Abstract
D-cycloserine (DCS) may facilitate fear extinction learning, but the behavioral consequences and mechanisms behind this effect are not well understood at present. In this article, we re-analyze data from previously-reported null-result experiments and find that rats showing above-median extinction learning during DCS treatment benefited from the drug, whereas rats showing below-median (and in this case little) extinction learning did not. Two additional experiments found that DCS facilitated extinction learning when specifically combined with a moderate, but not a small, number of extinction trials. DCS thus facilitates extinction learning only if the behavioral procedure first engages the extinction learning process. The benefits of the drug, however, were specific to the context in which extinction was learned—i.e., DCS did not prevent or influence the renewal of fear observed when the extinguished cue was tested in the original conditioning context.
Keywords: Extinction, D-cycloserine, relapse, renewal, fear
When a conditional stimulus (CS, e.g., a tone) has been associated with an aversive unconditional stimulus (US, e.g., footshock), presentation of the CS will evoke fear. This form of conditioning may play a role in the etiology of many anxiety disorders (e.g., Barlow, 2002; Bouton, Mineka, & Barlow, 2001; Mineka & Zinbarg, 2006). Importantly, conditioned fear can be reduced by repeated presentation of the CS without the US. This phenomenon, known as extinction, is widely used as a tool in therapy. However, although extinction seems to eliminate fear, it does not reflect an erasure of the original fear learning (e.g., Bouton, 2004; Bouton, Westbrook, Corcoran, & Maren, 2006; Rescorla, 2001). For example, fear of the CS returns if the context is changed after extinction, a phenomenon known as the renewal effect (e.g., Bouton & King, 1983). This result, among others, suggests that extinction depends at least partly on new learning that depends on the context for retrieval. This principle has a number of implications for the success of therapies that rely on extinction for their beneficial effects (e.g., Bouton, 2002).
If extinction involves new learning, then the loss of fear should be enhanced if the organism is given a drug that can facilitate learning. Consistent with this view, administration of D-cycloserine (DCS), a partial agonist of the NMDA receptor involved in long-term potentiation (a cellular model of learning), facilitates extinction (e.g., Walker, Ressler, Lu, & Davis, 2002; Ledgerwood, Richardson, & Cranney, 2003). Rats given DCS with a small number of extinction trials show less fear than control subjects that receive the same number of trials without DCS during tests of the CS conducted the next day. The results have clinical significance. In humans, DCS administration can likewise facilitate the loss of fear resulting from exposure that causes incomplete fear loss in controls (acrophobia: Ressler, Rothbaum, Tannenbaum, Anderson, Graap, Zimand, Hodges, & Davis, 2004; social phobia: Guastella, Richardson, Lovibond, Rapee, Gaston, Mitchell, & Dadds, 2008; Hofmann, Meuret, Smits, Simon, Pollack, Eisenmenger, Shiekh, & Otto, 2006; obsessive-compulsive disorder: Kushner, Kim, Donahue, Thuras, Adson, Kotlyar, McCabe, Peterson, & Foa, 2007).
Although DCS can lead to faster extinction, we currently know little about the boundary conditions of its effect. What are the best conditions for delivering DCS to facilitate extinction learning? And does extinction with DCS cause a more permanent, or fundamentally different, form of extinction learning? Woods and Bouton (2006) found that although DCS facilitated fear loss in extinction, it did not weaken the renewal effect. Rats received CS-shock pairings in one context and then four extinction trials (CS – no shock presentations) in a second context. The four extinction trials were preceded by an injection of saline or DCS (15 mg/kg or 30 mg/kg). Subsequent tests in the context of extinction revealed that rats that had received the 30 mg/kg dose of DCS were less afraid of the CS than controls that had received extinction with saline. However, when the CS was then tested in the original conditioning context, the DCS group showed a substantial renewal of fear that was similar in strength to the one observed in the saline controls. Thus, although DCS facilitated extinction learning, it did not change extinction’s fundamental dependence on the context.
