the identical surgical exposure without transection of the spinal nerves. Finally, DRGs of 14 intact mice were studied. Following surgery mice were maintained in standard cages bedded with wood shavings, with a 12/12 h light/dark cycle and with food and water available ad libitum.
Fluorescence tracer labeling of cutaneous and muscular afferents
DRG cell bodies giving rise to cutaneous afferent fibers were identified by retrograde labeling with Fluoro-Gold (Fluorochrome, Englewood, CO). Thirty to 40 μl of a 4% solution of Fluoro-Gold dissolved in sterile distilled water was injected intradermally in the lateral region of the hindpaw 1 wk before sacrifice (Honmou et al. 1994). To label muscle afferent neurons, an incision was made in the skin of the anesthetized mouse to expose the gastrocnemius and soleus muscle of the leg. True Blue (Sigma, total 40 μl in distilled water) was injected directly into the muscles at three to five points. Fluoro-Gold-and True-Blue-labeled neurons were identified in vitro by yellow and blue fluorescence emission respectively, on brief exposure of the cells to ultraviolet light.
DRG neuron dissociation and culture
One to 2 days after spinal nerve transection, mice were exsanguinated under Nembutal (60 mg/kg ip) anesthesia, and the left lumbar ganglia L5 and L4 were excised in ice-cold sterile calcium-free Kreb’s solution. The ganglia were gently minced before being incubated in HBSS containing 1 mg/ml collagenase A (Boehringer-Mannheim, Indianapolis, IN). They were then incubated with gentle shaking in solution D, which consisted of HBSS, 1 mg/ml collagenase D (Boehringer-Mannheim), 0.4 mg/ml 1:250 trypsin (Sigma), and 0.1 mg/ml DNase-1 (Sigma). The HBSS contained (in mM) 137 NaCl, 4.2 NaHCO3, 0.4 Na2HPO4, 5.4 KCl, 0.4 KH2PO4, 5.5 glucose, and 10 HEPES, pH: 7.3. Digested DRG were carefully transferred to culture medium (DMEM and F12 in a ratio of 1:1) containing 1 mg/ml bovine serum albumin (Sigma) and 1.3 mg/ml trypsin inhibitor (Sigma). The enzymatically treated DRG were gently triturated using a fire-polished Pasteur pipette and then distributed onto uncoated glass coverslips. Neurons were then kept in a 5% CO2-95% O2 incubator at 37°C. No antibiotic or NGF was added to the medium.
Whole cell patch-clamp recordings
Whole cell patch-clamp recordings were obtained soon after plating (2–6 h). Neurons, plated on glass cover slips, were placed in a recording chamber on the stage of an inverted microscope (Nikon) and continuously superfused with a modified Krebs’ solution [composition (in mM): 124 NaCl, 26 NaHCO3, 3 KCl, 1.3 NaH2PO4, 2 MgCl2, 2 CaCl2, and 10 dextrose, pH 7.4; osmolarity, 305–315 mOsm) at room temperature (~23°C)] bubbled continuously with 95% O2-5% CO2 using a flow rate of 0.5–1 ml/min. Only medium to large neurons (31– 60 μm diameter) were studied. Micropipettes were pulled from borosilicate glass (World Precision Instruments) with a P97 micropipette puller (Sutter Instrument, San Rafael, CA) and polished with a microforge (Narishige, Tokyo). Electrode resistances ranged from 3 to 5 MΩ. The pipette solution contained (in mM): 140 KCl, 2 MgCl2, 1 CaCl2, 11 EGTA, 2 Mg-ATP, and 10 HEPES, pH 7.3; osmolarity, 300–310 mOsm. Tight seals of 1–2 GΩ were established in the cell-attached configuration. The whole cell configuration was established by giving a further suction pulse, having previously compensated capacitative transients with an Axopatch-1D patch-clamp amplifier (Axon Instruments). Once the gigaseal was established, the voltage-clamp mode was changed to current-clamp mode. The voltages were filtered at 10 kHz and acquired at 50 kHz using Clampex 8 software (Axon Instruments). The Digidata 1200B interface (Axon Instruments) was used for A–D conversion. Series resistance was balanced with the amplifier.
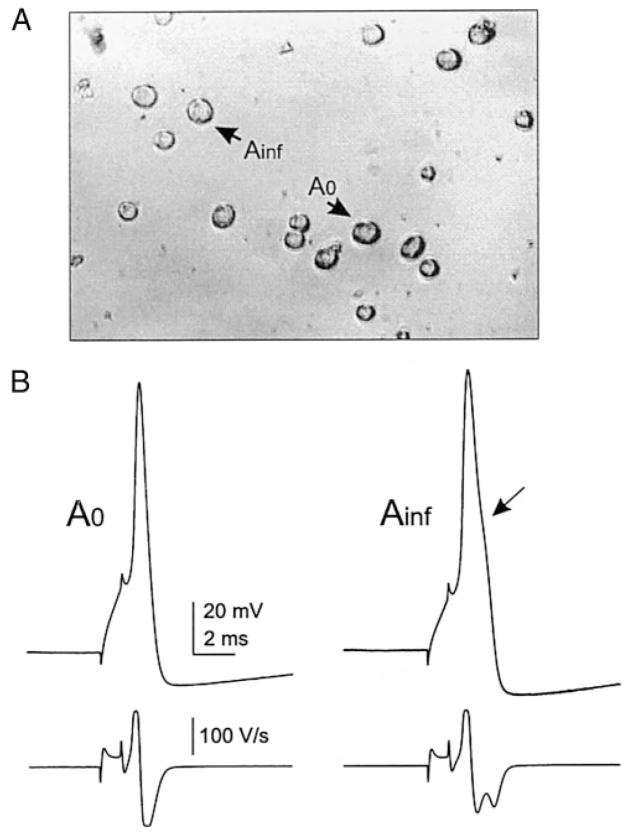
Action potentials were elicited from Vr levels by delivering depolarizing step pulses of 1- or 40-ms duration generated by Clampex 8. Neurons were examined in order, as patched, accepting only those exhibiting Vr more negative than −45 mV and an overshooting action potential. Ramp depolarization pulses of 4-s duration from Vr to −20 mV were applied manually to detect subthreshold oscillations and firing. In cells in which subthreshold oscillations were present, peak-to-peak oscillation amplitude was measured by averaging a sample of 30–40 cycles. Oscillations were usually obvious, but when necessary, we used the criterion of amplitude peaks of at least 1.5 times the amplitude of the background noise level present during brief pauses in the oscillations and the voltage dependence of subthreshold oscillation. The following additional parameters were measured: spike amplitude, measured from the baseline Vr to the positive peak of the spike; spike duration at half-amplitude; slopes of the rising and falling limbs of the action potential (dV/dt); afterhyperpolarization (AHP) amplitude, measured from the baseline; AHP duration measured at 75% decay; characteristics of a brief depolarizing potential that follows many DRG A0 neuron spikes. Amir et al. (2002b), who describe this potential in detail, refer to it as the depolarizing afterpotential (DAP). We refer to this same potential as the rebound depolarization potential (RDP) measured from the baseline after the AHP. The final parameter was the current threshold for evoking a single spike using 1-ms depolarizing pulses. The neurons were further characterized as Ainf if digital differentiation (Excel, Microsoft) indicated two peaks on the falling limb of the spike, or A0 if there was only one peak (Fig. 1B).
