Abstract
Mechanisms underlying the virulence of H5N1 influenza viruses in humans are poorly understood, though evidence of hyperinflammation and systemic viral replication has been reported. Plasmacytoid dendritic cells (PDCs), a major source of type I interferon, potentially affect host defense against influenza viruses. To analyze how influenza virus infection alters PDC function, we measured cytokine secretion from primary human PDCs infected with high- or low-pathogenicity influenza viruses. IFN-α responses induced by H5N1 viruses were several-fold higher than those induced by low-pathogenicity strains; differences in the secretion of the proinflammatory cytokines TNF-α and IP-10 were less pronounced, which marks a contrast with findings from human macrophage studies. Reassortant viruses bearing H5N1-derived NS genes did not elicit enhanced IFN-α secretion by PDCs; thus, other H5N1 gene(s) are responsible for the heightened response. Their central role in the induction of an effective antiviral immune response and the finding that they respond differently to influenza viruses of different pathogenicity suggests that PDCs may play a role in the hypercytokinemia associated with H5N1 infection in humans.
Keywords: H5N1 influenza virus, plasmacytoid dendritic cells, type I interferon
Introduction
During the first human H5N1 outbreak in 1997, 18 cases of H5N1 influenza A infection in humans were confirmed, and more than 300 infected individuals have been diagnosed since 2003. H5N1 influenza infection of humans causes severe pneumonia, systemic disease, and mortality in more than 50% of patients. H5N1 avian influenza A viruses (IAVs) are potential agents of pandemic influenza, but existing strains are incapable of efficient human-to-human transmission. Defining the viral and host factors that underlie these viruses’ high pathogenicity is crucial for the development of strategies to prevent and treat infection. Studies of patients infected with H5N1 influenza have shown that systemic viral replication can occur (e.g., RNA detection in rectal swabs, blood, and postmortem tissue specimens) (de Jong et al., 2005; de Jong et al., 2006; Uiprasertkul et al., 2005). However, viral antigen has not been detected consistently outside the respiratory tract of humans.
Several lines of evidence indicate that H5N1 influenza infection triggers a massive inflammatory response (or hypercytokinemia) that contributes to systemic tissue damage. First, data from H5N1 IAV–infected patients reveal that inflammatory cytokine levels in blood can elevate dramatically during severe infection (de Jong et al., 2006; Peiris et al., 2004; To et al., 2001), and viral replication in the upper respiratory tract correlates with increased concentrations of several of these cytokines (de Jong et al., 2006). Second, the expression of genes that encode many cytokines and chemokines (e.g., IFN-α and -β, interleukin (IL)-1β, MIP-1α, RANTES, TNF-α) is strongly induced in human monocyte-derived macrophages infected with human H5N1 isolates (Cheung et al., 2002), and high levels of TNF-α and IP-10 proteins are secreted from macrophages infected with several H5N1 IAVs (Cheung et al., 2002; Guan et al., 2004). Third, in vitro H5N1 influenza infection of primary respiratory epithelial cells also induces abundant transcription and expression of a subset of cytokines and chemokines (e.g., IP-10, IL-6, and RANTES) (Chan et al., 2005). Thus, there is in vivo and in vitro evidence for profuse release of cytokines and chemokines during H5N1 IAV infection in humans.
The elevation of cytokine levels in H5N1 influenza patients suggests that highly pathogenic H5N1 viruses cause skewing of the innate immune response. Noteworthy among the innate immune mediators involved in defense against influenza infection are the type I interferons (IFNs), namely IFN-α, which restrict influenza virus replication (Isaacs and Lindenmann, 1957) and promote the induction of influenza-specific adaptive immune responses (Coro, Chang, and Baumgarth, 2006; Ito et al., 2004). Plasmacytoid dendritic cells (PDCs) are potent producers of IFN-α in response to infection with IAV or other enveloped viruses (Siegal et al., 1999). PDCs are the principal source of IFN-α in the lungs of mice infected with the influenza A strain WSN (Jewell et al., 2007). PDCs are present in human lung tissues and bronchoalveolar lavage fluids (Demedts et al., 2005) and can be recruited to the nasal mucosa during respiratory viral infection (Gill et al., 2005). Considering these characteristics, PDCs may have an important role in the host response to IAV infection. Likewise, PDCs could have a pivotal influence on the regulation of inflammation during H5N1 IAV infection.