Woods and Bouton (2006) also mentioned other results suggesting further boundary conditions for the effects of DCS. In two unpublished experiments, DCS had no demonstrable effect on extinction learning. Such null results are consistent with reports in humans suggesting that DCS does not necessarily facilitate the extinction of spider fear (Guastella, Dadds, Lovibond, Mitchell, & Richardson, 2007), electrodermal conditioning (Guastella, Lovibond, Dadds, Mitchell, & Richardson, 2007), or at least one example of obsessive-compulsive disorder (Storch, Merlo, Bengtson, Murphy, Lewis, Yang, Jacob, Larson, Hirsh, Fernandez, Geffken, & Goodman, 2007). Such findings suggest there is a need to isolate the variables that modulate DCS’s effects on extinction learning.
One clue is provided by two recent reports. Weber, Hart, and Richardson (2007) examined the effects of DCS on the extinction of fear to an odor CS. Consistent with the research just described, DCS did not always facilitate the extinction of fear. However, the authors noted that not all subjects showed evidence of learning extinction during the session when the drug was given. When they separated subjects that had learned some extinction from those that had not, only the rats that had demonstrably learned some extinction benefited from the DCS. Unfortunately, the amount of extinction learning was not manipulated experimentally, and DCS’s effectiveness with rats that had learned some extinction might therefore be explained by an unidentified third variable that also distinguished rats that had learned some extinction from those that had not. In contrast, Lee, Milton, and Everitt (2006) manipulated the amount of extinction learning experimentally. After fear conditioning with an auditory CS, they found that (1.) when DCS was combined with minimal exposure to the CS, there was an increase in fear during a subsequent test, and (2.) when DCS was combined with more extinction exposure to the CS, there was less subsequent fear. They suggested that DCS enhanced memory “reconsolidation” (Nader, Schafe, & LeDoux, 2000) when it was combined with minimal CS exposure and enhanced extinction when it was combined with more. The results suggest that the amount of nonreinforced exposure to the CS, and extinction learning, may be an important factor in predicting the effects of DCS. But the experimental design did not guarantee that the specific combination of drug and CS re-exposure produces the effects, because the control groups that had received equivalent drug exposure had no re-exposure to the CS between conditioning and testing. Isolation of a role for the drug + CS exposure combination would require a control that receives equal but separate exposure to both the drug and the CS.
The research presented in the present article further extends the analysis of the role of extinction learning on the effectiveness of DCS. We began by reanalyzing the null results of Woods and Bouton (2006) to ask whether the effectiveness of DCS was correlated with the success of extinction. We then asked experimentally whether more extinction learning allows facilitation of extinction by DCS using an experimental design that uniquely isolated the importance of the combination of DCS and CS reexposure. Having established a role for DCS, we also further characterized its effect by asking whether it reduces the context-dependence of extinction, or merely enhances the rate of normal extinction learning, which can be highly dependent on the context.
Experiment 1
In the first experiment, we fully report the two null experiments mentioned by Woods and Bouton (2006) and also ask whether there was a correlation between extinction learning during the drug session and the effectiveness of the drug. Woods and Bouton (2006) used the conditioned suppression method, in which fear of a CS is indexed by the CS’s ability to suppress an ongoing operant lever-pressing baseline reinforced by food. This method has a long history in the study of fear conditioning (e.g., Estes & Skinner, 1941; Kamin, 1969; Rescorla, 1968) and has been use extensively in research on fear extinction (e.g., Bouton, 2004; Bouton & King, 1983).
Method
Subjects
The two experiments involved a total of 48 female Wistar rats (Charles River, Quebec, Canada), 75–90 days old at the start of the experiment. The rats were individually housed and food-deprived to 80% of their baseline body weights. Water was available ad lib, and the experiments were run on consecutive days during the light portion of a 16:8-hr light-dark cycle.
