Abstract
ATP-sensitive K+ (KATP) channel openers are vasodilators that activate both plasma membrane and mitochondrial KATP channels. Here, we investigated the molecular mechanisms by which diazoxide and pinacidil induce vasodilation by studying diameter regulation of wild-type [SUR2(+/+)] and sulfonylurea receptor (SUR) 2-deficient [SUR2(−/−)] mouse myogenic mesenteric arteries. Ryanodine (10 µM), a ryanodine-sensitive Ca2+ release (RyR) channel blocker; iberiotoxin (100 nM), a large-conductance Ca2+- activated K+ (KCa) channel blocker; 4-aminopyridine (4-AP; 1 mM), a voltage-gated K+ (KV) channel blocker; manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP; 100 µM), an antioxidant; and a combination of ryanodine and 4-AP reduced diazoxide (100 µM)-induced dilation in pressurized (60 mm Hg) SUR2(+/+) arteries by 45 to 77%. In contrast, these inhibitors did not alter pinacidil (5 µM)-induced dilation in SUR2(+/+) arteries. Reverse transcription-polymerase chain reaction indicated that SUR2B was the only SUR isoform expressed in SUR2(+/+) mesenteric artery smooth muscle cells, whereas SURs were absent in SUR2(−/−) cells. In SUR2(−/−) arteries, pinacidil-induced vasodilation was ~10% of that in SUR2(+/+) arteries, whereas diazoxide-induced vasodilation was similar in SUR2(+/+) and SUR2(−/−) arteries. Atpenin (1 µM), a selective electron transport chain (ETC) complex II inhibitor, dilated arteries similarly to diazoxide, and this effect was attenuated by MnTMPyP and ryanodine + 4-AP. Atpenin also attenuated diazoxide-, but not pinacidil-induced vasodilation. In summary, data indicate that pinacidil-induced vasodilation requires SUR2B, whereas diazoxide-induced vasodilation does not require SURs. Rather, diazoxide-induced vasodilation involves ETCII inhibition; a smooth muscle cell-reactive oxygen species elevation; and RyR, KCa, and KV channel activation. These data indicate that KATP channel openers regulate arterial diameter via SUR-dependent and -independent pathways.
Plasma membrane ATP-sensitive K+ (pmKATP) channels couple changes in cellular metabolic activity to membrane electrical excitability (Ashcroft and Ashcroft, 1990). KATP channels are composed of pore-forming Kir6.x and regulatory sulfonylurea receptor (SUR) subunits (Aguilar-Bryan et al., 1998). The assembly of four Kir6.x and four SUR subunits results in tissue-specific KATP channel complexes with different functional, electrophysiological, and pharmacological properties (Aguilar-Bryan et al., 1998).
SURs are members of the ATP-binding cassette transporter protein superfamily that are predicted to form 17 transmembrane-spanning helices and two intracellular nucleotide binding domains (Tusnády et al., 1997). Two distinct SUR isoforms (SUR1 and SUR2) have been identified that are ~70% identical (Aguilar-Bryan et al., 1998). Alternative splicing of the SUR2 gene at the 3′ end results in two additional isoforms, SUR2A and SUR2B, that have different pharmacological profiles (Isomoto et al., 1996). SURs are the molecular target of pharmacologically diverse and clinically important agonists and antagonists. Sulfonylureas, including glibenclamide and tolbutamide, block KATP channels and are used in the clinic to treat type-2 diabetes because they depolarize pancreatic β-cells and induce insulin secretion (Aguilar-Bryan et al., 1998). KATP channel openers, including pinacidil and cromakalim, activate vascular smooth muscle cell KATP channels, resulting in membrane hyperpolarization and vasodilation (Brayden, 2002). KATP channel openers have been used in the treatment of hypertension and angina, and they can mimic ischemic preconditioning, which protects organs, including the heart, against the harmful effects of transient ischemia (Grover, 1994).
Mitochondria KATP (mitoKATP) channels have also been described previously (O’Rourke, 2004). Several KATP channel openers activate both pmKATP and mitoKATP channels. In cardiac myocytes, diazoxide is a more effective mitoKATP than pmKATP activator, whereas pinacidil similarly activates both pmKATP and mitoKATP channels (Liu et al., 1998). We have shown that in rat cerebral artery smooth muscle cells, diazoxide induces a mitochondrial depolarization, leading to reactive oxygen species (ROS) generation (Xi et al., 2005). The mitochondria-derived ROS activate localized intracellular calcium (Ca2+) transients, termed “sparks,” and large-conductance Ca2+-activated K+ (KCa) channels, leading to vasodilation (Xi et al., 2005). In contrast, pinacidil does not modulate smooth muscle cell mitochondrial potential, ROS, or KCa channel activity (Xi et al., 2005). This study and earlier investigations demonstrating that KATP channel openers activate pmKATP channels indicate that KATP channel openers can induce vasodilation by activating two different signaling mechanisms, one pathway that is mitochondrial and another pathway that involves pmKATP channel activation.
The goal of the present investigation was to study the molecular mechanisms by which KATP channel openers induce vasodilation. First, we determined whether KATP channel openers induce vasodilation via a ROS- and KCa channel-dependent mechanism in systemic (i.e., noncerebral) arteries and in another species—mouse. Second, we investigated molecular targets for KATP channel openers in the vasculature. To study this aim, we measured SUR isoforms that are expressed in mesenteric artery smooth muscle cells and used arteries of wild-type [SUR2(+/+)] and SUR2 deficient [SUR2(−/−)] mice. We show that mesenteric artery smooth muscle cells of SUR2(+/+) mice express only SUR2B, whereas cells of SUR2(−/−) mice do not express SURs. SUR2B is essential for pinacidil-induced vasodilation, whereas SURs are not required for diazoxide-induced vasodilation. Our data indicate that diazoxide induces vasodilation by inhibiting electron transport chain (ETC) complex II, leading to ROS-dependentKCa and voltage-gatedK+ (KV) channel activation. This study identifies two distinct molecular targets by which KATP channel openers regulate arterial diameter, namely, SUR2B and mitochondria ETCII.
Materials and Methods
Animals
Animal protocols used were reviewed and approved by the Animal Care and Use Committee at the University of Tennessee Health Science Center, an Association for Assessment and Accreditation of Laboratory Animal Care-accredited institution. SUR2(−/−) mice used in the present study were generated by targeted disruption of nucleotide binding domain 1 of SUR2, as described previously (Chutkow et al., 2001). Heterozygous SUR2-deficient mice were then bred onto the FVB mouse substrain for more than five generations. Heterozygous mice were interbred or bred into SUR(+/+) mice to generate homozygous null [SUR2(−/−)] or wild-type [SUR2(+/+)] mice, respectively. Mice were genotyped using genomic DNA isolated from tail clips of 3-week-old littermates.
