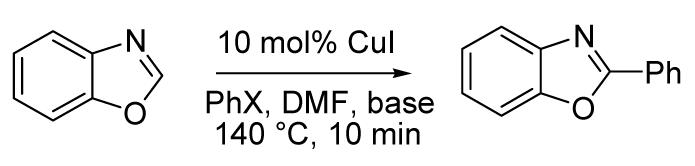
Because many pharmaceuticals contain heterocycle-aryl linkages, arylation of heterocycles has received significant attention in the recent years.1 The shortest and most efficient routes to these compounds involve direct functionalization of heterocycle C-H bonds.2 In general, most efforts in cross-coupling methodologies currently are geared toward the replacement of aryl iodides with cheaper aryl chlorides.3 However, for realistic catalyst loadings it is more cost-efficient to replace the expensive transition metal catalyst, usually palladium or rhodium, with a cheaper one.4 Use of copper catalysts for the amination and Stilleor Suzuki-type couplings has been demonstrated.5 Copper-catalyzed direct heterocycle C-H arylation reactions are unknown.6,7 We report here a general method for the copper-catalyzed heterocycle arylation by aryl iodides. In addition to electron-rich five membered heterocycles, electron-deficient pyridine oxides can also be arylated. Preliminary mechanistic studies of the arylation are also reported.
Our attention was drawn to the observation that copper salts can affect the regioselectivity of palladium-catalyzed electron-rich heterocycle arylation. Pioneering work in this field was performed by Miura and coworkers who demonstrated that N-methylimidazole is arylated in 2-position if a combination of catalytic Pd and stoichiometric Cu is used, and in 5-position if catalytic Pd is used.7 This effect may arise from the involvement of organocopper intermediates in the reaction. If the presumed intermediate could be generated without a palladium cocatalyst, a cheap and efficient method for the heterocycle arylation would be achieved. The organocopper species could be generated by using a stronger base instead of the commonly used cesium or potassium carbonates. Several amide and alkoxide bases were screened in the phenylation of benzoxazole. The best results were obtained by using lithium or potassium t-butoxides (Table 1), with LiOtBu/aryl iodide combination affording the highest yields. Equally good results can be obtained in DMF, DMA, DMPU or toluene-DMF mixtures. Commercial, non-anhydrous DMF can be used in all reactions.
Table 1.
Optimization of the arylation conditionsa
 | |||
|---|---|---|---|
| Entry | Base | PhX | Yield, % |
| 1 | KOtBu | PhF or PhOTs | No arylation |
| 2b | KotBu | PhCl | 40 |
| 3 | KotBu | PhBr | 51 |
| 4 | KotBu | PhI | 61 |
| 5 | LiOtBu | PhCl, PhBr or PhOTs | No arylation |
| 6 | LiOtBu | PhI | 93 |
Substrate (1 equiv), aryl halide (3 equiv), base (2 equiv). Yields are isolated yields.
PhCl (4 equiv), base (3 equiv).
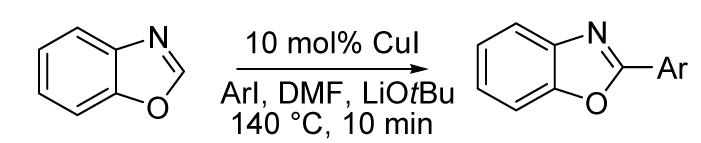
The scope with respect to aryl iodide is presented in Table 2. The arylation of benzoxazole shows that electron deficient (entries 1-2) as well as electron-rich (entries 3-7) aryl iodides are reactive. Substantial steric hindrance is tolerated on the aryl iodide (entries 5 and 6). Heteroaryl iodides are also reactive (entry 8). Yields are uniformly excellent, with the exception of mesityl iodide (entry 6).
Table 2.
Arylation scope with respect to aryl iodidesa
 | |||
|---|---|---|---|
| Entry | ArI | Product | Yield, % |
| 1 | 4-CF3C6H4I |
|
91 |
| 2 | 4-FC6H4I |
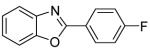
|
90 |
| 3 | 4-MeOC6H4I |
|
80 |
| 4 | 3,5-Me2C6H3I |
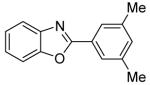
|
85 |
| 5 | 2-MeC6H4I |
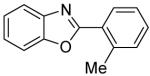
|
91 |
| 6 | 2,4,6-Me3C6H2I |
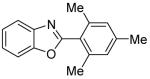
|
55 |
| 7 | 1-Iodonaphthalene |
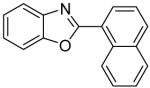
|
90 |
| 8 | 2-Iodopyridine |
|
89 |
Substrate (1 equiv), aryl iodide (3 equiv), base (2 equiv). Yields are isolated yields.