The primary objective of these studies was to ascertain whether high-pathogenicity (H5N1) or low-pathogenicity (H1N1 or H3N2) influenza viruses affect the function of primary human PDC. Toward this end, primary PDC were isolated and infected with either high or low-pathogenicity influenza viruses. As a measure of PDC function, the differentially infected PDC were assessed for the ability to secrete several cytokines, including IFN-α. Having observed significant differences in cytokine production, we investigated potential mechanisms for the strain-to-strain disparity in PDC stimulation. Results of our experiments reveal novel modulatory effects of H5N1 IAVs upon the innate immune response. These data also suggest a role for PDCs in establishing the amplified cytokine response observed in clinical studies of H5N1 IAV infection.
Results
Secretion of IFN-α from influenza virus–infected PDCs
We analyzed the capacity of influenza viruses to induce secretion of IFN-α from PDCs isolated from human blood. To determine virus dose effects, we infected PDCs with PR8 virus at an MOI of 0.05, 1, 5, and 25. IFN-α yields from cells infected at MOI of 1, 5, or 25 were similar, whereas the amount of IFN-α detected upon infection at an MOI of 0.05 was markedly lower, suggesting that PDCs require infection with only one virus particle for full induction of the IFN-α response (data not shown). To determine the kinetic properties of the PDC response to infection with PR8 virus, we analyzed IFN-α levels in supernatants at various time points. Primary PDCs infected with PR8 at an MOI of 5 produced readily detectable IFN-α, whereas noninfected cells secreted no detectable IFN-α. The concentration of IFN-α in supernatants of PR8-infected cells increased from 14 to 32 hours after infection before a plateau in concentration was reached (Fig. 1A).
Figure 1.
Characterization of IFN-α secretion from influenza-infected PDCs. IFN-α concentrations were measured by ELISA in supernatants of primary human PDCs infected in vitro with 5 MOI. (A) PDCs were either mock infected (squares) or infected with the laboratory strain PR8 (diamonds) and supernatants were sampled over a 44 hour timecourse. (B) PDCs were infected with PR8, A/Memphis/14/98, A/Hong Kong/213/03, or A/Vietnam/1203/04 and supernatants were sampled at 8, 20, and 32 hours. (C) PDCs were stimulated with mock infection, live A/Vietnam/1203/04 or BPL-inactivated A/Vietnam/1203/04 (data shown are from one representative experiment out of three performed with cells from different individuals).
A sufficient number of PDC were isolated from one donor to compare the kinetics of parallel infections with influenza strains PR8 (H1N1), A/Memphis/14/98 (H3N2), A/Hong Kong/213/03 (H5N1), or A/Vietnam/1203/04 (H5N1). IFN-α levels were determined by ELISA at 8, 20, and 32 hours postinfection to compare the kinetic response elicited by different strains. IFN-α production in response to the two H5N1 and two human-lineage strains followed roughly similar kinetics. IFN-α levels were minimal at 8 hours (0.2 – 5.6% of peak), increased most sharply between 8 and 20 hours (reaching 62 – 89% of peak), and increased more modestly between 20 and 32 hours (Fig 1B). PDCs isolated from additional donors were infected with the same influenza virus strains for cytokine quantification at 20 hours (Fig 2). IFN-α secreted from PR8-infected cells at 20 hours ranged from 0.65 to 5.15 ng/ml (median, 2.65 ng/ml; n=6). Because the magnitude of responsiveness to identical infections varied among individuals, cytokine levels induced by each virus were normalized to those found in PDCs contemporaneously infected with PR8 (Fig. 2A). The level of IFN-α produced in response to infection with A/Memphis/14/98, a low-pathogenicity virus, was similar to that produced with PR8. Significantly higher amounts of IFN-α were secreted from PDCs infected with H5N1 virus A/Hong Kong/213/03 than with either PR8 (P < 0.05) or A/Memphis/14/98 (P < 0.005). Similarly, A/Vietnam/1203/04 induced a higher IFN-α response than either low-pathogenicity virus (P < 0.005). Responses to the two H5N1 viruses did not differ from each other (P = 0.08), but A/Vietnam/1203/04 elicited the greatest IFN-α response in all six experiments.
Figure 2.

Secretion of cytokines from PDCs in response to low- and high-pathogenicity IAVs. PDCs isolated from the peripheral blood of six human donors were aliquoted and infected at an MOI of 5 with PR8, A/Memphis/14/98, A/Hong Kong/213/03, or A/Vietnam/1203/04 viruses. Supernatants were collected 20 hours postinfection, and the concentrations of (A) IFN-α, (B) TNF-α, and (C) IP-10 were analyzed by ELISA. To account for donor-to-donor variation, cytokine concentrations produced in response to each virus are shown as percentages of the concentration produced in response to PR8 within the same experiment. Each data point is denoted (diamonds), and the median cytokine response to each virus is presented (horizontal bar). Statistically significant differences are denoted by * (P < 0.05) or ** (P < 0.005).