Tissue Preparation and Arterial Smooth Muscle Cell Isolation
Mice (~20 g) were euthanized with an overdose of sodium pentobarbital (130 mg/kg i.p.). The mesenteric arterial bed was removed from the abdominal cavity and placed into oxygenated ice-cold (4°C) physiological saline solution (PSS) of the following composition: 112 mM NaCl, 4.8 KCl mM, 24 mM NaHCO3, 1.8 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 10 mM glucose, which was gassed with 21% O2, 5% CO2, 74% N2 to pH 7.4. Third- and fourth-order mesenteric artery branches (100–150 µm in diameter) were dissected and cleaned of adventitial connective tissue. Smooth muscle cells were enzymatically dissociated from mesenteric arteries using a method similar to that described previously (Jaggar, 2001). In brief, mesenteric arteries were placed into HEPES-buffered isolation solution: 55 mM NaCl, 80 mM sodium glutamate, 5.6 mM KCl, 2 mM MgCl2, 10 mM HEPES, and 10 mM glucose; pH 7.3 with NaOH) containing 0.3 mg/ml papain, 1 mg/ml dithioerythritol, and 1 mg/ml bovine serum albumin for 20 min (at 37°C) and immediately transferred to isolation solution containing 1 mg/ml collagenase F and 0.5 mg/ml collagenase H, 100 µM CaCl2, and 1 mg/ml BSA for 8 min (at 37°C). Arteries were subsequently washed in ice-cold isolation solution and triturated using a fire-polished glass Pasteur pipette to yield single smooth muscle cells. Smooth muscle cells were maintained in ice-cold (4°C) isolation solution and used for experiments within 2 h after isolation.
Reverse Transcription Polymerase Chain Reaction
Arterial smooth muscle cells were placed in a chamber mounted on the stage of an inverted TS 100 microscope (Nikon, Tokyo, Japan). Spindle-shaped smooth muscle cells were identified and aspirated into a borosilicate glass micropipette as we have done previously (Cheng et al., 2007). Approximately 100 cells were used for each RT-PCR experiment. Total RNA was prepared from arterial smooth muscle cells and whole hearts using Absolutely RNA nanoprep kit (Stratagene, La Jolla, CA) and TRIzol reagent (Invitrogen, Carlsbad, CA), respectively. RNA concentration and purity was measured using a DU 650 spectrophotometer (Beckman Coulter, Fullerton, CA). cDNA was synthesized from DNase-treated RNA samples using Affinity- Script Multiple Temperature Reverse Transcriptase (Stratagene). Anchored oligo(dT)20 primer (100-ng/µl reaction volume; Invitrogen) was used for the RT reactions. cDNA products were amplified by nested PCR using gene-specific oligonucleotide primer pairs. To determine the purity of collected smooth muscle cell populations, cDNA was screened for platelet/endothelial cell adhesion molecule (Pecam) 1 myosin, heavy polypeptide (Myh) 11, ubiquitin carboxyl-terminal hydrolase L1 (PGP9.5), and fatty acid binding protein (FABP) 4, which are endothelial cell, smooth muscle cell, neural cell, and adipocyte markers, respectively. The oligonucleotide primer sequences used for RT-PCR are shown in Table 1. Specific primers for first-round and nested PCR amplification of SUR1 were designed to generate cDNA fragments of 413 and 169 bp, respectively. To detect SUR2A and SUR2B transcripts, forward and reverse primers were designed within a region that is conserved in both splice variants. This approach generates different size cDNA fragments for SUR2A (first-round PCR, 451 bp; nested PCR, 320 bp) and SUR2B (first-round PCR, 275 bp; nested PCR, 144 bp). PCR amplification was performed in a Mastercycler (Eppendorf North America, New York, NY) with the following reaction conditions: an initial denaturation at 94°C for 2 min, followed by 40 cycles (denaturation at 94°C for 30 s, annealing at 57°C for 30 s, and extension at 72°C for 45 s), with a final extension at 72°C for 10 min. Reaction conditions for first-round PCR and nested PCR were the same. PCR products were separated by agarose gel (2%) electrophoresis, followed by ethidium bromide staining, UV transillumination, and documentation on Chemi- Imager 4000 (Alpha Innotech, San Leandro, CA).
TABLE 1.
Oligonucleotide primer sequences
| Primers | Sequence | GenBank Accession Numbers |
|---|---|---|
| SUR1 | NM_011510 | |
| First-round | ||
| PCR | ||
| Forward | 5′-CGAGAGTCCCTTCAATAAGCAA-3′ | |
| Reverse | 5′-AATGCTCAAAGAGCTGGCACTC-3′ | |
| Nested PCR | ||
| Forward; | 5′-CCCTCTACCAGCACACCAA-3′ | |
| Reverse | 5′-CAGTCTGCATGAGGCAGGTA-3′ | |
| SUR2 | D86037 (SUR2A) D86038 (SUR2B) | |
| First-round | ||
| PCR | ||
| Forward | 5′-TGGACAGAGACAGCTGTTCTG-3′ | |
| Reverse | 5′-AGGCAAACACTCCATCTTCCTG-3′ | |
| Nested PCR | ||
| Forward | 5′-AGCCACTGCTTCCATCGACA-3′ | |
| Reverse | 5′-CCTCTCTTCATCACAATAACCAG-3′ | |
| β-actin | NM_007393 | |
| First-round | ||
| PCR | ||
| Forward | 5′-CTACGAGGGCTATGCTCTCC-3′ | |
| Reverse | 5′-CTTCTGCATCCTGTCAGCAA-3′ | |
| Nested PCR | ||
| Forward | 5′-GCTACAGCTTCACCACCACA-3′ | |
| Reverse | 5′-AAGGAAGGCTGGAAAAGAGC-3′ | |
| Pecam-1 | NM_001032378 | |
| First-round | ||
| PCR | ||
| Forward | 5′-AGAGACGGTCTTGTCGCAGT-3′ | |
| Reverse | 5′-AATGGCAATTATCCGCTCTG-3′ | |
| Nested PCR | ||
| Forward | 5′-TGCTCTCGAAGCCCAGTATT-3′ | |
| Reverse | 5′-TGTGAATGTTGCTGGGTCAT-3′ | |
| Myh11 | NM_013607 | |
| First-round | ||
| PCR | ||
| Forward | 5′-AGCCGGAAAGACAGAGAACA-3′ | |
| Reverse | 5′-ACTTTCTGAGCCGCTGTGTT-3′ | |
| Nested PCR | ||
| Forward | 5′-GACAACTCCTCTCGCTTTGG-3′ | |
| Reverse | 5′-GCTCTCCAAAAGCAGGTCAC-3′ | |
| PGP9.5 | NM_011670 | |
| First-round | ||
| PCR | ||
| Forward | 5′-CGAAGATAGAGCCAAGTGTT-3′ | |
| Reverse | 5′-GAAAGTGCTCAGCCTGGTGT-3′ | |
| Nested PCR | ||
| Forward | 5′-GAATGCCCTTTCCAGTGAAC-3′ | |
| Reverse | 5′-GCTAAAGCTGCAAACCAAGG-3′ | |
| FABP4 | NM_024406 | |
| First-round | ||
| PCR | ||
| Forward | 5′-CAGCCTTTCTCACCTGGAAG-3′ | |
| Reverse | 5′-TGGCTCATGCCCTTTCATAA-3′ | |
| Nested PCR | ||
| Forward | 5′-CATCAGCGTAAATGGGGATT-3′ | |
| Reverse | 5′-TCGACTTTCCATCCCACTTC-3′ |
Pressurized Artery Diameter Measurements
An arterial segment ~2 mm in length was cannulated at each end in a temperature-controlled perfusion chamber (Living Systems Instrumentation, Burlington, VT). The chamber was continuously perfused with PSS equilibrated with a mixture of 21% O2, 5% CO2, 74% N2, and maintained at 37°C. Arteries were observed with a charge-coupled device camera attached to an inverted microscope (Nikon TE 200). Arterial diameter was measured by using the automatic edge-detection function of IonWizard software (Ionoptix, Milton, MA) and digitized at 1 Hz using a personal computer. Steady-state changes in intravascular pressure were achieved by elevating and lowering an attached reservoir and monitored using a pressure transducer. Intraluminal flow was absent during experiments. Tested compounds were applied via chamber perfusion. Where appropriate, the endothelium was denuded by introducing an air bubble into the artery lumen for 1 min followed by a wash with PSS. Successful endothelium-denudation was assessed by the lack of arterial dilation to 10 µM acetylcholine, an endothelium-dependent vasodilator. Effects of antioxidant, ion channel blockers, and ETCII inhibitor on diazoxide- and pinacidi-linduced vasodilations were compared with dilations induced by the same KATP channel opener in control in the same artery (i.e., in paired experiments).
