Abstract
Bone morphogenetic proteins (BMPs) are known to promote periodontal tissue regeneration, while noggin inhibits the biological activities of BMP-2, -4, and -7. To investigate the effect of BMPs and noggin gene transfer on cementogenesis,we used cloned murine cementoblasts (OCCM). Cells were transduced using adenoviruses encoding BMP-7 (Ad-BMP-7), noggin devoid of the heparin binding site (Ad-NOGΔB2), or a control adenovirus encoding green fluorescent protein (Ad-GFP). Cells were seeded into 3D polymer scaffolds and implanted into SCID mice to determine the in vivo mineral-inducing ability of the cells. Cells transduced with Ad-NOG.B2 at 3 and 6 weeks postimplantation exhibited reduced mineral formation compared with all other groups. Although gene expression of osteocalcin and bone sialoprotein increased after Ad-BMP-7 transduction in vitro, following BMP-7 gene transfer in vivo, transcripts for OCN and BSP were not significantly different from controls, and mineral density was not significantly increased compared with Ad-GFP and NT groups. These results indicate that in mature cementoblast populations, gene transfer of noggin inhibits biomineralization induced by cementoblasts, whereas exogenous BMP has minimal effects on mineralization.
Keywords: Bone Morphogenetic Proteins, Cementoblasts, Gene Therapy, Mineralization, Noggin
INTRODUCTION
Periodontal diseases, among the most common infectious diseases in the world today, are characterized by the destruction of several tissues surrounding the tooth, including alveolar bone, cementum, and the periodontal ligament [1]. A challenge in periodontal therapy is to promote the regeneration of these tooth-supporting structures. Cementogenesis is required for regeneration of periodontal tissues [2]. Existing clinical therapies aimed at restoring lost periodontal support are unpredictable and often result in incomplete regeneration [3]. A better under-standing of the cellular and molecular mechanisms regulating cementogenesis should aid in improving periodontal regenerative outcomes.
Bone morphogenetic proteins (BMPs) are multifunctional growth factors belonging to the transforming growth factor-β superfamily of polypeptide signaling molecules [4]. Not only do selected BMPs have the ability to induce ectopic bone formation, but they also play crucial roles in embryogenesis and fracture repair [5]. BMPs are differently expressed during tooth development and periodontal repair [6, 7]. BMP-2 triggers the differentiation of dental follicle cells into cementoblasts or osteoblasts in vitro [8]. Moreover, BMPspotently stimulate alveolar bone regeneration around teeth [9], as well as initiate cementogenesis in periodontal wounds [10, 11]. Therefore, BMP signaling appears to be critical in regulating periodontal tissue formation in pre- and postnatal life.
Several proteins are known to govern BMP activities such as the antagonists noggin, chordin and follistatin [12]. Among them, noggin initially identified in the Spemann organizer region of the Xenopus can bind BMP and prevent BMP from binding to its cognate receptors [13]. In addition to the requirement of noggin for normal skeletogenesis [14], noggin plays an important role in tooth development, because application of noggin to embryonic tooth bud explants can transform tooth type from incisors to molars [15]. Furthermore, noggin gene transfer to periodontal wounds has been shown to inhibit alveolar bone repair [11].
Therefore, to investigate further the specific role of BMP signaling on cementogenesis, an ex vivo SCID mouse model was utilized. Cloned cementoblasts were transduced in vitro using recombinant adenoviruses encoding BMP-7 (Ad-BMP-7), noggin (Ad-NOGΔB2), or a control adenovirus encoding green fluorescent protein (Ad-GFP). The transduced cells were subsequently seeded onto 3D biodegradable polymer scaffolds and then implanted subcutaneously into the dorsa of SCID mice for 3 and 6 weeks. The results indicated that BMP-7 gene transfer had a minimal impact on cementoblast-induced mineralization, whereas noggin gene delivery blocked cementoblast differentiation and subsequent mineralization in polymer scaffolds in vivo.
MATERIALS AND METHODS
Cell Isolation and Immortalization
These studies were performed according to protocols approved by the University of Michigan Unit for Laboratory and Animal Medicine. A murine first molar was used to isolate root-lining cells (cementoblasts). OC-TAg mice were used to obtain cementoblasts, which contain a transgene that consists of the protein-coding region of SV-40 large T- and small t-antigens, under control of the rat osteocalcin gene promoter [16]. The presence of the osteocalcin promoter driving the transgene ensures that cells expressing osteocalcin (for our purposes, cementoblasts) are preferentially immortalized. To isolate cementoblasts, day 40–42 (day 0 = vaginal plug, day 19 = birth) OC-TAg mice were selected based on our previous data in situ, and in vitro [16], which indicated that as the roots are starting to develop, cells along the root surface, cementoblasts, express high levels of cementoblast markers: bone sialoprotein (BSP) and osteocalcin (OCN) mRNA, while PDL cells do not express these genes. Expression of these genes by cementoblasts, but not PDL cells, in vitro was used to confirm isolation of appropri-ate cell populations [16]. To isolate cementoblasts, mandibulae were dissected from surrounding tissues, hemisected by incision through the midline symphysis, and first molars were extracted using a dissecting microscope. Molars were removed by cutting into the follicle or periodontal ligament region. To release cells, molars with surrounding tissues were digested using collagenase/trypsin solution. After enzymatic digestion to release cells, OCCM cells were subcloned, and selected clonal populations, expressing OCN and BSP, were used for these studies.