To test whether IFN-α induction depends on viral replication, we incubated PDCs with either live A/Vietnam/1203/04 or a beta-propiolactone inactivated preparation of the same virus stock. Inactivation of the virus reduced its capacity to stimulate IFN-α secretion from PDCs by approximately ten-fold (n=3, Fig. 1C), which indicates that viral replication must at least be initiated to trigger abundant IFN-α release. This is consistent with the over eight-hour lag before IFN-α is readily detected from infected PDCs. We assessed the replication of PR8 and A/Vietnam/1203/04 in PDCs by quantitative PCR based on amplification of a target sequence in M1 gene mRNA, normalized to GAPDH mRNA level, eight hours postinfection. This experiment was performed in duplicate using purified PDCs from two distinct donors. The relative level of M1 transcription in A/Vietnam/1203/04-infected cells exceeded that in PR8-infected cells by a factor of 2.3 in one experiment and 2.8 in the other. Titers of both viruses in 20-hour supernatants were very low (approximately 1 PFU / 100 cells) and could not be differentiated from residual inoculum (data not shown).
Secretion of inflammatory cytokines from influenza virus–infected PDCs
Infection of human macrophages with highly pathogenic H5N1 influenza viruses triggers an unusually high production of TNF-α and the chemokine IP-10 (Guan et al., 2004). Therefore, we analyzed the concentrations of these two inflammation mediators in the supernatants of PDCs infected with low- or high-pathogenicity influenza viruses. PDCs secreted readily detectable amounts of TNF-α in response to infection with all of the viruses (Fig. 2B). Differences in TNF-α induction by the different viruses were less pronounced than those in IFN-α induction, though the virus that elicited the highest median TNF-α response was also A/Vietnam/1203/04. The TNF-α response to stimulation with A/Vietnam/1203/04 was significantly greater than responses to A/Memphis/14/98 (P < 0.05) or A/Hong Kong/213/03 (P < 0.005). The levels of IP-10 in supernatants of infected PDCs were also low in comparison with the levels of IFN-α. The concentration of IP-10 induced by low-pathogenicity viruses did not differ from that induced by high-pathogenicity viruses (Fig. 2C).
Role of NS genes in differential cytokine responses of infected PDCs
Because IAV NS1 protein is a known antagonist of type I IFN production, we examined the effects of different NS genes on the modulation of PDC function. The NS gene of PR8 was replaced with that of A/Memphis/14/98, A/Hong Kong/213/03, or A/Vietnam/1203/04 to generate reassortant viruses. Reassortant viruses were then used to infect human PDCs. As was the case in experiments with wild-type viruses, we observed across experiments large variations in the magnitude of IFN-α released by infected PDCs (Fig. 3A). Thus, the levels of IFN-α induced by reassortant viruses are presented in proportion to that induced by PR8 in cells from the same individual. NS genes derived from H5N1 or H3N2 viruses and inserted into the PR8 background failed to enhance IFN-α production by infected PDCs, relative to PR8 (Fig. 3A & 3B). Rather, significantly less IFN-α secretion was induced by infection with PR8-NSMem/14/98 or PR8-NSVN/1203/04 (P < 0.05) than by infection with nonreassortant PR8. However, PR8-NSHK/213/03 infection typically resulted in an IFN-α response comparable to that elicited by nonreassortant PR8.
Figure 3.
IFN-α production by PDCs infected with reassortant PR8 viruses bearing different NS genes. PDCs from five donors were aliquoted and infected with PR8 or recombinant viruses at an MOI of 5 (n=3 for PR8-NSMem/14/98 infection). (A) Supernatants were collected at 20 hours postinfection, and IFN-α concentrations were determined by multiplex assay. Cytokine concentrations produced in response to each virus are plotted as changes relative to the concentrations of cytokines elicited by infection with PR8. Horizontal bars denote the median levels of IFN-α induced by each virus. Statistically significant differences in IFN-α induction between parent virus PR8 and reassortant PR8 viruses are indicated by * (P < 0.05). (B) The IFN-α concentrations resulting from infection of PDCs with each wild-type influenza virus (black bars) are compared with those elicited with reassortant PR8 viruses bearing the corresponding NS gene segments (gray bars).