Data Analysis
GraphPad Instat software (GraphPad Inc., San Diego, CA) was used for statistical analysis. Results are expressed as mean ± S.E.M. Statistical significance was calculated using Student’s t tests for paired or unpaired data. p < 0.05 was considered significant. The magnitude of myogenic tone was calculated using the following equation: myogenic tone (%) = (1 − active diameter/ passive diameter) × 100. Concentration-response curves were fit with a Boltzmann function to derive half-maximal effective concentration (EC50) values.
Chemicals
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Atpenin A5 (atpenin), iberiotoxin, ryanodine, and manganese (III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP) were purchased from Axxora Life Sciences, Inc. (San Diego, CA), California Peptide Research, Inc. (Napa, CA), A.G. Scientific (San Diego, CA), and Calbiochem (San Diego, CA), respectively.
Results
Diazoxide-Induced Mesenteric Artery Vasodilation Is Endothelium-Independent and Occurs Because of RyR, KCa, and KV Channel Activation
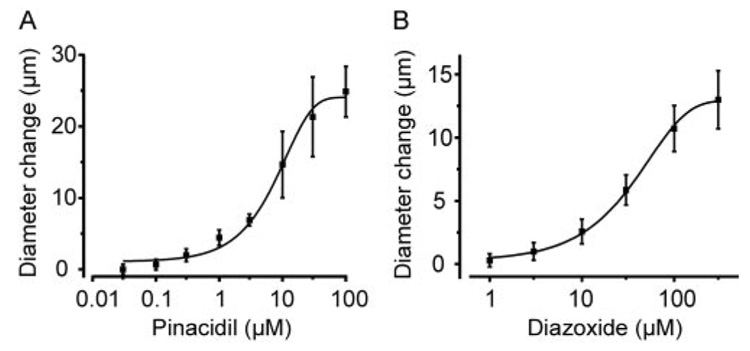
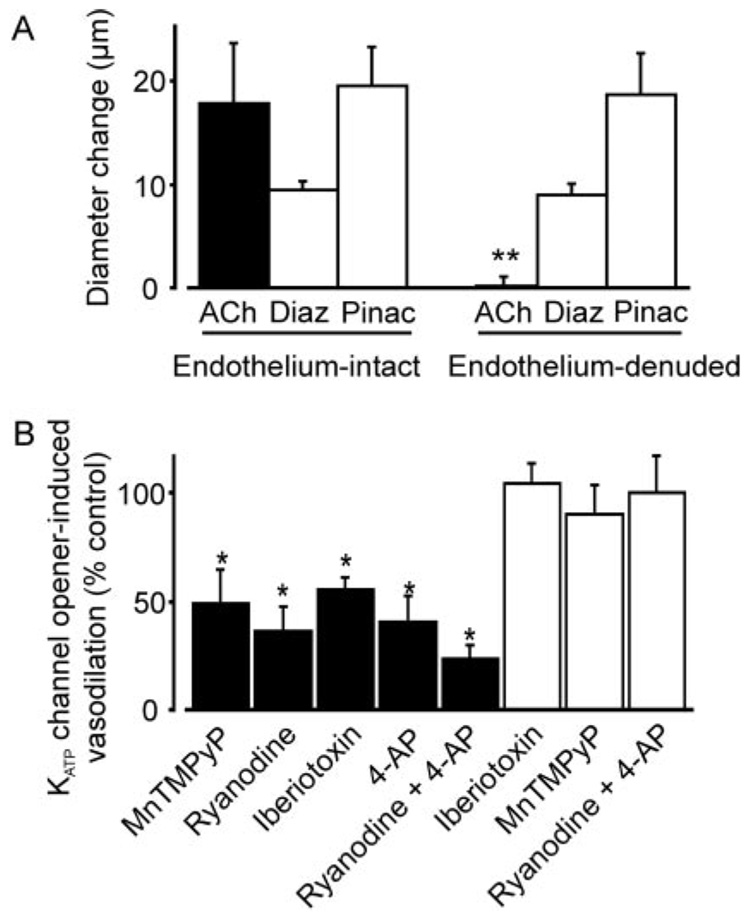
Murine mesenteric arteries were pressurized to 60 mm Hg to develop stable myogenic tone. Pinacidil and diazoxide caused vasodilation, with EC50 values of 11 and 53 µM, respectively (Fig. 1, A and B). Endothelium denudation did not alter diazoxide- or pinacidil-induced vasodilation (Fig. 2A). In contrast, MnTMPyP, a superoxide dismutase and catalase mimetic; ryanodine, a ryanodine-sensitive Ca2+ release (RyR) channel blocker; iberiotoxin, a Kca channel blocker; and 4-aminopyridine (4-AP), a KV channel blocker, reduced diazoxide-induced vasodilation in the same arteries by ~45 to 64% (Fig. 2B). A combination of ryanodine and 4-AP further reduced diazoxide-induced vasodilation by ~77%. In contrast, iberiotoxin, MnTMPyP, or a combination of ryanodine + 4-AP did not alter pinacidil-induced vasodilation (Fig. 2B). Changes in arterial diameter induced by MnTMPyP and channel blockers were MnT-MPyP, 7 ± 2 µm (n = 19); ryanodine, −10 ± 2 µm (n = 7); iberiotoxin, −7 ± 1 µm (n = 12); 4-AP, −12 ± 4 µm (n = 6); and ryanodine + 4-AP, −21 ± 3 (n = 19). These data indicate that in murine mesenteric arteries, diazoxide-induced vasodilation occurs because of a ROS elevation, and RyR, KCa, and KV channel activation. In contrast, pinacidil-induced vasodilation occurs through a ROS, KCa channel, and KV channel-independent mechanism.
Fig. 1.
Concentration-dependent dilation by pinacidil and diazoxide in pressurized (60 mm Hg) murine mesenteric arteries. Concentration-response effect of pinacidil (n = 8) (A) and diazoxide (n = 6) (B).
Fig. 2.
Diazoxide-induced mesenteric artery dilation is endothelium-independent and attenuated by RyR, KCa, and KV channel blockers. A, diameter responses to 10 µM acetylcholine (ACh; n = 7), 100 µM diazoxide (Diaz; endothelium-intact, n = 28 and endothelium-denuded, n = 7), and 5 µM pinacidil (Pinac; endothelium-intact, n = 19 and endothelium-denuded, n = 7) in pressurized (60 mm Hg) mesenteric arteries. B, diameter responses to diazoxide and pinacidil in endothelium-intact arteries. Filled bars illustrate the average effect of 100 µM MnTMPyP (n = 6), 10 µM ryanodine (n = 6), 100 nM iberiotoxin (n = 6), 1 mM 4-AP (n = 6), and ryanodine + 4-AP (n = 7) on dilation induced by 100 µM diazoxide. Empty bars show the mean effects of 100 nM iberiotoxin (n = 6), 100 µM MnTMPyP (n = 7), and ryanodine + 4-AP (n = 7) on dilation induced by 5 µM pinacidil. **, p < 0.05 compared with endothelium-intact arteries; *, p < 0.05 compared with the control.
