Abstract
Carbonic anhydrase XII (CA XII) is a transmembrane enzyme that is associated with neoplastic growth. CA XII has been proposed to be involved in acidification of the extracellular milieu, creating an appropriate microenvironment for rapid tumor growth. Because RNA sequence databases have indicated that two isoforms of CA XII might exist in human tissues, and because alternatively spliced protein forms have been linked to aggressive behavior of cancer cells, we designed a study to evaluate the presence of the two forms of CA XII in diffuse astrocytomas, a tumor type known for its aggressive and often noncurable behavior. Reverse transcription PCR of tumor samples surprisingly revealed that CA XII present in diffuse astrocytomas is mainly encoded by a shorter mRNA variant. We further showed by Western blotting that anti-CA XII antibody recognized both isoforms in the glioblastoma cell lines, and we then evaluated the expression of CA XII in astrocytomas using immunohistochemistry and correlated the results with various clinicopathological and molecular factors. Of 370 diffusely infiltrating astrocytomas, 363 cases (98%) showed immunoreactions for CA XII. Importantly, CA XII expression correlated with poorer patient prognosis in univariate (p = 0.010, log-rank test) and multivariate survival analyses (p = 0.039, Cox analysis). From these results, we conclude that CA XII is commonly expressed in diffuse astrocytomas and that it might be used as a biomarker of poor prognosis. The absence of 11 amino acids in the shorter isoform, which seems to be common in astrocytomas, may affect the normal quaternary structure and biological function of CA XII.
Keywords: alternative splicing, astrocytoma, cancer, carbonic anhydrase, glioblastoma, prognosis
Alternative splicing, a process in which identical pre-mRNA molecules are spliced in different ways, is a fundamental mechanism in differential gene expression. Variations in expression and/or activity of splicing factors could lead to changes in the splicing patterns of certain mRNAs whose protein products are involved in different stages of tumor progression. The alternative splicing may affect cell growth, adhesion, migration, invasion, and apoptosis as well as the connections between signaling pathways, all important steps in tumorigenesis. Recently, there have been several reports on alternative splicing in different cancers and the effect of splicing on patient prognosis.1,2
Carbonic anhydrase XII (CA XII) is a transmembrane enzyme that was originally identified by the over-expression of its mRNA in human renal cancer cells.3,4 Since then, it has been associated with several human neoplasms, as has the other transmembrane isoenzyme, CA IX.5–10 Both CA XII and CA IX are regulated by similar mechanisms. They are both induced at the transcriptional level via the hypoxia-inducible factor-1 (HIF-1)–mediated pathway, which is activated in tumor cells by hypoxia or by a mutation in the gene encoding the von Hippel-Lindau (VHL) tumor-suppressor protein.4,11 High expression of CA IX and CA XII in certain tumors has supported the idea that these isozymes may functionally participate in the invasion process. Acidification of the extracellular milieu surrounding the cancer cells, a process in which CAs ultimately participate, creates a microenvironment that leads to activation of proteolytic enzymes and favors tumor growth and spread.4,12 In favor of this hypothesis, it has been shown in vitro that CA inhibitors can reduce the invasion capacity and/or proliferation of cancer cells.12,13
The information obtained from GenBank suggested that CA XII may have two alternative isoforms. The spliced mRNA was predicted to lack exon 9, which has 33 nucleotides (coding for 11 amino acid residues). Because these alternative isoforms had not been studied in any of the previous publications on CA XII, we wanted to investigate whether they are detectable in diffuse astrocytoma samples, which are known to represent a highly malignant tumor type with an extremely poor prognosis. The forward and reverse primers for the PCR reaction were designed in a way that the predicted PCR amplification product covered exon 9, and therefore the alternatively spliced isoforms became identifiable by the difference in the length of the PCR product. The expression of CA12 mRNA was analyzed from one normal brain sample, six diffuse astrocytomas (grades II–IV, two samples of each grade), and one hemangioblastoma by reverse transcription (RT)-PCR. We chose diffuse astrocytomas, because they are the most common highly malignant glial cell–derived brain tumors. They are difficult to remove surgically because of their infiltrating and diffuse growth pattern, and thus the prognosis of patients is still very poor.14,15
Materials and Methods
mRNA Analysis
Total RNA was extracted from seven brain tumors and one normal brain sample using the RNeasy Mini-Kit (Qiagen, Hilden, Germany). RT was performed with Moloney murine leukemia virus reverse transcriptase (Finnzymes, Espoo, Finland) using random primers (400 μg/ml). The primers for the PCR were designed using the published information on CA12 mRNA in Gen-Bank (accession nos. NM_001218 and NM_206925). These two accession numbers represent alternatively spliced CA XII isoforms. The primers were designed in a way that both spliced forms could be identified from the samples as separate bands. The forward primer (F1) was 5′-CAACTTCCGGCAGGTCCAGA-3′ (nucleotides 972–991 in NM_001218), and the reverse primer (R1) was 5′-TTGAGGTGTCGCAAGTGTCCAG-3′ (nucleotides 1287–1308 in NM_001218 and 1254–1275 in NM_206925). The predicted amplification products were 336 bp for isoform 1 and 303 bp for isoform 2. The control PCR reaction was performed with the following primers for human β-actin (accession no. NM_001101): forward primer (F2) 5′-CACGGCATCGTCACCA-ACTG-3′ (nucleotides 290–309) and reverse primer (R2) 5′-GCCTGGATAGCAACGTACATGGC-3 (nucleotides 464–486), producing an amplification product of 197 bp. The PCR was performed using 1.1× ReddyMix PCR Master Mix (Abgene, Epsom, UK), and 20 ng cDNA was used as a template in a 25-μl reaction. The PCR reaction was performed on a thermal cycler (PTC 200 Thermal Cycler, MJ Research, Inc., Waltham, MA, USA); the protocol consisted of a 94°C denaturation step for 1 min followed by 33 cycles of denaturation at 94°C for 30 s, annealing at 65°C for 30 s, and extension at 72°C for 90 s, followed by final extension at 72°C for 3 min. The results of the PCR reaction were analyzed using 1.5% agarose gel containing 0.1 μg/ml ethidium bromide with DNA standard (100 bp DNA Ladder, New England Biolabs, Beverly, MA, USA).