Apparatus
There were two counterbalanced sets of four Skinner boxes located in separate rooms of the laboratory. Boxes from both sets measured 31.75 × 24.13 × 29.21 cm (l × w × h) and were housed in sound-attenuation chambers. The front and back walls were aluminum; the side walls and ceiling were clear acrylic plastic. There was a 5.08 × 5.08 cm recessed food cup centered in the front wall near floor-level. A 4.8-cm stainless steel operant lever was located to the left of the food cup, 6.2 cm above the floor. Ventilation fans provided background noise of 60 dB, and illumination was provided by two 7.5-W incandescent bulbs on the ceiling of the sound-attenuation chamber. The light-off CS (60 s) was created by terminating the houselights. The US was a 0.5-s, scrambled 1-mA shock provided by Med Associates shock sources. Lever pressing was reinforced with 45-mg food pellets.
In one set of boxes, the floor consisted of 0.48-cm diameter stainless steel grids spaced 3.81 cm and mounted parallel to the front wall. The ceiling and a side wall had black horizontal stripes (3.81 cm wide). A dish containing a 2% anise solution (McCormick) was placed outside each box to provide an odor. In the other set of boxes, the floor consisted of alternating stainless steel grids with different diameters (0.48 and 1.27 cm), spaced 1.59 cm. The ceiling and left sidewall were covered with dark dots (1.9 cm in diameter). A dish containing 5 ml of a 4% coconut solution (McCormick) was placed outside each box to provide odor.
Procedure
The procedure was the same as that described by Woods and Bouton (2006) through baseline training, fear conditioning, extinction, and testing.
Baseline training and conditioning
The rats were first trained to lever press on a variable-interval (VI) 90-s reinforcement schedule. There were eight daily 60-min sessions; half occurred in Context A (Days 1, 2, 3, 7) and the other half occurred in Context B (Days 4, 5, 6, 8). On Day 9, there was one 84-min session in which the rats received fear conditioning in Context A. Each of 12 presentations of the CS terminated in the onset of the US. The interval between trials (ITI) averaged 6 min. On Day 10, there was one 60-min baseline-recovery session in Context B in which the rats merely lever pressed on the VI-90 s schedule. The two groups of rats (Saline or DCS 30 mg/kg) were then matched on their baseline rates.
Extinction and testing
On Day 11, there was one 64-min session containing four CS alone (extinction) trials in Context B. The ITI averaged 15 min. DCS or saline was administered 15 min prior to the session. On Days 12 and 13, there were two similar test sessions (one per day) in Context B, each involving four more extinction trials with the CS. The Day 11 session, when the drug was administered, is referred to as the “drug session,” while the other two sessions are referred to as “test sessions.”
Drug administration
D-cycloserine (Sigma-Aldrich, St. Louis, MO) was mixed (immediately prior to use) with 0.9% physiological saline and injected subcutaneously in a volume of 1 ml/kg. Saline was injected in the same volume for the control group. Injections occurred in the colony room.
Dependent measure
The computer recorded the number of lever presses for each rat during the 60-s CS as well as the 60 s preceding the CS. These data were used to calculate a suppression ratio, C/(C+P), where C is the number of responses made during the CS and P is the number of responses made during the pre-CS period. A score of 0.5 indicates no response suppression during the CS (no fear), whereas a score of 0 indicates complete suppression (maximal fear). Statistical analysis was accomplished by analysis of variance; the rejection criterion was p < .05.