Wild-Type Murine Mesenteric Artery Myocytes Express Only SUR2B, whereas SUR2(−/−) Myocytes Do Not Express SUR Subunits
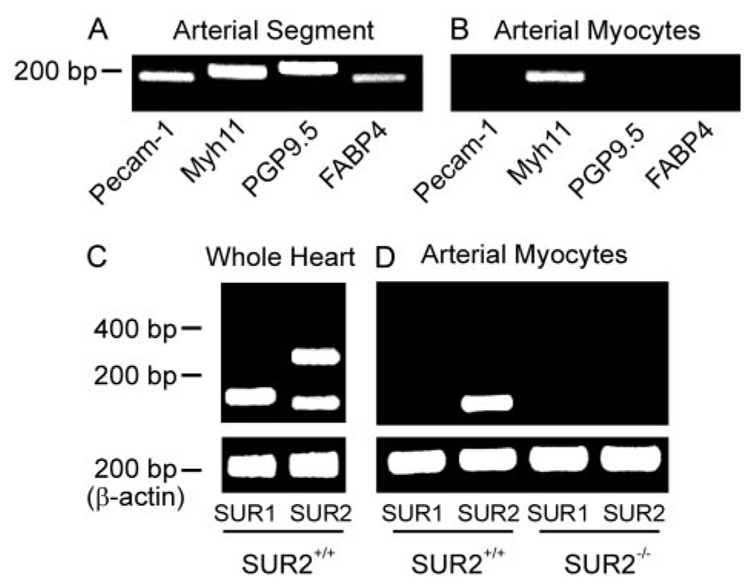
To investigate functional molecular targets for diazoxide and pinacidil in the vasculature, we sought to detect which SUR isoforms are expressed in mesenteric artery smooth muscle cells by using RT-PCR. To determine SUR subunits that are expressed specifically in mesenteric artery smooth muscle cells, isolated cells were placed in a chamber, visualized using an inverted microscope, and individually collected using a micropipette. Intact mesenteric artery segments contain multiple cell types, as shown by the amplification of transcripts for Pecam-1, Myh11, PGP9.5, and FABP4, which are endothelial-, smooth muscle-, neural-, and adipocyte-specific cell markers, respectively. In contrast, isolated mesenteric artery smooth muscle cell cDNA only amplified transcripts for Myh11, but not Pecam-1, PGP9.5, and FABP4. These data indicate that pure smooth muscle cell populations were collected (Fig. 3, A and B). Although all three sulfonylurea receptor subtypes (SUR1, SUR2A, and SUR2B) were detected in SUR2(+/+) heart lysate cDNA (Fig. 3C), only transcripts for SUR2B were amplified from SUR2(+/+) mouse arterial smooth muscle cell cDNA (Fig. 3D).
Fig. 3.
SUR2(+/+) murine mesenteric artery myocytes express SUR2B, whereas SUR2(−/−) myocytes do not express SUR subunits. A, mesenteric artery segments express transcripts for Pecam-1, Myh11, PGP9.5, and FABP4. B, in contrast, isolated, selected mesenteric artery smooth muscle cells only express transcript for Myh11. C, RT-PCR indicates that transcripts for SUR1, SUR2A, and SUR2B are amplified from cDNA generated from wild-type heart lysate (control; n = 4 for each). D, mesenteric artery smooth muscle cells isolated from SUR2(+/+) mice express only SUR2B, whereas mesenteric artery smooth muscle cells from SUR2(−/−) mice do not express any SURs (n = 4 for each). Transcript-specific primers for Pecam-1, Myh11, PGP9.5, FABP4, SUR1, SUR2A, SUR2B, and β-actin generated cDNA fragments of 176, 201, 219, 182, 169, 320, 144, and 208 bp, respectively.
SUR2 Is Not Required for Diazoxide-Induced Vasodilation, but It Is Essential for Pinacidil-Induced Vasodilation
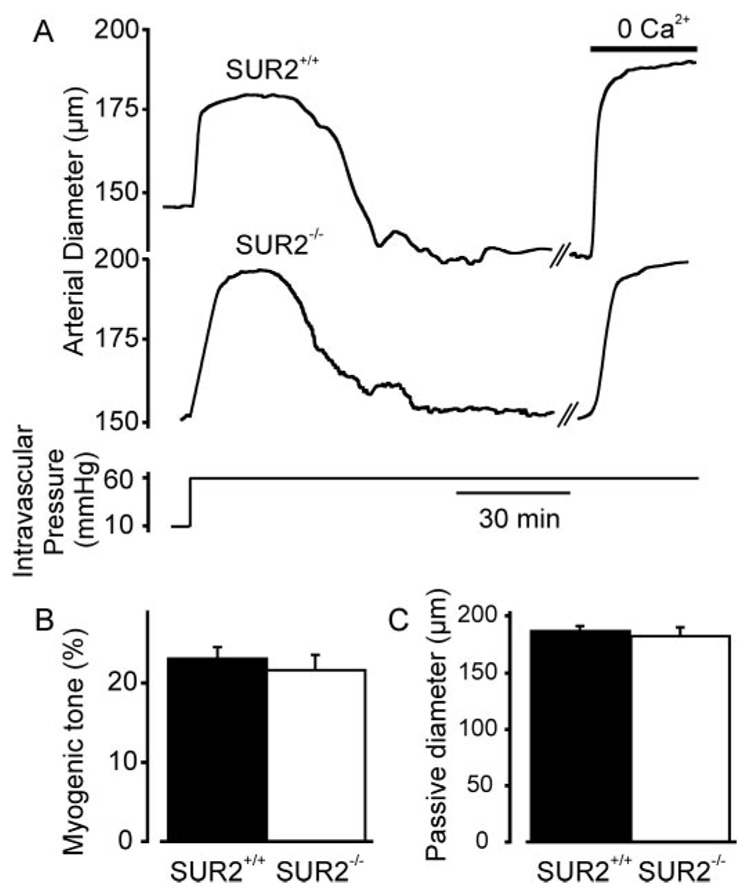
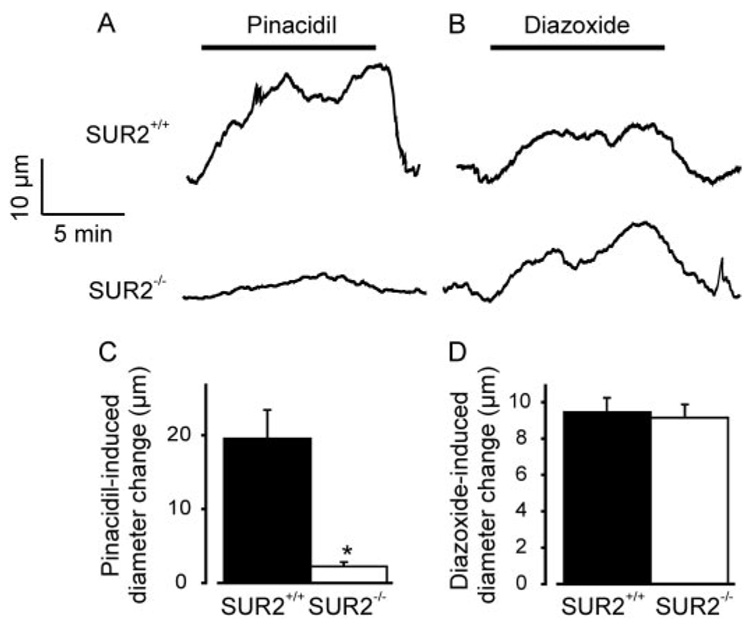
To determine whether SUR2 expression is required for diazoxide- and pinacidil-induced vasodilation, pressurized (60 mm Hg) mesenteric arteries from SUR2(+/+) and SUR2(−/−) mice were studied. Myogenic tone and passive diameter were similar in SUR2(+/+) and SUR2(−/−) arteries (Fig. 4, A–C). In SUR2(+/+) arteries, 5 µM pinacidil increased mean diameter by ~20 µm. In SUR2(−/−) arteries, pinacidil-induced dilation was largely absent, although a small dilation (2 ± 1 µm) still occurred (Fig. 5, A and C). In contrast, diazoxide (100 µM) similarly dilated SUR2(+/+) and SUR2(−/−) arteries (Fig. 5, B and D). Together, these data indicate that SUR2 is required for pinacidil-induced vasodilation and that diazoxide-induced vasodilation does not result from the activation of SUR-containing KATP channels.