Because the RT-PCR results showed evidence of CA XII splicing variants in human astrocytomas, we further expanded our PCR studies to include three glioblastoma cell lines, two renal cancer cell lines, and two normal tissues (colon and kidney) that are known to express CA XII. Total RNA was extracted from the human glioblastoma cell lines U-87 MG, U-373 MG, and CCF-STTG1 (European Collection of Cell Cultures, Salisbury, UK). The renal cancer cell lines used for the study were Caki-1 and A-498 (American Type Culture Collection, Manassas, VA, USA). RNA was isolated using the RNeasy Mini-Kit, and reverse transcription was performed with the Fermentas First Strand cDNA Synthesis Kit (MBI Fermentas, St. Leon-Rot, Germany). The PCR reaction was performed as described for tumor samples with a few exceptions, as follows. The control PCR reaction was performed with the following primers for human β-2-microglobulin (NM_004048): forward primer (F3) 5′-TCCAGCGTACTCCAAAGAT-TCAGG-3′ (nucleotides 122–145), reverse primer (R3) 5′-ATGCGGCATCTTCAAACCTCC-3′ (nucleotides 431–451); the resulting PCR product was 330 bp. Two nanograms of cDNA was used as a template in the PCR reactions performed from the commercial cDNA panel (Human MTC panel I, BD Biosciences, Palo Alto, CA, USA). The results of the PCR reaction were analyzed using 1.5% agarose gel.
To confirm the presence of the alternatively spliced mRNA, PCR products from a grade IV astrocytoma sample (showing a double band) were purified from the gel with a GFX PCR DNA and Gel Band Purification Kit (Amersham Biosciences, Poole, UK). The sequencing was performed using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reactions Kit, version 3.1 (Applied Biosystems, Foster City, CA, USA). The sequencing was performed in both directions with primers F1 and R1. Purified PCR product (5 μl) was mixed with 2 μl Big Dye mix and 2 μl sequencing buffer (400 mM Tris-HCl, 10 mM MgCl2, pH 9.0), and 1.6 pmol primers was added. The reactions were amplified by cycle sequencing on a PTC 200 Thermal Cycler according to the manufacturer’s protocol. The products were purified by ethanol precipitation, resuspended in HiDi formamide (Applied Biosystems), and denatured according to the manufacturer’s instructions. The sequencing was performed with an ABI PRISM Genetic Analyser 9100 (Applied Biosystems).
Western Blotting
Western blotting was performed to evaluate the presence of CA XII isoforms in five cell lines: U-87 MG, CCF-STTG1, Caki-1, A-498, and a human renal cell carcinoma cell line (UMRC6; generously provided by Dr. Sergey V. Ivanov). The cells were cultured under normoxia for 4 days in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and isolated from the cell culture plates, and total cell homogenates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting as previously described.16
Study Materials for Immunohistochemistry
Because there have been no earlier reports on alternative spliced isoforms of CA XII, and because we found that malignant astrocytomas and cell lines derived from glioblastomas expressed mainly the shorter form of CA XII, we wanted to study a large series of diffuse astrocytomas and correlate the level of CA XII expression with clinicopathological features and tumor-relevant molecular factors, including CA IX and CA II, cell proliferation (Ki-67/MIB-1), p53, vascular endothelial growth factor (VEGF), and epidermal growth factor receptor (EGFR).