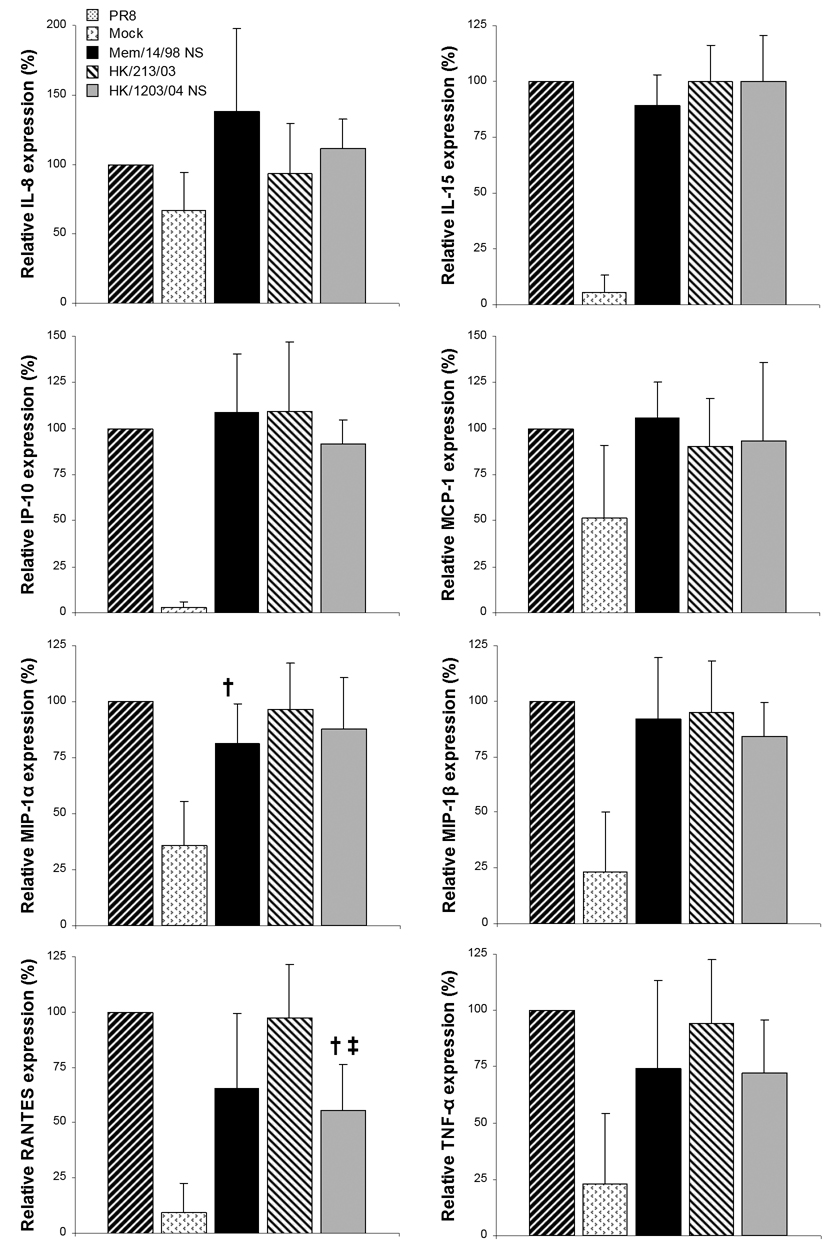
To broaden our analysis of the effects of the NS gene on the cytokine response of infected PDCs, we also used a multiplex assay to examine TNF-α, IP-10, IL-8, IL-15, MIP-1α, MIP-1β, MCP-1, and RANTES (Fig. 4). NS genes contributed by H5N1 or H3N2 viruses were not associated with enhanced secretion of these molecules by PDCs, nor were they generally associated with reduced production of these cytokines or chemokines. A notable exception was that PDCs infected with PR8-NSVN/1203/04 secreted significantly less RANTES than cells infected with PR8 or PR8-NSHK/213/03 (P < 0.05). In addition, PR8-NSMem/14/98 elicited significantly less MIP-1α than did PR8 (P < 0.05).
Figure 4.
Cytokine and chemokine production by PDCs infected with reassortant PR8 viruses bearing different NS genes. PDCs from five donors were aliquoted and infected with parent PR8 or recombinant viruses at an MOI of 5 for 20 hours, followed by multiplex evaluations of cytokines (n=3 for PR8-NSMem/14/98 infection). The concentrations of IL-8, IL-15, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α produced in response to each virus are normalized to their respective concentrations elicited by PR8. Statistically significant differences between the effects of parent PR8 virus and reassortant PR8 viruses are indicated by † (P < 0.05) and that between PR8-NSMem/14/98 and PR8-NSVN/1203/04 is indicated by ‡ (P < 0.05).