Gene Transfer for In Vitro Analysis
The procedures for gene transfer of cementoblasts have been published previously [17]. Briefly, cloned cementoblasts were transduced with one of three different recombinant adenoviruses driven by the cytomegalovirus promoter, encoding green fluo-rescent protein (Ad-GFP) (kind gift of Genzyme, Cambridge, MA, USA); bone morphogenetic protein-7 (Ad-BMP-7, kind gift of Drs. R. B. Rutherford and R.T. Franceschi, University of Michigan); and noggin devoid of the heparin binding site (Ad-NOGΔB2, Regeneron Pharmaceuticals, Tarrytown, NY, USA) [18]. Cementoblasts were transduced at a multiplicity of infection (MOI) of 100 in serum-free Dulbecco’s modified Eagle medium (DMEM) (Gibco BRL, Life Technologies, Grand Island, NY, USA) for 5 hr while shaking, and then the same volume of DMEM supplemented with 4% FBS was added and measured by flow activated cell sorting (FACS) to determine transduction efficiency. After 24 hr incubation in a humidified atmosphere of 5% CO2 in air at 37°C, the medium was changed to DMEM containing 10% FBS for an additional 24 hr. For the final 24 hr, the medium was changed to serum-free DMEM and then it was collected for analysis by Western blotting, while cells were used to extract RNA for analysis by Northern blotting. Transduced cementoblasts seeded onto polymer scaffolds are described below.
Western Blotting to Determine BMP-7 and Noggin Release
The serum-free media (above) were dialyzed against distilled water containing proteinase inhibitors and lyophilized. The lyophilized samples were dissolved in 50 µl SDS-PAGE loading buffer (2% SDS, 4M urea, 5% β-mercaptoethanol, 7% glycerol, 10 mM Tris-HCl, 0.002% bromophenol blue pH 6.8). Then 50 µl of each sample were fractionated by SDS-PAGE on 15% gels for BMP-7 detection, while 1 µl of each sample was fractionated by the same gel for noggin detection. As positive controls 5 Ng of recombinant human BMP-7 or recombinant human noggin were used. The fractionated proteins in the gels were then electrophoretically transferred to Immun-Blot™ PVDF Membranes (Bio-Rad Laboratories, Hercules, CA, USA). Rabbit anti-BMP-7 antibody (Kind gift of D. Rueger, Stryker Biotech, Hopkinton, MA, USA) was used at a dilution of 1:4000 and secondary antibody of horseradish peroxidase-conjugated goat antirabbit immunoglobulin G was used at a dilution of 1:35,000. Rat antihuman noggin antibody (RP57-16; Regeneron) was used at a concentration of 20 ng/ml and a secondary antibody of horseradish peroxidase-conjugated goat antirat immunoglobulin G was used at a dilution of 1:12,000. Immunoreactivity was determined using an ECL™ kit (Amer-sham Pharmacia Biotech, Piscataway, NJ, USA).
RNA Extraction and Northern Blotting
Total cellular RNA from OCCM cells, cultured in vitro for 3 days after transduction by the three different adenoviruses at MOI of 100, was extracted using a modified guanidine thiocyanate procedure with Trizol reagent. Then 10 µg of total RNA was denatured, fractionated on 6% formaldehyde/1.2% agarose gels, transferred onto nylon membranes (Duralon-UV, Strata-gene, LA Jolla, CA, USA), and immobilized using Stratalink (Stratagene). Blots were then hybridized with 32P-labeled osteocalcin and bone sialopreotein probes, generated from randomly primed cDNA (Rediprime, Amersham, Arlington Heights, IL, USA) and exposed to Kodak X-OMAT film with intensifying screens at −70°C for 6–24 hr. To evaluate relative loading of RNA samples, 18S was used as a standard. Mouse BSP cDNA (kind gift from Dr. M. Young) was 1-kb polymerase chain reaction (PCR) product of mouse BSP inserted in PCR II vector, whereas mouse OCN cDNA was obtained from pS65 cloning vector containing mouse OCN cDNA (kind gift from Dr. J. Wozney).
PLGA Scaffold Fabrication
Poly DL-lactic-co-glycolic acid (PLGA) 3D scaffolds were processed into porous foams by an established solvent-casting, particulate-leaching technique as described previously [19]. These composites were cut into 5×5×2 mm blocks, sterilized with ultraviolet light, and stored until use. The resultant PLGA blocks were porous scaffolds containing 95% porosity and pore sizes ranging from 250–425 µm.
In Vivo Ectopic Model of Mineral Neogenesis
The procedures for seeding and implantation have been previously reported in detail [20]. In brief, 1 million OCCM cells were transduced in vitro at an MOI of 100 with one of three adenoviruses as above (Ad-GFP, Ad-NOGΔB2, or Ad-BMP-7) or without adenovirus transduction (NT). The infected cells were seeded onto each PLGA scaffold and incubated in vitro for 24 hr in DMEM at 37°C. Next, PLGA blocks were inserted into surgical pockets created in immunodeficient (SCID) mice dorsa under general anesthesia using methoxyfluorane. Four blocks were placed in each animal and the implants were harvested at 3 and 6 weeks. For histological analysis (n = 3 animals/each time point/group), the cell-PLGA implants were fixed in 10% neutral buffered formalin and embedded in paraffin. The paraffin blocks were cut into 4 or 5-µm thick sections and stained with hematoxylin and eosin An additional 3 cell-PLGA implants were retrieved from SCID mice at 3 and 6 weeks, and immediately snap frozen in liquid N2, ground into small pieces, and RNA extracted using Trizol reagent (the samples from each group were pooled to allow for Northern blot analysis).