FIG. 1.
Action potentials of cultured mouse dorsal root ganglion (DRG) A0 and Ainf neurons under whole cell current-clamp recording conditions. Action potentials elicited in response of a 1-ms depolarizing current pulse (at threshold magnitude) injection into A0 and Ainf neurons (A) cultured for 3.5 h from DRG of mouse that had been subjected to spinal nerve injury 1 day previously. Electrophysiologically recorded A0 and Ainf neurons had diameters of 38 and 35 μm, respectively. The action potential induced in A0 neuron (B, left) exhibits a non-inflected falling phase of the action potential that is indicated by a single peak in the differentiated waveform (bottom trace, left), while the action potential in Ainf neuron (B, right) exhibits a “hump” in the falling phase of action potential (arrow, B, right) with 2 peaks in the differentiated waveform (bottom trace, right).
Data processing
Electrophysiological data were processed by using Clampfit (Axon Instruments) and Excel (Microsoft). Data are presented as means ± SD. Statistical evaluations were based on two-tailed t-tests, Mann-Whitney U tests, and significant of the χ2 or Fisher exact probabilities test (SigmaStat, SPSS; criterion, P < 0.05).
RESULTS
Action potential and subthreshold oscillation characteristics
Whole cell patch-clamp recordings were obtained from 135 dissociated medium to large A-type DRG neurons from control (non-axotomized) mouse DRG. The dissociated cells did not have neurites. Examples of two cells recorded from are shown in Fig. 1A. Two types of action potentials were observed in these neurons: a narrow non-inflected action potential (A0) was present in 63 neurons and a broader inflected spike was present in 72 neurons (Ainf; Fig. 1B). Under the action potentials in Fig. 1B are differentiated traces, more clearly demonstrating the point of inflection on the falling phase of the Ainf spike (arrow).
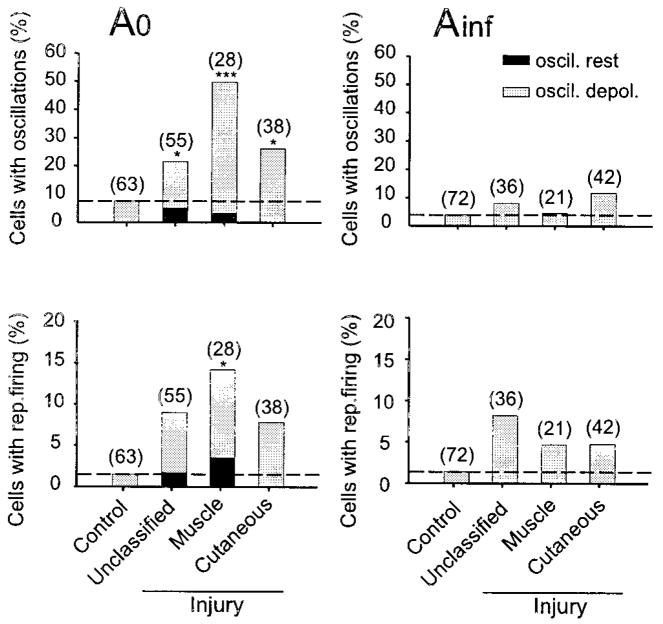
We succeeded in documenting subthreshold oscillations in dissociated DRG neurons. As in prior studies using excised whole rat DRG, oscillations occurred primarily in a subset of A0 neurons, which are the focus of this report. The A0 neurons dissociated from intact or sham-operated mice had a stable membrane potential (Vr) (Fig. 2A, left, top). The amplitudes and durations of action potentials, and the afterhyperpolarizations, the resting potentials, and neuronal size (diameter) for control A0 neurons are listed in Table 1. Some neurons exhibited modest irregular fluctuations of the membrane potential on strong depolarization as shown in Fig. 2A (left). About 8% (5 of a total of 63) of A0 neurons in the control group exhibited high-frequency subthreshold sinusoidal oscillations when the neurons were depolarized to an average of −24 ± 8 mV (Fig. 3, left). Oscillations were generally sustained at a given depolarization level, but some had intermittent brief pauses. The prevalence of oscillations among dissociated A0 neurons observed here was very similar to that reported previously in excised whole ganglia in rats (Amir et al. 1999; Liu et al. 2000a). Repetitive ectopic action potential firing triggered by subthreshold oscillations was observed in only one control DRG A0 neuron (of 63 A0 neurons) on intense depolarization to −10 mV.
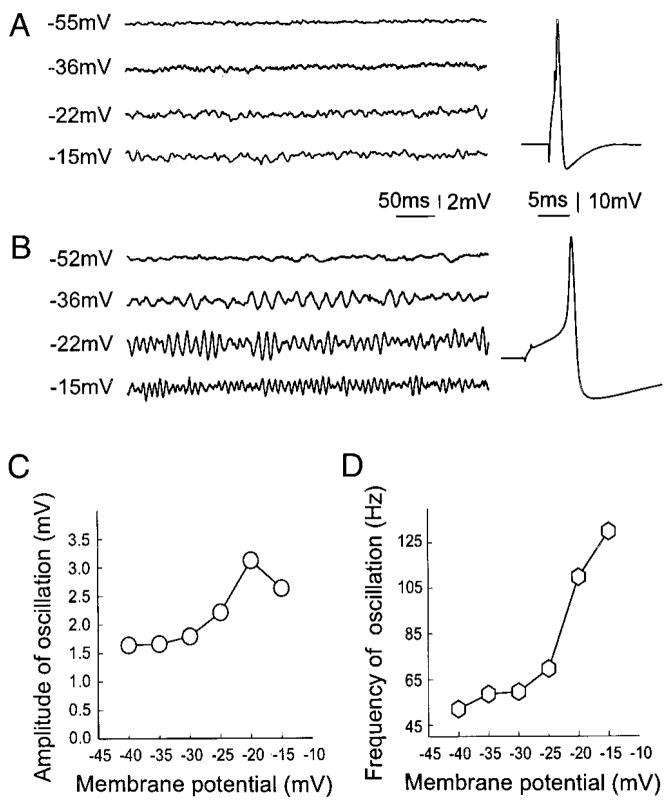
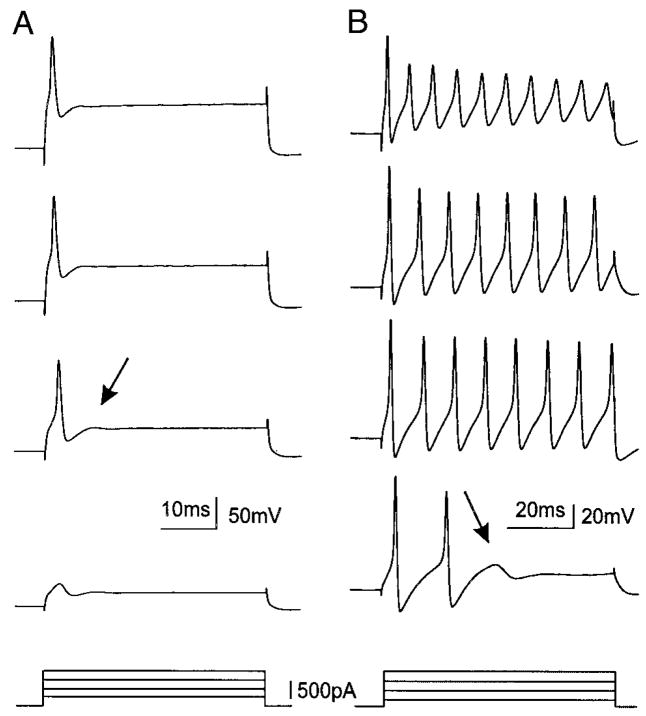
FIG. 2.