The study material consisted of 370 diffusively infiltrating astrocytic gliomas (grades II, III, and IV) obtained from patients who underwent surgery in Tampere University Hospital (Tampere, Finland) between 1983 and 2001. There were 287 primary tumors (39 grade II, 30 grade III, 218 grade IV) and 83 recurrences (15 grade II, 17 grade III, 51 grade IV). The tumors were radically resected when possible, and most patients with high-grade astrocytomas were also treated with radiotherapy. Ages of patients with primary tumors ranged between 3 and 82 years (median 55 ± SD 15 years), and ages of patients with recurrent tumors, between 3 and 75 years (median 44 ± SD 12 years). Overall survival was known for 287 patients (39 grade II, 30 grade III, and 218 grade IV), of whom only two were younger than 18 years. During the 5-year follow-up, 237 patients died and 50 patients were still alive.
After fixation in 4% phosphate-buffered formaldehyde, brain tumor specimens were processed into paraffin blocks. A neuropathologist (H.H.) evaluated the hematoxylin and eosin–stained slides on the basis of the WHO criteria,17 which divide diffusely infiltrating astrocytomas into three grades (II–IV). Evaluation was made according to the presence of atypia, mitotic activity, necrosis, and endothelial proliferation. Then samples from histologically representative tumor regions were placed into the recipient multitissue blocks, which were constructed with a custom-built instrument (Beecher Instruments, Silver Spring, MD, USA). The diameter of the tissue cores was 600 μm.
Antibodies and Immunohistochemistry
Monoclonal antibody M75, which recognizes the N-terminal domain of human CA IX, has been described previously.18 The rabbit antihuman CA XII serum used for both immunohistochemistry and Western blotting has been characterized by Karhumaa et al.16 The polyclonal antiserum was raised against the truncated form of recombinant human CA XII, and its specificity was confirmed under both denaturing and native conditions. The recombinant CA XII protein did not include the segment that is potentially deleted due to the alternative mRNA splicing process according to our present data. Therefore, the produced antibody should recognize both the intact CA XII and the spliced form. Rabbit antiserum against human CA II had been produced and characterized previously.19 Normal rabbit serum was used for control staining.
Immunohistochemical analysis for CA II, CA IX, and CA XII was performed as previously described.9,20,21 The stained sections were examined and photographed using a Zeiss Axioskop 40 microscope (Carl Zeiss, Göttingen, Germany). The intensity of the staining reaction for CA XII was scored from the sections using a four-category assessment: 0, no reaction; 1, weak reaction; 2, moderate reaction; 3, strong reaction. The extent of the CA XII staining was also scored on a scale from 0 to 3: 0, no positive cells; 1, <25% positive cells; 2, 25–50% positive cells; 3, >50% positive cells.
When simultaneous expression of CAs was evaluated in survival analysis, negative and weak stainings were considered CA-negative (CA −ve), and moderate and strong stainings were considered CA-positive (CA +ve), on each enzyme separately. Analyses on simultaneous expression of transmembrane CAs (CA XII and CA IX) were categorized as follows: 0, CA XII and CA IX both were CA −ve; 1, only one enzyme was CA +ve; 2, both enzymes were CA +ve. The variable obtained we refer to as the CA IX/XII index. Simultaneous expression of tumor-associated CAs (CA XII, CA IX, and CA II) was evaluated as follows: 0, all three enzymes were CA −ve; 1, one of the three enzymes was CA +ve; 2, two of the three enzymes were CA +ve; and 3, all three enzymes were CA +ve. This variable is called the CA II/IX/XII index.
Proliferation by Ki-67 (MIB-1) immunostaining,22,23 VEGF immunostaining,24 EGFR amplification by chromogenic in situ hybridization,25 and p53 immunostain-ing26 were performed as previously described.
Statistical Analysis
All statistical analyses were performed using SPSS 12.0 for Windows (SPSS, Chicago, IL, USA). The significance of associations was defined using the chi-square test, Mann-Whitney test, and Kruskal-Wallis test. The log-rank test and Kaplan-Meier curves were used in univariate survival analyses, and Cox multivariate regression analysis was used in the multivariate survival analyses.
Results
CA12 Variant mRNA Is Expressed in Astrocytic Tumors
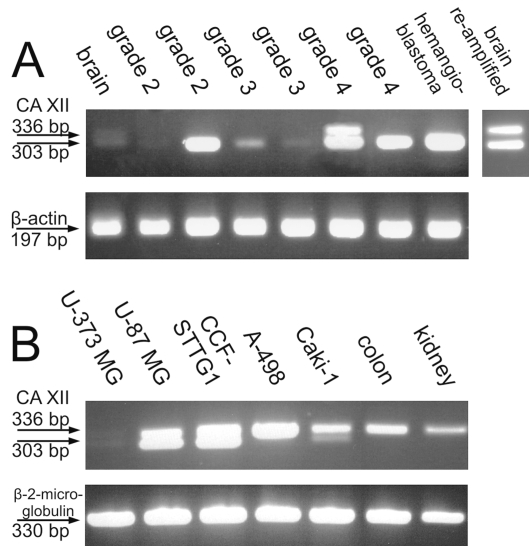
All astrocytoma samples except for one grade II tumor were positive for CA XII in the PCR reaction (Fig. 1). The two grade IV samples showed especially strong bands for CA12 mRNA. The hemangioblastoma sample served as a positive control for the PCR reaction, since defects in VHL protein often cause hemangioblastomas and also up-regulate the expression of CA XII.4 Also, the hemangioblastoma sample was positive for CA12 mRNA, as expected. The normal brain sample showed a very faint doublet, suggesting that it expresses both alternative isoforms. When it was reamplified with the original primers, the two bands became clearly visible. The tumor samples expressed mainly the shorter isoform of CA XII. Only one grade IV sample showed both bands. The bands from this sample were isolated from the gel and sequenced in order to confirm their identity. The sequencing results showed that these two bands indeed represented the two variants of CA12 mRNA whose sequences can be found in GenBank.