Measurement of Sustained Transgene Expression
Reverse transcription (RT) PCR was performed to determine the prolonged expression of the transgene GFP by adenovirus infection in vivo. After total RNA, extracted from PLGA-cell implants at 3 and 6 weeks, was digested with DNase I (Gibco BRL, Gaithersburg, MD, USA), 1 µg RNA from each specimen was reverse transcribed using a Retroscript kit (Ambion, Austin, TX, USA). A primer pair was specifically designed to determine the expression of GFP. The forward primer of the GFP gene was 5′-TACGGCGTGCAGTGCTT-3′.The reverse primer was 5′-TCGGCCATGATATAGAC-3′. The housekeeping gene, β-actin, was used to assess the loading of the samples. A total of 25 µl of PCR reaction was mixed with 5 µl of RT product, PCR buffer (10 mM Tris –HCl, pH 9.0, 50 mM KCl, and 0.1%Triton X-100), 0.2 mM dNTPs, 2.5 mM MgCl2, 20 pM of each primer, 0.5 Unit of Taq DNA polymerase (Promega, Madison, WI, USA) using Perkin Elmer thermocycler 9600 (Perkin Elmer, Norwalk, CT, USA). The PCR conditions for detecting the expression of adenoviruses encoding GFP were 95°C for 5 min, 40 cycles of 95°C for 1 min, 50°C for 1 min, followed by 72°C for 2 min. The expected PCR product was 265 bp for GFP and 248 bp for β-actin.
Histomorphometric Analysis of Mineral Neogenesis
Sections were cut in the central region of the specimens obtained from SCID mice at 3 and 6 weeks and coded. Then images of sections were captured at a 2X and 4X magnification, using a Nikon Eclipse E800 microscope (Nikon, Melville, NY, USA) fitted with a SPOT-2 Camera (Diagnostic Instruments, Sterling Heights, MI, USA). Image Pro Plus™ software (Media Cybernetics, Silver Spring, MD, USA) was used for histomor-phometric analysis. The specimen circumference was measured to obtain the implant total cross-sectional area, while the mineral regions were selected by thresholding mineralized tissue to determine mineralized area by a single calibrated examiner. The mineral density was displayed as the ratio of the mineral area to total area of each specimen. The coded specimens were then analyzed using an ANOVA and a Fisher’s protected least significant difference (PSLD) multiple comparison procedure to measure statistical differences among groups.
RESULTS
Efficiency of Gene Transfer
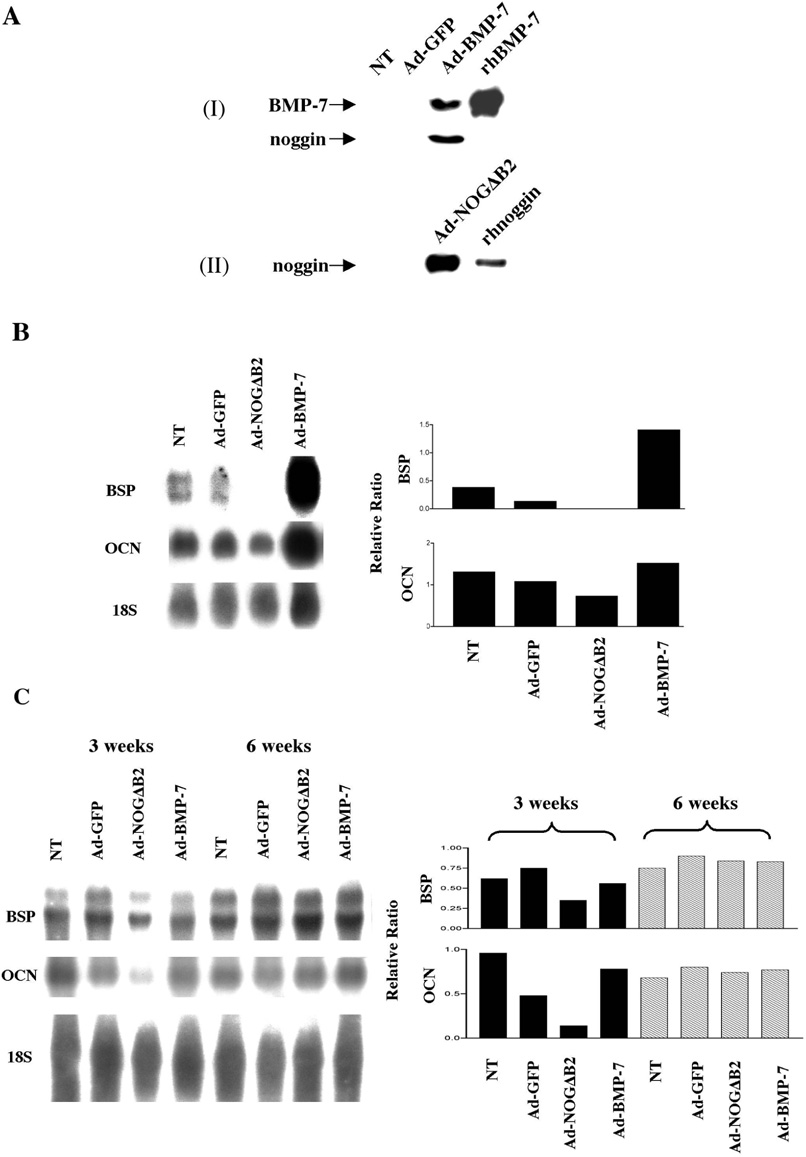
To choose the appropriate MOI for maximal protein release from OCCM cells, a time course by FACS was performed using the control virus Ad-GFP. FACS revealed initial GFP fluorescence as early as 6–8 hr after Ad-GFP exposure, with >95% of the cells exhibiting GFP protein by 24 hr after transduction using an MOI = 100 (data not shown). OCCM cells were cultured for 3 days after transduction with Ad-BMP-7, Ad-NOGΔB2, or Ad-GFP at an MOI of 100. OCCM cells without any adenovirus transduction (NT) were used as an additional control. The recombinant protein produced by each adenovirus released into the culture medium was determined by Western blot analysis. Detectable levels of BMP-7 and noggin were found only in OCCM cells transduced with Ad-BMP-7 and Ad-NOGΔB2, respectively, whereas cells transduced with Ad-GFP or NT failed to reveal measurable levels of BMP-7 and noggin protein (Figure 1A). Furthermore, OCCM cells transduced by Ad-BMP-7, in addition to producing BMP-7, also exhibited increased expression of noggin protein (Figure 1A).