Spinal nerve injury induces an increase in the number of cells with subthreshold oscillations on depolarization (B), which was rare in control DRG A0 neurons even on strong depolarization (A). Most of the oscillating axotomized cells have sharp and infection-free action potentials following smaller current injection (B, right), while the control A0 neurons usually have a larger threshold for action potentials (A, right). C: the amplitude of oscillations as a function of membrane potential depolarization. The amplitude increased progressively as this neuron was depolarized beyond −35 mV. When the depolarization reached an “optimal ” membrane potential (−22 mV), the amplitude of oscillation was maximal (B). Further depolarization resulted in decrease in the amplitude. However, the frequency of oscillations was voltage dependent in monotonic manner (D).
TABLE 1.
Properties of DRG A0 neurons in various groups
| Action Potential
|
AHPf |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vr, −mV | Amplitude, mV | Duration, ms | dV/dt rise, V/s | dV/dt fall, V/s | Culture Time, h | Amplitude, mV | Duration, ms | RDP, mV | Size, μm | Single Spike Threshold, nA | Multiple Spikes, % | |
| Control | 57.5 ± 5.5 (63) | 85.1 ± 11.8 (63) | 0.88 ± 0.30 (63) | 191.2 ± 71.2 (63) | 134.3 ± 40.6 (63) | 4.0 ± 1.6 (63) | 15.9 ± 5.3 (60) | 11.2 ± 4.5 (33) | 2.1 ± 1.8 (38) | 41.7 ± 5.3 (63) | 1.9 ± 1.1 (63) | 2/57 (4) |
| Chung | ||||||||||||
| Unclassified | 54.1 ± 6.3 (55) | 82.9 ± 15.8 (55) | 0.83 ± 0.44 (55) | 206.6 ± 80.0 (55) | 146.5 ± 50.3 (55) | 4.2 ± 1.7 (55) | 17.4 ± 4.3 (55) | 11.8 ± 3.9 (51) | 3.9 ± 2.6 (47) | 41.1 ± 5.5 (55) | 0.9 ± 0.5 (55) | 8/47 (17) |
| Muscle | 52.3 ± 5.9 (28) | 79.6 ± 17.7 (28) | 0.94 ± 0.47 (28) | 176.7 ± 94.8 (28) | 129.7 ± 50.2 (28) | 4.7 ± 1.6 (28) | 16.8 ± 2.2 (28) | 12.9 ± 3.6 (28) | 3.8 ± 2.2 (24) | 42.0 ± 5.8 (28) | (0.8 ± 0.528) | 18/21 (86) |
| Cutaneous | 54.1 ± 5.0 (38) | 83.8 ± 8.8 (38) | 0.90 ± 0.36 (38) | 180.0 ± 58.3 (38) | 120.4 ± 33.5 (38) | 4.4 ± 1.5 (38) | 15.4 ± 4.0 (38) | 12.4 ± 4.3 (37) | 2.5 ± 1.4 (27) | 41.2 ± 3.7 (38) | 1.1 ± 0.6 (38) | 6/33 (18) |
| P | ||||||||||||
| Muscle/cutaneous | N* | N | N | N | N | N | N | N | 0.014 | N | 0.035 | <0.001† |
| Chung | ||||||||||||
| All | 53.7 ± 5.8 (121) | 82.4 ± 14.4 (121) | 0.88 ± 0.42 (121) | 191.3 ± 78.4 (121) | 134.4 ± 46.8 (121) | 4.4 ± 1.6 (121) | 16.6 ± 3.9 (121) | 12.3 ± 4.0 (116) | 3.5 ± 2.3 (98) | 41.3 ± 5.1 (121) | 0.9 ± 0.5 (121) | 32/101 (32) |
| P | ||||||||||||
| Chung/control | <0.001 | N | N | N | N | N | N | N | <0.001 | N | <0.001 | <0.001† |
All values are means ± SD with n in parentheses.
N: P > 0.05, 2-tailed t-test;
χ2 test. DRG, dorsal root ganglion; AHP, afterhyperpolarization; RDP, rebound depolarization potential.
FIG. 3.
Prevalence of subthreshold oscillations at Vr (■) and on depolarization (
 ) among A0 and Ainf neurons dissociated from control and axotomized DRG. Bottom: the prevalence of oscillation-triggered repetitive firing at Vr (■) and on depolarization (
) among A0 and Ainf neurons dissociated from control and axotomized DRG. Bottom: the prevalence of oscillation-triggered repetitive firing at Vr (■) and on depolarization (
 ) among the A0 and Ainf cells. The numbers in the brackets are the numbers of neurons tested. *: P < 0.05; ***: P < 0.001, compared with that of control.
) among the A0 and Ainf cells. The numbers in the brackets are the numbers of neurons tested. *: P < 0.05; ***: P < 0.001, compared with that of control.