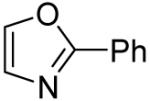
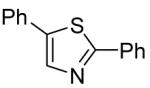
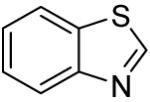
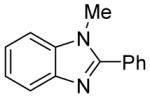
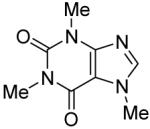
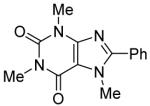
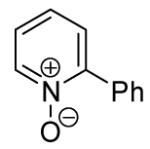
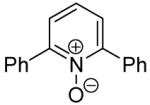
The scope with respect to the heterocycles is presented in Table 3. Oxazole can be monoarylated in 59% yield, with 7% of the diarylated product isolated (entry 1). 1,3-Thiazole is diarylated in 59% yield (entry 2). 4,5-Dimethylthiazole and benzothiazole are also reactive (entries 3 and 4). 1,2,4-Triazole, benzimidazole, and caffeine are arylated in good yields (entries 5, 6 and 7). Interestingly, electron-deficient 2-phenylpyridine oxide is arylated in 6-position in a 70% yield.8 2-Phenylpyridine and Nmethylindole were found to be unreactive under these reaction conditions. In most cases, LiOtBu affords the best yields. However, in the case of imidazole or triazole derivatives (entries 5, 6, and 7) use of KOtBu or KOtBu/LiOtBu mixture as a base afforded higher yields.
Table 3.
Arylation scope with respect to heterocyclesa
Substrate (1 equiv), iodobenzene (3 equiv), base (2 equiv). Yields are isolated yields.
2,5-Diphenyloxazole also isolated (7%).
2-Phenylthiazole also isolated (37%).
KOtBu base.
LiOtBu/KOtBu base (1:1).
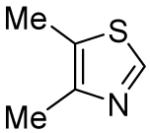
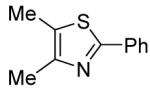
We have carried out preliminary mechanistic investigations of the coupling process (Scheme 1). The arylation employing KOtBu base is successful for aryl iodides, bromides and chlorides, although the yields are moderate. If 4,5-dimethylthiazole is reacted with iodo- or bromobenzene-d5 using KOtBu as a base (Scheme 1A), tetradeuterated product 1-1 is obtained. A single hydrogen is introduced at the ortho-position of the phenyl group. This observation can be explained by assuming that the reaction proceeds via a copper-assisted benzyne-type mechanism.9,10 No H-D exchange is observed if pentadeuterated 1-2 is submitted to the reaction conditions of Scheme 1A. If LiOtBu is used as a base, hydrogen incorporation is not observed (Scheme 1B, 1-2). Involvement of benzyne intermediate is unlikely in this case. Presumably heterocycle deprotonation by t-butoxide (perhaps assisted by copper precoordination to the heterocycle)2h followed by lithium-copper transmetallation and reaction of the organocopper species with aryl iodide leads to the arylation product. No product (LiOtBu base; PhI) or only a trace of the product (<2%; KOtBu base; PhI) was obtained if CuI was omitted from the reaction of Scheme 1.
Scheme 1.
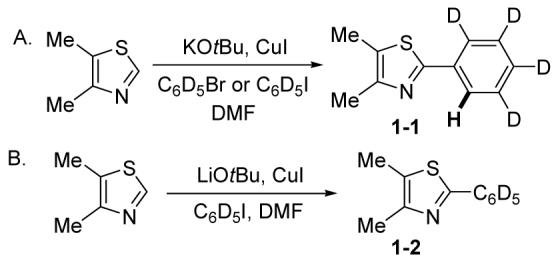
Mechanistic investigations.
In conclusion, a new method for the direct, copper-catalyzed arylation of heterocycle C-H bonds by aryl halides has been developed. In addition to electron-rich five-membered heterocycles, electron-poor pyridine oxides can also be arylated. The best results are obtained by using a combination of lithium t-butoxide base and aryl iodide coupling partner. The generality and ready availability of stating materials should make this method useful for organic synthesis.
ACKNOWLEDGMENTS
We thank the Welch Foundation (Grant No. E-1571) and National Institute of General Medical Sciences (Grant No. R01GM077635) for supporting this research.