Discussion
Since the initial outbreak of highly virulent H5N1 in humans in 1997, deregulated cytokine responses have been linked with severe disease in clinical and animal studies. The cytokine hyperinduction in humans has been further characterized by ex vivo infection of immune cell populations, chiefly human macrophages, and respiratory epithelial cells (Chan et al., 2005; Cheung et al., 2002; Zhou et al., 2006). These studies have involved a relatively narrow phylogenic range of viruses and cellular specimens sampled from a small number of human subjects. Nonetheless, a trend has been established: H5N1 viruses typically elicit higher levels of certain cytokines such as TNF-α, RANTES, and IP-10 compared with less virulent influenza A strains. The degree to which type I IFNs are induced and affect the immune response to H5N1 influenza infection is less clear, perhaps due to the diversity and tissue-specific distribution of IFN-secreting cells. The effects of type I IFNs on viral replication and the downstream inflammatory cascades might have a marked impact on disease severity in human H5N1 patients. PDCs are prolific producers of type I IFNs, and as such, may have a pivotal influence on the host response during low- or high-pathogenicity influenza virus infection.
PDCs are uniquely equipped to respond to viral stimuli, including influenza virus. In vitro studies have established that influenza virus elicits strong IFN-α production from PDCs (Diebold et al., 2004), and PDCs are reportedly less prone than conventional dendritic cells to NS1-mediated suppression of the IFN-α response (Diebold et al., 2003). TLR7/9 receptors of PDCs recognize single-stranded viral RNA, such as the influenza virus genome, and trigger IFN-α production (Asselin-Paturel and Trinchieri, 2005; Diebold et al., 2004), but replication-dependent IFN-α responses to viral stimuli have also been reported (Hornung et al., 2004). Constitutive expression of the transcription factor IRF-7 and optimal retention of MyD88–IRF-7 complexes in the endosome also facilitate the potent cytokine response of virus-infected PDCs. PDCs migrate to lymphoid organs where they modulate the activation of lymphocytes (Cella et al., 2000; Jego et al., 2003). PDCs also traffic through respiratory tissues (Gill et al., 2005; Tsoumakidou et al., 2006), suggesting that these cells function as sentinels and early antiviral effectors in the respiratory tract.
In a previous study, a highly pathogenic avian H5N1 IAV elicited ample IFN-α secretion from human PDCs (Thitithanyanont et al., 2007). In our study, we compared low-pathogenicity human-lineage viruses with two highly pathogenic H5N1 virus isolates. Among replicate experiments with PDCs from multiple donors, A/Vietnam/1203/04 (H5N1) frequently elicited an IFN-α response several-fold higher than that elicited by the less virulent H1N1 or H3N2 viruses. We demonstrated that induction of IFN-α from PDCs by A/Vietnam/1203/04 is largely dependent on the virus’ viability. Quantification of viral messenger RNA in cells infected with PR8 versus A/Vietnam/1203/04 (low versus high IFN-α-inducing viruses, respectively) revealed evidence of a possible association between viral gene replication efficiency and the intensity of IFN-α induction. It is noteworthy that others previously reported a relatively high efficiency of A/Vietnam/1203/04 polymerase complex in mammalian cells and linked this to the virus’ virulence in ferrets (Salomon et al. 2006). We also measured the concentrations of the inflammatory mediators TNF-α and IP-10, both of which are secreted in abundance from human monocyte-derived macrophages after infection with various H5N1 influenza viruses (Cheung et al., 2002; Guan et al., 2004). We observed that PDCs, in contrast to macrophages, secrete similar quantities of TNF-α and IP-10 in response to H5N1 viruses as to low-pathogenicity IAV strains. The fact that exceptional IFN-α production was not coupled with especially high TNF-α or IP-10 production in H5N1 influenza–infected PDCs probably reflects the differences in cytokine specialization between PDCs and macrophages.