Effects of axotomy on membrane oscillations and action potential characteristics
Axotomy substantially augmented oscillatory behavior and ectopic spike discharge. We studied 121 A0 neurons cultured from mice that previously had undergone spinal nerve section; 28 of these were identified as muscle afferents, 38 as cutaneous afferents, and 55 were not classified. Of the total population of 121 neurons, 3% exhibited high-frequency subthreshold oscillation and repetitive firing at their Vr (−45 to −50 mV). An additional 27% (n = 33) neurons exhibited subthreshold sinusoidal oscillations when they were depolarized to an average of 33 ± 7 mV (Fig. 2B, left; Table 2). The increase in the prevalence of oscillations to 31% in the injured group from 8% in controls (P = 0.001) was very similar to that seen previously in rat DRG neurons recorded from whole excised ganglia (Liu et al. 2000a).
TABLE 2.
Comparison of oscillation parameters among DRG A0 neurons from control and various nerve injury mice
| Chung
|
|||||||
|---|---|---|---|---|---|---|---|
| Groups | Control | Unclassified | Muscle | Cutaneous | P (Muscle/Cutaneous) | P Chung (All) | P (Chung/Control) |
| Threshold of oscillation, mV | −23.8 ± 7.5 (5) | −34.2 ± 7.1 (10) | −32.0 ± 7.3 (13) | −32.2 ± 7.2 (10) | N* | −32.8 ± 7.0 (33) | 0.012 |
| Threshold of oscillation-triggered firing, mV | — | −35 (1) | −30.0 ± 9.0 (3) | −33.0 ± 7.0 (3) | N | −32.0 ± 6.9 (7) | N/A |
| Frequency of oscillation at threshold, Hz | 107.3 ± 34.7 (5) | 91.7 ± 23.3 (10) | 73.2 ± 31.6 (13) | 60.3 ± 17.3 (10) | N | 74.9 ± 27.7 (33) | 0.024 |
| Amplitude of oscillation at threshold, mV | 1.5 ± 1.1 (5) | 1.0 ± 0.4 (10) | 1.3 ± 0.5 (13) | 1.1 ± 0.3 (10) | N | 1.2 ± 0.5 (33) | N |
| Optimal membrane potential for oscillation, mV | −18.2 ± 5.3 (5) | −28.5 ± 6.1 (10) | −25.5 ± 6.6 (13) | −26.4 ± 6.7 (10) | N | −26.7 ± 6.4 (33) | 0.008 |
| Best frequency at optimal potential, Hz | 129.5 ± 35.1 (5) | 109.3 ± 26.1 (10) | 95.0 ± 27.1 (13) | 68.6 ± 25.0 (10) | 0.026 | 91.3 ± 30.2 (33) | 0.014 |
| Amplitude of oscillation at optimal potential, mV | 2.4 ± 1.9 (5) | 2.0 ± 1.0 (10) | 2.4 ± 1.1 (13) | 1.8 ± 0.5 (10) | N | 2.1 ± 1.0 (33) | N |
All values are means ± SD with n in parentheses.
N: P > 0.05, 2-tailed t-test.
In the control DRGs none of the Ainf neurons showed oscillations at Vr, and only 4% (3 of 72) showed slow sub-threshold oscillations on strong depolarization when the membrane potential reached about −20 mV (Fig. 3, right). The oscillation frequency was much lower than in A0 neurons (16–23 Hz) and the amplitude was larger (2.0–2.2 mV). After spinal nerve injury there were more Ainf neurons with oscillations (9/99 vs. 3/73), but the difference was not statistically significant (9 vs. 4%, P > 0.2, Fig. 3, right). Axotomy also did not change the threshold for evoking oscillations.
Threshold for evoking oscillations shifts toward the resting membrane potential following spinal nerve injury
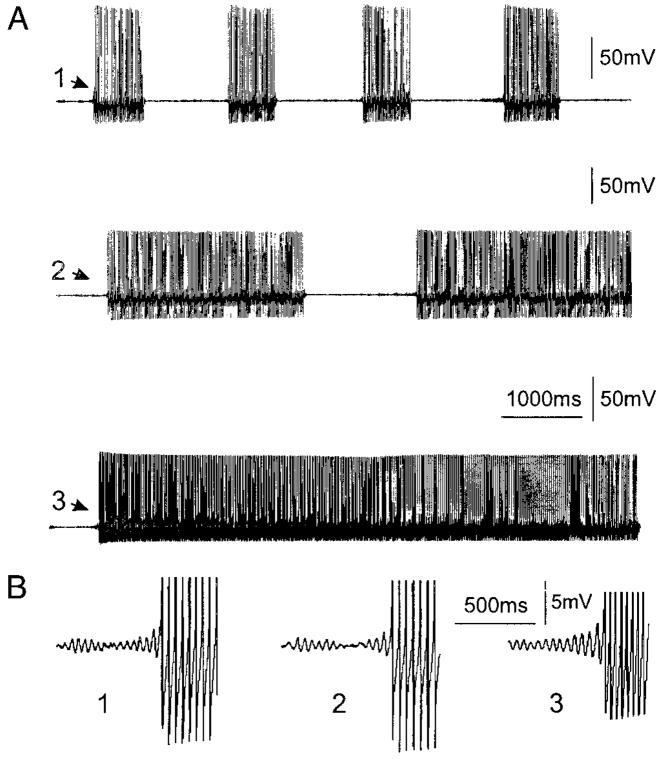
As noted in the preceding text, ongoing subthreshold oscillations at Vr occurred in only a small fraction (3%, 4/121) of the A0 DRG neurons even after spinal nerve injury. The majority of the neurons that exhibited oscillations did so only when the neurons were depolarized by constant current injection. In control DRG neurons (A0), threshold depolarization for evoking oscillations averaged −24 ± 8 mV (Table 2). However, in neurons isolated from axotomized mice, depolarization to −33 ± 7 mV was sufficient to evoke subthreshold oscillation. This voltage difference is statistically significant (P < 0.05, Table 2). In addition, the “optimal membrane potential,” the potential at which oscillations had their maximal peak-to-peak amplitude (Amir et al. 1999), was also shifted toward the resting potential (−27 ± 6 mV vs. −18 ± 5 mV, P < 0.01, Table 2). In some of the axotomized neurons (n = 7), the oscillations gave rise to repetitive action potential firing in a burst pattern on depolarization (−32 ± 7 mV, Fig. 4).
FIG. 4.
Burst (A, top and middle) and tonic (A, bottom) ectopic action potential trains are often seen at Vr (A, top) or on ramp current injection depolarization (A, middle and bottom) after spinal nerve injury. Note that in all of the trains, the first spikes (A, 1–3) are always triggered by a subthreshold oscillation peak as seen in the expanded time and voltage scale (B, 1–3). The action potentials in B are truncated.
Changes in amplitude and frequency of the subthreshold oscillations
The subthreshold oscillation amplitude changed systematically when the cells were depolarized (Fig. 2, B and C). Depolarization from the membrane potential at which subthreshold oscillations were first observed always led to an increase in the peak-to-peak oscillation amplitude. This continued until a maximal amplitude was reached. Depolarization beyond this “optimal membrane potential” caused the amplitude to decline until oscillations were no longer discernable above the background noise. The amplitude of the oscillations at the optimal membrane potential was not appreciably different (P > 0.2) after spinal nerve injury (Table 2).