Fig. 1.
Reverse transcription PCR results showing the expression of CA12 mRNA in diffuse astrocytomas, hemangioblastoma, and normal brain (A) and in human tissues and cancer cell lines (B). The normal brain sample contains a faint double band representing alternatively spliced isoforms of carbonic anhydrase XII (CA XII; A). Hemangioblastoma, which served as a positive control, confirms that the PCR reaction has succeeded. The result of one grade II sample can be considered negative, whereas all the other astrocytoma samples contain CA12 mRNA. One grade IV sample contained a double band, but in all the other positive samples the shorter form of CA XII was expressed. The last lane in A shows reamplified PCR products from the normal brain. Normal human tissues (colon and kidney) expressed the longer isoform of CA XII (B). This was also the dominant form in renal carcinoma cell lines (A-498 and Caki-1). However, Caki-1 also produced the shorter isoform. Two of the glioblastoma cell lines expressed both isoforms, and one was almost negative (the gel contained a very faint double band that is barely detectable in the picture taken from the gel).
We also analyzed some additional samples to see how common the spliced form is in different tissues known to express CA XII, for example, in the normal kidney and colon.8,10 CA XII is also overexpressed in renal carcinomas and in several other tumors.3–5 Therefore, RT-PCR was performed for the normal human kidney and colon and two renal carcinoma cell lines (Caki-1 and A-498). We also examined three glioblastoma multiforme (grade IV astrocytoma) cell lines (U-373 MG, U-87 MG, and CCF-STTG1). The results are shown in Fig. 1B. Normal human kidney and colon produced only the longer isoform of CA XII. The A-498 renal carcinoma cell line also produced the longer isoform, whereas the Caki-1 cell line showed bands representing both isoforms. One glioblastoma cell line was almost negative for CA XII, while the other two cell lines expressed both isoforms of CA XII.
Our conclusion from the PCR results is that the normal human colon and kidney express only the longer isoform of CA XII. The longer isoform may be dominant also in renal carcinoma cell lines, although Caki-1 cells also contained a faint band representing the shorter isoform. The normal human brain used in our study weakly expressed both isoforms, whereas the brain tumors expressed greater quantities of the spliced isoform.
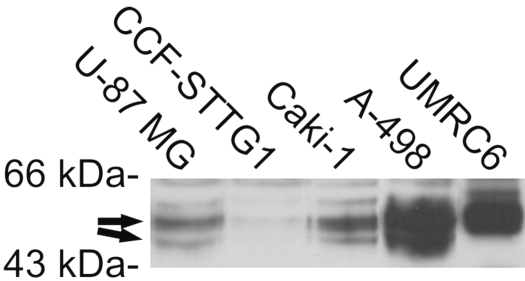
Western blotting of two glioblastoma and the Caki-1 cell lines suggests that both CA12 mRNA variants are translated into protein isoforms in these cell lines (Fig. 2). A VHL-defective cell line, A-498, showed an intense broad polypeptide band, which may include the shorter form in addition to the intact longer form of CA XII protein. Another cell line with VHL deletions, UMRC6, also showed an intense band, but only the longer isoform was evident.
Fig. 2.
Western blotting of carbonic anhydrase XII (CA XII) in glioblastoma and renal cancer cell lines. The arrows indicate the polypeptide bands representing the two isoforms of CA XII.