The influenza NS1 protein has been characterized as a type I IFN antagonist that is crucial for efficient viral replication (Wang et al., 2000; Solorzano et al., 2005). How the abundant IFN-α secretion noted in our study harmonizes with the high pathogenicity of H5N1 viruses remains unclear. One explanation could be inferred from a previous report that the NS1 genes of H5N1 influenza viruses mediate resistance to the antiviral effects of IFN-α and TNF-α in infected porcine lung epithelial cells (Seo, Hoffmann, and Webster, 2002). In another study, NS1 of highly pathogenic A/Hong Kong/486/97 (H5N1) contributed to the robust TNF-α response of monocyte-derived macrophages infected in vitro (Cheung et al., 2002). Taken together, these findings suggest that unique NS1 properties may permit highly pathogenic H5N1 viruses to replicate efficiently in mammalian hosts despite an exceptional cytokine response.
To further characterize the role of NS1 in H5N1 viral infections, we infected PDCs with reassortant viruses differing only in the NS gene segment and assessed cytokine production. This revealed that an H5N1-derived NS1 gene alone cannot confer an elevated cytokine response by PDCs in the context of the PR8 backbone. Hence, we conclude that the exceptional IFN-α–inducing property of the H5N1 viruses in PDCs is not caused by ineffective NS1 proteins with weak IFN antagonism properties. This is consistent with a recent report that NS1 proteins of several diverse avian IAV strains retain inhibitory properties against IFN gene expression in mammalian cells (Hayman et al., 2007). Rather, it appears that other genes of the highly pathogenic H5N1 viruses are required for enhanced cytokine secretion, and identifying the roles of these genes will require further study. Of note, recombinant viruses bearing the NS gene segments of A/Memphis/14/98 (H3N2) and A/Vietnam/1203/04 (H5N1) elicited significantly lower IFN-α responses by PDCs than the nonreassortant PR8 virus. In an analogous finding, others showed that differences in IFN-β induction by several human H3N2 IAV strains in tested cell lines do not always correspond with differences in IFN antagonism by their respective NS1 proteins (Hayman et al., 2006). One reason for this lack of correlation between NS1 antagonism and PDC IFN-α responses could be the fitness of a given influenza virus strain, suggesting that avian H5N1 strains crossing a species barrier to humans have not adapted in this regard.
We have shown that two pathogenic H5N1 viruses induce higher levels of type I IFN secretion by PDCs than low-pathogenicity human-lineage IAVs. The collective evidence that H5N1 influenza viruses elicit a potent type I IFN response from human PDCs and a robust TNF-α response from macrophages is consistent with the idea that the severe pathogenesis of H5N1 infection in humans is driven, in part, by inflammation. Natural mutations causing an H5N1 strain to better suppress the type I IFN response of PDCs might reduce its pathogenicity and increase its fitness for replication and transmission in humans.
Materials and Methods
Cells
Peripheral blood from healthy donors was purchased from Lifeblood Biological Services (Memphis, TN). Components of peripheral blood were separated by counter-flow centrifugal elutriation with the Elutra cell separation system (Gambro BCT, Inc., Lakewood, CO). Under this protocol, immature PDCs were coenriched with monocytes. PDCs were magnetically labeled with BDCA-4 microbeads (Miltenyi Biotec, Auburn, CA) and separated by positive selection with an autoMACS™ Separator (Miltenyi) in accordance with the manufacturer’s instructions. The purity of cell preparations was assessed by flow cytometry with directly conjugated anti–BDCA-2 monoclonal antibodies (Miltenyi). The purity of the PDC populations ranged from 84% to 96% among the 11 donors; median purity was 93%.
Viruses
Human H5N1 IAVs included in this study, A/Hong Kong/213/03 and A/Vietnam/1203/04, were obtained from the World Health Organization H5 Reference Laboratory Network. The human H3N2 virus A/Memphis/14/98 was obtained from the repository of St. Jude Children's Research Hospital. Recombinant influenza viruses that contained NS genes from each of these viruses and a background of genes isolated from the laboratory strain A/Puerto Rico/8/34 (PR8) (H1N1) were rescued by eight-plasmid reverse genetics (Hoffmann et al., 2000). A/Vietnam/1203/04 virus was chemically inactivated with 0.05% β-propiolactone (BPL) for 24 hours and subsequently the BPL was inactivated for 30 minutes at 37°C.