As with oscillation amplitude, oscillation frequency increased systematically when the cells were depolarized (Fig. 2, B and D). However, unlike amplitude, there was no optimum value. Rather, oscillation frequency continued to increase with depolarization in a monotonic manner. Spinal nerve injury caused a decrease of the frequency of oscillation at both optimal membrane potential (best frequency; 91 vs. 130 Hz; P < 0.05) and at threshold membrane potentials for oscillation (75 vs. 107 Hz; P < 0.05; Table 2). These characteristics were seen previously in whole excised DRGs in rats (Amir et al. 1999).
Changes in oscillation-related electrophysiological properties after spinal nerve injury
Dissociated DRG neurons from control and nerve injury mice were characterized for a range of electrophysiological parameters in addition to their propensity to oscillate and to fire repetitively. On spike repolarization there is an undershoot in potential to several millivolts below resting potential (the fast afterhyperpolarization, AHPf). As reported by Amir et al. (2002b), the AHPf is often followed by a brief rebound depolarization (Fig. 5B, ↓). This rebound depolarization potential [RDP, referred to as the depolarizing afterpotential (DAP) by Amir et al. 2002b] following the action potential and the AHPf may be related to oscillation behavior, because it only appears in the A0 neurons, especially in the oscillating neurons (Table 1). The prevalence of RDP in the pA0 neuron population increased significantly following nerve injury from 60% in control (38/63) to 81% (98/121, P < 0.01) at Vr. Moreover, there was a significant increase in the amplitude of RDP at Vr after nerve injury [3.5 ± 2.3 mV (n = 98) vs. 2.1 ± 1.8 mV (n = 38), P < 0.001, Table 1].
FIG. 5.
Spinal nerve injury results in a propensity of cells to generate multiple action potentials in response to step depolarization, with a higher frequency in response to a larger depolarization (right). In contrast, only 1 spike is seen when the control DRG A0 neurons is depolarized with similar stimuli (left). Spinal nerve injury also increases the “RDP” following the action potentials (arrow in right vs. that in left).
In addition to an association with the RDP, subthreshold oscillations were observed in neurons that displayed an AHPf. All the oscillating neurons manifested large fast AHPs and did not show slow or prolonged AHPs. The prevalence of A0 neurons with only AHPf increased from 43% (27/63) to 84% (102/121, P < 0.001) following axotomy.
The oscillating neurons often had a lower threshold for single action potential induction. The threshold decreased significantly (P < 0.001) from 1.9 ± 1.1 nA (n = 63) in control A0 cells to 0.9 ± 0.5 nA (n = 121) in axotomized neurons. In addition, the ability of A0 cells to fire repetitively in response to a sustained suprathreshold step depolarizing stimulus (40- to 80-ms duration) was increased from 4% (2/57) in controls to 32% (32/101) after nerve injury (P < 0.001, Fig. 5, Table 1).
The resting potential in the DRG A0 neurons in the nerve injury group was less negative (−54 ± 6 vs. −58 ± 6 mV, P < 0.001). Interestingly, no changes were observed in the amplitude and duration of action potential or in the slopes of the rising and falling phases of action potentials (Table 1). Nerve injury did not change the relative proportion of A0 and Ainf neurons in the whole population; A0/Ainf was 121/99 for the injury group and 63/72 in controls (P > 0.05). The distribution of cutaneous afferents within the population also did not change following spinal nerve injury (38/42 vs. 63/72, P > 0.2).
Subthreshold oscillations develop preferentially in A0 neurons innervating muscles
To determine whether muscle or cutaneous afferents were more prone toward oscillations, we compared the occurrence of subthreshold oscillations in axotomized DRG neurons that innervated skin (Fluoro-Gold labeled) or innervated skeletal muscle (True Blue labeled). The number of A0 cells with oscillations at resting potential (black) and on depolarization (gray) in control, unclassified cells, and identified muscle and cutaneous afferents are shown in Fig. 3 (left). The axotomized muscle afferent group showed a clear increase in the prevalence of oscillations at Vr and on depolarization (P < 0.001). The axotomized cutaneous afferents did not display oscillations at Vr but on depolarization the proportion of oscillating cells more than doubled compared with controls (P < 0.05). The number of axotomized cells with membrane oscillations was greater in muscle afferent than in cutaneous neurons (50 vs. 26%, P = 0.09, Fig. 3). However, there was no apparent difference between the oscillating cutaneous afferents and muscle afferents with regard to most of the parameters of subthreshold oscillations except for lower oscillation frequency at the optimal membrane potential (Table 2). The percentage of cells after injury that showed repetitive firing arising from the oscillations was increased in all groups after injury, but the increase only reached significance in the muscle afferent group of A0 neurons (Fig. 3, bottom left). Comparable data for Ainf neurons in control and injury groups are shown in Fig. 3, right. There was no significant difference between control and injury groups of Ainf neurons in the prevalence of oscillations and in most other electrophysiological properties (Table 3).
TABLE 3.
Properties of DRG Ainf neurons in various groups
| Action Potential
|
AHPf |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vr, −mV | Amplitude, mV | Duration, ms | dV/dt rise, V/s | dV/dt fall1, V/s | dV/dt fall2, V/s | Amplitude, mV | Duration, ms | Culture Time, h | Size, μm | Single Spike Threshold, nA | |
| Control | 59.2 ± 6.0 (72) | 102.6 ± 9.2 (72) | 1.77 ± 0.83 (72) | 171.4 ± 69.2 (72) | 65.9 ± 28.9 (72) | 66.2 ± 21.0 (72) | 14.4 ± 4.2 (72) | 16.7 ± 10.4 (7) | 4.2 ± 1.6 (72) | 40.9 ± 4.1 (72) | 2.0 ± 0.9 (72) |
| Chung | |||||||||||
| Unclassified | 57.1 ± 5.9 (36) | 101.1 ± 12.2 (36) | 1.54 ± 0.58 (36) | 182.4 ± 87.2 (36) | 71.8 ± 29.5 (36) | 83.5 ± 26.3 (36) | 15.3 ± 4.7 (36) | 18.1 ± 4.4 (11) | 4.4 ± 1.8 (36) | 40.6 ± 4.3 (36) | 1.5 ± 0.5 (36) |
| Muscle | 57.5 ± 4.0 (21) | 97.0 ± 11.6 (21) | 2.28 ± 1.18 (21) | 164.1 ± 71.0 (21) | 48.4 ± 25.7 (21) | 70.2 ± 26.8 (21) | 14.9 ± 4.1 (21) | 18.8 ± 7.2 (10) | 4.4 ± 1.4 (21) | 40.1 ± 3.3 (21) | 1.8 ± 0.4 (21) |
| Cutaneous | 55.1 ± 5.9 (42) | 95.0 ± 12.1 (42) | 2.15 ± 0.99 (42) | 142.0 ± 68.3 (42) | 48.9 ± 23.4 (42) | 66.1 ± 20.3 (42) | 16.7 ± 3.0 (42) | 17.5 ± 5.6 (23) | 5.1 ± 1.5 (42) | 39.6 ± 3.1 (42) | 1.5 ± 0.6 (42) |
| P | |||||||||||
| Muscle/cutaneous | N* | N | N | N | N | N | N | N | N | N | 0.043 |
| Chung | |||||||||||
| All | 56.3 ± 5.6 (99) | 97.6 ± 12.2 (99) | 1.96 ± 0.96 (99) | 161.4 ± 77.6 (99) | 57.1 ± 28.3 (99) | 73.3 ± 25.1 (99) | 15.8 ± 4.0 (99) | 18.0 ± 5.6 (44) | 4.7 ± 1.6 (99) | 40.7 ± 3.7 (99) | 1.6 ± 0.5 (99) |
| P | |||||||||||
| Chung/control | 0.001 | 0.004 | N | N | N | N | 0.028 | N | N | N | =<0.001 |
All values are means ± SD with n in parentheses.