CA XII Protein Is Expressed in Most Astrocytic Tumors
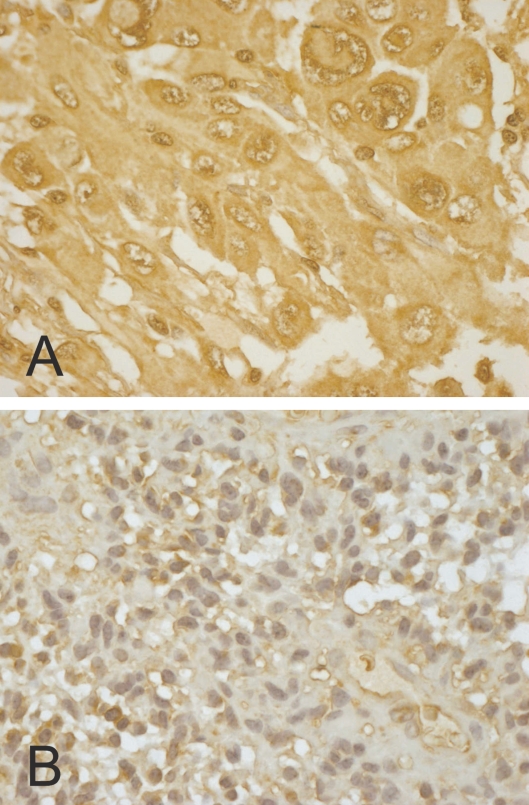
Because we found by RT-PCR and Western blotting the spliced isoform of CA XII in malignant astrocytomas and cell lines derived from glioblastomas, we wanted to study by immunohistochemistry whether CA XII is expressed in many or most astrocytomas, and whether the levels of CA XII expression correlate with clinico-pathological features and with tumor-relevant molecular factors in diffuse astrocytoma specimens. In our large series of diffusely infiltrating astrocytomas, 363 (98%) of 370 cases showed positive immunostaining for CA XII. When the tissue sections were evaluated by four-category assessment, CA XII intensities were as follows: 39 (11%) were strongly stained, 169 (46%) moderately stained, 155 (42%) weakly stained, and 7 (2%) cases were negative. The score for CA XII extent describing the relative area of the positive staining was found to be 3 (>50% positive cells) in 207 (56%), 2 (25%–50% positive cells) in 47 (13%), 1 (<25% positive cells) in 109 (29%), and 0 (no positive cells) in 7 (2%) cases. The distribution of CA XII immunostaining was usually quite homogeneous in tumor tissue (Fig. 3). In some cases, immunoreactivity was also observed in the nuclei and cytoplasm of tumor cells. The nuclear staining was considered unspecific. The cytoplasmic staining most probably represents newly produced enzyme and was taken into account in the present scoring method. When morphologically evaluated, neither endothelial proliferation nor necrosis correlated statistically with CA XII intensity (p = 0.264 and p = 0.619, respectively, chi-square test).
Fig. 3.
Immunohistochemical staining of carbonic anhydrase XII in two astrocytomas. A and B show strong and weak immunoreactions, respectively. Original magnification, ×400.
The present results indicate that the CA XII intensity was higher in tumors with higher malignancy grade (p = 0.006, chi-square test; Table 1). The association was significant even when the intensities were grouped as CA-positive and CA-negative tumors (p = 0.032, chi-square test). Increasing patient age and CA XII intensity significantly correlated with each other in all tumors combined (primary tumors and recurrences) (p = 0.022, variance analysis) as well as in primary tumors (p = 0.016, variance analysis). This finding may be related to the higher age of patients with the most malignant gliomas.
Table 1.
Comparison between carbonic anhydrase XII intensity and WHO grade (p = 0.006, chi-square test)
| WHO Grade
|
||||
|---|---|---|---|---|
| Carbonic Anhydrase XII Intensity | II | III | IV | Total |
| 0 | 4 | 0 | 3 | 7 |
| 1 | 28 | 22 | 105 | 155 |
| 2 | 19 | 23 | 127 | 169 |
| 3 | 3 | 2 | 34 | 39 |
| Total | 54 | 47 | 269 | 370 |
When we compared the typical molecular pathological features of astrocytomas with CA XII immunohistochemistry, we found no association between proliferation by Ki-67/MIB-1 and CA XII expression (359 cases, Mann-Whitney test). There was a significant association between positive CA XII intensity and positive VEGF status (322 cases, p = 0.032, chi-square test). Neither EGFR amplification (249 cases) nor p53 (141 cases) correlated with CA XII intensity (chi-square test).
CA XII Expression Correlates with Poor Prognosis
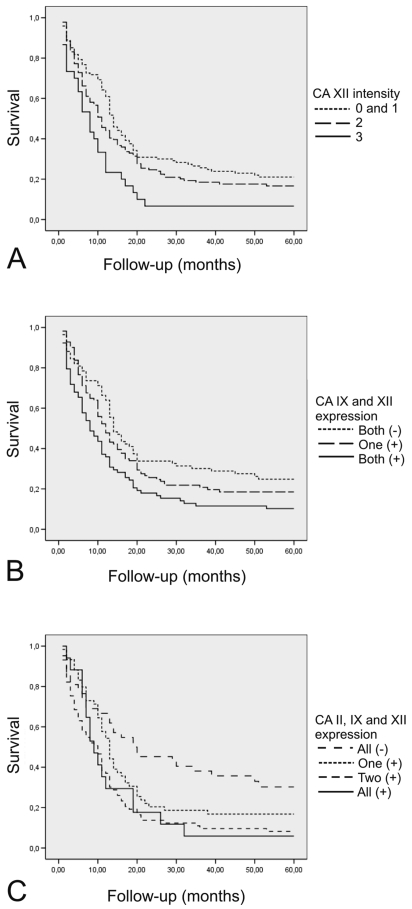
Overall survival data were known for 287 patients. When the patient survival was tested by the log-rank test in primary tumors, CA XII intensity divided the tumors into four significantly differing prognostic subsets (p = 0.010, log-rank test). The difference was even more significant when the cases with intensities 0 (negatively stained) and 1 (weakly stained) were pooled to make only three categories (p = 0.004, log-rank test) (Fig. 4A).