Plasmids encoding each of the PR8 genes were described previously (Hoffmann et al., 2002). RNA was isolated from A/Hong Kong/213/03, A/Vietnam/1203/04, and A/Memphis/14/98 and reverse transcribed into DNA templates for amplification of NS genes, as previously described (Hoffmann et al., 2001). NS genes of A/Hong Kong/213/03 (NSHK/213/03), A/Vietnam/1203/04 (NSVN/1203/04), and A/Memphis/14/98 (NSMem/14/98) were ligated into the plasmid pHW2000. These recombinant plasmids and the seven complementary plasmids encoding all PR8 genes except NS were used to cotransfect mixed cultures of Madin-Darby canine kidney (MDCK) and 293T cells (Hoffmann et al., 2000). PR8 virus with its native NS gene was generated by the same protocol. Wild-type and recombinant viruses were propagated in 10-day-old embryonated chicken eggs, except for A/Memphis/14/98, which required culture in MDCK cell culture. NS genes from the egg-grown recombinant viruses were sequenced and confirmed to match those of the original wild-type H5N1 viruses. The control for mock infection of PDCs was allantoic fluid from noninfected eggs.
In vitro infections
PDC growth medium consisted of serum-free CellGenix™ DC medium (CellGenix, Frieburg, Germany) plus penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (250 ng/ml) (Invitrogen, Carlsbad, CA). PDCs suspended in growth medium were infected with influenza viruses at a multiplicity of infection (MOI) of 5 plaque-forming units (PFUs) per cell and allowed to incubate 45 minutes at 37 °C. Cells were washed in fresh medium, resuspended to 7 × 105/ml, and dispensed into flat-bottom cell culture plates. Supernatants were sampled at the indicated times to examine the kinetics of the PDC response. In vitro PDC infection experiments with H5N1 IAV isolates were performed under BSL-3+ laboratory conditions. Trypan blue exclusion was used to compare the viability of mock-infected PDCs and PR8-infected PDCs maintained in culture at 20 hours postinfection. The viability of influenza-infected PDCs (range, 82%–97%) was higher than that of mock-infected PDCs (range, 49%–95%; three experiments). This is consistent with a report that H5N1 IAV–infected PDCs maintain high viability (Thitithanyanont et al., 2007).
Measurement of cytokine concentrations
The concentration of IFN-α in supernatant from PDCs was determined with a commercial ELISA kit (PBL Biomedical Laboratories, Piscataway, NJ). Concentrations of other secreted cytokines were measured using the human Cytokine 25-Plex AB Bead Kit (Biosource International, Inc., Camarillo, CA). The multiplex assay was analyzed on a Bio-Plex array reader (Bio-Rad Laboratories, Inc., Hercules, CA). For experiments carried out under BSL-3+ conditions, TNF-α and IP-10 were analyzed by ELISA (Quantikine Immunoassays, R&D Systems, Minneapolis, MN).
Real-time reverse transcriptase PCR
Real-time reverse transcriptase polymerase chain reaction was used to assess influenza virus replication in PDC samples. Eight hours after infection of PDC from two donors with A/Vietnam/1203/04 and A/PR/8/34 virus (MOI = 5) RNA was extracted using a Qiagen RNA extraction kit (Qiagen, Valencia, CA). cDNA was produced from the RNA using dT primers and Superscript RT III (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Matrix protein 1 mRNA was quantified using a previously described M1 primer-probe set (Di Trani et al., 2006) and compared to that of a house-keeping control gene (GAPDH). Delta Ct values were determined for both virus-PDC samples and the difference between delta Ct values was used to determine the difference in amount of M1 mRNA between PR8 and A/Vietnam/1203/04 virus infected PDCs.
Statistics
The differences in cytokine induction by different viruses were analyzed by using SAS software (SAS Institute, Cary, NC) and the mixed model for randomized block design and blocking on donor. The distribution of the concentrations of each cytokine was normalized by logarithm transformation.