Footnotes
Supporting Information Available: Detailed experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1 (a).Dalvie DK, Kalgutkar AS, Khojasteh-Bakht SC, Obach RS, O’Donnell JP. Chem. Res. Toxicol. 2002;15:269. doi: 10.1021/tx015574b. [DOI] [PubMed] [Google Scholar]; (b) Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]
- 2 (a).Akita Y, Inoue A, Yamamoto K, Ohta A, Kurihara T, Shimizu M. Heterocycles. 1985;23:2327. Park C-H, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V. Org. Lett. 2004;6:1159. doi: 10.1021/ol049866q. Yokooji A, Okazawa T, Satoh T, Miura M, Nomura M. Tetrahedron. 2003;59:5685. Rieth RD, Mankad NP, Calimano E, Sadighi JP. Org. Lett. 2004;6:3981. doi: 10.1021/ol048367m. Bellina F, Cauteruccio S, Mannina L, Rossi R, Viel S. J. Org. Chem. 2005;70:3997. doi: 10.1021/jo050274a. Deprez NR, Kalyani D, Krause A, Sanford MS. J. Am. Chem. Soc. 2006;128:4972. doi: 10.1021/ja060809x. Okazawa T, Satoh T, Miura M, Nomura M. J. Am. Chem. Soc. 2002;124:5286. doi: 10.1021/ja0259279. Bellina F, Cauteruccio S, Rossi R. Eur. J. Org. Chem. 2006:1379. doi: 10.1021/jo701496p. Bowie AL, Jr., Hughes CC, Trauner D. Org. Lett. 2005;7:5207. doi: 10.1021/ol052033v. Lewis JC, Wu JY, Bergman RG, Ellman JA. Angew. Chem., Int. Ed. 2006;45:1589. doi: 10.1002/anie.200504289. Chiong HA, Daugulis O. Org. Lett. 2007;9:1449. doi: 10.1021/ol0702324. Lu J, Tan X, Chen C. J. Am. Chem. Soc. 2007;129:7768. doi: 10.1021/ja072844p. Stuart DR, Fagnou K. Science. 2007;316:1172. doi: 10.1126/science.1141956. Wang X, Lane BS, Sames D. J. Am. Chem. Soc. 2005;127:4996. doi: 10.1021/ja050279p. Dwight TA, Rue NR, Charyk D, Josselyn R, DeBoef B. Org. Lett. 2007;9:3137. doi: 10.1021/ol071308z. Review:Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/b606984n.
- 3.Review: Littke AF, Fu GC. Angew. Chem., Int. Ed. 2002;41:4177. doi: 10.1002/1521-3773(20021115)41:22<4176::AID-ANIE4176>3.0.CO;2-U.
- 4.The following is the cost breakdown of a molar scale reaction run with three equiv of aryl halide and 5 mol% of palladium acetate or 10 mol% of copper iodide catalyst. The cost of palladium-catalyzed reaction is $11 (chlorobenzene) + $403 (Pd(OAc)2). The cost of copper-catalyzed reaction is $150 (iodobenzene) + $3 (CuI; Aldrich prices). An expensive ligand is usually used for palladium-catalyzed reactions.
- 5 (a).Klapars A, Antilla JC, Huang X, Buchwald SL. J. Am. Chem. Soc. 2001;123:7727. doi: 10.1021/ja016226z. [DOI] [PubMed] [Google Scholar]; (b) Allred GD, Liebeskind LS. J. Am. Chem. Soc. 1996;118:2748. [Google Scholar]; (c) Thathagar MB, Beckers J, Rothenberg G. J. Am. Chem. Soc. 2002;124:11858. doi: 10.1021/ja027716+. [DOI] [PubMed] [Google Scholar]; (d) Ma D, Liu F. Chem. Comm. 2004:1934. doi: 10.1039/b407090a. [DOI] [PubMed] [Google Scholar]
- 6 (a).Cu-catalyzed oxidation of C-H bonds in 2-phenylpyridines:Chen X, Hao X-S, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:6790. doi: 10.1021/ja061715q. Uemura T, Imoto S, Chatani N. Chem. Lett. 2006;35:842. Cu-catalyzed reaction of indoles with tetrahydroisoquinolines: Li Z, Li C-J. J. Am. Chem. Soc. 2005;127:6968. doi: 10.1021/ja0516054.
- 7 (c).Heterocycle arylation under Pd, Pd/Cu catalysis or by using several equiv Cu: Pivsa-Art S, Satoh T, Kawamura Y, Miura M, Nomura M. Bull.Chem. Soc. Jpn. 1998;71:467.
- 8.Palladium-catalyzed pyridine oxide arylation: Campeau L-C, Rousseaux S, Fagnou K. J. Am. Chem. Soc. 2005;127:18020. doi: 10.1021/ja056800x.
- 9 (a).Silver-benzyne complex reactions with arene nucleophiles: Friedman L. J. Am. Chem. Soc. 1967;89:3071. doi: 10.1021/ja00994a025. Review about Cu-catalyzed nucleophilic substitution: Lindley J. Tetrahedron. 1984;40:1433. Aryne substitution: Pellissier H, Santelli M. Tetrahedron. 2003;59:701. Benzyne reactions with Pd species: Liu Z, Larock RC. J. Org. Chem. 72:2007, 223. doi: 10.1021/jo0619534.
- 10.Arylation of 4,5-dimethylthiazole by p-tolyl bromide under conditions of Scheme 1A affords a mixture of m-tolyl- and p-tolylderivatives. See Supplementary information for details.