Fig. 4.
Survival of patients with diffuse astrocytomas grouped according to carbonic anhydrase immunostaining intensity. (A) Carbonic anhydrase XII (CA XII) expression. Reaction strength: 0, no reaction; 1, weak; 2, moderate; 3, strong. (B) Simultaneous expression of transmembrane CA IX and CA XII. (C) Simultaneous expression of tumor-associated CA II, CA IX, and CA XII.
The prognostic significance of CA XII intensity was also evaluated in a multivariate analysis when important clinicopathological factors such as patient age, WHO grade, and proliferation by MIB-1 were included in the analysis. Most important, Cox multivariate analysis revealed that patient age (p < 0.001; odds ratio, 1.959; 95% confidence intervals [95% CIs] for odds ratio, 1.629–2.356), tumor grade (p < 0.001; odds ratio, 2.202; 95% CI, 1.697–2.858), and CA XII intensity (p= 0.033; odds ratio, 1.250; 95% CI, 1.018–1.535) all had independent prognostic value.
The expression levels of other tumor-associated CA isozymes, CA II and CA IX, were recently studied in the same patients.20,21 In the present study, we evaluated both CA XII and CA IX intensities in parallel in 352 cases. When we compared the intensities of these enzymes, we found that CA XII positively correlated with CA IX intensity (p < 0.001, chi-square test). Simultaneous expression of CA XII and endothelial CA II was evaluated in 258 cases as well as in 260 cases between CA XII and cytoplasmic CA II, and no association was seen (p = 0.872 and p = 0.778, respectively, chi-square test). As described in “Materials and Methods,” CA IX/XII and CA II/IX/XII indices describing simultaneous expression of CA II, CA IX, and CA XII were established. The CA IX/XII index significantly correlated with the WHO grade of the tumors (p = 0.002, chi-square test). When the CA IX/XII index was evaluated by the log-rank test, it classified the tumors into three prognostically differing subsets (p = 0.003, log-rank test; Fig. 4B). Remarkably, the simultaneous expression of transmembrane CAs (CA XII and CA IX) was significant when evaluated in Cox multivariate analysis. The analysis revealed that patient age (p < 0.001; odds ratio, 1.992; 95% CI for odds ratio, 1.655–2.397), WHO grade (p < 0.001; odds ratio, 2.176; 95% CI, 1.674–2.829), and the CA IX/XII index (p = 0.048; odds ratio, 1.196; 95% CI, 1.002–1.427) had independent prognostic value. The prognostic significance of the CA II/IX/XII index was studied in 191 cases. This index resulted in four significant prognostic subsets of tumors (p = 0.002, log-rank test; Fig. 4C). However, the CA II/IX/XII index was not significant in multivariate analyses that included the abovementioned variables.
Discussion
The present studies were initiated when we found novel sequence data on an alternatively spliced CA XII isoform from GenBank. This information seemed important, because alternative mRNA splicing has been linked with oncogenic processes and found to correlate with patient survival.1,2 In addition, CA XII overexpression has been associated with several types of cancer, but its prognostic significance has not been evaluated thoroughly. The only published data available are from analyses of breast cancer patients, in whom CA XII expression was found to be associated with good prognosis.6
The present RT-PCR analyses were designed to evaluate whether an alternatively spliced variant of CA12 mRNA was detectable in astrocytoma samples. Normal human brain showed faint positive signals for both isoforms. All astrocytoma tumor samples except for one grade II sample contained the smaller, spliced isoform. Only one astrocytoma tumor sample and two glioblastoma cell lines contained the longer isoform in addition to the shorter one, whereas normal human kidney and colon contained only the longer isoform of CA XII. In conclusion, normal human tissues samples expressed mainly the longer isoform and little or none of the shorter isoform. In contrast, the shorter isoform of CA XII appeared to be the predominant form in the brain tumors examined.
The results obtained in the present study raise a question about the possible functional consequences related to the alternatively spliced CA XII transcript. Exon 9, which is missing from the shorter isoform, has 33 nucleotides and encodes for 11 amino acid residues. Thus, the alternative splicing does not alter the reading frame in this splicing variant. Interestingly, the shorter isoform lacks residues that are located adjacent to the predicted membrane-spanning helix on the extracellular side of the membrane (Fig. 5). The CA XII transmembrane domain contains the GXXXG and GXXXS motifs that have been considered to be important for the dimerization of the native enzyme.27–29 Importantly, the GXXXG motif is disrupted in the shorter isoform, and therefore these alternatively spliced protein isoforms could have different properties in the oligomerization of CA XII. The structural prediction by Motifscan software (http://scansite.mit.edu) suggests that the intracellular C-tail of CA XII contains a binding site for protein kinases C and A. Disruption of the GXXXG motif could potentially change the quaternary structure of dimeric CA XII protein and thereby affect the signaling cascades involving protein kinase–driven phosphorylation in the C-tail of CA XII molecule.