Fig. 4.
Myogenic response and passive diameter are similar in mesenteric arteries of SUR2(+/+) and SUR2(−/−) mice. A, representative traces illustrating that an elevation in intravascular pressure from 10 to 60 mm Hg induces a similar myogenic constriction in SUR2(+/+) and SUR2(−/−) arteries. B, mean myogenic tone in SUR2(+/+) (n = 49) and SUR2(−/−) arteries (n = 24) pressurized to 60 mm Hg. C, Mean passive diameter of SUR2(+/+) (n = 49) and SUR2(−/−) arteries (n = 24), as determined in a Ca2+-free bath solution.
Fig. 5.
SUR deficiency inhibits pinacidil-induced dilation, but does not alter diazoxide-induced dilation in mesenteric arteries. A and B, representative traces illustrating a pinacidil (5 µM) and diazoxide (100 µM)- induced dilation in SUR2(+/+) and SUR2(−/−) arteries. C and D, mean dilation induced by pinacidil (SUR2(+/+), n = 19; SUR2(−/−), n = 16) and diazoxide (SUR2(+/+), n = 28; SUR2(−/−), n = 15). *, p < 0.05 compared with SUR2(+/+).
Electron Transport Chain Complex II Inhibition Produces Vasodilation Mechanistically Similar to Diazoxide and Attenuates Diazoxide-Induced Vasodilation
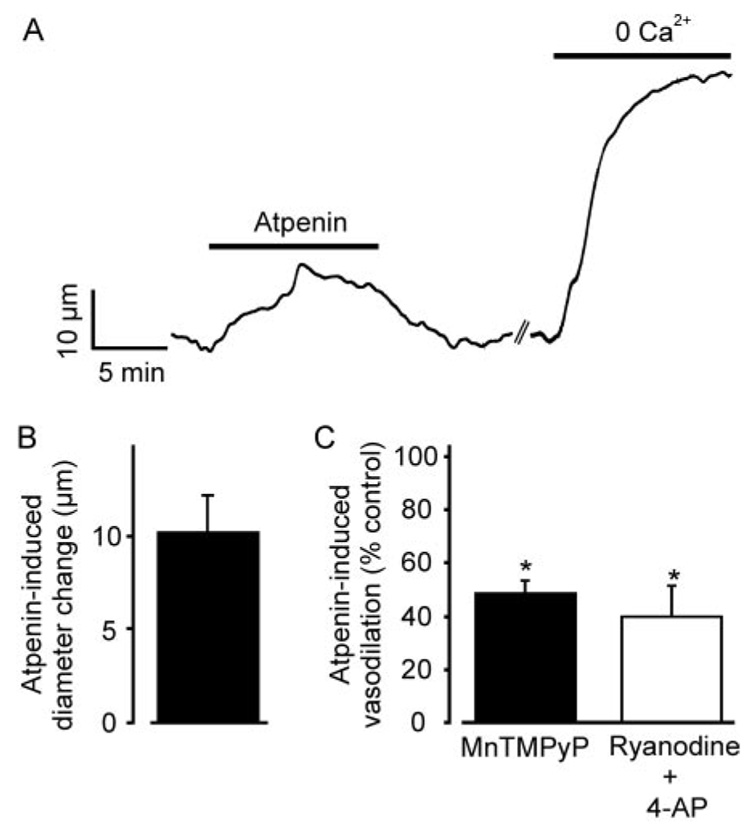
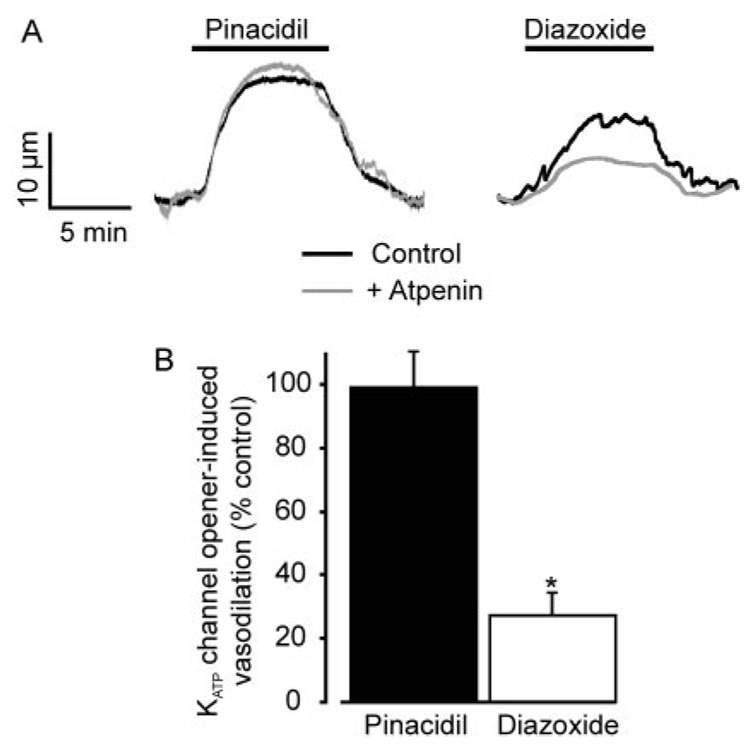
We tested the hypothesis that diazoxide induces vasodilation by inhibiting ETCII (Hanley et al., 2002). Atpenin (1 µM), a potent and selective ETCII inhibitor (Miyadera et al., 2003), increased the mean diameter of pressurized (60 mm Hg) mesenteric arteries by ~10 µm, which is similar to the dilation induced by diazoxide (Fig. 5D and Fig 6, A and B). MnTMPyP or a combination of ryanodine + 4-AP reduced mean atpenin-induced vasodilation by ~52 and 60%, respectively (Fig. 6C). Atpenin also reduced mean diazoxide-induced vasodilation by ~73%, but did not alter vasodilation induced by pinacidil (Fig. 7, A and B). These data indicate that atpenin and diazoxide regulate arterial diameter through similar mechanisms, implicate the arterial smooth muscle cell ETCII as the molecular target through which diazoxide induces vasodilation, and indicate that pinacidil does not induce vasodilation by inhibiting the ETC.