Figure 1.
In vitro production of BMP-7 and noggin by adenoviral gene transfer and effects of prolonged delivery of BMP-7 and noggin on gene expression of bone sialoprotein (BSP) and osteocalcin (OCN) in OCCM cells in vitro and in vivo. (AI)OCCM cells transduced in vitro by Ad-BMP-7 produced immunodetectable BMP-7 and noggin. No measurable BMP-7 or noggin protein was found in NT and Ad-GFP-treated samples. (AII) OCCM cells transduced by Ad-NOGΔB2 secreted recombinant noggin into the culture medium. Furthermore, noggin was not detected in the conditioned media of OCCM cells in Ad-GFP and NT groups. (B) 72 hr following gene transfer of Ad-BMP-7, Ad-NOGΔB2, Ad-GFP, or NT to OCCM cells, BSP and OCN expression were examined by Northern blotting (B, left panel). The density of each band was measured by image analysis, and the values normalized against 18S rRNA (B, right panel). BMP-7 gene transfer increased BSP gene expression of OCCM cells by ~3-fold, compared with NT and Ad-GFP, while Ad-NOGΔB2 gene transfer inhibited BSP gene expression. OCN gene expression was modestly enhanced by Ad-BMP-7 gene transfer. Ad-NOGΔB2 gene transfer decreased OCN expression by ~50% compared with controls. (C) In vivo BSP and OCN expression in OCCM-PLGA implants at 3 and 6 weeks examined by Northern blotting. BSP and OCN expressions by Ad-NOGΔB2 treatment at 3 weeks were downregulated by ~2- and >3.5-fold, respectively, when compared with NT and Ad-GFP groups, while no differences in BSP and OCN gene expression were noted at 6 weeks.
Effects of BMP-7 and Noggin Gene Transfer on Cementoblast Differentiation
In Vitro
Total RNA extracts from OCCM cells, transduced by Ad-BMP-7, Ad-NOGΔB2, or Ad-GFP in vitro for 3 days, were analyzed for expression of putative cementoblast/osteoblast markers: OCN and BSP by Northern blotting. As shown in Figure 1B, BMP-7 gene transfer increased BSP transcripts by 2–3-fold, compared with NT or Ad-GFP groups. Ad-NOGΔB2 gene transfer downregulated BSP gene expression markedly to undetectable levels. Ad-NOGΔB2 gene transfer resulted in a ~50% decrease in OCN gene expression, while Ad-BMP-7 treatment enhanced OCN message only slightly when compared with NT and Ad-GFP groups (Figure 1B). Ad-GFP treatment decreased BSP expression by ~2-fold and had no obvious effect on OCN expression, compared with NT (Figure 1B).
In Vivo
Transcripts for OCN and BSP also were determined in implants retrieved at 3 and 6 weeks. At 3 weeks postgene transfer and cell implantation, Ad-NOGΔB2 treatment downregulated BSP expression by ~2-fold and OCN expression >3.5-fold when compared with NT and Ad-GFP groups parallels in vitro results. However, by 6 weeks, no significant alterations in BSP or OCN gene expression were noted (Figure 1C). Interestingly, BMP-7 gene transfer had no significant effect on either OCN or BSP gene expression at either 3 or 6 weeks, compared with NT and Ad-GFP groups. Ad-GFP treatment decreased OCN expression by ~2-fold and had no significant effect on BSP expression at 3 weeks. At 6 weeks, Ad-GFP has no effect on BSP and OCN gene expression when compared with NT (Figure 1C).
Sustained Transgene Expression in OCCM-Polymer Implants
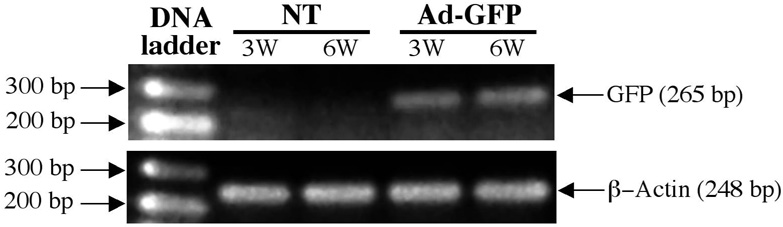
Implant specimens retrieved at 3 and 6 weeks demonstrated prolonged expression of GFP as analyzed by RT-PCR (Figure 2). No such expression was detectable in the NT, Ad-NOGΔB2, or Ad-BMP7 groups (data not shown).
Figure 2.
RT-PCR analysis of prolonged transgene expression in vivo. RNA extracted from each implant group was subjected to RT-PCR with primer pairs specifically designed for detecting the expression of the GFP, in comparison to β-actin at 3 and 6 weeks. PCR products were analyzed on an ethidium bromide-stained 1.5% agarose gel. Transcripts for GFP were noted up to 6 weeks and were not detected in NT group (n = 3 animals/group).