Fig. 5.
The complete sequence of carbonic anhydrase XII. The 11 amino acid residues missing in the shorter isoform are shown in boldface and italics. The GXXXG and GXXXS motifs are underlined. The transmembrane region was predicted with three programs (TMHMM [http://www.cbs.dtu.dk/services/TMHMM-2.0/], TMpred [http://www.ch.embnet.org/software/TMPRED_form.html], TopPred [http://bioweb.pasteur.fr/seqanal/interfaces/toppred.html]), and the N-terminal helix prediction of these programs differed slightly: the area that showed differences is shown in light gray, and the common transmembrane sequence prediction by these programs is shown in dark gray.
The alternative splicing event in the CA12 gene seems to be conserved during evolution. Messenger RNAs representing these alternatively spliced isoforms can be found in the mouse databases (e.g., accession no. BC035941 codes for isoform 1 and BC031385 for isoform 2). Interestingly, the mouse and human 9th exons do not contain the same amino acids, except for the GXXXG motif (GLSLG), which is exactly the same in both species. The conservation of alternative splicing and disruption of the GXXXG motif raise the possibility that these two isoforms may have important functional roles, which can be evaluated using mammalian expression systems.
According to our immunohistochemical results, CA XII is also expressed at the protein level in most diffuse astrocytomas, and increasing cellular immunopositivity was associated with higher WHO grade and older patient age. Previous studies have established that age is an important prognostic factor in patients with primary astrocytomas and brain metastases30–32 and is still considered in the assessment of treatment strategies.33 Elderly patients have a worse outcome, which has been shown in numerous retrospective, prospective, and epidemiological studies.17 Our study suggests that CA XII is another prognostic factor showing a positive correlation with patient age. Remarkably, in multivariate analyses, CA XII expression was an independent prognostic factor in addition to patient age and WHO grade.
Endothelial vascular proliferation due to angiogenic factors is one criterion that is used to distinguish grade IV astrocytomas from lower-grade tumors. We recently reported the induction of CA II expression in the tumor endothelium of diffuse astrocytomas.20 This phenomenon was also associated with poor prognosis. Thus, we wanted to evaluate the simultaneous expression of hypoxia-inducible CA XII and CA IX as well as CA II as prognostic indicators. Simultaneous expression of CA II, CA IX, and CA XII predicted an extremely poor prognosis for the patients, and the multivariate analysis further revealed that simultaneous expression of both CA XII and CA IX was an independent prognostic factor. According to our data, parallel evaluation of these isozymes using an immunohistochemical method could be of clinical value when predicting the prognoses of astrocytomas in patients. Most notably, the survival data indicated that CA XII itself was an independent prognostic factor in astrocytomas, which seemed to express predominantly the alternatively spliced CA12 mRNA. This finding agrees well with the previous observations that the mRNA splicing events are commonly associated with poor prognosis in cancer patients.1,2 Further studies are warranted to evaluate whether the presence of the spliced CA12 mRNA could be clinically useful as a prognostic marker in cancer diagnostics.
Acknowledgments
We thank Mrs. Aulikki Lehmus and Mrs. Reija Randen for skillful technical assistance. This work was supported by grants from the Cancer Society of Finland, the Sigrid Juselius Foundation, the Academy of Finland, the Finnish Medical Foundation, the European Union DeZnIT project, the Medical Research Fund of Tampere University Hospital, the Orion-Farmos Research Foundation, the AstraZeneca Foundation, and the Ida Montin Foundation.