Acknowledgments
We thank the SJCRH cytokine assay lab for expert assistance with multiplex assays. Assistance with statistical analysis was provided by Xiaoping Xiong, Chenghong Li, and Shengjie Wu. Funding was provided by the Children’s Infection Defense Center at St. Jude Children's Research Hospital and the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J. Exp. Med. 2005;202(4):461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 2000;1(4):305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- Chan MC, Cheung CY, Chui WH, Tsao SW, Nicholls JM, Chan YO, Chan RW, Long HT, Poon LL, Guan Y, Peiris JS. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360(9348):1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J. Immunol. 2006;176(7):4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, Beld M, Le TP, Truong HK, Nguyen VV, Tran TH, Do QH, Farrar J. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 2005;352(7):686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir. Cell. Mol. Biol. 2005;32(3):177–184. doi: 10.1165/rcmb.2004-0279OC. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, Al-Shamkhani A, Flavell R, Borrow P, Reis e Sousa C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424(6946):324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- Di Trani L, Bedini B, Donatelli I, Campitelli L, Chiappini B, De Marco MA, Delogu M, Buonavoglia C, Vaccari G. A sensitive one-step real-time PCR for detection of avian influenza viruses using a MGB probe and an internal positive control. BMC Infect. Dis. 2006;6:87. doi: 10.1186/1471-2334-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MA, Palucka AK, Barton T, Ghaffar F, Jafri H, Banchereau J, Ramilo O. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J. Infect. Dis. 2005;191(7):1105–1115. doi: 10.1086/428589. [DOI] [PubMed] [Google Scholar]
- Guan Y, Poon LL, Cheung CY, Ellis TM, Lim W, Lipatov AS, Chan KH, Sturm-Ramirez KM, Cheung CL, Leung YH, Yuen KY, Webster RG, Peiris JS. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. U S A. 2004;101(21):8156–8161. doi: 10.1073/pnas.0402443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman A, Comely S, Lackenby A, Hartgroves LC, Goodbourn S, McCauley JW, Barclay WS. NS1 proteins of avian influenza A viruses can act as antagonists of the human alpha/beta interferon response. J. Virol. 2007;81(5):2318–2327. doi: 10.1128/JVI.01856-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman A, Comely S, Lackenby A, Murphy S, McCauley J, Goodbourn S, Barclay W. Variation in the ability of human influenza A viruses to induce and inhibit the IFN-beta pathway. Virology. 2006;347(1):52–64. doi: 10.1016/j.virol.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20(25–26):3165–3170. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. U S A. 2000;97(11):6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001;146(12):2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 2004;173(10):5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B. Biol. Sci. 1957;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Ito T, Amakawa R, Inaba M, Hori T, Ota M, Nakamura K, Takebayashi M, Miyaji M, Yoshimura T, Inaba K, Fukuhara S. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J. Immunol. 2004;172(7):4253–4259. doi: 10.4049/jimmunol.172.7.4253. [DOI] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Jewell NA, Vaghefi N, Mertz SE, Akter P, Peebles RS, Jr., Bakaletz LO, Durbin RK, Flano E, Durbin JE. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J. Virol. 2007;81(18):9790–9800. doi: 10.1128/JVI.00530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF, Nicholls JM, Ng TK, Chan KH, Lai ST, Lim WL, Yuen KY, Guan Y. Reemergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363(9409):617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Franks J, Govorkova EA, Ilyushina NA, Yen HL, Hulse-Post DJ, Humberd J, Trichet M, Rehg JE, Webby RJ, Webster RG, Hoffmann E. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J. Exp. Med. 2006;203(3):689–697. doi: 10.1084/jem.20051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 2002;8(9):950–954. doi: 10.1038/nm757. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Solorzano A, Webby RJ, Lager KM, Janke BH, Garcia-Sastre A, Richt JA. Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. J. Virol. 2005;79(12):7535–7543. doi: 10.1128/JVI.79.12.7535-7543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thitithanyanont A, Engering A, Ekchariyawat P, Wiboon-ut S, Limsalakpetch A, Yongvanitchit K, Kum-Arb U, Kanchongkittiphon W, Utaisincharoen P, Sirisinha S, Puthavathana P, Fukuda MM, Pichyangkul S. High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-alpha and TLR ligands. J. Immunol. 2007;179(8):5220–5227. doi: 10.4049/jimmunol.179.8.5220. [DOI] [PubMed] [Google Scholar]
- To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, Tang NL, Tsang DN, Sung RY, Buckley TA, Tam JS, Cheng AF. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 2001;63(3):242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Tsoumakidou M, Tzanakis N, Papadaki HA, Koutala H, Siafakas NM. Isolation of myeloid and plasmacytoid dendritic cells from human bronchoalveolar lavage fluid. Immunol. Cell. Biol. 2006;84(3):267–273. doi: 10.1111/j.1440-1711.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- Uiprasertkul M, Puthavathana P, Sangsiriwut K, Pooruk P, Srisook K, Peiris M, Nicholls JM, Chokephaibulkit K, Vanprapar N, Auewarakul P. Influenza A H5N1 replication sites in humans. Emerg. Infect. Dis. 2005;11(7):1036–1041. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J. Virol. 2000;74(24):11566–11573. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Law HK, Cheung CY, Ng IH, Peiris JS, Lau YL. Differential expression of chemokines and their receptors in adult and neonatal macrophages infected with human or avian influenza viruses. J. Infect. Dis. 2006;194(1):61–70. doi: 10.1086/504690. [DOI] [PMC free article] [PubMed] [Google Scholar]