Descriptive Histologic and Histomorphometric Analysis
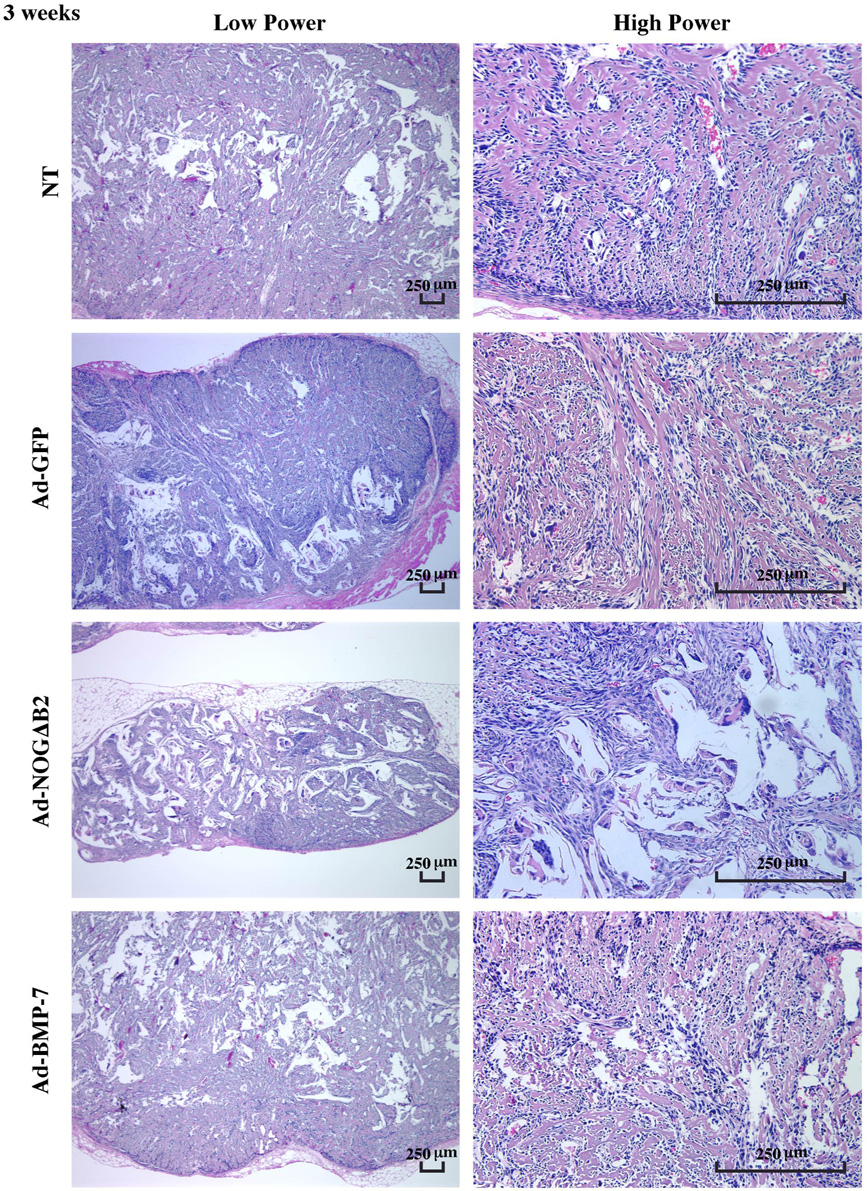
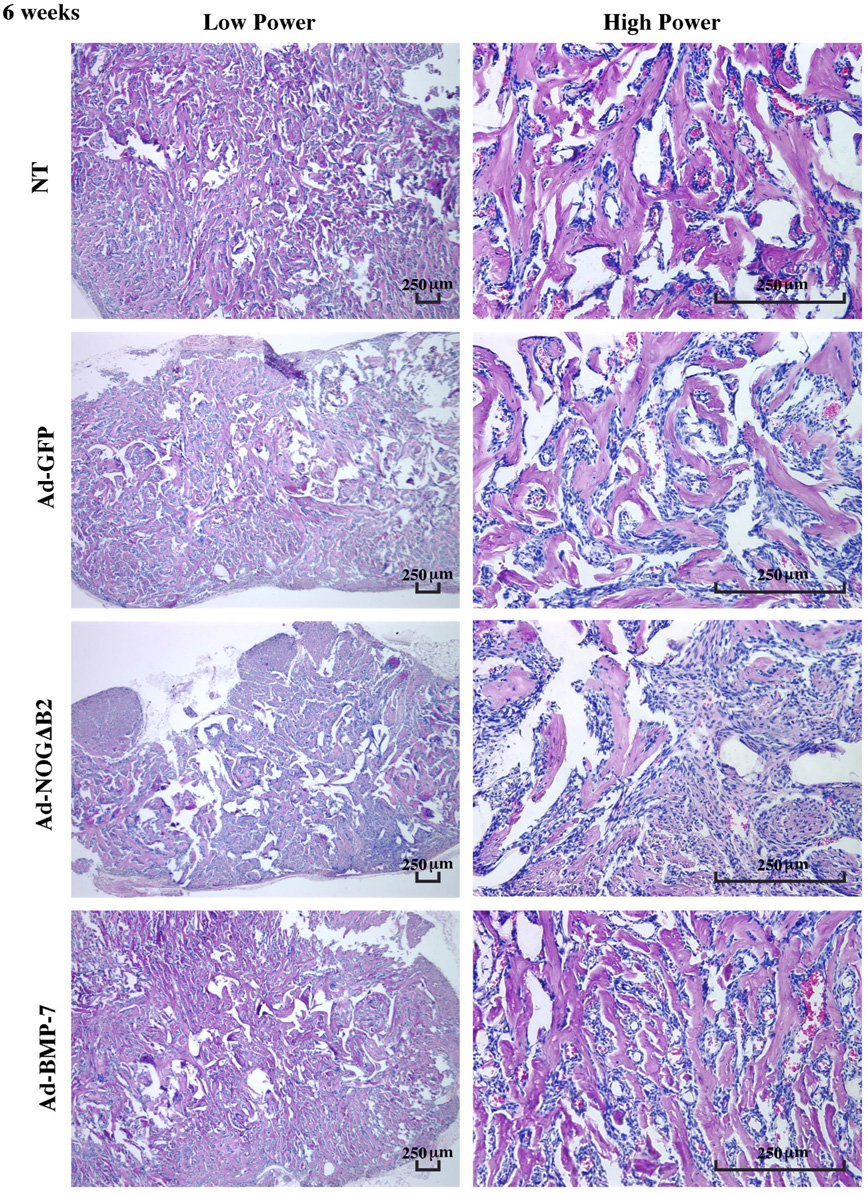
In the specimens retrieved from SCID mice at 3 and 6 weeks, mineral formation was found in all groups (Figure 3 and Figure 4). The mineralized tissues were, in general, woven in appearance. Mineral spicules became progressively denser and lamellar from 3 to 6 weeks (Figure 3 and Figure 4). The majority of the mineralized tissues in the Ad-NOGΔB2–treated implants were localized at the peripheral regions of implants at 3 weeks (Figure 3), while the arrangement of mineral spicules at 6 weeks was more evenly distributed, although remained less dense as compared with other groups (Figure 4). Although no bone marrow-like tissue was observed at 6 weeks, there was significant evidence of vascular-ization throughout the mineralized tissues (Figure 4).