N: P > 0.05, 2-tailed t-test.
DISCUSSION
We examined oscillatory membrane potential behavior and associated biophysical properties in dissociated mouse DRG neurons in short-term culture, comparing control and axotomized neurons in a preparation in which we could identify cutaneous and muscle afferents. We found that a small number of A0 neurons taken from intact DRGs showed subthreshold oscillations. None of the Ainf neurons exhibited oscillatory behavior at resting potential, but a few (4%) did so on depolarization. The neurons in culture were aneuritic and spatially isolated on the culture dishes. This confirms that the oscillatory mechanism is intrinsic to the soma of the neuron and does not depend on metabolic or chemical cooperation from neighboring neurons or glia. Spinal nerve transection induced a nearly fourfold increase (8–30%) in the prevalence of oscillations in A0 neurons. This change occurred within 48 h of axotomy (see Liu et al. 2000a). There was no significant change in Ainf neurons giving rise to oscillations.
We found that, while axotomy facilitates oscillations in both cutaneous and muscle afferents, the majority of the A0 neurons with axotomy-enhanced oscillations were muscle afferents. Accompanying these changes in oscillatory behavior were changes in specific electrophysiological properties of the axotomized neurons. Previous reports using intracellular recordings from whole excised DRGs in rats that underwent spinal nerve injury showed an increase in oscillatory behavior in heterogeneous samples of A0 neurons (Amir et al. 1999; Liu et al. 2000a), and teased fiber recordings in vivo indicated that there is a bias of hyperexcitability toward muscle afferents (Michaelis et al. 2000; Tal et al. 1999). Our results confirm that medium to large muscle and cutaneous afferents develop axotomy-induced membrane oscillations, but muscle afferents are the predominant functional class of neurons giving rise to oscillations following nerve injury (50 vs. 26%). Unfortunately, because of the very small sample of oscillating cells from nerve-intact preparations, we do not know whether the bias favoring of oscillations in muscle afferents is a normal characteristic or one acquired following axotomy. It is not unlikely that cells that normally fire with a steady, tonic rhythm such as muscle proprioceptors have intrinsic resonance and hence a preexisting bias toward oscillations.
Characteristics of subthreshold oscillation in the dissociated mouse DRG neurons
Dissociation of DRG neurons with enzymatic and mechanical treatments results in the shearing of the axon, removal of adherent satellite glia, and loss of the surrounding tissue milieu, in addition to potential effects of mechanical damage and enzymatic action on the neurons that remain. In spite of these isolation procedures, oscillatory behavior remained and was quite similar to that observed in the excised whole DRG preparation in which cell and tissue integrity was preserved. This confirms that oscillation behavior in these neurons is a property of the cell body membrane. In both preparations, oscillations occurred almost exclusively in A0 neurons, and both the proportion of A0 neurons with oscillations and spiking (on depolarization) and the effects of axotomy were nearly identical. Interestingly, oscillation frequency at threshold and at the optimal membrane potential were also identical in mouse and rat. Likewise, all of the biophysical properties measured closely resembled prior observations in whole rat ganglia [Tables 1 and 3, Liu et al. (2000a)]. This indicates that the dissociation procedure, and particularly its effects on ion channels, is relatively benign and suggests that the mouse DRG culture system provides a tractable model for investigation of injury-induced changes in membrane oscillations. Given the ability to develop transgenic mice models, future studies examining the oscillations in specific knock-out mice may be valuable.
Some differences were noted, however, which might be attributable to the cell dissociation procedure. First, fewer neurons showed oscillations and firing at Vr. Second, oscillation amplitude exhibited less voltage dependence. Third, both the oscillation threshold and “optimal membrane potential” were more positive than those observed in rat DRG neurons following similar nerve injury treatment. Note, however, that we cannot rule out the possibility that one or more of these differences is due to species (mouse vs. rat) or recording method employed (patch-clamp in this study vs. sharp micro-electrode penetration in the other) rather than to the dissociation procedure.
Enhancement of subthreshold oscillations
Axotomy increased the incidence of oscillations and firing at Vr. It also increased the population of neurons with oscillations when depolarized (Fig. 3). In addition, the threshold for induction of action potentials by step depolarization was more negative in the injury group. While the mechanisms for these changes are unclear, the results indicate that the excitability of the injured neurons was greater than that of the controls, possibly due to relative changes in the expression of various sodium channel subtypes (see e.g., Amir et al. 2002a; Waxman et al. 1999).
Possible mechanisms of oscillations
The mechanisms underlying the oscillations are becoming progressively clearer (Amir et al. 2002a). It is known that oscillations are dependent on TTX-sensitive sodium currents (Amir et al. 1999) and that blockade of K+ channels can both initiates oscillations and spontaneous ectopic discharge and modulate their frequency (Amir et al. 2002; Devor 1983; Kajander and Bennett 1992). There is likely an interaction between inward Na+ currents and outward K+ currents both in the development and the frequency properties of the oscillations (Amir et al. 2002a). It is interesting in this regard that neurons that have fast hyperpolarizing afterpotentials followed by a RDP (Amir et al. 2002b), which can elicit an action potential, are the only cells that show the high-frequency oscillations. This suggests that on rapid repolarization during the AHP, Na+ channels may be reprimed sufficiently to rapidly reactivate. Under certain conditions, this interaction between Na+ channel activation and inactivation, with repriming of the Na+ channel from K+ channel-induced repolarization, could repeat and lead to membrane oscillations.