Fig. 6.
Atpenin, an ETCII inhibitor, dilates pressurized arteries, and this effect is attenuated by MnTMPyP and ryanodine + 4-AP. A, representative trace illustrating atpenin (1 µM)-induced vasodilation and dilation induced by removal of bath Ca2+ in the same artery. B, mean arterial dilation to 1 µM atpenin (n = 10). C, MnTMPyP (100 µM; n = 6) and ryanodine + 4-AP (n = 5) attenuate 1 µMatpenin-induced dilation. *, p < 0.05 compared with the control.
Fig. 7.
Atpenin reduces diazoxide-, but not pinacidil-induced, vasodilation. A, representative traces illustrating vasodilation to 100 µM diazoxide and 5 µM pinacidil in the same arteries in control (black trace) and in the presence of 1 µM atpenin (gray trace). B, mean effects of atpenin on dilation induced by 100 µM diazoxide (n = 9) and 5 µM pinacidil (n = 7). *, p < 0.05 compared with the control.
Discussion
In the present study, we investigated functional molecular targets for KATP channel openers in smooth muscle cells of small, resistance-size, mesenteric arteries. We demonstrate that diazoxide-induced dilation of pressurized mesenteric arteries is independent of SURs but involves smooth muscle cell ETC inhibition, a ROS elevation and RyR, KCa, and KV channel activation. In contrast, pinacidil-induced vasodilation requires SUR2B and does not result from ROS generation, or RyR, KCa, or KV channel activation. These findings indicate that pinacidil and diazoxide induce vasodilation by two distinct mechanisms: one mechanism that requires SUR2B and another mechanism that targets the mitochondria ETC.
KATP channels are expressed in the vascular endothelium and may modulate vasodilation in response to shear stress, acidosis, and vasodilators, including adenosine (Brayden, 2002; Yoshida et al., 2004). Here, pinacidil and diazoxide similarly dilated endothelium-intact and -denuded arteries. These data indicate that in mesenteric arteries, endothelial cell-mediated mechanisms do not significantly contribute to the vasodilation induced by these KATP channel openers.
In arterial smooth muscle cells, Ca2+ sparks occur as a result of the opening of several RyR channels on the sarcoplasmic reticulum. A Ca2+ spark activates several KCa channels, resulting in a transient KCa current. Asynchronous transient KCa currents within the arterial wall induce membrane hyperpolarization, which reduces voltage-dependent Ca2+ channel activity, leading to vasodilation (Jaggar et al., 2000). We have shown that in smooth muscle cells of rat resistance-size cerebral arteries, diazoxide induces mitochondrial depolarization, leading to a ROS elevation that activates Ca2+ sparks and KCa channels (Xi et al., 2005). The resulting membrane hyperpolarization leads to vasodilation. In contrast, in rat cerebral arteries, pinacidil-induced vasodilation does not result from mitochondrial ROS generation or Ca2+ spark and KCa channel activation (Xi et al., 2005). Data here and in our previous study collectively indicate that in both rat cerebral and mouse mesenteric arteries, diazoxide- induced vasodilation occurs as a result of mitochondria-derived ROS-mediated RyR and KCa channel activation, whereas pinacidil-induced vasodilation occurs via a ROS, Ca2+ spark, and KCa channel-independent mechanism (Xi et al., 2005). These studies suggest that the mechanisms by which diazoxide and pinacidil induce vasodilation are similar in arteries from different species and vascular beds.
Ryanodine and iberiotoxin reduced diazoxide-induced vasodilation less in mesenteric arteries than in cerebral arteries (Xi et al., 2005). These data suggested that in addition to KCa channel activation, other mechanisms mediated diazoxide-induced vasodilation in mesenteric arteries. In rat mesenteric and canine coronary artery smooth muscle cells, hydrogen peroxide (H2O2) activated 4-AP-sensitive K+ currents (Gao et al., 2003; Rogers et al., 2007). Likewise, 4-AP blocked H2O2-induced relaxation of mesenteric and coronary artery rings (Gao et al., 2003; Rogers et al., 2006). These studies suggested that ROS relaxed mesenteric and coronary arteries by activating KV channels. Therefore, we determined whether diazoxide-induced ROS dilated mesenteric arteries by activating 4-AP-sensitive KV channels. 4-AP significantly reduced diazoxide-induced vasodilation in murine mesenteric arteries, and a combination of 4-AP and ryanodine, which would simultaneously block KV and RyR channels, reduced diazoxide-induced vasodilation more than did each blocker when applied alone. These data suggest that in mesenteric arteries, diazoxide-induced ROS generation activates both KCa and KV channels, resulting in membrane hyperpolarization and vasodilation. In contrast, iberiotoxin and a combination of ryanodine and 4-AP did not alter pinacidil-induced vasodilation, indicating that this response is RyR, KCa, and KV channel-independent. In cerebral artery myocytes, diazoxide (100 µM)-induced KCa channel activation was abolished by CCCP, a mitochondrial uncoupler (Xi et al., 2005). Thus, diazoxide activates smooth muscle cell KCa channels by a mitochondria- dependent mechanism (Xi et al., 2005). We did not determine here whether diazoxide directly activates KV channels, but previous studies have demonstrated that KV channels are activated by ROS, supporting our proposal (Gao et al., 2003; Rogers et al., 2006; Rogers et al., 2007).
ROS have been demonstrated to induce both vasoconstriction and vasodilation (Lyle and Griendling, 2006). These apparently disparate findings may relate to the vascular tissue and species studied, the experimental techniques used to measure contractility, whether ROS were applied exogenously or generated endogenously, and whether the ROS elevation was acute or prolonged. In general, our data are consistent with previous evidence that KV channels mediate ROS-induced relaxation in nonpressurized coronary and mesenteric artery rings, although differences are also apparent. In nonpressurized mesenteric and coronary artery rings that were precontracted with U46619 and phenylephrine, KCa channel activation did not mediate H2O2-induced relaxation (Gao et al., 2003; Rogers et al., 2006). Intravascular pressure activates Ca2+ sparks, whereas vasoconstrictors block Ca2+ sparks and thus KCa channels (Jaggar and Nelson, 2000; Jaggar, 2001). The inability of KCa channel blockers to prevent ROS-induced relaxation in mesenteric and coronary artery rings (Gao et al., 2003; Rogers et al., 2006) likely reflects a combination of the absence of pressure-induced KCa channel activation and KCa channel inhibition by the vasoconstrictor used to induce tone. Mitochondria-derived ROS and exogenous H2O2 application may also activate different K+ channel subtypes. Regardless of these differences, our data indicate that KV channel activation makes a larger contribution to diazoxide-induced, ROS-mediated vasodilation in pressurized murine mesenteric arteries than in pressurized rat cerebral arteries. Whether this mechanistic difference is due to the species or anatomical origin of the arteries remains to be determined.