References
- 1.Srebrow A, Kornblihtt AR. The connection between splicing and cancer. J Cell Sci. 2006;119:2635–2641. doi: 10.1242/jcs.03053. [DOI] [PubMed] [Google Scholar]
- 2.Venables JP. Unbalanced alternative splicing and its significance in cancer. Bioessays. 2006;28:378–386. doi: 10.1002/bies.20390. [DOI] [PubMed] [Google Scholar]
- 3.Tureci O, Sahin U, Vollmar E, et al. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci U S A. 1998;95:7608–7613. doi: 10.1073/pnas.95.13.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanov SV, Kuzmin I, Wei MH, et al. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci U S A. 1998;95:12596–12601. doi: 10.1073/pnas.95.21.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson PH, Chia SK, Wykoff CC, et al. Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br J Cancer. 2003;88:1065–1070. doi: 10.1038/sj.bjc.6600796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivela AJ, Kivela J, Saarnio J, Parkkila S. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. World J Gastroenterol. 2005;11:155–163. doi: 10.3748/wjg.v11.i2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kivela A, Parkkila S, Saarnio J, et al. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol. 2000;156:577–584. doi: 10.1016/S0002-9440(10)64762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kivela AJ, Parkkila S, Saarnio J, et al. Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol. 2000;114:197–204. doi: 10.1007/s004180000181. [DOI] [PubMed] [Google Scholar]
- 10.Parkkila S, Parkkila AK, Saarnio J, et al. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem. 2000;48:1601–1608. doi: 10.1177/002215540004801203. [DOI] [PubMed] [Google Scholar]
- 11.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 12.Parkkila S, Rajaniemi H, Parkkila AK, et al. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci U S A. 2000;97:2220–2224. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Supuran CT, Briganti F, Tilli S, et al. Carbonic anhydrase inhibitors: sulfonamides as antitumor agents? Bioorg Med Chem. 2001;9:703–714. doi: 10.1016/s0968-0896(00)00288-1. [DOI] [PubMed] [Google Scholar]
- 14.Boudreau CR, Yang I, Liau LM. Gliomas: advances in molecular analysis and characterization. Surg Neurol. 2005;64:286–294. doi: 10.1016/j.surneu.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Hou LC, Veeravagu A, Hsu AR, Tse VC. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20:E5. doi: 10.3171/foc.2006.20.4.2. [DOI] [PubMed] [Google Scholar]
- 16.Karhumaa P, Parkkila S, Tureci O, et al. Identification of carbonic anhydrase XII as the membrane isozyme expressed in the normal human endometrial epithelium. Mol Hum Reprod. 2000;6:68–74. doi: 10.1093/molehr/6.1.68. [DOI] [PubMed] [Google Scholar]
- 17.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 18.Pastorekova S, Zavadova Z, Kostal M, et al. A novel quasi-viral agent, MaTu, is a two-component system. Virology. 1992;187:620–626. doi: 10.1016/0042-6822(92)90464-z. [DOI] [PubMed] [Google Scholar]
- 19.Parkkila AK, Parkkila S, Juvonen T, Rajaniemi H. Carbonic anhydrase isoenzymes II and I are present in the zona glomerulosa cells of the human adrenal gland. Histochemistry. 1993;99:37–41. doi: 10.1007/BF00268018. [DOI] [PubMed] [Google Scholar]
- 20.Haapasalo J, Nordfors K, Jarvela S, et al. Carbonic anhydrase II in the endothelium of glial tumors: a potential target for therapy. Neuro-Oncology. 2007;9:308–313. doi: 10.1215/15228517-2007-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haapasalo JA, Nordfors KM, Hilvo M, et al. Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res. 2006;12:473–477. doi: 10.1158/1078-0432.CCR-05-0848. [DOI] [PubMed] [Google Scholar]
- 22.Sallinen PK, Haapasalo HK, Visakorpi T, et al. Prognostication of astrocytoma patient survival by Ki-67 (MIB-1), PCNA, and S-phase fraction using archival paraffin-embedded samples. J Pathol. 1994;174:275–282. doi: 10.1002/path.1711740407. [DOI] [PubMed] [Google Scholar]
- 23.Sallinen P, Haapasalo H, Kerttula T, et al. Sources of variation in the assessment of cell proliferation using proliferating cell nuclear antigen immunohistochemistry. Anal Quant Cytol Histol. 1994;16:261–268. [PubMed] [Google Scholar]
- 24.Soini Y, Salo T, Satta J. Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Hum Pathol. 2003;34:756–763. doi: 10.1016/s0046-8177(03)00245-4. [DOI] [PubMed] [Google Scholar]
- 25.Jarvela S, Helin H, Haapasalo J, et al. Amplification of the epidermal growth factor receptor in astrocytic tumours by chromogenic in situ hybridization: association with clinicopathological features and patient survival. Neuropathol Appl Neurobiol. 2006;32:441–450. doi: 10.1111/j.1365-2990.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 26.Haapasalo H, Isola J, Sallinen P, et al. Aberrant p53 expression in astrocytic neoplasms of the brain: association with proliferation. Am J Pathol. 1993;142:1347–1351. [PMC free article] [PubMed] [Google Scholar]
- 27.Whittington DA, Waheed A, Ulmasov B, et al. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc Natl Acad Sci U S A. 2001;98:9545–9550. doi: 10.1073/pnas.161301298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol. 2000;296:911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 29.Senes A, Gerstein M, Engelman DM. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J Mol Biol. 2000;296:921–936. doi: 10.1006/jmbi.1999.3488. [DOI] [PubMed] [Google Scholar]
- 30.Surawicz TS, Davis F, Freels S, et al. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–160. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 31.Lutterbach J, Bartelt S, Momm F, et al. Is older age associated with a worse prognosis due to different patterns of care? A long-term study of 1346 patients with glioblastomas or brain metastases. Cancer. 2005;103:1234–1244. doi: 10.1002/cncr.20895. [DOI] [PubMed] [Google Scholar]
- 32.Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 33.Laigle-Donadey F, Delattre JY. Glioma in the elderly. Curr Opin Oncol. 2006;18:644–647. doi: 10.1097/01.cco.0000245324.19411.19. [DOI] [PubMed] [Google Scholar]