Figure 3.
Effect of BMP-7 and noggin gene transfer on cementoblast-induced mineralization in polymer scaffolds at 3 weeks. Left panels display low power photomicrographs, whereas the right panels show high power images of OCCM cell-polymer implants treated by BMP-7 or noggin gene transfer. Mineral spicules were formed in all groups and were covered with spindle-like cells. However, implants retrieved from Ad-NOGΔB2–treated OCCM cells exhibited a paucity of mineral induction. There was vascularization among the mineral spicules; however, no bone marrow-like tissue was formed in any group (H&E staining; bar = 250 µm; n = 3 animals/group).
Figure 4.
Effect of BMP-7 and noggin gene transfer on mineralization in polymer scaffolds in vivo at 6 weeks. Left panels display low power photomicrographs; right panels show high power images. The mineral spicules became denser and more lamellar from 3–6 weeks. Noggin gene transfer decreased mineral area and implant size, while BMP gene transfer had minimal effects on mineralization compared with GFP treatment. Vascularization was formed among the mineral spicules. No bone marrow-like tissue was noted (H&E staining; bar = 250 µm; n = 3 animals/group).
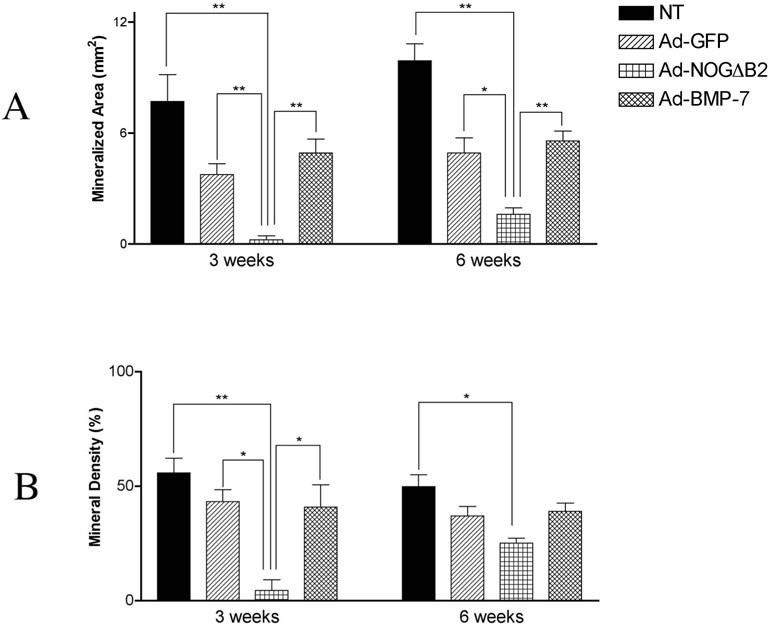
Ad-NOGΔB2 treatment of cementoblasts resulted in a potent decrease in mineralized tissue area by >16-fold and >3-fold at 3 weeks and 6 weeks, respectively when compared with NT and Ad-GFP groups (Figure 5). Ad-NOGΔB2 transduction of OCCM cells in polymer implants resulted in reductions in mineral density by >13-fold and ~2-fold at 3 weeks and 6 weeks, respectively, when compared with NT and Ad-GFP groups (Figure 5). Ad-BMP-7 treatment of cementoblasts seeded in PLGA scaffolds had no significant effect on mineralized area or mineral density at 3 and 6 weeks as compared with either NT or Ad-GFP groups (Figure 3– Figure 5). Furthermore, there were no significant differences at 3 and 6 weeks between NT and Ad-GFP groups for either mineralization area or mineral density (Figure 5).
Figure 5.
Histomorphometric analysis of mineralized tissue area and density of the PLGA-cell implants at 3 and 6 weeks following gene transfer. Histomorpho-metric analysis was performed to measure. (A) Mineralized area of implants at 3 and 6 weeks. Implants from Ad-NOGΔB2 treatment of OCCM cells resulted in a reduction in mineral area compared with NT and Ad-GFP groups (*p < .05, **p < .01), while Ad-BMP-7 treatment had no effect on the induction of mineral. (B) Mineral density of implants from each group at 3 and 6 weeks. The mineral density of Ad-NOGΔB2 treated specimens were greatly reduced at 3 weeks compared with NT and Ad-GFP groups (*p< .05,** p <.01), at 6 weeks, Ad-NOGΔB2 treatment demonstrated slightly reduced mineral density when compared with NT (**p < .01), although it was not different compared with the implants from Ad-BMP-7 or Ad-GFP gene transfer.