Previous studies have identified SUR isoforms expressed in intact vessels (Cao et al., 2002; Ploug et al., 2006). Because SURs are expressed in multiple vascular wall cell types, including endothelial cells and perivascular neurons, it was unclear which SUR isoforms were present specifically in smooth muscle cells (Aguilar-Bryan et al., 1998; Yoshida et al., 2004). We performed RT-PCR on isolated smooth muscle cells to avoid mRNA contamination from other vascular wall cell types. As a positive control, SUR subtypes expressed in SUR(+/+) whole heart were examined. Transcripts for SUR1, SUR2A, and SUR2B were amplified from whole heart cDNA, consistent with previous studies (Isomoto et al., 1996; Morrissey et al., 2005), whereas only SUR2B was detected in mouse arterial smooth muscle cells. These data indicate that SUR2B is the only SUR subtype that is expressed in mesenteric artery smooth muscle cells.
Plasma membrane KATP currents have been recorded in a wide variety of vascular smooth muscle cell types, including cells from mesenteric artery (Quayle et al., 1995; Brayden, 2002). We have previously shown that genetic ablation of SUR2 abolishes vascular smooth muscle cell KATP currents (Kakkar et al., 2006). In the present study, myogenic tone and passive diameter were similar in SUR2(−/−) and SUR2(+/+) arteries. These data suggest that KATP channels do not contribute significantly to resting myogenic tone and that genetic ablation of SUR2 does not result in significant remodeling of the mesenteric vasculature. pmKATP channels formed from Kir6.2/SUR1 or Kir6.2/SUR2B subunits were activated by diazoxide and pinacidil (Babenko et al., 1998). In contrast, Kir6.2/SUR2A channels were activated by pinacidil and insensitive to diazoxide (Babenko et al., 1998). These data suggest that pinacidil activates KATP channels containing all SUR subtypes whereas diazoxide activates only channels containing SUR1 or SUR2B. In the present study, pinacidil-induced dilation of pressurized SUR2(−/−) mesenteric arteries was virtually absent, indicating that SUR2B is the primary vasodilatory target for pinacidil. However, a small pinacidil-induced dilation was present in SUR2(−/−) arteries, indicating that a SUR-independent target also exists. Because pinacidil-induced vasodilation was not attenuated by iberiotoxin, MnTMPyP, or ryanodine + 4-AP, the signaling pathway mediating the SUR-independent vasodilation to pinacidil is unclear. In contrast, diazoxide similarly dilated SUR2(+/+) and SUR2(−/−) arteries. These findings indicate that plasma membrane SUR2B-containing KATP channels mediate pinacidil-induced vasodilation. In contrast, diazoxide-induced vasodilation is entirely independent of SUR-containing plasma membrane KATP channels.
MitoKATP channels have been studied in several tissues, including liver, heart, and brain (O’Rourke, 2004). Although heterologous expression of Kir6.1 and SUR1 subunits generates currents with similar pharmacology to mitoKATP, the precise molecular composition of mitoKATP is unclear (Liu et al., 2001). Western blot analysis of mitochondria isolated from liver and skeletal muscle detected Kir6.1 (Suzuki et al., 1997). A low-affinity binding site for glibenclamide in purified mitochondria preparations has been proposed to be a mitochondrial SUR (Szewczyk et al., 1997). Furthermore, mitoKATP reconstituted in liposomes consists of 55- and 63-kDa proteins that may be mitoKir and mitoSUR, respectively (Bajgar et al., 2001; Mironova et al., 2004). These studies suggest that mitoKATP channels may be similar to pmKATP channels and consist of Kir and SUR subunits. Other studies have suggested that mitochondrial respiratory chain complexes are targets for diazoxide-induced cardioprotection (Ovide-Bordeaux et al., 2000; Dröse et al., 2006). Mitochondrial ETCII (succinate dehydrogenase) and four mitochondrial inner membrane proteins (mitochondrial ATP-binding cassette (mABC) protein 1, phosphate carrier, adenine nucleotide translocator, and ATP synthase) reconstituted cardiac myocyte mitoKATP channels that were activated by diazoxide and ETCII inhibitors and blocked by glibenclamide and 5-HD (Ardehali et al., 2004). Thus, the ETCII may be a functional component of mitoKATP channels that contain mABC1, but not SURs (Ardehali et al., 2004). KATP channel-independent modulation of mitochondrial metabolism by glib-enclamide has been described previously (Engbersen et al., 2005), and 5-HD is a substrate for acyl-coA synthetase (Hanley et al., 2002). We have previously reported that glibenclamide, a pmKATP and mitoKATP channel blocker, and 5-HD, a mitoKATP channel blocker, attenuated diazoxide-induced mitochondrial depolarization and transient KCa current activation in cerebral artery myocytes (Xi et al., 2005). Data here and in our previous study collectively indicate that SUR subunits do not mediate diazoxide-induced mitochondrial depolarization, ROS generation, transient KCa current activation, and vasodilation, or inhibition of these effects by glibenclamide and 5-HD (Xi et al., 2005). In summary, our data provide evidence that if mitoKATP channels in arterial myocytes are targets of diazoxide and KATP channel blockers, these channels do not contain SUR subunits.
The ETC is a primary source of ROS in many cell types, including smooth muscle cells (Lesnefsky et al., 2001). Both diazoxide and pinacidil stimulate ROS generation in cardiac myocytes, which may underlie their cardioprotective effects (Forbes et al., 2001; Lesnefsky et al., 2001; Lembert et al., 2003). In contrast, diazoxide elevated mitochondria-derived ROS in rat cerebral artery smooth muscle cells, whereas pinacidil had no effect (Xi et al., 2005). To investigate whether diazoxide dilated mesenteric arteries by inhibiting ETCII, we studied diameter regulation by atpenin. Atpenin blocks ETCII at concentrations ~3 orders of magnitude lower than other commonly used ETCII inhibitors, including carboxin and 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione (Miyadera et al., 2003). In the present study, 1 µM atpenin dilated pressurized mesenteric arteries similarly to 100 µM diazoxide. We also tested the hypothesis that similarly to diazoxide, ETCII inhibition by at-penin causes vasodilation via ROS and RyR and KV channel activation. MnTMPyP or a combination of ryanodine + 4-AP caused a similar reduction in atpenin- and diazoxide-induced induced vasodilation. Furthermore, atpenin attenuated diazoxide-induced vasodilation, but it did not alter pinacidil-induced vasodilation. These data suggest that ETCII inhibition by either atpenin or diazoxide dilates mesenteric arteries via a ROS, Ca2+ spark, KCa, and KV channel pathway. Our results also indicate that the mitochondrial ETC and not a SUR-containing KATP channel, is the functional molecular target for diazoxide-induced vasodilation. Because diazoxide targets the ETC, ATP/ADP may decrease. This would be expected to activate KATP channels and contribute to the dilation. However, diazoxide similarly dilated SUR2(+/+) and SUR2(−/−) arteries. Therefore, any ATP/ADP shift does not seem to be sufficient to activate KATP channels. These data indicate that pinacidil and diazoxide induce vasodilation by SUR-dependent and independent pathways, respectively. Other structurally unrelated KCOs, including minoxidil sulfate, nicorandil, and aprikalim, may also act through these pathways and induce vasodilation by other mechanisms, including nitric oxide generation (Rosenblum, 2003).
In conclusion, our study indicates that pinacidil and diazoxide dilate resistance-size mesenteric arteries by two molecularly distinct signaling pathways. Pinacidil-induced vasodilation requires SUR2B. In contrast, diazoxide-induced vasodilation occurs independently of SUR-containing KATP channels and involves mitochondrial ETCII inhibition; a smooth muscle cell ROS elevation; and RyR, KCa channel, and KV channel activation.
Acknowledgments
We thank Drs. Zheng Fan and John Bannister for critical reading of the manuscript.