There are other axotomy-enhanced changes in DRG neurons, in rats at least, that could contribute to the oscillations. Axotomy is known to differentially alter the expression of certain Na+ channel subtypes (see Waxman et al. 1999 for review) and alter action potential waveform (Abdulla and Smith 2001a; Kim et al. 1998; Liu et al. 2000a; Oyelese and Kocsis 1996; Stebbing et al. 1999). It is known that a kinetically fast TTX-sensitive type III Na+ channel is up-regulated following sciatic nerve (Cummins and Waxman 1997; Rizzo et al. 1995; Waxman et al. 1994) and spinal nerve axotomy (Kim et al. 2001) and that the slower TTX-resistant channels are downregulated (Cummins and Waxman 1997; Dib-Hajj et al. 1998; Novakovic et al. 1998). Moreover, TTX-sensitive Na+ channels of DRG neurons reprime faster after axotomy, presumably due, at least in part, to the upregulated expression of type III Na+ channels (Cummins and Waxman 1997; Everill et al. 2001). K+ currents (sustained and transient) of DRG neurons are reduced to about half of normal amplitude after peripheral axotomy (Abdulla and Smith 2001b; Everill and Kocsis 1999) and potassium channel density is reduced as well (Ishikawa et al. 1999). A reduction in K+ current could account for both lower threshold and increased repetitive discharge of the injured neurons as reported here. However, these changes in Na+ and K+ channel organization were observed in DRG neurons several days to weeks postaxotomy (sciatic nerve), and we are not certain if these changes are present at the shorter times following spinal nerve injury as reported here.
Axotomy-enhanced subthreshold oscillations and neuropathic pain
Afferent discharge arising ectopically in DRG neuronal somata is believed to contribute to spontaneous dysaesthesias, pain on movement, and tissue tenderness in patients with neuropathy and nerve injury (Devor and Seltzer 1999). During the first days after nerve injury in the Chung model of neuropathic pain, essentially all of the ectopic activity that originates in the DRG occurs in neurons with myelinated axons (A neurons) (Boucher et al. 2000; C. N. Liu et al. 2000b; X. Liu et al. 2000; Michaelis et al. 2000), although spontaneous firing at very low frequency also arises in C fibers in adjacent nerves that were not injured (Wu et al. 2001). In the present study, we recorded from dissociated A neurons in excised DRGs in which we could identify the neurons as cutaneous or muscle afferents. We found a substantial number of oscillating cells, and in all cases depolarization enhanced the oscillations and promoted repetitive firing. In vivo, depolarization of neurons within the DRG might come about in a number of ways. These include, among others, ischemia, mechanical forces applied during relative movement of adjacent vertebrae, traction forces from nerves (e.g., during walking or straight leg lifting), chemically mediated cross-excitation among the DRG A and/or C neurons (Amir and Devor 1996, 2000; Liu et al. 1999) and sympathetic sprouting in the DRG (Devor et al. 1994; McLachlan et al. 1993). Each of these processes is thought to contribute to neuropathic pain. Note that ectopic discharge originating ectopically in the DRG may contribute to neuropathic pain in two ways. It may be directly responsible for ongoing and movement-related paresthesias and pain, and it may also trigger allodynia and hyperalgesia in skin and deep tissue due to the triggering and maintenance of central sensitization (Devor and Seltzer 1999).
Oscillations and enhanced firing occurred in both cutaneous and muscle A afferents but were more frequent in muscle afferents. Some of these afferents, from skin and from muscle, were undoubtedly Aδ nociceptors whose activity is expected to evoke pain. However, there are at least three ways in which activity in larger diameter A afferents, including proprioceptors, which make up the bulk of A-afferents innervating muscle, might contribute to neuropathic pain. First, activity in Aβ afferents can cross-excite A- and C-fiber nociceptors both at the site of nerve injury and within the DRG (Amir and Devor 2000; Devor and Wall 1990; Lisney and Devor 1987). Second, following axotomy, A afferents undergo changes in key anatomical and neurochemical characteristics that render them capable of directly activating spinal pain-signaling neurons in both superficial and deep laminae of the spinal cord (Kohama et al. 2000; Woolf and Doubell 1994). Finally, in the presence of central sensitization, afferent A-fiber activity, both ectopic activity originating in the DRG and natural input originating in skin and deep tissue, may be felt as painful. Central sensitization in the Chung model may be due to activity in injured Aδ nociceptors, activity in neighboring intact C fibers, or ectopic activity in the injured A-afferents themselves (Liu et al. 2000b; Wu et al. 2001). It is interesting to speculate as to whether under these circumstances proprioceptive input from muscle and joints might be felt as deep, aching pain.
Acknowledgments
We thank the Paralyzed Veterans of America and the Eastern Paralyzed Veterans Association for support. We also thank Y.-F. Liu for preparing mouse dorsal root ganglion cultures.
This work was supported in part by the Medical Research and the Rehabilitation Research and Development Services of the Department of Veterans Affairs, the National Multiple Sclerosis Society (RG 2135, RG1912), National Institute of Neurological Disorders and Stroke Grant NS-10174, the United States-Israel Binational Science Foundation, and the German-Israel Foundation for Research and Development.