Results
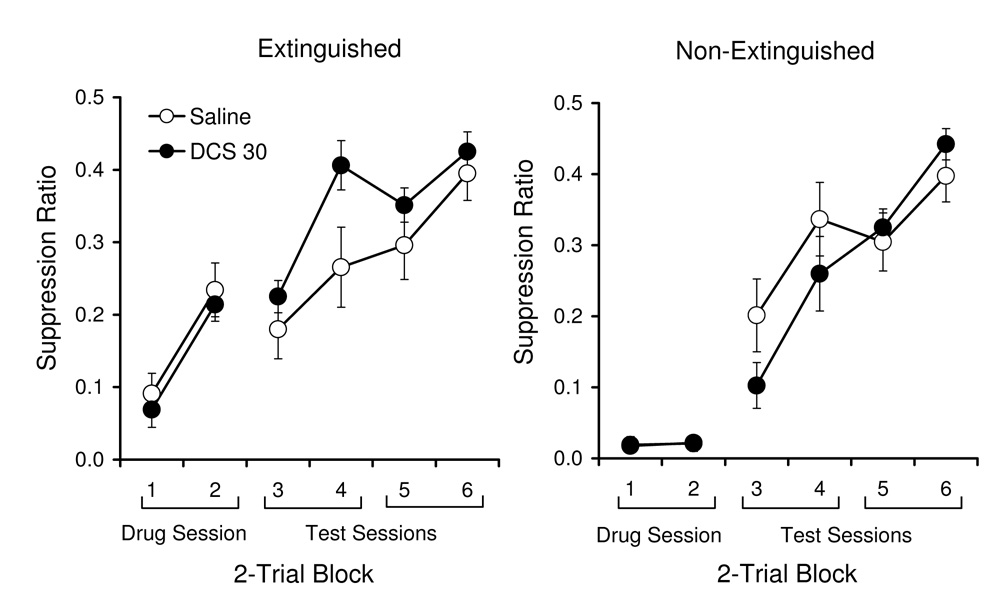
Following Weber et al. (2007), we performed a median split for each group on suppression ratios from the last two-trial block of the drug session. Rats above the median had learned more extinction during the drug session than rats below the median. The results for the above-median and below-median rats are summarized in the left- and right-hand panels of Figure 1. With the methods used here, rats that were above the median learned some extinction during the drug session, as indicated by a significant decrease in suppression across the trial blocks, F(1, 22) = 54.80, whereas rats below the median did not, F(1, 22) < 1. When we compared data across two-trial blocks of the test sessions, there was less suppression to the CS in the DCS group for the above-median groups, as indicated by a significant group × block interaction, F(3, 66) = 2.95. Planned comparisons revealed that the groups differed significantly only on the second block, F(1, 22) = 4.73, other Fs(1, 22) ≤ 1.11. There was no such DCS effect during testing for the groups that had shown below-median extinction. In fact, the saline group initially showed more extinction, which produced a group × block interaction, F(3, 66) = 3.47. Further comparisons revealed that the groups did not differ significantly on any block, Fs(1, 22) ≤ 2.67. A positive effect of DCS on extinction depended on whether the animals had learned some extinction on the drug day.
Figure 1.
Mean suppression (±SEM) of above-median (left) and below-median (right) groups during the drug session and the test sessions of Experiment 1. Extinguished = rats that showed above-median extinction during the drug session; Non-Extinguished = rats below the median.
There was no difference between the groups in their lever pressing rates during the 60 s period before each CS during the Drug and Test sessions. The Saline and DCS groups averaged pre-CS scores of 17.8 and 20.7 on the drug session and 19.4 and 19.9 during the test sessions.
Discussion
The procedure used by Woods and Bouton (2006) did not produce extinction learning in all animals during the drug session. However, animals that did learn some extinction later demonstrated facilitation by DCS. In contrast, animals that showed no evidence of extinction learning did not benefit from DCS. In fact, in the latter rats, there was a suggestion that DCS enhanced fear of the CS relative to the saline control. This result is reminiscent of data suggesting that after a single re-exposure to the CS, DCS can enhance fear of the CS, as if it enhances the reconsolidation of fear (Lee et al., 2006). The amount of extinction learning evident during the drug session predicts the effect of DCS.