This study was upported by National Institutes of Health grants HL077678 and HL67061 (to J.H.J.) and HL78926 (to E.M.M.). A.A. is an American Heart Association postdoctoral fellow.
ABBREVIATIONS
- pmKATP
plasma membrane ATP-sensitive K+
- KATP
ATP-sensitive K+
- SUR
sulfonylurea receptor
- mitoKATP
mitochondria ATP-sensitive K+
- ROS
reactive oxygen species
- KCa
large-conductance Ca2+-activated K+
- ETC
electron transport chain
- KV
voltage-gated K+
- PSS
physiological saline solution
- RT
reverse transcription
- PCR
polymerase chain reaction
- Pecam
platelet/endothelial cell adhesion molecule
- Myh
myosin, heavy polypeptide
- PGP9.5
ubiquitin carboxyl-terminal hydrolase L1
- FABP
fatty acid binding-protein
- bp
base pair(s)
- MnTMPyP
manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin
- RyR
ryanodine-sensitive Ca2+ release
- 4-AP
4-aminopyridine
- U46619
15-hydroxy-11 α,9 α-(epoxymethano)prosta-5,13-dienoic acid
- 5-HD
5-hydroxydecanoate
References
- Aguilar-Bryan L, Clement JP, 4th, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- Ardehali H, Chen Z, Ko Y, Mejía-Alvarez R, Marbán E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci U S A. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft SJ, Ashcroft FM. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of Sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369–33374. doi: 10.1074/jbc.M103320200. [DOI] [PubMed] [Google Scholar]
- Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:312–316. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- Cao K, Tang G, Hu D, Wang R. Molecular basis of ATP-sensitive K+ channels in rat vascular smooth muscles. Biochem Biophys Res Commun. 2002;296:463–469. doi: 10.1016/s0006-291x(02)00892-6. [DOI] [PubMed] [Google Scholar]
- Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel CaV1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem. 2007;282:29211–29221. doi: 10.1074/jbc.M610623200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF. Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci U S A. 2001;98:11760–11764. doi: 10.1073/pnas.201390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröse S, Brandt U, Hanley PJ. K+-Independent actions of diazoxide question the role of inner membrane KATP channels in mitochondrial cytoprotective signaling. J Biol Chem. 2006;281:23733–23739. doi: 10.1074/jbc.M602570200. [DOI] [PubMed] [Google Scholar]
- Engbersen R, Masereeuw R, van Gestel MA, van der Logt EM, Smits P, Russel FG. Glibenclamide depletes ATP in renal proximal tubular cells by interfering with mitochondrial metabolism. Br J Pharmacol. 2005;145:1069–1075. doi: 10.1038/sj.bjp.0706275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Hirota S, Zhang DW, Janssen LJ, Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol. 2003;138:1085–1092. doi: 10.1038/sj.bjp.0705147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover GJ. Protective Effects of ATP-sensitive potassium-channel openers in experimental myocardial ischemia. J Cardiovasc Pharmacol. 1994;24:S18–S27. [PubMed] [Google Scholar]
- Hanley PJ, Mickel M, Löffler M, Brandt U, Daut J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-Sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Jaggar JH. JH intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by utp in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1528–C1539. doi: 10.1152/ajpcell.2000.279.5.C1528. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res. 2006;98:682–689. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- Lembert N, Idahl LA, Ammon HP. KATP channel independent effects of pinacidil on ATP production in isolated cardiomyocyte or pancreatic beta-cell mitochondria. Biochem Pharmacol. 2003;65:1835–1841. doi: 10.1016/s0006-2952(03)00179-5. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ren G, O’Rourke B, Marbán E, Seharaseyon J. Pharmacological comparison of native mitochondrial Katp channels with molecularly defined surface Katp channels. Mol Pharmacol. 2001;59:225–230. [PubMed] [Google Scholar]
- Liu Y, Sato T, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- Mironova GD, Negoda AE, Marinov BS, Paucek P, Costa AD, Grigoriev SM, Skarga YY, Garlid KD. Functional distinctions between the mitochondrial ATP-dependent K+ channel (mitoKATP) and its inward rectifier subunit (mitoKIR) J Biol Chem. 2004;279:32562–32568. doi: 10.1074/jbc.M401115200. [DOI] [PubMed] [Google Scholar]
- Miyadera H, Shiomi K, Ui H, Yamaguchi Y, Masuma R, Tomoda H, Miyoshi H, Osanai A, Kita K, Omura S. Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase) Proc Natl Acad Sci U S A. 2003;100:473–477. doi: 10.1073/pnas.0237315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey A, Parachuru L, Leung M, Lopez G, Nakamura TY, Tong X, Yoshida H, Srivastiva S, Chowdhury PD, Artman M, et al. Expression of ATP-sensitive K+ channel subunits during perinatal maturation in the mouse heart. Pediatr Res. 2005;58:185–192. doi: 10.1203/01.PDR.0000169967.83576.CB. [DOI] [PubMed] [Google Scholar]
- O’Rourke B. Evidence for mitochondrial K+ channels and their role in cardio-protection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovide-Bordeaux S, Ventura-Clapier R, Veksler V. Do modulators of the mitochondrial KATP channel change the function of mitochondria in situ? J Biol Chem. 2000;275:37291–37295. doi: 10.1074/jbc.M005772200. [DOI] [PubMed] [Google Scholar]
- Ploug KB, Edvinsson L, Olesen J, Jansen-Olesen I. Pharmacological and molecular comparison of KATP channels in rat basilar and middle cerebral arteries. Eur J Pharmacol. 2006;553:254–262. doi: 10.1016/j.ejphar.2006.09.053. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Bonev AD, Brayden JE, Nelson MT. Pharmacology of ATP-sensitive K+ currents in smooth muscle cells from rabbit mesenteric artery. Am J Physiol. 1995;269:C1112–C1118. doi: 10.1152/ajpcell.1995.269.5.C1112. [DOI] [PubMed] [Google Scholar]
- Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive KV channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1404–H1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, Saitoh S, Tune JD, Chilian WM. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–H2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI. ATP-sensitive potassium channels in the cerebral circulation. Stroke. 2003;34:1547–1552. doi: 10.1161/01.STR.0000070425.98202.B5. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kotake K, Fujikura K, Inagaki N, Suzuki T, Gonoi T, Seino S, Takata K. Kir6.1: a possible subunit of ATP-sensitive K+ channels in mitochondria. Biochem Biophys Res Commun. 1997;241:693–697. doi: 10.1006/bbrc.1997.7891. [DOI] [PubMed] [Google Scholar]
- Szewczyk A, Wójcik G, Lobanov NA, Nałecz MJ. The mitochondrial sulfonylurea receptor: identification and characterization. Biochem Biophys Res Commun. 1997;230:611–615. doi: 10.1006/bbrc.1996.6023. [DOI] [PubMed] [Google Scholar]
- Tusnády GE, Bakos E, Váradi A, Sarkadi B. Membrane topology distinguishes a subfamily of the ATP-binding cassette (ABC) transporters. FEBS Lett. 1997;402:1–3. doi: 10.1016/s0014-5793(96)01478-0. [DOI] [PubMed] [Google Scholar]
- Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res. 2005;97:354–362. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA. KATP channels of primary human coronary artery endothelial cells consist of a hetero-multimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol. 2004;37:857–869. doi: 10.1016/j.yjmcc.2004.05.022. [DOI] [PubMed] [Google Scholar]