DISCUSSION
Cementoblasts are considered to originate from dental follicle cells during root development, or from periodontal cell precursors in the paravascular and endosteal areas of contiguous alveolar bone [21]. The differentiation of these mesenchymal cells into cementoblasts appears to require trigger factors such as BMPs. BMP-2 was shown to induce dental follicle cell differentiation into cementoblasts/osteoblasts in vitro [8]. BMP-2 and BMP-7 promote osteogenesis and cementogenesis in critical size periodontal defects in dogs [9, 22]. Although BMP’s effects on mesenchymal cell differentiation into hard tissue-forming cells (e.g., osteoblasts, cementoblasts, and odontoblasts) have been studied widely, the impact of BMPs and their antagonists on mature cementoblasts is not completely understood. Results of our studies indicate for the first time that BMP-7 gene delivery had minimal effects on mature cementoblasts in terms of modulation of mineral neogenesis, whereas its antagonist noggin inhibited mature cementoblasts from promoting mineralization in vivo.
In spite of many of the positive results reported with the use of BMPs for periodontal regeneration, varying degrees of ankylosis have been noted [22]. Therefore, the balance of BMP signaling and antagonist regulation appears to be important in the maintenance of the cementum-PDL-bone interface. Ad-BMP-7 induced noggin production in cementoblasts, which also may occur during tooth development and repair. This phenomenon has been noted in rat osteoblasts, the mesodermal pluripotent cell line C1, and in BMP-induced skeletogenesis [23, 24]. More recently, when dermal fibroblasts were transduced with Ad-BMP-7 and then transplanted to periodontal defects in rats, these cells not only expressed BMP-7 genes and corresponding protein, but also increased gene and protein expression of noggin [11]. Talwar et al. reported that both rapid and slow release of BMP did not contribute to the union of bone to the root surface [25]. They speculated that the preservation of the periodontal ligament space appears to be a function of intrinsic factors (possibly controlled by BMPs) and occlusal function. Therefore, BMP activation of noggin expression may serve as a regulatory mechanism to maintain appropriate cementogenesis and periodontal ligament formation without mineralized tissue anchoring directly from the bone to the tooth surface.
Noggin is a secreted glycoprotein that binds to selected BMPs and blocks the binding of BMP-2, -4, and -7 to cell surface receptors [13]. Noggin not only prevents stromal cell differentiation in vitro [26], but also inhibits BMP-2–induced alkaline phosphatase activity in C2C12 cells in vitro and membranous ossification in vivo [27]. The results in noggin gene transfer are more potent than those shown by Zhao et al. for inhibition of BSP transcripts in vitro; however, this effect may be due to the continuous delivery of noggin [28]. In the present study, noggin downregulated BSP and OCN gene expression of cementoblasts both in vitro and in vivo, and inhibited mineralization by cementoblasts, while it did not affect cementoblast proliferation or cell survival (data not show). These results further support the importance of BSP and OCN in the mineralization of extracellular matrix [29]. However, endogenous BMPs and corresponding receptors (BMP-2, -3, -4, -6, and –7, and BMP-RI and BMP-RII are expressed by OCCM cells; data not shown) are required for cementoblasts to induce mineral formation given the results of noggin’s inhibition of cementogenesis. Therefore, BMP is not only a trigger for mesenchymal precursor cell differentiation into cementoblast or osteoblasts, but it also appears to contribute to normal function of mature cementoblast populations.
Gene expression of recombinant adenoviruses in vivo is transient in immunocompetent animals, while more sustained in immunodeficiency [30]. Therefore, our study likely resulted in a longer expression pattern of transgene expression although the viruses are eventually eliminated by the host via liver excretion (Figure 2). Our 3-week results demonstrated no significant alterations in BSP and OCN expression by Ad-BMP-7, while Ad-NOGΔB2 decreased BSP and OCN gene expression in vivo. One plausible explanation is that since endogenous BMP exhibits maximum effects on OCCM biomineralization, exogenous BMP does not appreciably affect OCCM-mediated mineralization. In support of this theory, Zhao et al. showed that recombinant human BMP-2 had no effect on Cbfa1 transcripts from cultured OCCM cells [28]. However, by 6 weeks, no significant differences in the expression of these genes were noted among the groups. Nevertheless, the earlier alterations in gene expression most likely accounted for the continued morphologic changes observed among groups at 6 weeks. But Ad-GFP was noted to mildly affect BSP and OCN expression in vitro and in vivo compared with NT (Figure 1B and Figure 1C); however, Ad-GFP did not affect mineralization as measured by change in area or density (Figure 5).
SUMMARY
Our study demonstrated that cloned cementoblasts seeded into polymer scaffolds and implanted in SCID mice induced mineral formation; gene expression of noggin in cementoblasts via adenoviral delivery leads to inhibition of mineralization; and prolonged gene transfer of BMP-7 has minimal effects on cementoblast-induced mineralization in this model. This latter result may prove to have significant clinical implication. There is a growing awareness that the effect of cell signaling molecules on cell function are very sensitive to stage of maturation, and our results support these findings. At the clinical level, these finding suggest that beyond identifying appropriate factors for promoting regeneration, we must consider the cell population, including differentiation stage and/or capacity, at the healing site. Future studies are needed to clarify the mechanisms of BMP signaling in the host local periodontal wound environment.
ACKNOWLEDGMENTS
The authors thank Jan Berry and Sarah Webb for their technical and surgical assistance. The authors also appreciate the expert histological assistance of Christopher Strayhorn. This study was supported by NIH/NIDCR grants DE 11960 (WVG), DE 13397(WVG & MJS),DE09532 (MJS & WVG), andDE13047 (MJS).