Experiment 2
The results of Experiment 1 (and those of Weber et al., 2007) are correlational—they suggest that DCS’s effectiveness can be predicted from whether the animal had learned extinction during the session with the drug. However, like all correlational studies, it is possible that the results were controlled by a third (unidentified) variable. Experiment 2 was therefore designed to ask experimentally whether the effectiveness of DCS is stronger when animals are allowed to learn some extinction during the drug session. The experiment employed the design illustrated in Table 1. All rats received fear conditioning with two CSs in Context A; one CS was then extinguished with saline in Context B and the other was extinguished with DCS in Context C. Tests of the CSs in their respective contexts then assessed the drug’s effect on extinction. Notice that the within-subject comparison of fear of a CS extinguished with DCS and a second CS extinguished with Saline specifically isolates the role of the combination of DCS and CS re-exposure (cf. Lee et al., 2006). Importantly, different groups also received either 12 or 2 extinction trials. Prior research with the freezing method used in this experiment suggested that 12 trials would permit extinction learning, but two trials would not (Morris & Bouton, 2007). The hypothesis, therefore, was that DCS would only facilitate extinction when it was combined with 12 extinction trials. At the end of the experiment, the CSs were tested in Context A to ask whether DCS prevented relapse in the form of the renewal effect. We switched to the conditioned freezing method because of our recent success with it in studies of another potential facilitator of fear extinction, yohimbine (Morris & Bouton, 2007).
Table 1.
Design of Experiments 2A and 2B
| Phase |
||||
|---|---|---|---|---|
| Group | Conditioning | Extinction | B/C Test | Renewal Test |
| 12 Ext | AX+, AY+ | 12 BX− (Sal.), 12 CY− (DCS) | BX−, CY− | AX−, AY− |
| 2 Ext | AX+, AY+ | 2 BX− (Sal.), 2 CY− (DCS) | BX−, CY− | AX−, AY− |
Note: A, B, and C = Contexts; X and Y = Tone and clicker CSs (counterbalanced); + = shocked trial, − = no-shock trial; Sal. = Saline; DCS = D-cycloserine
Method
Subjects
The subjects were 64 (32 each in Experiments 2A and 2B) naive female 80 – 95-day-old Wistar rats from the same supplier. The rats were housed as in Experiment 1. Food and water were available ad lib.
Apparatus
The four coconut-scented boxes described previously served as Context A. Two more sets of four boxes housed in two other rooms served as Contexts B and C (counterbalanced). Boxes in the first of these new sets measured 26 × 25 × 19 cm. The front, back, and one side wall were made of aluminum; the remaining side wall and ceiling were clear acrylic plastic. The floor consisted of tubular steel bars, 16 mm in diameter and spaced 32 mm center-to-center. Odor was provided by 5 ml of white vinegar in a dish outside the front wall. Boxes in the second set measured 32 × 25 × 21; the front and rear walls, as well as the ceiling, were made of clear acrylic plastic, while the sidewalls were made of aluminum. The floor was made of stainless steel grids, 5 mm in diameter and spaced 15 mm apart. This set of chambers was scented with 0.5 ml of Vick’s Vaporub™ in a dish outside the chamber.
Both sets of boxes were housed in windowed sound attenuating chambers illuminated by two 7.5-watt incandescent bulbs mounted to the ceiling. A fan in each chamber provided 60 dB of background noise. A video camera was positioned approximately 1.22 m in front of each matrix of four sound attenuation chambers.
The CSs were a 3000-Hz tone (80 dB) and an intermittent white noise (clicker) (70 dB, 4 pulses / s) delivered through a 7.6 cm speaker mounted to the ceiling of the sound attenuation chamber.
Drugs
D-cycloserine was mixed with chilled physiological saline (0.9%) and kept on ice until administered. Two concentrations (15 and 30 mg/kg/ml) were prepared fresh on each day of extinction.