References
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol. 2001a;85:630–643. doi: 10.1152/jn.2001.85.2.630. [DOI] [PubMed] [Google Scholar]
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol. 2001b;85:644–658. doi: 10.1152/jn.2001.85.2.644. [DOI] [PubMed] [Google Scholar]
- Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Devor M. Functional cross-excitation between afferent A and C neurons in dorsal root ganglia. Neuroscience. 2000;95:189–195. doi: 10.1016/s0306-4522(99)00388-7. [DOI] [PubMed] [Google Scholar]
- Amir R, Liu CN, Kocsis JD, Devor M. Oscillatory mechanism in primary sensory neurons. Brain. 2002a;125:421–435. doi: 10.1093/brain/awf037. [DOI] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Burst discharge in primary sensory neurons: triggered by subthreshold oscillations, maintained by depolarizing afterpotentials. J Neurosci. 2002b;22:1187–1198. doi: 10.1523/JNEUROSCI.22-03-01187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Burchiel KJ. Spontaneous impulse generation in normal and denervated dorsal root ganglia: sensitivity to the alpha-adrenergic stimulation and hypoxia. Exp Neurol. 1984;85:257–272. doi: 10.1016/0014-4886(84)90139-0. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M. Potassium channels moderate ectopic excitability of nerve-end neuromas in rats. Neurosci Lett. 1983;40:181–186. doi: 10.1016/0304-3940(83)90299-9. [DOI] [PubMed] [Google Scholar]
- Devor M, Jänig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, editors. Textbook of Pain. 4. London, UK: Churchill Livingstone; 1999. pp. 129–164. [Google Scholar]
- Devor M, Wall PD. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. J Neurophysiol. 1990;64:1733–1746. doi: 10.1152/jn.1990.64.6.1733. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG. Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo. J Neurophysiol. 1998;79:2668–2676. doi: 10.1152/jn.1998.79.5.2668. [DOI] [PubMed] [Google Scholar]
- Everill B, Cummins TR, Waxman SG, Kocsis JD. Sodium currents of large (Abeta-type) adult cutaneous afferent dorsal root ganglion neurons display rapid recovery from inactivation before and after axotomy. Neuroscience. 2001;106:161–169. doi: 10.1016/s0306-4522(01)00258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent DRG neurons after axotomy. J Neurophysiol. 1999;82:700–708. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- Han HC, Lee DH, Chung JM. Characteristics of ectopic discharges in a rat neuropathic pain model. Pain. 2000;84:253–261. doi: 10.1016/s0304-3959(99)00219-5. [DOI] [PubMed] [Google Scholar]
- Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J Neurophysiol. 1994;71:1627–1637. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Tanaka M, Black J, Waxman SG. Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve. 1999;22:502–507. doi: 10.1002/(sici)1097-4598(199904)22:4<502::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oh Y, Chung JM, Chung K. The changes in expression of three subtypes of TTX-sensitive sodium channels in sensory neurons after spinal nerve ligation. Brain Res Mol Brain Res. 2001;95:153–161. doi: 10.1016/s0169-328x(01)00226-1. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kim YI, Na HS, Kim SH, Han HC, Yoon YW, Sung B, Nam HJ, Shin SL, Hong SK. Cell type-specific changes of the membrane properties of peripherally-axotomized dorsal root ganglion neurons in a rat model of neuropathic pain. Neuroscience. 1998;86:301–309. doi: 10.1016/s0306-4522(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Kirk EJ. Impulses in dorsal spinal nerve rootlets in cats and rabbits arising from dorsal root ganglia isolated from the periphery. J Comp Neurol. 1974;155:165–175. doi: 10.1002/cne.901550203. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Devor M. Altered excitability of large-diameter cutaneous afferents following nerve injury: consequences for chronic pain. In: Devor M, Rowbotham MC, Wiesenfeld-Hallin Z, editors. Proceedings of the 9th World Congress on Pain. Progress in Pain Research and Management. Vol. 16. Seattle, WA: IASP; 2000. pp. 119–135. [Google Scholar]
- Kohama I, Ishikawa K, Kocsis JD. Synaptic reorganization in the substantia gelatinosa after peripheral nerve neuroma formation: aberrant innervation of lamina II neurons by Abeta afferents. J Neurosci. 2000;20:1538–1549. doi: 10.1523/JNEUROSCI.20-04-01538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Liu X, Kim HT, Chung K, Chung JM. Receptor subtype mediating the adrenergic sensitivity of pain behavior and ectopic discharges in neuropathic Lewis rats. J Neurophysiol. 1999;81:2226–2233. doi: 10.1152/jn.1999.81.5.2226. [DOI] [PubMed] [Google Scholar]
- Lisney SJ, Devor M. Afterdischarge and interactions among fibers in damaged peripheral nerve in the rat. Brain Res. 1987;415:122–136. doi: 10.1016/0006-8993(87)90275-7. [DOI] [PubMed] [Google Scholar]
- Liu CN, Amir R, Devor M. Effect of age and nerve injury on cross-excitation among sensory neurons in rat dorsal root ganglia. Neurosci Lett. 1999;259:95–98. doi: 10.1016/s0304-3940(98)00909-4. [DOI] [PubMed] [Google Scholar]
- Liu CN, Michaelis M, Amir R, Devor M. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: relation to neuropathic pain. J Neurophysiol. 2000a;84:205–215. doi: 10.1152/jn.2000.84.1.205. [DOI] [PubMed] [Google Scholar]
- Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000b;85:503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- Liu X, Eschenfelder S, Blenk KH, Jänig W, Habler H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain. 2000;84:309–318. doi: 10.1016/s0304-3959(99)00211-0. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Jänig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Liu X, Jänig W. Axotomized and intact muscle afferents but no skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. J Neurosci. 2000;20:2742–2748. doi: 10.1523/JNEUROSCI.20-07-02742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic S, Tzoumaka E, McGivern J, Haraguchi M, Sangameswaran L, Gogas K, Eglen R, Hunter J. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Kocsis JD. GABAA receptor-mediated conductance and action potential waveform in cutaneous and muscle afferent neurons of the adult rat: differential expression and response to nerve injury. J Neurophysiol. 1996;76:2383–2392. doi: 10.1152/jn.1996.76.4.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M, Kocsis JD, Waxman SG. Selective loss of slow and enhancement of fast Na+ currents in cutaneous afferent dorsal root ganglion neurons following axotomy. Neurobiol Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Fields HL. The relationship of pain, allodynia and thermal sensation in post-herpetic neuralgia. Brain. 1996;119:347–354. doi: 10.1093/brain/119.2.347. [DOI] [PubMed] [Google Scholar]
- Sheen K, Chung JM. Signs of neuropathic pain depend on signals from injured nerve fibers in a rat model. Brain Res. 1993;610:62–68. doi: 10.1016/0006-8993(93)91217-g. [DOI] [PubMed] [Google Scholar]
- Stebbing MJ, Eschenfelder S, Habler HJ, Acosta MC, Jänig W, McLachlan EM. Changes in the action potential in sensory neurons after peripheral axotomy in vivo. Neuroreport. 1999;10:201–206. doi: 10.1097/00001756-199902050-00001. [DOI] [PubMed] [Google Scholar]
- Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65:235–242. doi: 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Tal M, Wall PD, Devor M. Myelinated afferent fiber types that become spontaneously active and mechanosensitive following nerve transection in the rat. Brain Res. 1999;824:218–223. doi: 10.1016/s0006-8993(99)01190-7. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and pain. Proc Natl Acad Sci USA. 1999;96:7635–7639. doi: 10.1073/pnas.96.14.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Black J. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons and is reexpressed following axotomy. J Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Doubell TP. The pathophysiology of chronic pain—increased sensitivity to low-threshold A beta-fiber inputs. Curr Opin Neurobiol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YW, Na HS, Chung JM. Contributions of injured and intact afferents to neuropathic pain in an experimental rat mode. Pain. 1996;64:27–36. doi: 10.1016/0304-3959(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Donnelly DF, Song XJ, LaMotte RH. Axotomy increases the excitability of dorsal root ganglion cells with unmyelinated axons. J Neurophysiol. 1997;78:2790–2794. doi: 10.1152/jn.1997.78.5.2790. [DOI] [PubMed] [Google Scholar]