REFERENCES
- 1.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States 1988–1994. J. Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Saygin NE, Giannobile WV, Somerman MJ. Molecular and cell biology of cementum. Periodontol.2000. 2000;24:73–98. doi: 10.1034/j.1600-0757.2000.2240105.x. [DOI] [PubMed] [Google Scholar]
- 3.Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr. Pharm. Biotechnol. 2002;3:129–139. doi: 10.2174/1389201023378391. [DOI] [PubMed] [Google Scholar]
- 4.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 5.Reddi AH. Bone morphogenetic proteins: From basic science to clinical applications. J. Bone Joint. Surg. Am. 2001;83-A Suppl 1:S1–S6. doi: 10.2106/00004623-200100001-00001. [DOI] [PubMed] [Google Scholar]
- 6.Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev. Dyn. 1997;210:383–396. doi: 10.1002/(SICI)1097-0177(199712)210:4<383::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Amar S, Chung KM, Nam SH, Karatzas S, Myokai F, Van Dyke TE. Markers of bone and cementum formation accumulate in tissues regenerated in periodontal defects treated with expanded polytetrafluoroethylene membranes. J. Periodont. Res. 1997;32:148–158. doi: 10.1111/j.1600-0765.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M, Xiao G, Berry J, Franceschi R, Reddi A, Somerman M. Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J. Bone Miner. Res. 2002;17:1441–1452. doi: 10.1359/jbmr.2002.17.8.1441. [DOI] [PubMed] [Google Scholar]
- 9.Giannobile WV, Ryan S, Shih MS, Su DL, Kaplan PL, Chan TC. Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. J. Periodontol. 1998;69:129–137. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- 10.Ripamonti U, Heliotis M, Rueger DC, Sampath TK. Induction of cementogenesis by recombinant human osteogenic protein-1 (hop-1/bmp-7) in the baboon (Papio ursinus) Arch. Oral. Biol. 1996;41:121–126. doi: 10.1016/0003-9969(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 11.Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J. Periodontol. 2003;74:202–213. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massague J, Chen YG. Controlling TGF-beta signaling. Genes. Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 13.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 14.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 15.Tucker AS, Matthews KL, Sharpe PT. Transformation of tooth type induced by inhibition of BMP signaling. Science. 1998;282:1136–1138. doi: 10.1126/science.282.5391.1136. [DOI] [PubMed] [Google Scholar]
- 16.D’Errico JA, Berry JE, Ouyang H, Strayhorn CL, Windle JJ, Somerman MJ. Employing a transgenic animal model to obtain cementoblasts in vitro. J. Periodontol. 2000;71:63–72. doi: 10.1902/jop.2000.71.1.63. [DOI] [PubMed] [Google Scholar]
- 17.Giannobile WV, Lee CS, Tomala MP, Tejeda KM, Zhu Z. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J. Periodontol. 2001;72:815–823. doi: 10.1902/jop.2001.72.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Economides AN, Stahl NE, Harland RM inventors; Regeneron Pharmaceuticals, Inc. Modified noggin polypeptides and compositions. 6,075,007. U.S. patent. 2000
- 19.Mooney DJ, Sano K, Kaufmann PM, Majahod K, Schloo B, Vacanti JP, Langer R. Long-term engraftment of hepatocytes transplanted on biodegradable polymer sponges. J. Biomed. Mater. Res. 1997;37:413–420. doi: 10.1002/(sici)1097-4636(19971205)37:3<413::aid-jbm12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Jin Q, Zhao M, Webb SA, Berry JE, Somerman MJ, Giannobile WV. Cementum engineering using three-dimensional polymer scaffolds. J. Biomed. Mater. Res. 2003;67A:54–60. doi: 10.1002/jbm.a.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCulloch CA, Nemeth E, Lowenberg B, Melcher AH. Paravascular cells in endosteal spaces of alveolar bone contribute to periodontal ligament cell populations. Anat. Rec. 1987;219:233–242. doi: 10.1002/ar.1092190304. [DOI] [PubMed] [Google Scholar]
- 22.Sigurdsson TJ, Nygaard L, Tatakis DN, Fu E, Turek TJ, Jin L, Wozney JM, Wikesjo UM. Periodontal repair in dogs: Evaluation of rhBMP-2 carriers. Int. J. Periodont. Restor. Dent. 1996;16:524–537. [PubMed] [Google Scholar]
- 23.Nifuji A, Noda M. Coordinated expression of noggin and bone morphogenetic proteins (BMPs) during early skeletogenesis and induction of noggin expression by BMP-7. J. Bone Miner. Res. 1999;14:2057–2066. doi: 10.1359/jbmr.1999.14.12.2057. [DOI] [PubMed] [Google Scholar]
- 24.Nifuji A, Kellermann O, Noda M. Noggin expression in a mesodermal pluripotent cell line C1 and its regulation by BMP. J. Cell Biochem. 1999;73:437–444. [PubMed] [Google Scholar]
- 25.Talwar R, Di Silvio L, Hughes FJ, King GN. Effects of carrier release kinetics on bone morphogenetic protein-2-induced periodontal regeneration in vivo. J. Clin. Periodontol. 2001;28:340–347. doi: 10.1034/j.1600-051x.2001.028004340.x. [DOI] [PubMed] [Google Scholar]
- 26.Gazzerro E, Du Z, Devlin RD, Rydziel S, Priest L, Economides AN, Canalis E. Noggin arrests stromal cell differentiation in vitro( small star, filled) Bone. 2003;32:111–119. doi: 10.1016/s8756-3282(02)00948-1. [DOI] [PubMed] [Google Scholar]
- 27.Aspenberg P, Jeppsson C, Economides AN. The bone morphogenetic proteins antagonist Noggin inhibits membranous ossification. J. Bone Miner. Res. 2001;16:497–500. doi: 10.1359/jbmr.2001.16.3.497. [DOI] [PubMed] [Google Scholar]
- 28.Zhao M, Berry JE, Somerman MJ. Bone morphogenic protein-2 inhibits differentiation and mineralization of cementoblasts in vitro. J. Dent. Res. 2003;82:23–27. doi: 10.1177/154405910308200106. [DOI] [PubMed] [Google Scholar]
- 29.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 30.Wu JC, Sundaresan G, Iyer M, Gambhir SS. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol. Ther. 2001;4:297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]