Procedure
Experiment 2A
The rats were handled daily for five days prior to the experiment. On Day 1, fear was conditioned in Context A to the two CSs in two 10-min sessions separated by 3 hours. In the first session, the rats received two 30-sec clicker CSs that coterminated with shock (1.0 mA, 0.5-s duration). The trials were presented five minutes apart. Two tone CSs were similarly paired with the shock during the second session.
Extinction training occurred on Days 2 and 3 in Contexts B and C, respectively. On each day, all rats received a 30-min extinction session with one of the CSs in one context (CS sequence and contexts counterbalanced). Before one of the sessions (counterbalanced), the rats were subcutaneously injected with one of two doses of DCS (15 or 30 mg/kg) in a volume of 1 ml/kg. Prior to the other session, they were injected with equivolume saline. The injections occurred in the colony room 15 minutes before the start of the session. One group receiving each dose (n = 8) received 12 extinction trials with each CS with a 2-min variable ITI. The other group (n = 8) received 2 extinction trials that were presented at the same times as the first and last trials given the 12-trial group.
On Days 4 and 5, the clicker and tone CSs were tested in the contexts in which they had been extinguished. On Day 4, the rats were returned to Context B for 10 minutes. Two CS-alone trials were presented 5 minutes apart. On Day 5, the rats were similarly tested in Context C. On Day 6, the rats were returned to Context A for renewal testing. There were two 10-min sessions separated by 3 hours. Two clicker-alone trials were presented five minutes apart in the first session, and two tone-alone trials were similarly presented in the second session.
Experiment 2B
was identical to 2A except that, in order to equate the 2-trial and 12-trial groups on their freezing levels during testing, the 2-trial group received its two pairings of tone and clicker CSs with a weaker 0.5-mA, 0.5-s shock.
Scoring
Freezing was scored from videotape with a time-sampling procedure in which the rat’s behavior was scored as freezing or not every 2 s. Freezing was defined as the absence of all movement, except that related to breathing (Fanselow, 1980). The percentage of all samples scored as freezing during each CS presentation was calculated for each rat and averaged over 2-trial blocks. A second observer (blind to treatment) scored all data from testing in Contexts B and C. The correlations between the observers’ scores (each rat in both B and C) were r (62) = .97 and .92 for Experiments 2A and 2B.
Results
Experiment 2A
The results of extinction, testing in Contexts B and C, and then the renewal tests in Context A are summarized in Figure 2. DCS dose and CS identity had no effects or interactions in any phase, ps > .05, and so the figure collapses over these variables. There was clear extinction over the six two-trial blocks in the 12-trial group, F (5,75) = 32.90; there was no main effect or interaction of the drug effect over block, Fs < 1. During the B/C test sessions, there was a significant effect of drug (DCS vs. saline) in the 12-trial group, F (1,15) = 4.76, but none in the 2-trial group, F(1,15) <1. Although these results indicate that DCS does facilitate extinction (loss of freezing) in rats given the larger number of trials, DCS did not influence the strength of the renewal effect observed when the animals were tested with the CSs in Context A. An ANOVA comparing the 12-trial group’s freezing to the drug- and saline-extinguished CSs revealed a significant difference between the tests, F (1,15) = 14.58, and no effect or interaction with the drug factor, Fs ≤ 2.01.
Figure 2.
Mean freezing of the groups during extinction (left) and testing (middle) of Experiment 2A. The renewal test, when the CS was returned to the original conditioning context, Context A, is shown at right. DCS = d-cycloserine. Note: because the crucial comparisons are within-subject, standard error bars are not shown.
Freezing during the pre-CS periods during the B/C test were 9.8% and 8.3% for saline and DCS-tested CS in the 12-trial group and 8.3% and 10.3% for the 2-trial group. During the renewal test, the 12-trial group had pre-CS freezing of 6.9% and 7.9% for the same CSs.
Experiment 2B
The results of Experiment 2B are summarized in Figure 3. DCS dose and CS identity had no effects or interactions in any phase, ps > .05, except for an interaction between CS identity and test order during the B/C test, F (1,12) = 5.02. (Because the same data pattern was evident at both levels of these factors, the figure collapses over these variables for simplicity.) Once again there was significant extinction over the six two-trial blocks in the 12-trial group, F (5,75) = 10.50, with no main effect or interaction of the drug effect over block, Fs < 1. During the B/C test session, there was a difference in freezing between the DCS- and saline-extinguished CSs, F(1,15) = 3.87, p = .03, one-tailed; when the data were combined with the identical 12-trial group in Experiment 2A, the difference was highly significant, F(1,31) = 8.69, two-tailed p = .006. In contrast, the difference in the 2-trial group did not approach significance, F < 1. There was no overall difference in freezing between the 2-trial and 12-trial groups, F(1,30) = 1.10.
Figure 3.
Mean freezing of the groups during extinction (left) and testing (middle) of Experiment 2B. The renewal test is shown at right. DCS = d-cycloserine. Experiment 2B was the same as Experiment 2A except that the 2-trial extinction group originally received conditioning with a weaker shock in order to produce a similar level of freezing during testing in Contexts B and C. Note: because the crucial comparisons are within-subject, standard error bars are not shown.
As in Experiment 2, DCS did not influence the strength of the renewal effect observed when the animals were tested with the CSs in Context A. An ANOVA comparing the drug- and saline-extinguished CS in the B/C test and Context A revealed no drug effects or interactions, Fs ≤ 1.71. Freezing increased when the rats were returned to and tested in Context A, F (1,15) = 3.97, p = .03, one-tailed. When the data were pooled with those of the identical group in Experiment 2A, the increase was highly significant, F (1, 31) = 14.41, two-tailed p = .001.
Freezing during the pre-CS periods during the B/C test were 11.5% and 10.6% for saline and DCS-tested CS in the 12-trial group and 9.6% and 7.9% for the 2-trial group. During the renewal test, the 12-trial group had pre-CS freezing of 8.3% and 9.2% for the same CSs.
Discussion
As predicted, rats that received 12 extinction trials with DCS showed extinction facilitation by DCS, whereas rats that received only 2 extinction trials showed no facilitation. These experimental results confirm the correlational results reported in Experiment 1: DCS facilitates extinction learning if learning has already begun. Importantly, although DCS produced a better reduction of freezing in the 12-trial group, this reduction was specific to the CS-context combination that had been combined with drug during extinction. In addition, DCS did not influence the strength of the renewal effect. The benefits of DCS may thus be restricted to the extinction context (Woods & Bouton, 2006).
Experiment 2B addressed the possibility that Experiment 2A’s 2-trial group showed no effect of DCS because the higher level of freezing at testing was less sensitive to group differences (e.g., if freezing was at a response ceiling). The fact that the 2-trial group had the same low level of freezing during the test, but still showed no DCS effect, indicates that the effect of the number of extinction trials was not merely an artifact of differential levels of fear during testing.
General Discussion
The data reported here help clarify the effects of DCS on extinction learning. They indicate that the effect of DCS is to boost or enhance extinction learning that is already in progress. This conclusion was supported by (1.) the retrospective and correlational analysis of our earlier null results (Experiment 1), which indicated that the effectiveness of DCS was predicted by the extent to which animals learned extinction during the drug session (while the drug was in the brain), and (2.) the experimental results indicating that DCS influenced extinction learning when it was specifically combined with a moderate but not a minimal amount of extinction training (Experiments 2A and 2B). From a practical perspective, the data clearly suggest that DCS would be best administered when a patient is given enough extinction training to engage the extinction learning process while the drug is on board.
From a theoretical perspective, extinction learning is thought to be an error correction process that begins when the organism detects a difference between what the CS predicts (e.g., shock) and what actually occurs on the trial (e.g., no shock) (e.g., see Bouton, 2004; Pearce, 1994; McNally & Westbrook, 2003). The new, inhibitory, learning to the CS is thought to accrue on each trial in proportion to the predictive error until the error is corrected over trials. The present results imply that DCS facilitates error correction rather than error detection, which could presumably influence extinction as early as the first extinction trial. DCS apparently has an effect once extinction learning has begun.
The present experiments did not replicate Lee et al.’s (2006) finding that DCS increased fear, rather than decreased fear, when it was administered with minimal extinction training. This was even true in Experiment 2B, when the group that received relatively little extinction was at a low level of the freezing scale—eliminating ceiling and scaling effects. There are many differences between the present studies and those of Lee et al., including the number of CS re-exposures in the minimally re-exposed group (2 as opposed to 1), the duration of the re-exposure session, and the context in which re-exposure occurred (different from the conditioning context versus the same). In addition, Experiments 2A and 2B used an experimental design that more conclusively isolated the effect of the combination of DCS and CS exposure. However, it is important to emphasize that the present results are consistent with those of Lee et al. in showing that the effectiveness of DCS as a facilitator of extinction depends on the number of extinction trials. Moreover, in the cocaine place preference conditioning preparation, Kelley, Anderson, and Itzhak (2007) found no facilitation of reconsolidation by DCS given with one CS re-exposure (which otherwise appeared to induce reconsolidation); in fact, DCS appeared to facilitate extinction, rather than increase conditioned performance, after a single re-exposure trial. One implication is that the effect of DCS combined with very few extinction trials may depend on a fairly delicate balance between its enhancement of extinction learning (which would serve to decrease conditioned responding) and its enhancement of whatever learning underlies the “reconsolidation” effect (which would serve to increase conditioned responding).
Importantly, the current results further indicate that when DCS is effective at facilitating extinction, its effects might not transfer to the original conditioning context. That is, whether extinction was combined with DCS or saline, there was equivalent renewal of fear when the CS was removed from the extinction context and tested in the original conditioning context (Experiment 2). That result replicates, in the freezing preparation, the results of Woods and Bouton (2006) in the conditioned suppression preparation. Despite its positive effect on increasing the loss of responding given a controlled number of extinction trials, DCS does not necessarily change the fundamental context-dependence of extinction learning, which may leave fear vulnerable to relapse in a different context.
Acknowledgments
Supported by Grant R01 MH064847 from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. 2nd edition. New York: Guilford Press; 2002. [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:248–265. [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychological Review. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and brain mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Estes WK, Skinner BF. Some quantitative properties of anxiety. Journal of Experimental Psychology. 1941;29:390–400. [Google Scholar]
- Fanselow MS. Conditioned and unconditioned components of post-shock freezing. Pavlovian Journal of Biological Sciences. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-Cycloserine on exposure therapy for spider fears. Journal of Psychiatric Research. 2007;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial for the effect of D-Cycloserine on conditioning and extinction in humans. Behaviour Research and Therapy. 2007;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biological Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JAJ, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Archives of General Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. Predictability, surprise, attention, and conditioning. In: Campbell BA, Church RM, editors. Punishment and aversive behavior. New York: Appleton-Century-Crofts; 1969. pp. 279–296. [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: Disruption and reinstatement. NeuroReport. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biological Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: Inhibition and potentiation. The Journal of Neuroscience. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behavioral Neuroscience. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behavioral Neuroscience. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. American Psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: A role for context. Behavioral Neuroscience. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Pearce JM. Similarity and discrimination: A selective review and a connectionist model. Psychological Review. 2004;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. Journal of Comparative and. Physiological Psychology. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Experimental extinction. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: Erlbaum; 2001. pp. 119–154. [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cogntive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobics to facilitate extinction of fear. Archives of General Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, Jacob ML, Larson M, Hirsh A, Fernandez M, Geffken GR, Goodman WK. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. International Clinical Psychopharmacology. 2007;22:230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. Journal of Neuroscience. 2002;15:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiology of Learning and Memory. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behavioral Neuroscience. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]